Role of High-Fat Diet Alone on Lipids, Arterial Wall and Hippocampal Neural Cell Alterations in Animal Models and Their Implications for Humans
Simple Summary
Abstract
1. Introduction
1.1. Obesity Outcomes—Childhood to Adulthood
1.2. Aim
1.3. Research Question
2. Materials and Methods
2.1. Keywords
2.2. Search Criteria
3. Results
4. Discussion
4.1. High-Fat Diet in Animal Models
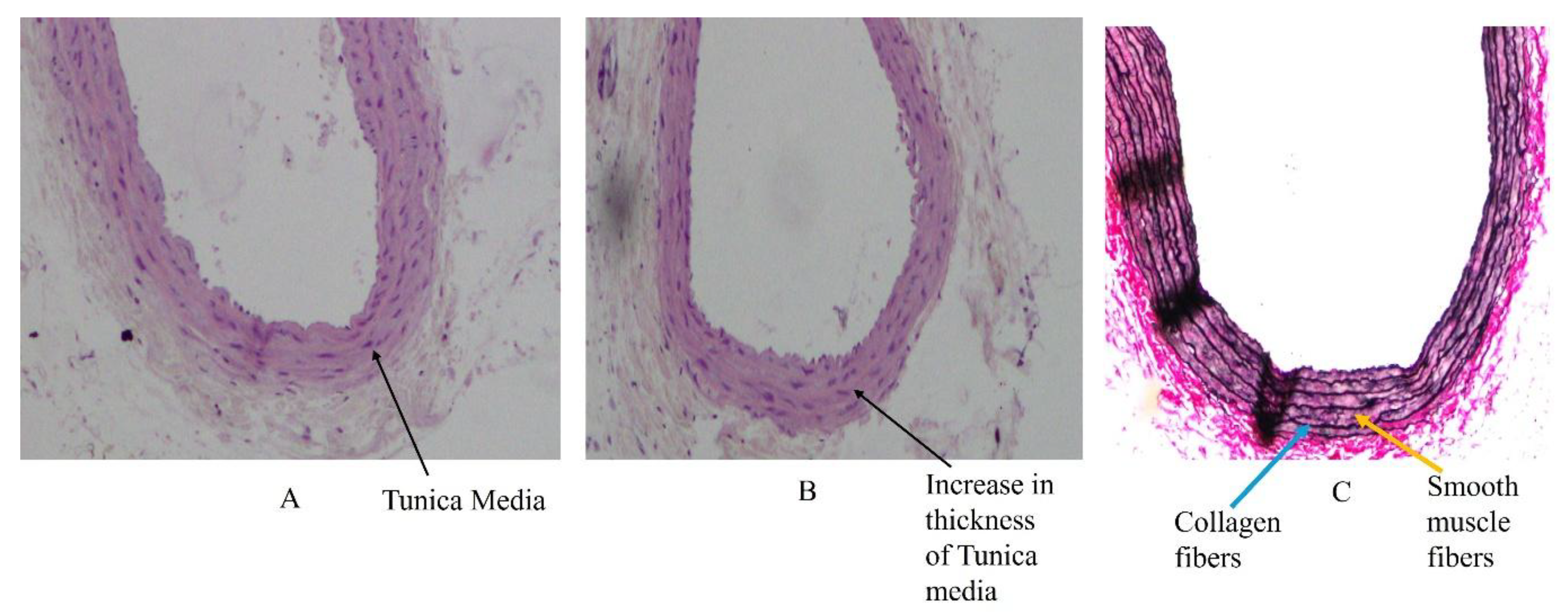
4.2. High-Fat Diet and Changes in Arterial Wall Structure
4.3. Basic Structure of Medium-Sized Arteries
4.4. Primary Changes in the Tunica Intima and Tunica Media of Arteries Due to High-Fat Diet
4.5. Oxidative Stress on Arterial Wall, Endothelial Dysfunction and Hippocampal Neuronal Cell Alterations
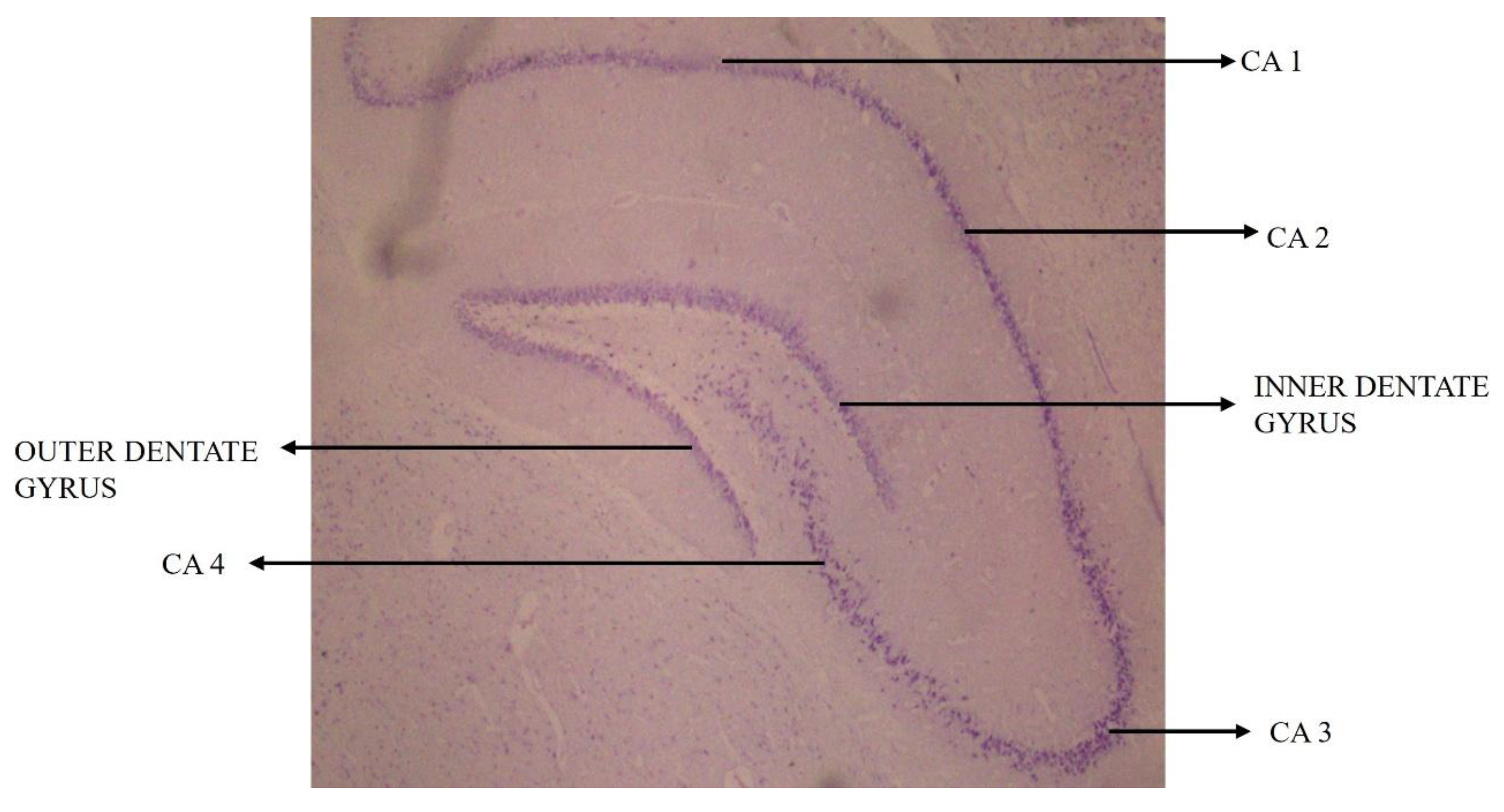
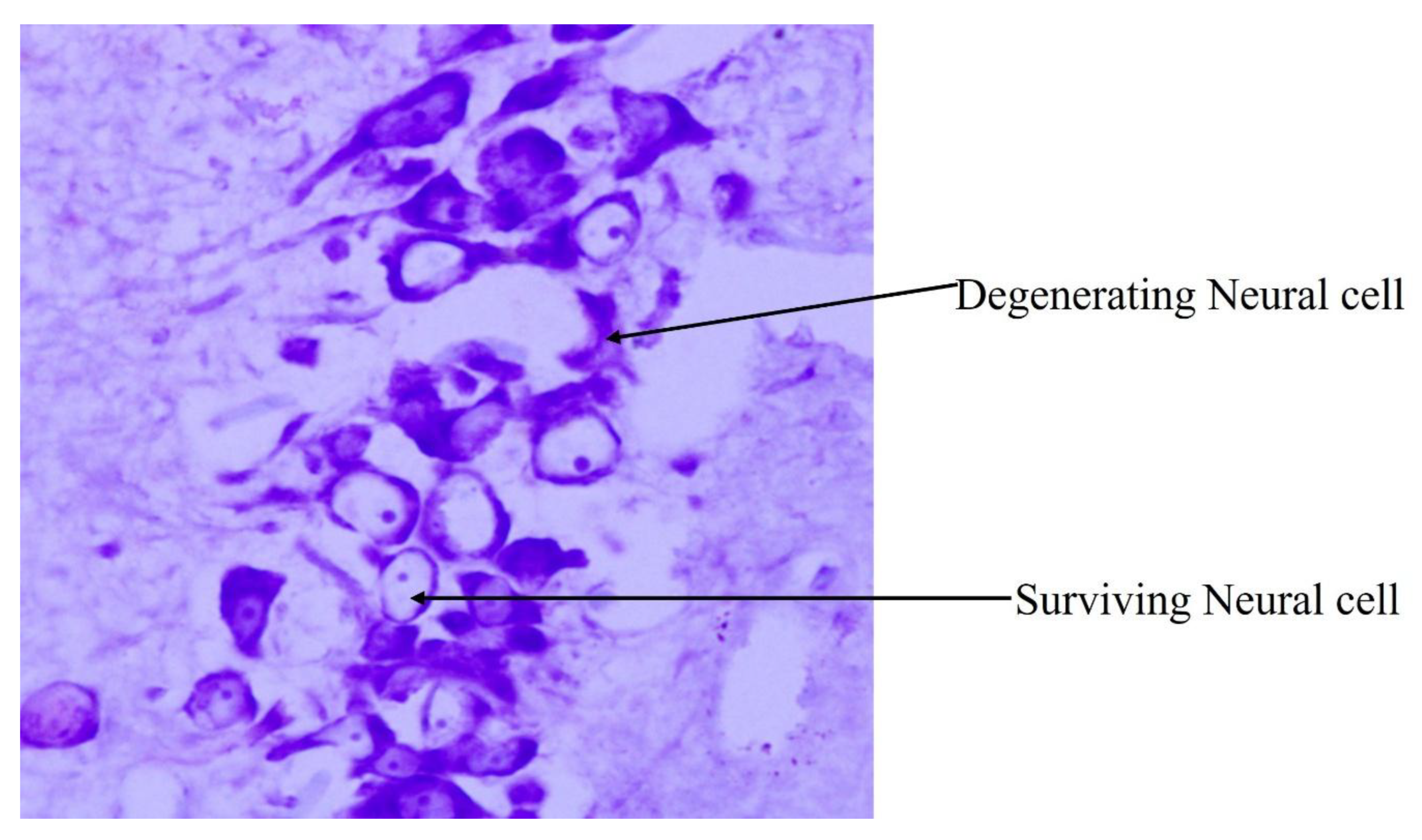
4.6. Influence of High-Fat-Diet-Induced Arterial Wall Changes on Neural Cells in Hippocampal Subregions
4.7. High-Fat Diet and Hippocampal Dysfunctions
4.8. High-Fat-Diet Feeding and CA1 and CA3 Subregions of the Hippocampus
4.9. Correlation Between High-Fat-Diet-Induced Arterial Wall and Hippocampal Neural Cell Changes
4.10. Limitations of High-Fat Diet in Animal Models for Human Studies
4.11. Present Prevention and Treatment Insights for Increased Lipid Profiles, Arterial Wall and Hippocampal Neuronal Cell Alterations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metab. Clin. Exp. 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Luhar, S.; Timæus, I.M.; Jones, R.; Cunningham, S.; Patel, S.A.; Kinra, S.; Clarke, L.; Houben, R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE 2020, 15, e0229438. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxid. Med. Cell Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.G.; Kebede, M.A.; Meoli, C.C.; Stöckli, J.; Whitworth, P.T.; Wright, A.L.; Hoffman, N.J.; Minard, A.Y.; Ma, X.; Krycer, J.R.; et al. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 2018, 293, 5731–5745. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.S.; Kishore, A.; Prasad, K.; Mohandas Rao, K.G.; Rai, K.S. Protective role of Dietary Choline and Docosahexaenoic acid or Environmental enrichment in attenuating high fat diet-induced arterial wall changes in obese rats. Res. J. Pharm. Technol. 2022, 15, 665–670. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Guiducci, L.; Campani, D.; Rosa, F.L.; Insilla, A.C.; Bartoli, A.; Cabiati, M.; Sena, V.D.; Ry, S.D.; Burchielli, S.; et al. Leptin resistance before and after obesity: Evidence that tissue glucose uptake underlies adipocyte enlargement and liver steatosis/steatohepatitis in Zucker rats from early-life stages. Int. J. Obes. 2022, 46, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Darios, E.S.; Andres Contreras, G.; Garver, H.; Fink, G.D. Male and female high-fat diet-fed Dahl SS rats are largely protected from vascular dysfunctions: PVAT contributions reveal sex differences. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H15–H28. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.D.S.; Claudio, E.R.G.; da Silva, V.L.; Cordeiro, J.P.; Domingos, L.F.; da Cunha, M.R.H.; Mauad, H.; do Nascimento, T.B.; Lima-Leopoldo, A.P.; Leopoldo, A.S. High-Fat Diet-Induced Obesity Model Does Not Promote Endothelial Dysfunction via Increasing Leptin/Akt/eNOS Signaling. Front. Physiol. 2019, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Putro, P.S.; Indarto, D.; Purwanto, B.; Soewondo, W.; Kusumaningrum, S. The Comparative Effects of Branded and Local High Fat Foods on Body Mass Index and Vascular Wall Thickness in Male Wistar Rats for Development of Atherosclerosis Animal Model. In Proceedings of the 4th International Conference on Life Sciences and Biotechnology (ICOLIB 2021), Jember, Indonesia, 15–16 November 2021; Atlantis Press International BV: Paris, France, 2023; Volume 27, pp. 572–578. [Google Scholar]
- Ismail, B.S.; Mahmoud, B.; Abdel-Reheim, E.S.; Soliman, H.A.; Ali, T.M.; Elesawy, B.H.; Zaky, M.Y. Cinnamaldehyde Mitigates Atherosclerosis Induced by High-Fat Diet via Modulation of Hyperlipidemia, Oxidative Stress, and Inflammation. Oxid. Med. Cell Longev. 2022, 2022, 4464180. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Bizzarri, M.; Tir, M.; Aldawood, N.; Alobaid, H.; Allagui, M.S.; Dahmash, W.; Tlili, N.; Mnafgui, K.; Alwasel, S.; et al. High-fat diet-induced aggravation of cardiovascular impairment in permethrin-treated Wistar rats. Ecotoxicol. Environ. Saf. 2021, 222, 112461. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liao, X.X.; Wu, X.Y.; Wang, R.; Hu, Z.W.; Liu, S.Y.; He, W.F.; Zhou, J.J. Effects of the Lipid Metabolites and the Gut Microbiota in ApoE−/− Mice on Atherosclerosis Co-Depression from the Microbiota-Gut-Brain Axis. Front. Mol. Biosci. 2022, 9, 786492. [Google Scholar] [CrossRef] [PubMed]
- Elabi, O.F.; Cunha, J.P.M.C.M.; Gaceb, A.; Fex, M.; Paul, G. High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease. J. Neuroinflammation 2021, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Serrano, A.M.; Mohr, A.A.; Philippe, J.; Skoug, C.; Spégel, P.; Duarte, J.M.N. Cognitive Impairment and Metabolite Profile Alterations in the Hippocampus and Cortex of Male and Female Mice Exposed to a Fat and Sugar-Rich Diet are Normalized by Diet Reversal. Aging Dis. 2022, 13, 267–283. [Google Scholar] [CrossRef] [PubMed]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood-Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef] [PubMed]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn MDPh, D.N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.X.; Sun, J.; Chen, H.C.; Chen, A.D.; Gao, L.P.; Yin, J.; Jing, Y.H. High-Fat Diet Increases Amylin Accumulation in the Hippocampus and Accelerates Brain Aging in hIAPP Transgenic Mice. Front. Aging Neurosci. 2019, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.S.; KG Rao, M.; Rai, K.S. Hippocampal neural cell degeneration and memory deficit in high-fat diet-induced postnatal obese rats–exploring the comparable benefits of choline and DHA or environmental enrichment. Int. J. Neurosci. 2021, 131, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, M.; Shea, T.B. A High-fat and High-Cholesterol Diet Potentiates Oxidative Damage in Hippocampus of Mice Lacking Apolipoprotein, E. Open Neurol. J. 2018, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Khazen, T.; Hatoum, O.A.; Ferreira, G.; Maroun, M. Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids. Sci. Rep. 2019, 9, 12270. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front. Neurosci. 2019, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.R.; Granholm, A.C.E. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J. Cereb. Blood Flow Metab. 2012, 32, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-C.; Lee, H.-C.; Liu, Y.-C.; Yen, P.S.-Y.; Liu, C.-K.; Chen, C.-H.; Hsieh, T.-H.; Chen, S.-L. Long-Term High-Fat Diet Consumption Depletes Glial Cells and Tyrosine Hydroxylase–Containing Neurons in the Brain of Middle-Aged Rats. Cells 2022, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, M.; Cheng, S.; Li, F.; Zhang, B.; Sun, X.; Hu, H.; Chen, L.; Zhao, Z.; Hu, H.; et al. Low-dose alcohol ameliorated high fat diet-induced anxiety-related behavior: Via enhancing adiponectin expression and activating the Nrf2 pathway. Food Funct. 2021, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Spagnuolo, M.S.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Gatto, C.; Iossa, S.; Cigliano, L. Effect of Initial Aging and High-Fat/High-Fructose Diet on Mitochondrial Bioenergetics and Oxidative Status in Rat Brain. Mol. Neurobiol. 2019, 56, 7651–7663. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; Milenkovic, D.; Rutledge, J.; Villablanca, A. The Western Diet Regulates Hippocampal Microvascular Gene Expression: An Integrated Genomic Analyses in Female Mice. Sci. Rep. 2019, 9, 19058. [Google Scholar] [CrossRef] [PubMed]
- Khedr, S.A.; Elmelgy, A.A.; El-Kharashi, O.A.; Abd-Alkhalek, H.A.; Louka, M.L.; Sallam, H.A.; Aboul-Fotouh, S. Metformin potentiates cognitive and antidepressant effects of fluoxetine in rats exposed to chronic restraint stress and high fat diet: Potential involvement of hippocampal c-Jun repression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.S.; Prasad, K.; Mohandas Rao, K.G.; Rai, K.S. Efficacy of choline and DHA supplements or enriched environment exposure during early adult obesity in mitigating its adverse impact through aging in rats. Saudi J. Biol. Sci. 2021, 28, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tayebati, S.K.; Roy, P.; Micioni Di Bonaventura, M.V.; Moruzzi, M.; Cifani, C.; Amenta, F.; Tomassoni, D. Obesity-Related Brain Cholinergic System Impairment in High-Fat-Diet-Fed Rats. Nutrients 2022, 14, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ramlakhan, K.P.; Johnson, M.R.; Roos-Hesselink, J.W. Pregnancy and cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. Maternal High-Fat Diet and Offspring Hypertension. Int. J. Mol. Sci. 2022, 23, 8179. [Google Scholar] [CrossRef] [PubMed]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2020, 78, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Simpson, S.J. Protein Leverage: Theoretical Foundations and Ten Points of Clarification. Obes. Rev. 2019, 27, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Fontaine, M.A.; Mangat, R.; Fouhse, J.M.; Diane, A.; Willing, B.P.; Proctor, S.D. High Vaccenic Acid Content in Beef Fat Attenuates High Fat and High Carbohydrate Western Diet Induced Changes in Lipid Metabolism and Gut Microbiota in Pigs. Microorganisms 2021, 9, 2517. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.P.; Raja, R.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Desai, D.M.; Desai, A.; et al. Obesity and Coronary Artery Disease: An Updated Systematic Review 2022. Cureus 2022, 14, e29480. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.S. Protective Role of Essential Nutrients and Environmental Enrichment in Attenuating Postnatal Obesity-Induced Atherosclerosis and Spatial Memory deficits through Aging in Rats—A Behavioral, Biochemical and Histological Study. Ph.D. Thesis, Manipal Academy of Higher Education, Karnataka, India, 28 May 2021. [Google Scholar]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Cunha, P.; Carneiro, A.; Vila, I.; Cunha, C.; Silva, C.; Longatto-Filho, A.; Mesquita, A.; Cotter, J.; Correia-Neves, M.; et al. Is Obesity a Risk Factor for Carotid Atherosclerotic Disease?—Opportunistic Review. J. Cardiovasc. Dev. Dis. 2022, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Huwart, S.J.P.; Morales-Puerto, N.; Everard, A. Gut microbiota-related neuroinflammation at the crossroad of food reward alterations: Implications for eating disorders. Gut 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, B.; Zhou, J.; Gu, X.; Zhou, Y.; Yang, Y.; Guo, F.; Wei, X.; Wang, H.; Si, N.; et al. High-Fat Diet Alleviates Neuroinflammation and Metabolic Disorders of APP/PS1 Mice and the Intervention with Chinese Medicine. Front. Aging Neurosci. 2021, 13, 658376. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. High-Fat Diet, Epigenetics, and Atherosclerosis: A Narrative Review. Nutrients 2025, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Wayal, V.; Wang, S.D.; Hsieh, C.C. Novel bioactive peptides alleviate Western diet-induced MAFLD in C57BL/6J mice by inhibiting NLRP3 inflammasome activation and pyroptosis via TLR4/NF-κB and Keap1/Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2025, 148, 114177. [Google Scholar] [CrossRef] [PubMed]
- Tkaczenko, H.; Kurhaluk, N. Antioxidant-Rich Functional Foods and Exercise: Unlocking Metabolic Health Through Nrf2 and Related Pathways. Int. J. Mol. Sci. 2025, 26, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, C.; Chi, Y.; Su, B.; Chen, H.; Xie, H. Effect of peripheral adiponectin on perioperative neurocognitive disorder via regulation of glucose metabolism in aged rats. Neuroreport 2025, 36, 505–513. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, T.M.; Razolli, D.S.; Correa-da-Silva, F.; de Lima-Junior, J.C.; Gaspar, R.; Sidarta-Oliveira, D.; Victorio, S.C.; Donato, J., Jr.; Kim, Y.B.; Velloso, L.A. The partial inhibition of hypothalamic IRX3 exacerbates obesity. EBioMedicine 2019, 39, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Puppala, S.R.; Comuzzie, A.G.; Mahaney, M.C.; VandeBerg, J.L.; Olivier, M.; Cox, L.A. Analysis of serum changes in response to a high fat high cholesterol diet challenge reveals metabolic biomarkers of atherosclerosis. PLoS ONE 2019, 14, e0214487. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.; Ramos, M.; Marques, S.I.; Silva, A.; Pereira, P.A.; Madeira, M.D.; Mateus, N.; Cardoso, A. Effects of High-Fat and High-Fat High-Sugar Diets in the Anxiety, Learning and Memory, and in the Hippocampus Neurogenesis and Neuroinflammation of Aged Rats. Nutrients 2023, 15, 1370. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C. Hippocampal Vascular Supply and Its Role in Vascular Cognitive Impairment. Stroke 2023, 54, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sheikh, A.M.; Tabassum, S.; Iwasa, K.; Shibly, A.Z.; Zhou, X.; Wang, R.; Bhuiya, J.; Abdullah, F.B.; Yano, S.; et al. Effect of high-fat diet on cerebral pathological changes of cerebral small vessel disease in SHR/SP rats. Geroscience 2024, 46, 3779–3800. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Kundu, P.; Rooney, W.D.; Raber, J. The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review. Molecules 2021, 26, 3406. [Google Scholar] [CrossRef] [PubMed]
- Custers; Emma, E.M.; Kiliaan; Amanda, J. Dietary lipids from body to brain. Prog. Lipid Res. 2022, 85, 101–144. [Google Scholar] [CrossRef] [PubMed]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research—Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Ketelhuth, D.F.J.; Malin, S.G.; Hansson, G.K. Animal Models of Atherosclerosis-Supportive Notes and Tricks of the Trade. Circ. Res. 2022, 130, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Tsilingiris, D.; Liu, J.; Dalamaga, M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol. Open 2022, 15, 100208. [Google Scholar] [CrossRef] [PubMed]
- de Moura EDias, M.; Dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.O.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Peluzio, M.D.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Chmelir, T.; Chottova Dvorakova, M. Animal Models in Diabetic Research-History, Presence, and Future Perspectives. Biomedicines 2023, 11, 2852. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Chahuan, S.; Dhankhar, S.; Kaur, A.; Garg, N.; Saini, M.; Singh, T.G. Insights into therapeutic approaches for the treatment of neurodegenerative diseases targeting metabolic syndrome. Mol. Biol. Rep. 2025, 52, 260. [Google Scholar] [CrossRef] [PubMed]


| Author | Year of Publication | Animal Model | Key Result |
|---|---|---|---|
| Zeynep Tuzcu [4] | 2017 | Wistar Rats | HFD feeding increased body weight, visceral fat, and liver weight and reduced feed consumption, as compared to the control rats |
| Pettersson [5] | 2012 | C57Bl/6 Mice | HFD-induced metabolic changes were seen less in female mice HFD-fed male mice showed an increase in adipose tissue inflammation, glucose intolerance, and an increase in insulin levels and hypertrophy of islet cells |
| Burchfield JG [6] | 2018 | C57BL/6J Mice | High-fat/high-sucrose diet (HFHSD) mice showed metabolic changes, hyperleptinemia, reduced physical activity, glucose intolerance, peripheral insulin resistance, hyperglycemia, increase in lipid deposition, and bone weakening |
| Prabhu. S [7] | 2022 | Wistar Rats | HFD when given for 3 months showed a significant raise in body mass index as compared to normal control same-age matched rats |
| M.A. Guzzardi [8] | 2022 | fa/fa Zucker Rats | Leptin resistance led to glucose intolerance and an increase in hepatic glucose production. This also led to changes in pancreatic and intestinal hormones. Thus, fat deposits in adipocytes and hepatocytes were also seen |
| Author | Year of Publication | Animal Model | Key Result |
|---|---|---|---|
| S.W. Watts [9] | 2021 | DahlSS Rats | HFD, when given as a diet from weaning period, led to development of hypertension in both male and female DahlSS rats. Endothelial dysfunction, along with medial hypertrophy, was observed. |
| Rocha [10] | 2019 | Wistar Rats | High-unsaturated-fat diet showed vascular sensitivity to leptin and increasing Nitric oxide bioavailability. This leads to increase in Nitric oxide production through an increase in NOS activation, which is partly intervened with by the Akt pathway. |
| Putro [11] | 2021 | Wistar Rats | Supplementation of DYETs that contained 40% of calories as a fat, lard and cholesterol diet, showed a higher increased internal carotid-artery vascular wall thickness. |
| Basma S. Ismail [12] | 2022 | Wistar Rats | HFD-administered rats showed fat vacuoles in intracytoplasmic regions and cardiomyocytes, mononuclear cellular infiltration, and arterial wall fat deposition with degeneration. Central necrosis and cardiac muscle disintegration were also observed, along with loss of striations in myocytes leading to fragmented cardiomyocytes. |
| A. Feriani [13] | 2021 | Wister Rats | Subchronic postweaning exposure to HFD or permethrin (PER) exposure can change cardiac integrity and initiate fibrosis. In addition, also, raised aortic levels of ox-LDL are seen. |
| Author | Year of Publication | Animal Model | Result |
|---|---|---|---|
| Hu et al. [14] | 2022 | ApoE−/− mice | The HFB group displayed symptoms of depression and clinical markers associated with atherosclerosis. |
| Elabi [15] | 2021 | C57BL6 mice | HFD in mice resulted in a significant depletion of pericytes. |
| Garcia-Serrano AM [16] | 2022 | C57BL/6J mice | Mice fed HFHSD, both male and female, exhibited elevated anxiety-like behavior, memory impairment in object identification tasks, but intact working spatial memory. |
| de Paula [17] | 2021 | Swiss mice | HFD resulted in depressive-like behavior or astrocyte activation in the hippocampus, along with increased permeability of the blood–brain barrier (BBB) in the hippocampus. |
| N. Saiyasit [18] | 2020 | Wistar rats | Long-term HFD showed hippocampal synaptic deplasticity and decline in cognitive functions. |
| Xi [19] | 2019 | APP transgenic mice | Administration of HFD was associated with increased amylin deposits in the hippocampus, along with brain aging and Aβ42 deposition. |
| G.S. Prabhu [20] | 2021 | Sprague Dawley rats | Long-term HFD feed resulted in significant reduction in number of surviving neural cells in hippocampus. |
| Guaraldi M [21] | 2018 | C57BL/6 mice | Treatment with HDF showed increase in ROS in hippocampus, apolipoprotein E alteration, and adverse effects on learning and memory. |
| Khazen, T [22] | 2019 | Sprague Dawley rats | Synaptic plasticity in the hippocampus was eliminated by juvenile HFD. Adult HFD enhanced in vivo LTP and object-location memory. |
| Lizarbe [23] | 2019 | C57BL/6J mice | HFD disturbed the hippocampus-dependent spatial memory with reduced levels of vesicular glutamate transporter vGlut1 and vesicular GABA transporter. |
| Freeman LR [24] | 2012 | Fischer 344 rats | HFD therapy exacerbated hippocampal microgliosis and disrupted the integrity of the blood–brain barrier. |
| Chou, M [25] | 2022 | Wistar-Kyoto rats | Long-term HFD significantly decreased the number of astrocytes and tyrosine hydroxylase-containing neurons in the substantia nigra and locus coeruleus. |
| Cheng, J [26] | 2021 | C57BL/6 mice | Reduction in APN and Nrf2 expression in the hippocampus of the HFD group. |
| Crescenzo, R [27] | 2019 | Sprague Dawley rats | HFD was observed to lower antioxidant defenses and raise the amount of PGC-1α and UCP2 in the hippocampal regions. |
| Nuthikattu, S [28] | 2019 | Mice | The hippocampus microvasculature was regulated at multiple molecular levels with prolonged ingestion of the Western diet. |
| Khedr, S. A [29] | 2018 | Wistar rats | HFD exposure showed cognitive impairment and lesser CA1 synapses, along with reduced BDNF levels. |
| G.S. Prabhu [30] | 2021 | Sprague Dawley rats | HFD supplement from young age throughout childhood, increased the TG, cholesterol and LDL levels, thus resulting in significant reduction in surviving neural cells in CA3 subregion of hippocampus. |
| Martinelli I [31] | 2022 | Wistar rats | HFD resulted in downregulation of acetylcholinesterase (AChE) enzyme, both in the frontal cortex and hippocampus. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, G.S.; Rao KG, M.; Concessao, P.L.; Rai, K.S. Role of High-Fat Diet Alone on Lipids, Arterial Wall and Hippocampal Neural Cell Alterations in Animal Models and Their Implications for Humans. Biology 2025, 14, 971. https://doi.org/10.3390/biology14080971
Prabhu GS, Rao KG M, Concessao PL, Rai KS. Role of High-Fat Diet Alone on Lipids, Arterial Wall and Hippocampal Neural Cell Alterations in Animal Models and Their Implications for Humans. Biology. 2025; 14(8):971. https://doi.org/10.3390/biology14080971
Chicago/Turabian StylePrabhu, Gayathri S., Mohandas Rao KG, Preethi Lavina Concessao, and Kiranmai S. Rai. 2025. "Role of High-Fat Diet Alone on Lipids, Arterial Wall and Hippocampal Neural Cell Alterations in Animal Models and Their Implications for Humans" Biology 14, no. 8: 971. https://doi.org/10.3390/biology14080971
APA StylePrabhu, G. S., Rao KG, M., Concessao, P. L., & Rai, K. S. (2025). Role of High-Fat Diet Alone on Lipids, Arterial Wall and Hippocampal Neural Cell Alterations in Animal Models and Their Implications for Humans. Biology, 14(8), 971. https://doi.org/10.3390/biology14080971






