Marsupenaeus japonicus HSP90’s Function Under Low Temperature Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Shrimp Rearing
2.2. Cloning of the MjHSP90 cDNA
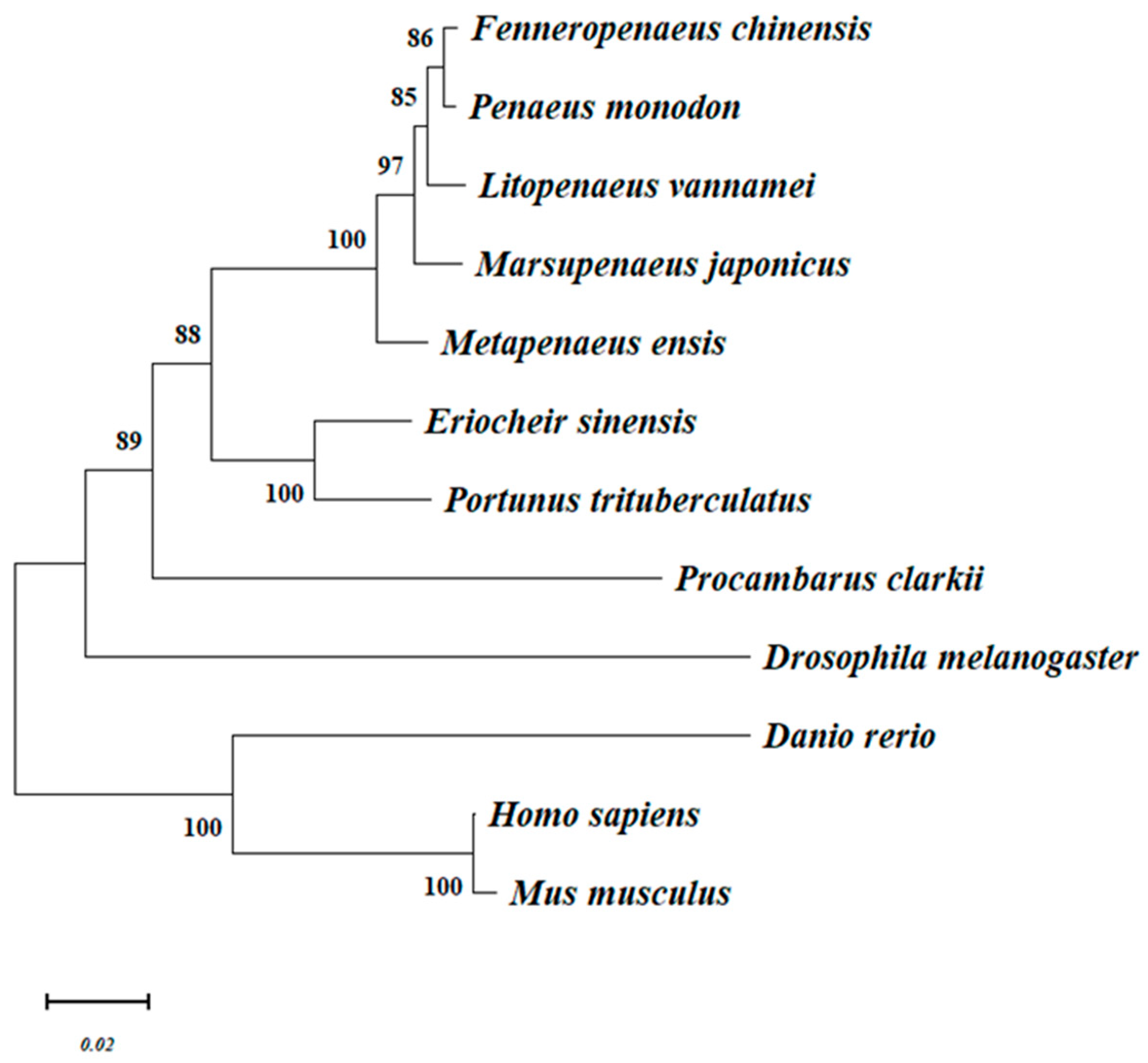
2.3. Sequence Characterization and Phylogenetic Tree Construction
2.4. Cold Stress and Sample Acquisition
2.5. Analysis of MjHSP90 Tissue Expression and Low-Temperature Expression Pattern
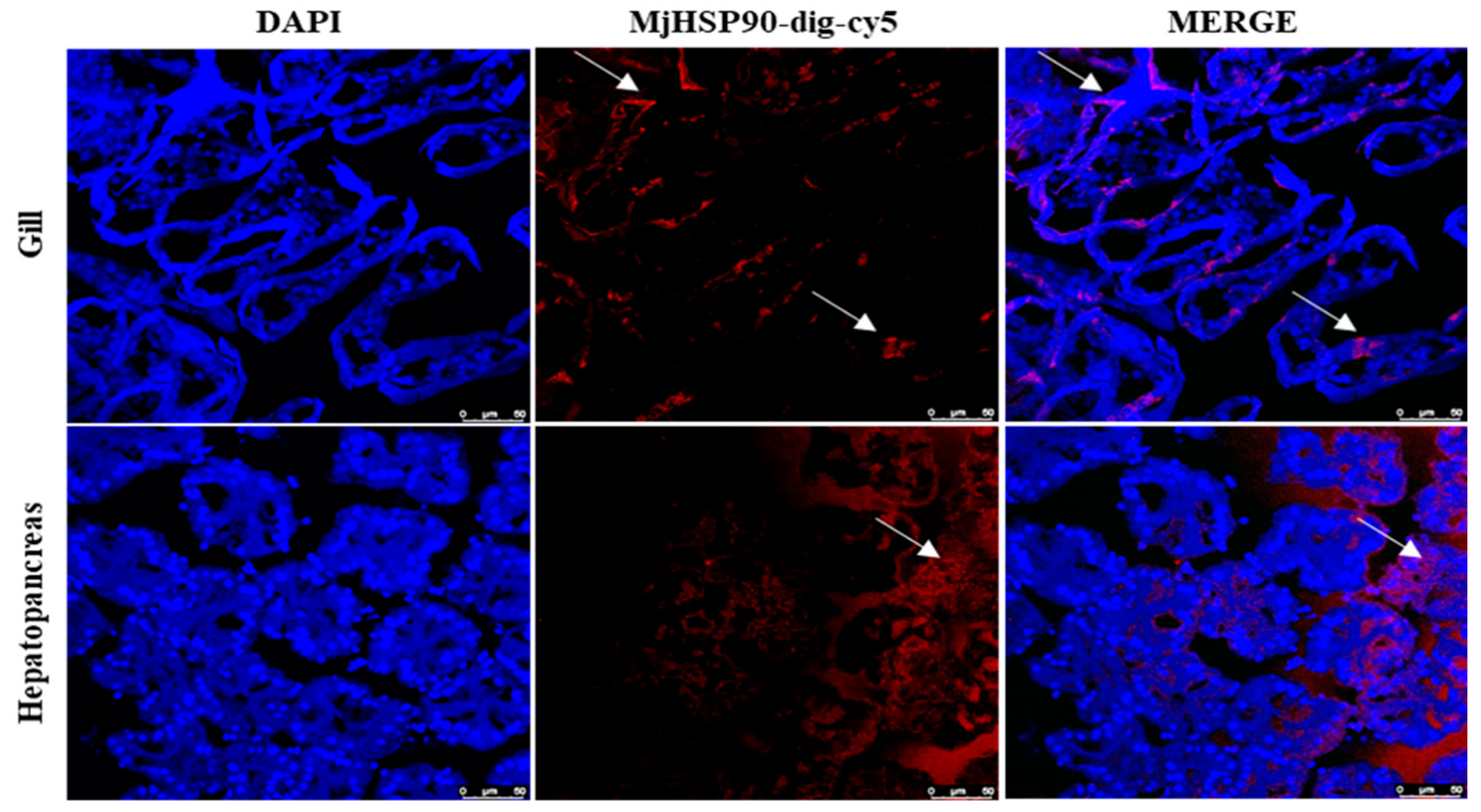
2.6. Analysis of the Localization of MjHSP90 in Gill and Hepatopancreas Tissue
2.6.1. Preparation of Frozen Sections
2.6.2. Synthesis of the RNA Probe
2.6.3. Fluorescence In Situ Hybridization (FISH)
2.7. The Impact of MjHSP90 Knockdown on the Expression Levels of Genes Associated with the Mitochondrial Apoptosis Pathway
2.7.1. Double-Stranded RNA (dsRNA) Synthesis
2.7.2. Detection of dsMjHSP90 Interference Efficiency
2.7.3. M. japonicus Mortality When Treated with Cold Stress After MjHSP90 Knockdown
2.7.4. Assessment of Gene Expression Associated with the Mitochondrial Apoptosis Pathway Following MjHSP90 Knockdown
2.8. TUNEL Assay Detection of Apoptosis
2.9. Statistical Considerations
3. Results
3.1. Analysis of the MjHSP90 Sequence
3.2. Multiple Sequence Alignment
3.3. Phylogenetics
3.4. Tissue Distribution of MjHSP90 in M. japonicus
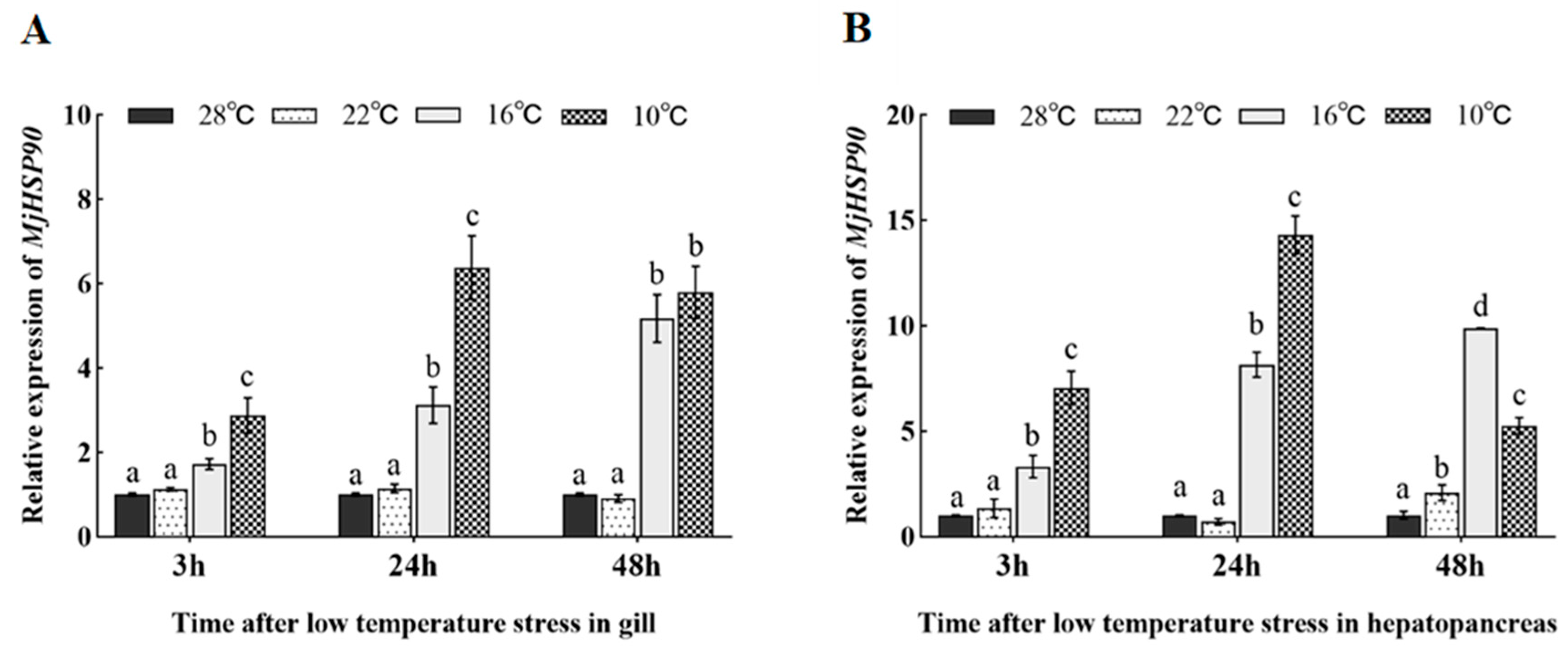
3.5. Expression Pattern of MjHSP90 in Cold Stress-Treated Shrimp
3.6. The Efficiency of the MjHSP90 dsRNA Interference
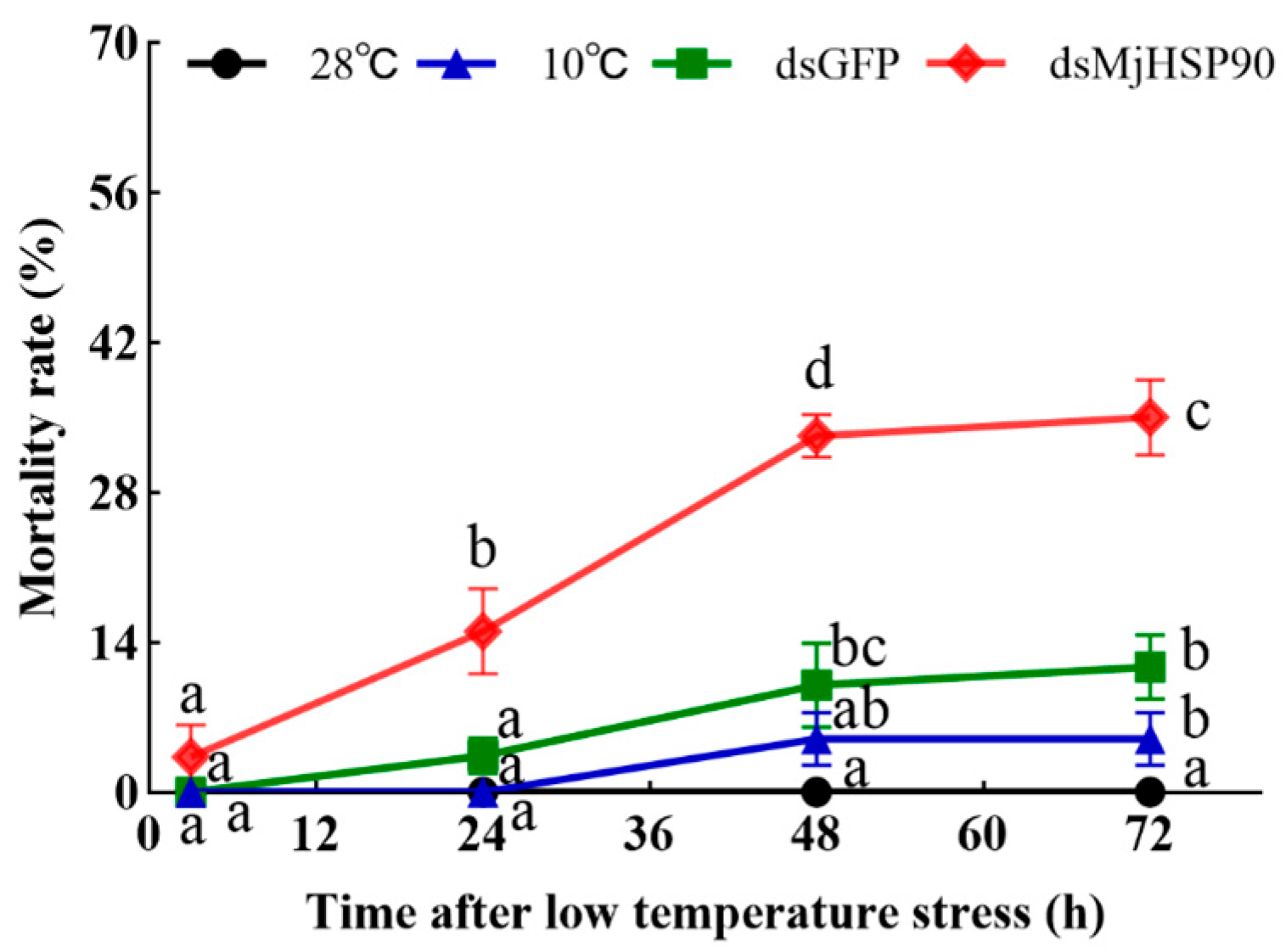
3.7. M. japonicus Mortality When Treated with Cold Stress After MjHSP90 Knockdown
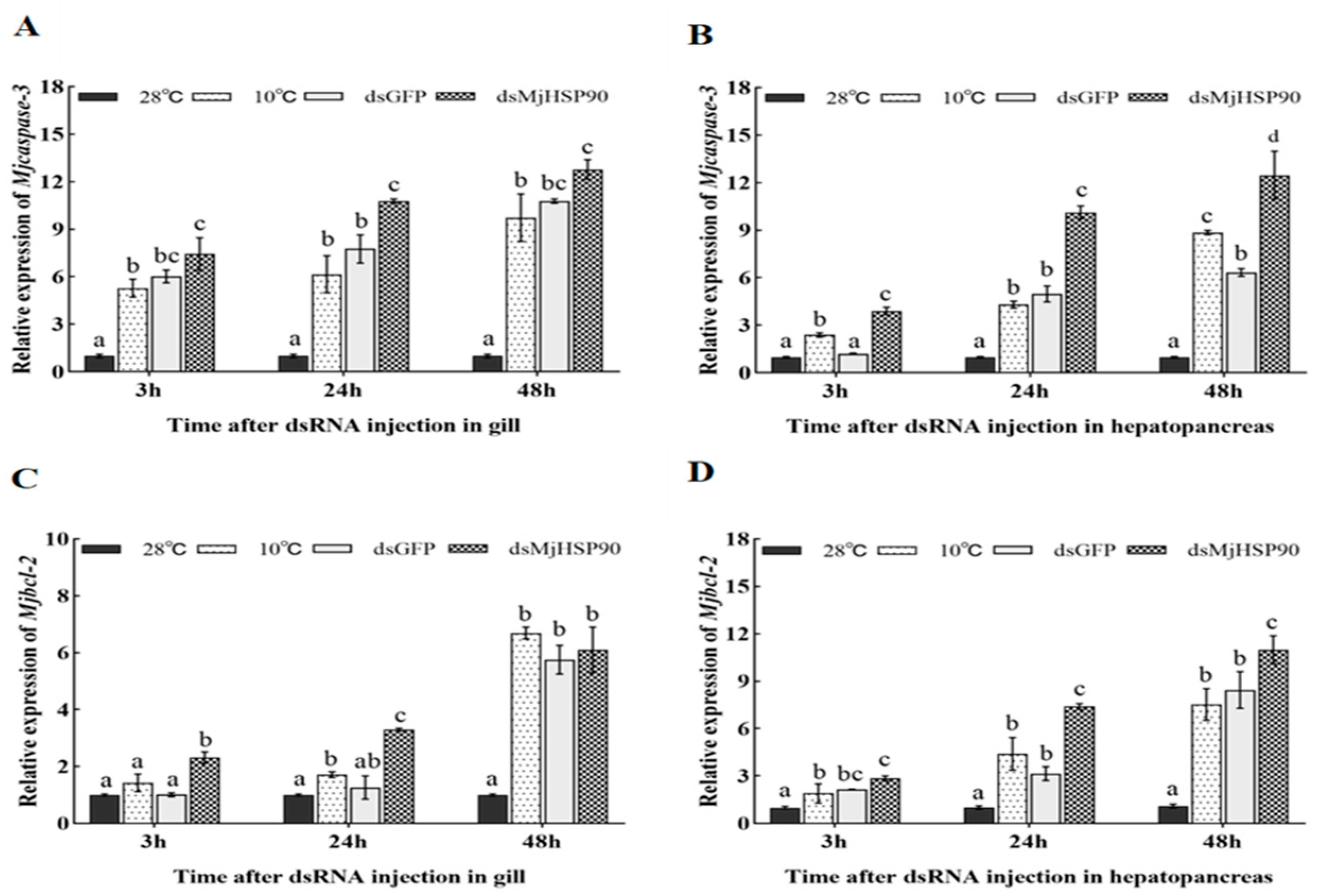
3.8. Differential Expression of M. japonicus Apoptotic Pathway Genes Under Cold Stress After MjHSP90 Knockdown
3.9. Cell Apoptosis Following MjHSP90 Knockdown
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forster, J.; Hirst, A.G.; Woodward, G. Growth and development rates have different thermal responses. Am. Nat. 2011, 178, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Lo, V.K.; Zillig, K.W.; Cocherell, D.E.; Todgham, A.E.; Fangue, N.A. Effects of low temperature on growth and metabolism of larval green sturgeon (Acipenser medirostris) across early ontogeny. J. Comp. Physiol. B 2024, 194, 427–442. [Google Scholar] [CrossRef]
- Rajesh, M.; Rehana, S. Impact of climate change on river water temperature and dissolved oxygen: Indian riverine thermal regimes. Sci. Rep. 2022, 12, 9222. [Google Scholar] [CrossRef]
- Mengal, K.; Kor, G.; Kozák, P.; Niksirat, H. Heat shock proteins adaptive responses to environmental stressors and implications in health management of decapods. Aquacult. Rep. 2023, 30, 101564. [Google Scholar] [CrossRef]
- Sornchuer, P.; Junprung, W.; Yingsunthonwattana, W.; Tassanakajon, A. Heat shock factor 1 regulates heat shock proteins and immune-related genes in Penaeus monodon under thermal stress. Dev. Comp. Immunol. 2018, 88, 19–27. [Google Scholar] [CrossRef]
- Weber, A.V.; Firth, B.L.; Cadonic, I.G.; Craig, P.M. Interactive effects of venlafaxine and thermal stress on zebrafish (Danio rerio) inflammatory and heat shock responses. J. Comp. Physiol. C 2023, 269, 109620. [Google Scholar] [CrossRef]
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259. [Google Scholar] [CrossRef]
- Storey, J.M.; Storey, K.B. Chaperone proteins: Universal roles in surviving environmental stress. Cell Stress Chaperones 2023, 28, 455–466. [Google Scholar] [CrossRef]
- Sung, Y.Y.; MacRae, T.H.; Sorgeloos, P.; Bossier, P. Stress response for disease control in aquaculture. Rev. Aquac. 2011, 3, 120–137. [Google Scholar] [CrossRef]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef]
- Lai, B.T.; Chin, N.W.; Stanek, A.E.; Keh, W.; Lanks, K.W. Quantitation and intracellular localization of the 85k heat shock protein by using monoclonal and polyclonal antibodies. Mol. Cell Biol. 1984, 4, 2802–2810. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef]
- Fatani, A.; Wu, X.; Gbotsyo, Y.; MacRae, T.H.; Song, X.; Tan, J. ArHsp90 is important in stress tolerance and embryo development of the brine shrimp, Artemia franciscana. Cell Stress Chaperones 2024, 29, 285–299. [Google Scholar] [CrossRef]
- Fishery and Fishery Administration Bureau of the Ministry of Agriculture and Rural Affairs. China Fisheries Statistical Yearbook; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Yu, Z.; Ren, X.; Shao, H.; Liu, P.; Li, J. Effect of Low Temperature Stress on Antioxidant System and Apoptosis of Marsupenaeus japonicus. Prog. Fish. Sci. 2022, 43, 157–166. [Google Scholar] [CrossRef]
- Peng, M.; Wang, H.; Wen, S.; Liang, Z.; Huang, Z.; Zhang, B.; Chen, T.; Liu, Q.; Li, Q.; Meng, Y.; et al. Identification and expression analysis of the heat shock proteins Hsp70, Hsp90, and Hsp90b in Litopenaeus vannamei under low-temperature stress. Aquac. Rep. 2025, 40, 102591. [Google Scholar] [CrossRef]
- Ju-Ngam, T.; McMillan, N.; Yoshimizu, M.; Kasai, H.; Wongpanya, R.; Srisapoome, P. Functional and Stress Response Analysis of Heat Shock Proteins 40 and 90 of Giant River Prawn (Macrobrachium rosenbergii) under Temperature and Pathogenic Bacterial Exposure Stimuli. Biomolecules 2021, 11, 1034. [Google Scholar] [CrossRef]
- Ren, X.; Lv, J.; Liu, M.; Wang, Q.; Shao, H.; Liu, P.; Li, J. A chromosome-level genome of the kuruma shrimp (Marsupenaeus japonicus) provides insights into its evolution and cold-resistance mechanism. Genomics 2022, 114, 110373. [Google Scholar] [CrossRef]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. In 2-D Proteome Analysis Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 1999; Volume 112, pp. 531–552. [Google Scholar] [CrossRef]
- An, Y.; Xing, H.; Zhang, Y.; Jia, P.; Gu, X.; Teng, X. The evaluation of potential immunotoxicity induced by environmental pollutant ammonia in broilers. Poult. Sci. 2019, 98, 3165–3175. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Chen, J.; Han, Q.; Xu, Y.; Ishfaq, M.; Teng, X. Ammonia inhalation impaired immune function and mitochondrial integrity in the broilers bursa of fabricius: Implication of oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2020, 190, 110078. [Google Scholar] [CrossRef] [PubMed]
- Krone, P.H.; Lele, Z.; Sass, J.B. Heat shock genes and the heat shock response in zebrafish embryos. Biochem. Cell Biol. 1997, 75, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Rungrassamee, W.; Leelatanawit, R.; Jiravanichpaisal, P.; Klinbunga, S.; Karoonuthaisiri, N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev. Comp. Immunol. 2010, 34, 1082–1089. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, Z.; Yu, S.; Yang, Y.; Li, Y.; He, C. Genome-wide identification of HSP70/110 genes in sea cucumber Apostichopus japonicus and comparative analysis of their involvement in aestivation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 162–171. [Google Scholar] [CrossRef]
- Han, B.; Luo, J.; Jiang, P.; Li, Y.; Wang, Q.; Bai, Y.; Chen, J.; Wang, J.; Zhang, J. Inhibition of Embryonic HSP 90 Function Promotes Variation of Cold Tolerance in Zebrafish. Front. Genet. 2020, 11, 541944. [Google Scholar] [CrossRef]
- Ren, X.; Bian, X.; Jia, S.; Gao, T.; Wang, J.; Wang, J.; Li, J. Heat-shock protein 70 reduces apoptosis in the gills and hepatopancreas of Marsupenaeus japonicus under low-temperature stress. Int. J. Biol. Macromol. 2025, 307, 141931. [Google Scholar] [CrossRef]
- Mottola, G.; Nikinmaa, M.; Anttila, K. Hsp70s transcription-translation relationship depends on the heat shock temperature in zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 240, 110629. [Google Scholar] [CrossRef]
- Song, L.; Li, C.; Xie, Y.; Liu, S.; Zhang, J.; Yao, J.; Jiang, C.; Li, Y.; Liu, Z. Genome-wide identification of Hsp70 genes in channel catfish and their regulated expression after bacterial infection. Fish Shellfish Immunol. 2016, 49, 154–162. [Google Scholar] [CrossRef]
- Kelly, N.I.; Wilson, C.C.; Currie, S.; Burness, G. Acclimation capacity of the cardiac HSP70 and HSP90 response to thermal stress in lake trout (Salvelinus namaycush), a stenothermal ice-age relict. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 53–60. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, P.; Liu, Y.; Song, M.; Li, F.; Ou, J.; Lai, J. Integrated metabolomic and transcriptomic responses to heat stress in a high-altitude fish, Triplophysa siluroides. Fish Shellfish Immunol. 2023, 142, 109118. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Jiang, Y.; Zhou, H.; Sun, M.; Ning, Z.; Mu, W. Identification, characterization, and function of GRP94 and HSP90β in cold stress response in cold water fish Phoxinus lagowskii. Aquacult. Rep. 2023, 28, 101443. [Google Scholar] [CrossRef]
- Liu, J.; Du, H.; Liu, T.; Chen, C.; Yan, Y.; Liu, T.; Liu, L.; Wang, E. Accurate reflection of hepatopancreas antioxidation and detoxification in Procambarus clarkii during virus infection and drug treatment: Reference gene selection, evaluation and expression analysis. Aquaculture 2022, 556, 738283. [Google Scholar] [CrossRef]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Fang, L.; Guan, C.; Xu, Y. Heat-shock protein 90 alleviates oxidative stress and reduces apoptosis in liver of Seriola aureovittata (yellowtail kingfish) under high-temperature stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 270, 110927. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, X.; Ren, X.; Jia, S.; Gao, T.; Wang, J.; Wang, J.; Liu, P.; Li, J.; Li, J. Marsupenaeus japonicus HSP90’s Function Under Low Temperature Stress. Biology 2025, 14, 966. https://doi.org/10.3390/biology14080966
Bian X, Ren X, Jia S, Gao T, Wang J, Wang J, Liu P, Li J, Li J. Marsupenaeus japonicus HSP90’s Function Under Low Temperature Stress. Biology. 2025; 14(8):966. https://doi.org/10.3390/biology14080966
Chicago/Turabian StyleBian, Xueqiong, Xianyun Ren, Shaoting Jia, Tian Gao, Junxia Wang, Jiajia Wang, Ping Liu, Jian Li, and Jitao Li. 2025. "Marsupenaeus japonicus HSP90’s Function Under Low Temperature Stress" Biology 14, no. 8: 966. https://doi.org/10.3390/biology14080966
APA StyleBian, X., Ren, X., Jia, S., Gao, T., Wang, J., Wang, J., Liu, P., Li, J., & Li, J. (2025). Marsupenaeus japonicus HSP90’s Function Under Low Temperature Stress. Biology, 14(8), 966. https://doi.org/10.3390/biology14080966





