The Role of Viral Infections in the Immunopathogenesis of Type 1 Diabetes Mellitus: A Narrative Review
Simple Summary
Abstract
1. Introduction
- Summarize the major viral pathogens implicated in T1DM;
- Explain proposed immunopathogenic mechanisms;
- Highlight key population studies and recent evidence.
2. Pathogenesis of T1DM
3. Viral Infections and Type 1 Diabetes (T1DM)
- Direct cytolysis: viral infection of pancreatic beta cells leading to functional impairment or lysis;
- Molecular mimicry: cross-reactivity between viral antigens and beta-cell autoantigens;
- Persistent infection and immune modulation: chronic low-grade viral presence that sustains immune activation or disrupts tolerance.
3.1. Direct Cytolysis: Enteroviruses—Coxsackie-B (CVBs), Mumps Virus, SARS-CoV-2
3.2. Molecular Mimicry: Coxsackie B Viruses, Rotavirus, Rubella, Influenza
3.3. Persistent Infection and Immune Modulation
4. Population Studies
5. Mechanisms of Virus-Induced T1DM
5.1. Direct Cytolysis
5.2. Persistent Infection
5.3. Molecular Mimicry and Sequence Homology
- The PEVKEK motif in Coxsackievirus B4 VP1 protein mimics an epitope in GAD65, a major autoantigen in beta cells [48];
- Rotavirus VP7 has shown homology with IA-2, another islet cell antigen implicated in T1DM [49].
5.4. Altered Antigen Presentation
5.5. Exosomes and Viral Antigen Transfer
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oikarinen, S.; Krogvold, L.; Edwin, B.; Buanes, T.; Korsgren, O.; Laiho, J.E.; Oikarinen, M.; Ludvigsson, J.; Skog, O.; Anagandula, M.; et al. Characterisation of enterovirus RNA detected in the pancreas and other specimens of live patients with newly diagnosed type 1 diabetes in the DiViD study. Diabetologia 2021, 64, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- The TEDDY Study Group the Environmental Determinants of Diabetes in the Young (TEDDY) study: Study design. Pediatr. Diabetes 2007, 8, 286–298. [CrossRef] [PubMed]
- Honeyman, M.C.; Coulson, B.S.; Stone, N.L.; Gellert, S.A.; Goldwater, P.N.; Steele, C.E.; Couper, J.J.; Tait, B.D.; Colman, P.G.; Harrison, L.C. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 2000, 49, 1319–1324. [Google Scholar] [CrossRef]
- Stene, L.C.; Oikarinen, S.; Hyöty, H.; Barriga, K.J.; Norris, J.M.; Klingensmith, G.; Hutton, J.C.; Erlich, H.A.; Eisenbarth, G.S.; Rewers, M. Enterovirus Infection and Progression from Islet Autoimmunity to Type 1 Diabetes. Diabetes 2010, 59, 3174–3180. [Google Scholar] [CrossRef]
- Morran, M.P.; Vonberg, A.; Khadra, A.; Pietropaolo, M. Immunogenetics of type 1 diabetes mellitus. Mol. Asp. Med. 2015, 42, 42–60. [Google Scholar] [CrossRef]
- Zorena, K.; Michalska, M.; Kurpas, M.; Jaskulak, M.; Murawska, A.; Rostami, S. Environmental Factors and the Risk of Developing Type 1 Diabetes—Old Disease and New Data. Biology 2022, 11, 608. [Google Scholar] [CrossRef]
- Christen, U.; Bender, C.; von Herrath, M.G. Infection as a cause of type 1 diabetes? Curr. Opin. Rheumatol. 2012, 24, 417–423. [Google Scholar] [CrossRef]
- Coppieters, K.T.; Boettler, T.; von Herrath, M. Virus Infections in Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2011, 2, a007682. [Google Scholar] [CrossRef]
- Dahl-Jørgensen, K. Virus as the cause of type 1 diabetes. Trends Mol. Med. 2024, 30, 1020–1027. [Google Scholar] [CrossRef]
- Bjerregaard-Andersen, M.; Da Silva, J.; Diogo, R.; Claro, A.R.; Ferro, I.; Romana, A.; Rocha, P.; Sá, B.; Lobarinhas, G.; Rolim, S.; et al. Association between COVID-19 and the incidence of type 1 diabetes in Portugal–A registry study. BMC Endocr. Disord. 2024, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Moin, A.S.M.; Butler, A.E. Virus-induced diabetes mellitus: Revisiting infection etiology in light of SARS-CoV-2. Metabolism 2024, 156, 155917. [Google Scholar] [CrossRef]
- Grieco, F.A.; Sebastiani, G.; Spagnuolo, I.; Patti, A.; Dotta, F. Immunology in the clinic review series; focus on type 1 diabetes and viruses: How viral infections modulate beta cell function. Clin. Exp. Immunol. 2012, 168, 24–29. [Google Scholar] [CrossRef]
- Krogvold, L.; Genoni, A.; Puggioni, A.; Campani, D.; Richardson, S.J.; Flaxman, C.S.; Edwin, B.; Buanes, T.; Dahl-Jørgensen, K.; Toniolo, A. Live enteroviruses, but not other viruses, detected in human pancreas at the onset of type 1 diabetes in the DiViD study. Diabetologia 2022, 65, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.; Cinek, O.; Rasmussen, T.; Witsø, E.; Grinde, B.; Stene, L.C.; Rønningen, K.S. Human Enterovirus RNA in Monthly Fecal Samples and Islet Autoimmunity in Norwegian Children with High Genetic Risk for Type 1 Diabetes. Diabetes Care 2010, 34, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, M.; Reunanen, A.; Leinikki, P.; Vesikari, T.; Lounamaa, R.; Tuomilehto, J.; the Childhoood Diabetes in Finland Study Group; Toivanen, L.; Kaprio, E.A.; Fagerlund, A.; et al. Decline of mumps antibodies in Type 1 (insulin-dependent) diabetic children and a plateau in the rising incidence of Type 1 diabetes after introduction of the mumps-measles-rubella vaccine in Finland. Diabetologia 1993, 36, 1303–1308. [Google Scholar] [CrossRef]
- Parkkonen, P.; Hyöty, H.; Koskinen, L.; Leinikki, P. Mumps virus infects Beta cells in human fetal islet cell cultures upregulating the expression of HLA class I molecules. Diabetologia 1992, 35, 63–69. [Google Scholar] [CrossRef]
- Honeyman, M. How robust is the evidence for viruses in the induction of type 1 diabetes? Curr. Opin. Immunol. 2005, 17, 616–623. [Google Scholar] [CrossRef]
- Aarnisalo, J.; Veijola, R.; Vainionpää, R.; Simell, O.; Knip, M.; Ilonen, J. Cytomegalovirus infection in early infancy: Risk of induction and progression of autoimmunity associated with type 1 diabetes. Diabetologia 2008, 51, 769–772. [Google Scholar] [CrossRef]
- Ekman, I.; Vuorinen, T.; Knip, M.; Veijola, R.; Toppari, J.; Hyöty, H.; Kinnunen, T.; Ilonen, J.; Lempainen, J. Early childhood CMV infection may decelerate the progression to clinical type 1 diabetes. Pediatr. Diabetes 2018, 20, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2009, 47, 193–199. [Google Scholar] [CrossRef]
- Roivainen, M. Enteroviruses: New findings on the role of enteroviruses in type 1 diabetes. Int. J. Biochem. Cell Biol. 2005, 38, 721–725. [Google Scholar] [CrossRef]
- Helfand, R.F.; Gary, H.E.; Freeman, C.Y.; Anderson, L.J.; Pittsburgh Diabetes Research Group; Pallansch, M.A. Serologic Evidence of an Association between Enteroviruses and the Onset of Type 1 Diabetes Mellitus. J. Infect. Dis. 1995, 172, 1206–1211. [Google Scholar] [CrossRef]
- Nigro, G.; Pacella, M.E.; Patanè, E.; Midulla, M. Multi-system Coxsackievirus B-6 infection with findings suggestive of diabetes mellitus. Eur. J. Pediatr. 1986, 145, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Szopa, T.M.; Titchener, P.A.; Portwood, N.D.; Taylor, K.W. Diabetes mellitus due to viruses—Some recent developments. Diabetologia 1993, 36, 687–695. [Google Scholar] [CrossRef]
- Cinek, O.; Witsø, E.; Jeansson, S.; Rasmussen, T.; Drevinek, P.; Wetlesen, T.; Vavrinec, J.; Grinde, B.; Rønningen, K. Longitudinal observation of enterovirus and adenovirus in stool samples from Norwegian infants with the highest genetic risk of type 1 diabetes. J. Clin. Virol. 2006, 35, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hyöty, H.; Hiltunen, M.; Knip, M.; Laakkonen, M.; Vähäsalo, P.; Karjalainen, J.; Koskela, P.; Roivainen, M.; Leinikki, P.; Hovi, T.; et al. A Prospective Study of the Role of Coxsackie B and Other Enterovirus Infections in the Pathogenesis of IDDM. Diabetes 1995, 44, 652–657. [Google Scholar] [CrossRef]
- Boddu, S.; Aurangabadkar, G.; Kuchay, M. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab. Syndr. 2022, 14, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Trevisani, V.; Cattini, U.; Bruzzi, P.; Lucaccioni, L.; Madeo, S.; Predieri, B. COVID-19 and Type 1 Diabetes: Concerns and Challenges. Acta Bio Medica Atenei Parm. 2020, 91, e2020033. [Google Scholar] [CrossRef]
- Genc, S. Could COVID-19 Trigger Type 1 Diabetes? Presentation of COVID-19 Case Presented with Diabetic Ketoacidosis. Acta Endocrinol. 2021, 17, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the UK. Diabetes Care 2020, 43, e170–e171. [Google Scholar] [CrossRef]
- Bramante, C.T.; Johnson, S.G.; Garcia, V.; Evans, M.D.; Harper, J.; Wilkins, K.J.; Huling, J.D.; Mehta, H.; Alexander, C.; Tronieri, J.; et al. Diabetes medications and associations with COVID-19 outcomes in the N3C database: A national retrospective cohort study. PLoS ONE 2022, 17, e0271574. [Google Scholar] [CrossRef]
- Fousteri, G.; Jhatakia, A.D. Viral Infections and Autoimmune Disease: Roles of LCMV in Delineating Mechanisms of Immune Tolerance. Viruses 2019, 11, 885. [Google Scholar] [CrossRef]
- Toor, S.M.; Saleh, R.; Nair, V.S.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2020, 162, 30–43. [Google Scholar] [CrossRef]
- Fignani, D.; Licata, G.; Brusco, N.; Nigi, L.; Grieco, G.E.; Marselli, L.; Overbergh, L.; Gysemans, C.; Colli, M.L.; Marchetti, P.; et al. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) Is Expressed in Human Pancreatic β-Cells and in the Human Pancreas Microvasculature. Front. Endocrinol. 2020, 11, 596898. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’aDdio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’aCqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Filippi, C.M.; von Herrath, M.G. Viral Trigger for Type 1 Diabetes. Diabetes 2008, 57, 2863–2871. [Google Scholar] [CrossRef]
- Gale, E.A.M. Congenital rubella: Citation virus or viral cause of type 1 diabetes? Diabetologia 2008, 51, 1559–1566. [Google Scholar] [CrossRef]
- Yue, Y.; Tang, Y.; Tang, J.; Shi, J.; Zhu, T.; Huang, J.; Qiu, X.; Zeng, Y.; Li, W.; Qu, Y.; et al. Maternal infection during pregnancy and type 1 diabetes mellitus in offspring: A systematic review and meta-analysis. Epidemiology Infect. 2018, 146, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liang, S.; Zhang, C. NK Cells in Autoimmune Diseases: Protective or Pathogenic? Front. Immunol. 2021, 12, 624687. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakami, A.M. Pattern of thyroid, celiac, and anti-cyclic citrullinated peptide autoantibodies coexistence with type 1 diabetes mellitus in patients from Southwestern Saudi Arabia. Saudi Med. J. 2016, 37, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Liao, J.-Y. Epidemiologic Implication of the Association between Herpes Simplex Virus Infection and the Risk of Type 1 Diabetes Mellitus: A Nationwide Case-Control Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 7832. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, I.-C.; Chen, H.-J.; Yeh, S.-Y.; Kao, C.-H.; Kaderali, L. Association of Herpes Zoster and Type 1 Diabetes Mellitus. PLoS ONE 2016, 11, e0155175. [Google Scholar] [CrossRef]
- Liu, X.-F.; Wang, X.; Yan, S.; Zhang, Z.; Abecassis, M.; Hummel, M. Epigenetic Control of Cytomegalovirus Latency and Reactivation. Viruses 2013, 5, 1325–1345. [Google Scholar] [CrossRef]
- Torne, A.S.; Robertson, E.S. Epigenetic Mechanisms in Latent Epstein-Barr Virus Infection and Associated Cancers. Cancers 2024, 16, 991. [Google Scholar] [CrossRef] [PubMed]
- Bougnères, P.; Le Fur, S.; Valleron, A.-J. Early varicella infection is associated with a delayed onset of childhood type 1 diabetes. Diabetes Metab. 2022, 48, 101394. [Google Scholar] [CrossRef]
- Parviainen, A.; But, A.; Sund, R.; Arffman, M.; Siljander, H.; Knip, M. Incidence of Type 1 Diabetes in Relation to Exposure to Rotavirus Infections in Pre- and Postvaccine Birth Cohorts in Finland. Diabetes Care 2023, 47, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Salminen, K.K.; Vuorinen, T.; Oikarinen, S.; Helminen, M.; Simell, S.; Knip, M.; Ilonen, J.; Simell, O.; Hyoty, H. Isolation of enterovirus strains from children with preclinical Type 1 diabetes. Diabet. Med. 2004, 21, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, G.R.; Geluk, A.; Ottenhoff, T.H.M.; Melchers, W.J.G.; Roep, B.O.; Galama, J.M.D. Molecular mimicry in diabetes mellitus: The homologous domain in coxsackie B virus protein 2C and islet autoantigen GAD 65 is highly conserved in the coxsackie B-like enteroviruses and binds to the diabetes associated HLA-DR3 molecule. Diabetologia 1998, 41, 40–46. [Google Scholar] [CrossRef]
- Honeyman, M.C.; Stone, N.L.; Falk, B.A.; Nepom, G.; Harrison, L.C. Evidence for Molecular Mimicry between Human T Cell Epitopes in Rotavirus and Pancreatic Islet Autoantigens. J. Immunol. 2010, 184, 2204–2210. [Google Scholar] [CrossRef]
- Insel, R.A.; Dunne, J.L.; Atkinson, M.A.; Chiang, J.L.; Dabelea, D.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Krischer, J.P.; Lernmark, Å.; et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015, 38, 1964–1974. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to Multiple Islet Autoantibodies and Risk of Progression to Diabetes in Children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.A.; von Herrath, M.G. Viruses and Type 1 diabetes: A dynamic labile equilibrium. Diabetes Manag. 2013, 3, 217–223. [Google Scholar] [CrossRef]
- Eshof, B.L.v.D.; Medfai, L.; Nolfi, E.; Wawrzyniuk, M.; Sijts, A.J.A.M. The Function of Immunoproteasomes—An Immunologists’ Perspective. Cells 2021, 10, 3360. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Regulation of Extracellular Vesicle-Mediated Immune Responses against Antigen-Specific Presentation. Vaccines 2022, 10, 1691. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.; Nampoothiri, M.; Lewis, S. Multifunctional role of exosomes in viral diseases: From transmission to diagnosis and therapy. Cell. Signal. 2022, 94, 110325. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
| Virus | Virus Family | Evidence Type | Mechanism of Action | Reference Studies |
|---|---|---|---|---|
| Coxsackie B (CVB) | Picornaviridae | Epidemiological, Histological, Animal | Beta-cell tropism, persistent infection, immune activation | [1,2,4] |
| Rotavirus | Reoviridae | Serological, Animal | Molecular mimicry, beta-cell damage | [3] |
| Mumps | Paramyxoviridae | Epidemiological, In vitro | Direct beta-cell infection, HLA I upregulation | [15,16] |
| Rubella | Togaviridae | Congenital Infection | Direct infection, molecular mimicry | [17] |
| CMV | Herpesviridae | Serological, Epidemiological | Possible protective modulation | [18,19] |
| SARS-CoV-2 | Coronaviridae | Receptor/Pathophysiology | ACE * 2-mediated beta-cell entry, transient hyperglycemia | [10,20] |
| Study/Cohort | Country | Virus | Sample Size | Study Design | Main Findings | Limitations | References |
|---|---|---|---|---|---|---|---|
| DAISY | USA | Enterovirus | ~2500 children | Prospective, genetic-risk stratified | Enteroviral RNA linked to islet autoimmunity | Modest event rate; limited diversity | [4] |
| DiViD | Norway | Enterovirus | 6 recent-onset adults | Pancreatic biopsy study | Viral RNA in pancreatic islets | Small sample size; no controls | [1] |
| TEDDY | EU/USA | Multiple viruses | >8000 children | Prospective, high-risk HLA | Temporal link between viruses and autoantibodies | Limited to genetically at-risk children | [2] |
| Finnish Registry | Finland | Rotavirus | >50,000 births | Retrospective cohort | Decline in T1DM after RV vaccine | Potential confounding; ecological analysis | [46] |
| N3C Database | USA | SARS-CoV-2 | >1 million children | Retrospective EHR-based | Increased risk of new-onset diabetes post-COVID-19 | Diabetes type often unspecified | [31] |
| DIPP | Finland | Enterovirus | >100,000 births | Prospective, genetically stratified | Strong temporal link between enterovirus and autoimmunity | Focused on high-risk genetic subgroups | [47] |
| ISIS-DIAB | France | Varicella | 157 children with T1DM | Retrospective observational | Early varicella linked to delayed T1DM onset | Retrospective, limited sample | [45] |
| COVID-19 Portugal Study | Portugal | SARS-CoV-2 | 885 children (new T1DM cases) | Retrospective, national registry | Spike in T1DM incidence during/after COVID-19 waves | Unclear T1DM vs. T2DM classification; ecological design | [10] |
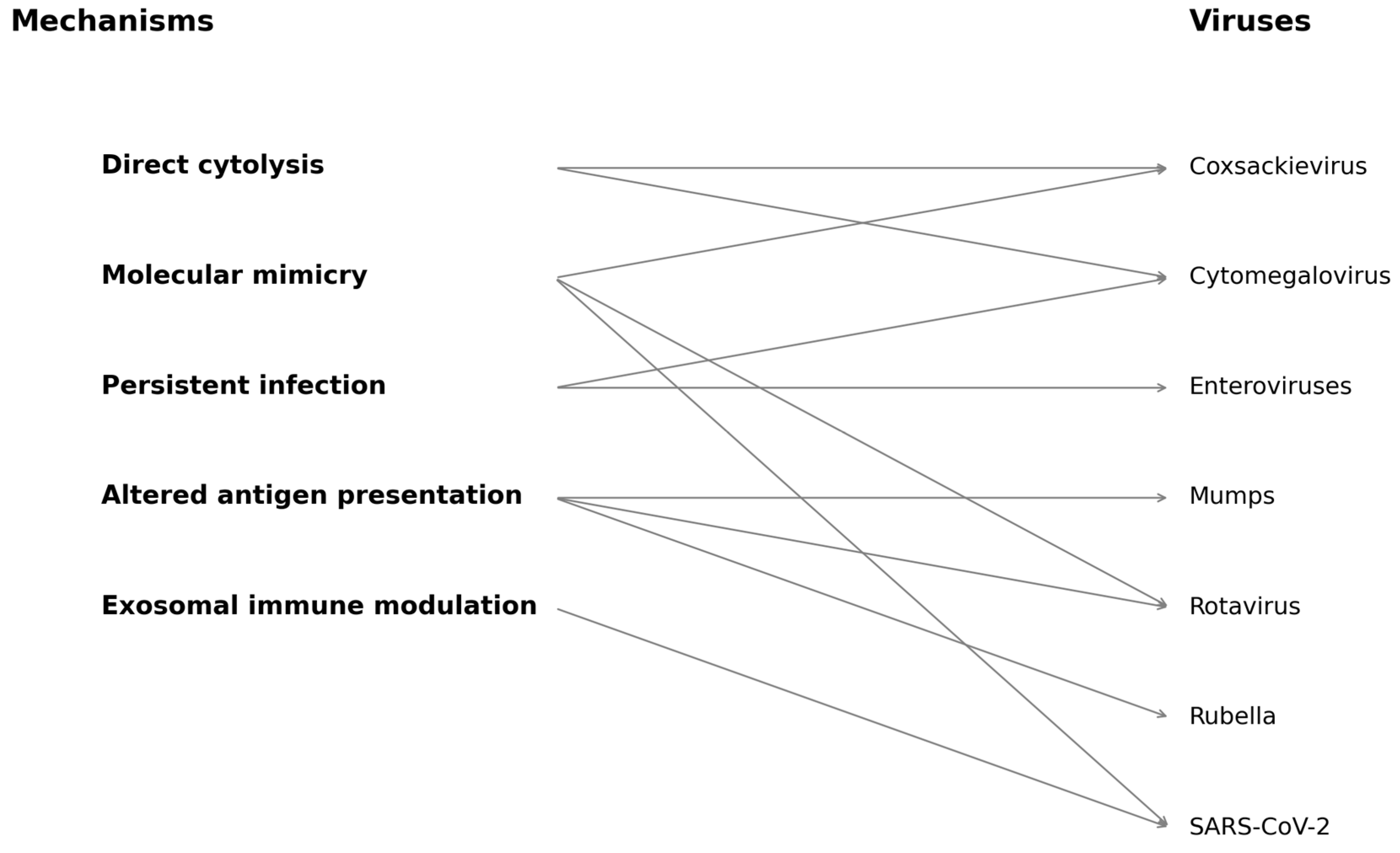
| Mechanism | Description | Representative Viruses |
|---|---|---|
| Direct Cytolysis | Lytic destruction of beta cells via productive viral infection, releasing autoantigens and triggering immune responses. | Coxsackievirus B, Rubella |
| Persistent Infection | Low-grade, chronic infection in pancreatic tissue sustaining inflammation and antigen presentation. | Enteroviruses (e.g., Coxsackie B), CMV |
| Molecular Mimicry | Viral peptides share homology with beta-cell antigens, promoting cross-reactive autoimmune responses. | Coxsackievirus B4 (PEVKEK/GAD65), Rotavirus (VP7/IA-2) |
| Altered Antigen Presentation | Virus-induced inflammatory signals upregulate MHC molecules and immunoproteasome processing, revealing neoepitopes. | Enteroviruses, SARS-CoV-2 |
| Exosomal Antigen Transfer | Viruses use exosomes to transfer RNA or protein to APCs, promoting immune priming without co-stimulation. | Enteroviruses, CMV, SARS-CoV-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiri, I.; Xanthi, M.; Domazinaki, C.-M.; Magiorkinis, E. The Role of Viral Infections in the Immunopathogenesis of Type 1 Diabetes Mellitus: A Narrative Review. Biology 2025, 14, 981. https://doi.org/10.3390/biology14080981
Kotsiri I, Xanthi M, Domazinaki C-M, Magiorkinis E. The Role of Viral Infections in the Immunopathogenesis of Type 1 Diabetes Mellitus: A Narrative Review. Biology. 2025; 14(8):981. https://doi.org/10.3390/biology14080981
Chicago/Turabian StyleKotsiri, Ioanna, Maria Xanthi, Charalampia-Melangeli Domazinaki, and Emmanouil Magiorkinis. 2025. "The Role of Viral Infections in the Immunopathogenesis of Type 1 Diabetes Mellitus: A Narrative Review" Biology 14, no. 8: 981. https://doi.org/10.3390/biology14080981
APA StyleKotsiri, I., Xanthi, M., Domazinaki, C.-M., & Magiorkinis, E. (2025). The Role of Viral Infections in the Immunopathogenesis of Type 1 Diabetes Mellitus: A Narrative Review. Biology, 14(8), 981. https://doi.org/10.3390/biology14080981







