Effects of Interspecific Competition on Habitat Shifts of Sardinops melanostictus (Temminck et Schlegel, 1846) and Scomber japonicus (Houttuyn, 1782) in the Northwest Pacific
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Fisheries Data
2.1.2. Environmental Data
2.2. Methods of Analysis
2.2.1. Constructing Single-Species Suitable Habitat Models (Single-Species SDM, ssSDM)
2.2.2. Construction of the Interspecific Competition Species Distribution Model (icSDM)
2.2.3. Building on the Framework of Interspecific Competition, the Temporal Variations in Suitable Habitats for Sardinops melanostictus and S. japonicus Were Analyzed Both on a Monthly and Annual Scale
3. Results
3.1. Development of the icSDM for S. melanostictus and S. japonicus
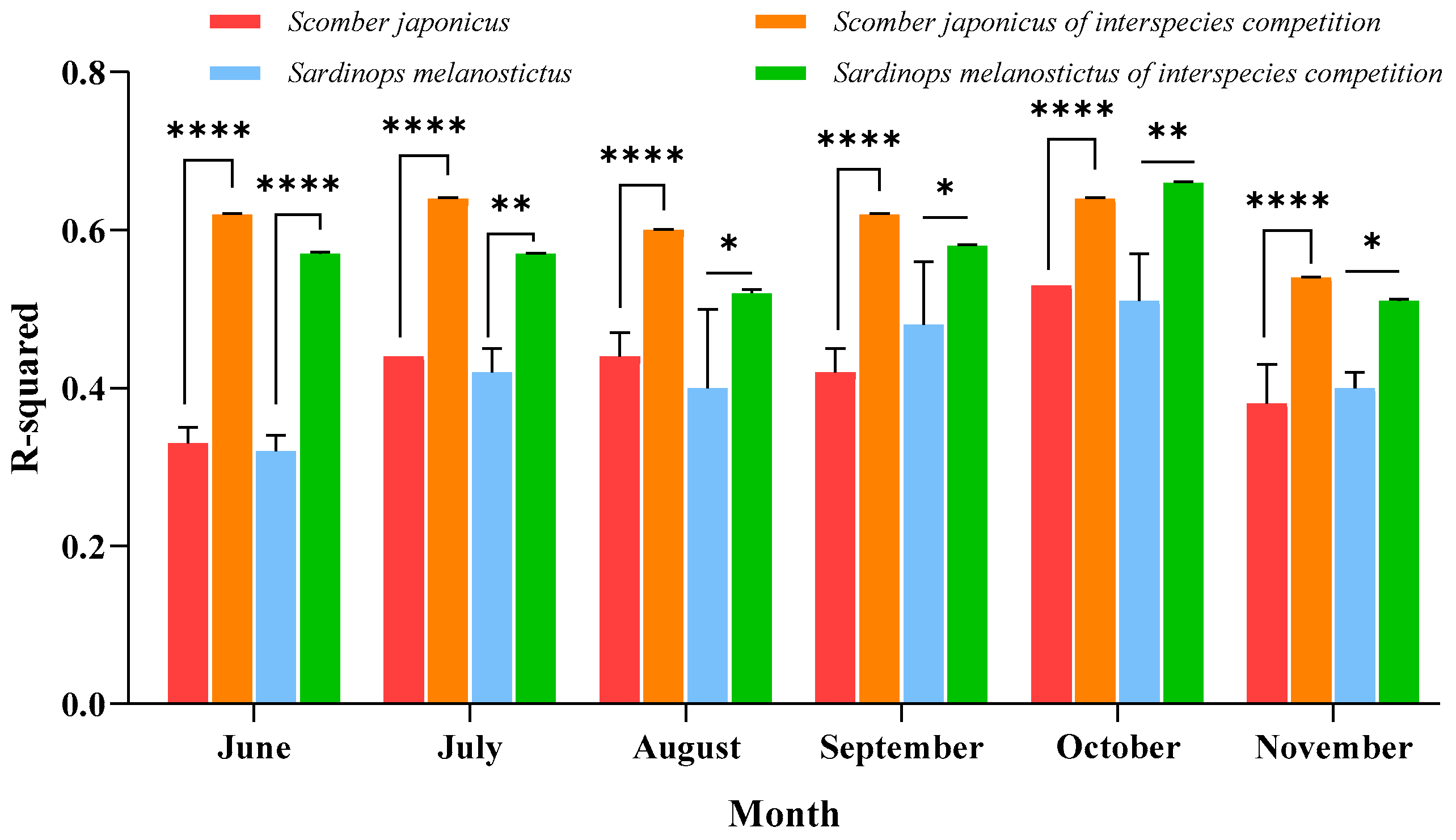
3.2. Validation of the Impact of Interspecific Competition on the Habitat Distribution of Both Species
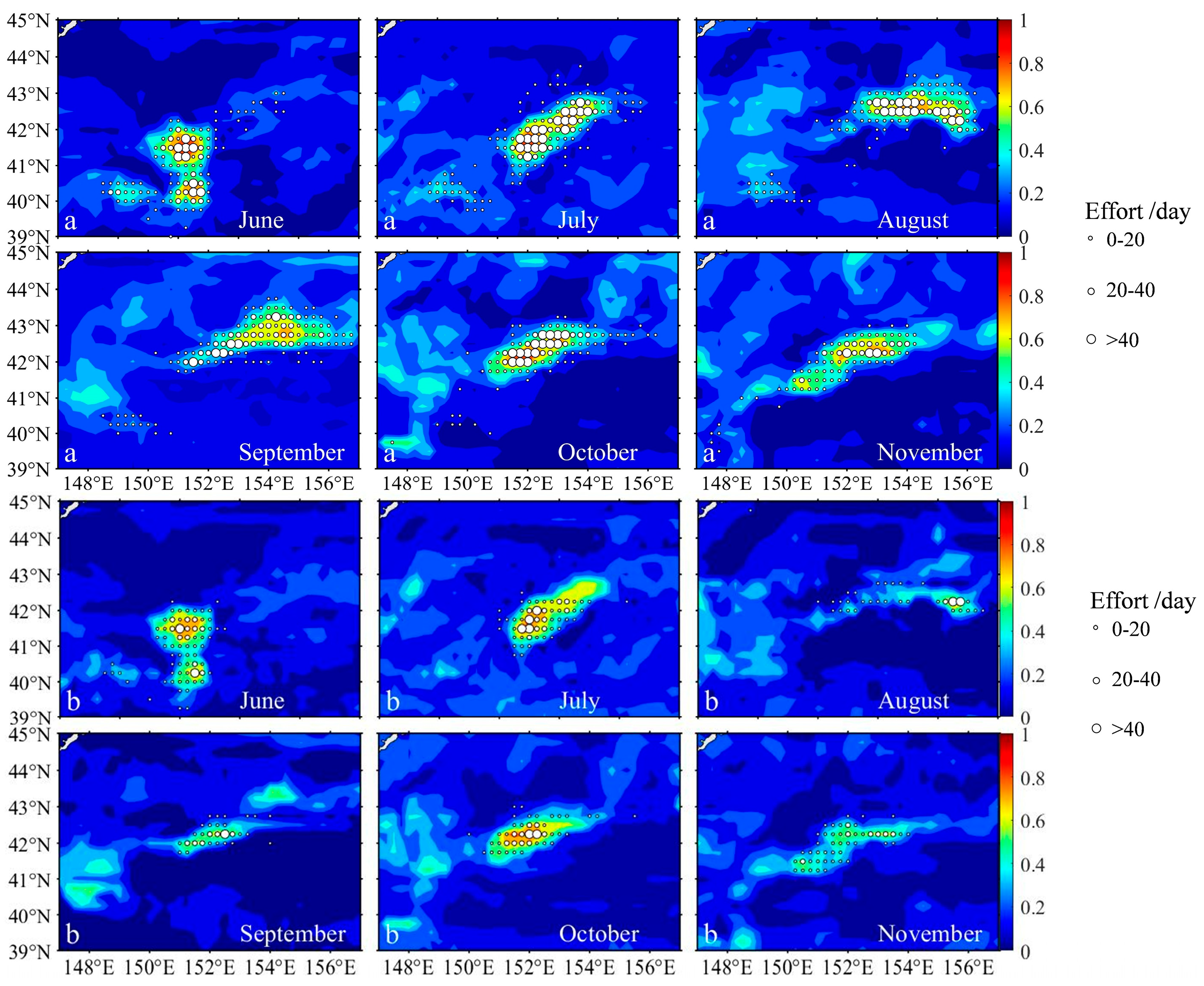
3.3. The Impact of Interspecific Competition on Habitat Dynamics in S. melanostictus and S. japonicus
3.4. The Correlation Between Habitat Variations of Sardinops melanostictus and S. japonicus
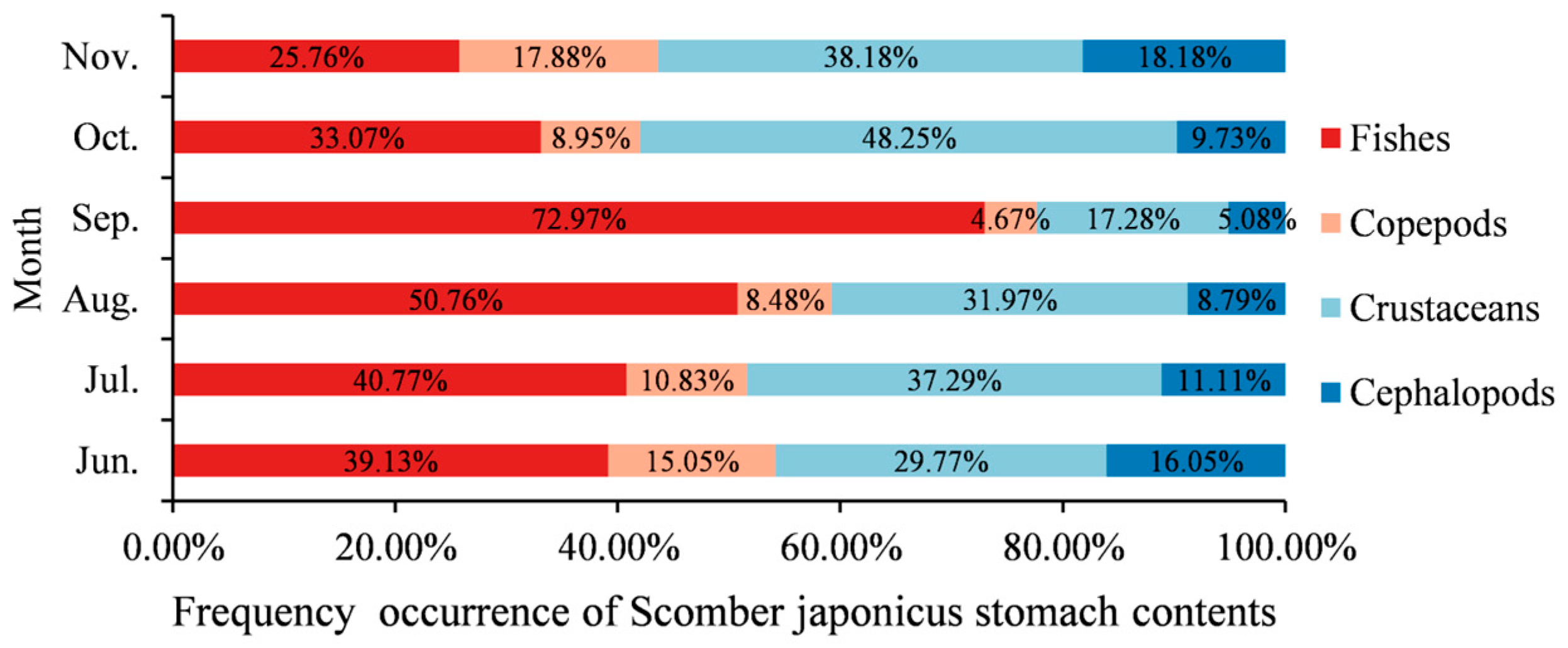
3.5. Fork Length, Body Weight, and Stomach Content Composition of Sardinops melanostictus and Scomber japonicus
4. Discussion
4.1. Advantage Analysis of Establishing an icSDM
4.2. The Impact of Interspecies Competitive Relationships Between S. melanostictus and S. japonicus on Habitat Changes
4.2.1. The Significance of Interspecies Competitive Relationships in Driving Habitat Changes Among Associated Species
4.2.2. Divergent Responses of Interspecific Competition to Habitat Changes for Both Species
4.2.3. The Effect of Predation and Being Preyed upon on the Interspecific Competitive Relationship Between the Two Species
4.3. Correlation Between Habitat Changes of Sardinops melanostictus and S. japonicus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klyashtorin, L.B. Long-term climate change and main commercial fish production in the Atlantic and Pacific. Fish. Res. 1998, 37, 115–125. [Google Scholar] [CrossRef]
- Fuji, T.; Nakayama, S.I.; Hashimoto, M.; Hashimoto, M.; Kamimura, Y.; Furuichi, S.; Oshima, K.; Suyama, S. Biological interactions potentially alter the large-scale distribution pattern of the small pelagic fish, Pacific saury Cololabis saira. Mar. Ecol. Prog. Ser. 2023, 704, 99–117. [Google Scholar] [CrossRef]
- Liu, S.H.; Tian, Y.J.; Liu, Y.; Alabia, I.D.; Cheng, J.H.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II Top. Stud. Oceanogr. 2023, 207, 105227. [Google Scholar] [CrossRef]
- Shi, Y.C.; Kang, B.; Fan, W.; Xu, L.L.; Zhang, S.M.; Cui, X.S.; Dai, Y. Spatio-Temporal Variations in the Potential Habitat Distribution of Pacific Sardine (Sardinops melanostictus) in the Northwest Pacific Ocean. Fishes 2023, 8, 86. [Google Scholar] [CrossRef]
- NPFC Secretariat. Summary Footprint of Chub Mackerel Fisheries; North Pacific Fisheries Commission: Tokyo, Japan, 2023; pp. 1–6. [Google Scholar]
- Nakayama, S.I.; Takasuka, A.; Ichinokawa, M.; Okamura, H. Climate change and interspecific interactions drive species alternations between anchovy and sardine in the western North Pacific: Detection of causality by convergent cross mapping. Fish. Oceanogr. 2018, 27, 312–322. [Google Scholar] [CrossRef]
- Yatsu, A.; Kawabata, A. Reconsidering Trans-Pacific “synchrony” in population fluctuations of sardines. Bull. Jpn. Soc. Fish. Oceanogr 2017, 81, 271–283. [Google Scholar]
- Liu, S.Y.; Zhang, H.; Yang, C.; Fang, Z. Differences in habitat distribution of Sardinops melanostictus and Scomber japonicus in the Northwest Pacific based on a maximum entropy model. J. Shanghai Ocean Univ. 2023, 32, 806–817. [Google Scholar]
- Shi, Y.S.; Zhang, X.M.; Yang, S.Y.; Dai, Y.; Cui, X.S.; Wu, Y.M.; Zhang, S.M.; Fan, W.; Han, H.B.; Zhang, H.; et al. Construction of CPUE standardization model and its simulation testing for chub mackerel (Scomber japonicus) in the Northwest Pacific Ocean. Ecol. Indic. 2023, 155, 111022. [Google Scholar] [CrossRef]
- Ma, S.Y.; Fu, C.H.; Li, J.C.; Sun, P.; Liu, Y.; Ye, Z.J.; Watanable, Y.; Tian, Y.G. Non-stationary effects of multiple drivers on the dynamics of Japanese sardine (Sardinops melanostictus, Clupeidae). Fish Fish. 2023, 24, 40–55. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lemoir, J. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Han, H.B.; Yang, C.; Jiang, B.H.; Shang, C.; Sun, Y.Y.; Zhao, X.Y.; Xiang, D.X.; Zhang, H.; Shi, Y.C. Construction of chub mackerel (Scomber japonicus) fishing ground prediction model in the northwestern Pacific Ocean based on deep learning and marine environmental variables. Mar. Pollut. Bull. 2023, 193, 115158. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, H.; Yang, C.; Fang, Z. Relationship between stock dynamics and environmental variability for Japanese sardine (Sardinops melanostictus) and chub mackerel (Scomber japonicus) in the Northwest Pacific Ocean: A review. J. Dalian Ocean Univ. 2023, 38, 357–368. [Google Scholar]
- Wang, Z.Q.; Ito, S.; Yabe, I.; Guo, C.Y. Development of a bioenergetics and population dynamics coupled model: A case study of chub mackerel. Front. Mar. Sci. 2023, 10, 1142899. [Google Scholar] [CrossRef]
- Chavez, F.P.; Ryan, J.; Lluch-Cota, S.E.; Ñiquen, C.M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Oozeki, Y.; Carranza, M.Ñ.; Takasuka, A.; Dejo, P.A.; Kuroda, H.; Malagas, J.T.; Okunishi, T.; Espinoza, L.V.; Aguilar, D.G.; Okamura, H.; et al. Synchronous multi-species alternations between the northern Humboldt and Kuroshio Current systems. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 11–21. [Google Scholar] [CrossRef]
- Sogawa, S.; Hidaka, K.; Kamimura, Y.; Takahshi, M.; Saito, H.; Okazaki, Y.; Setou, T. Environmental characteristics of spawning and nursery grounds of Japanese sardine and mackerels in the Kuroshio and Kuroshio Extension area. Fish. Oceanogr. 2019, 28, 454–467. [Google Scholar] [CrossRef]
- Gkanasos, A.; Schismenou, E.; Tsiaras, K.; Somarakis, S.; Giannoulaki, M.; Sofianos, S. A three dimensional, full life cycle, anchovy and sardine model for the North Aegean Sea (Eastern Mediterranean): Validation, sensitivity and climatic scenario simulations. Medit. Mar. Sci. 2021, 22, 653–668. [Google Scholar] [CrossRef]
- Umezawa, Y.; Ono, Y.; Okunishi, T.; Yukami, R.; Kamimura, Y.; Yoshimizu, C.; Tayasu, I. Investigation of inter-annual variation in the feeding habits of Japanese sardine (Sardinops melanostictus) and mackerels (Scomber spp.) in the Western North Pacific based on bulk and amino acid stable isotopes. Front. Mar. Sci. 2023, 10, 1225923. [Google Scholar] [CrossRef]
- Bai, X.; Gao, L.; Choi, S. Exploring the Response of the Japanese Sardine (Sardinops melanostictus) Stock-Recruitment Relationship to Environmental Changes under Different Structural Models. Fishes 2022, 7, 276. [Google Scholar] [CrossRef]
- Yen, K.W.; Lu, H.J.; Chang, Y.; Lee, M.A. Using remote-sensing data to detect habitat suitability for yellowfin tuna in the Western and Central Pacific Ocean. Int. J. Remote Sens. 2012, 33, 7507–7522. [Google Scholar] [CrossRef]
- Pavlov, Y.L. Random Forests; Karelian Centre Russia Academy of Sciences: Petrozavodsk, Russia, 1997; Volume 45, pp. 5–32. [Google Scholar]
- Gao, F.; Chen, X.J.; Guan, W.J.; Li, G. A new model to forecast fishing ground of Scomber japonicus in the Yellow Sea and East China Sea. Acta Oceanol. Sin. 2016, 35, 74–81. [Google Scholar] [CrossRef]
- Cai, K.; Kindong, R.; Ma, Q.Y.; Tian, S.Q. Stock Assessment of Chub Mackerel (Scomber japonicus) in the Northwest Pacific Using a Multi-Model Approach. Fishes 2023, 8, 80. [Google Scholar] [CrossRef]
- Mondal, S.; Wang, Y.C.; Lee, M.A.; Weng, J.S.; Mondal, B.K. Ensemble three-dimensional habitat modeling of Indian Ocean immature albacore tuna (Thunnus alalunga) using remote sensing data. Remote Sens. 2022, 14, 5278. [Google Scholar] [CrossRef]
- Akia, S. Du Global au Local: Étude des Interactions Entre les Thons de la ZEE de la Cote d’Ivoire et le Reste du Stock de l’Atlantique Est à Partir des Données du Programme de Marquage AOTTP de l’ICCAT et de Quelques Indicateurs Biologiques. Doctoral Dissertation, Université Montpellier, Montpellier, France, 2021. [Google Scholar]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Brooker, R.W. Plant–plant interactions and environmental change. New Phytol. 2006, 171, 271–284. [Google Scholar] [CrossRef]
- Meier, E.S.; Edwards Jr, T.C.; Kienast, F.; Dobbertin, M.; Zimmermann, N.Z. Co-occurrence patterns of trees along macro-climatic gradients and their potential influence on the present and future distribution of Fagus sylvatica L. J. Biogeogr. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Bakun, A.; Broad, K. Environmental ‘loopholes’ and fish population dynamics: Comparative pattern recognition with focus on El Niño effects in the Pacific. Fish. Oceanogr. 2003, 12, 458–473. [Google Scholar] [CrossRef]
- Yu, W.; Guo, A.; Zhang, Y.; Chen, X.J.; Qian, W.G.; Li, Y.S. Climate-induced habitat suitability variations of chub mackerel Scomber japonicus in the East China Sea. Fish. Res. 2018, 207, 63–73. [Google Scholar] [CrossRef]
- Bordalo-Machado, P. Fishing effort analysis and its potential to evaluate stock size. Rev. Fish. Sci. 2006, 14, 369–393. [Google Scholar] [CrossRef]
- Yang, R.M.; Zhang, G.L.; Liu, F.; Lu, Y.Y.; Yang, F.; Yang, F.; Yang, M.; Zhao, Y.G.; Li, D.C. Comparison of boosted regression tree and random forest models for mapping topsoil organic carbon concentration in an alpine ecosystem. Ecol. Indic. 2016, 60, 870–878. [Google Scholar] [CrossRef]
- Xue, Y.; Guan, L.S.; Tanaka, K.; Li, Z.G.; Chen, Y.; Ren, Y.P. Evaluating effects of rescaling and weighting data on habitat suitability modeling. Fish. Res. 2017, 188, 84–94. [Google Scholar] [CrossRef]
- Sakamoto, T.; Takahashi, M.; Chung, M.T.; Rzewski, R.R.; Komatsu, K.; Shirai, K.; Ishimura, T.; Higuchi, T. Contrasting life-history responses to climate variability in eastern and western North Pacific sardine populations. Nat. Commun. 2022, 13, 5298. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.W.; Tang, F.H.; Fan, W.; Zhang, H.; Cui, X.S.; Guo, G.G. Distribution of resource and environment characteristics of fishing ground of Scomber japonicas in the North Pacific high seas. Mar. Fish. 2017, 39, 372–382. [Google Scholar]
- Aono, T.; Sakamoto, T.; Ishimura, T.; Takahashi, M.; Yasuda, T.; Kitajima, S.; Nishida, K.; Matsuura, T.; Ikari, A.; Ito, S.I. Migration patterns of the Japanese sardine in the Sea of Japan by combining the microscale stable isotope analysis of otoliths and an ocean data assimilation model. Front. Mar. Sci. 2024, 11, 1394801. [Google Scholar] [CrossRef]
- Yang, C.; Han, H.B.; Zhang, H.; Shi, Y.C.; Su, B.; Jiang, P.W.; Xiang, D.; Sun, Y.Y.; Li, Y. Assessment and management recommendations for the status of Japanese sardine Sardinops melanostictus population in the Northwest Pacific. Ecol. Indic. 2023, 148, 110111. [Google Scholar] [CrossRef]
- Takasuka, A.; Kubota, H.; Oozeki, Y. Spawning overlap of anchovy and sardine in the western North Pacific. Mar. Ecol. Prog. Ser. 2008, 366, 231–244. [Google Scholar] [CrossRef]
- Kanamori, Y.; Takasuka, A.; Nishijima, S.; Okamura, H. Climate change shifts the spawning ground northward and extends the spawning period of chub mackerel in the western North Pacific. Mar. Ecol. Prog. Ser. 2019, 624, 155–166. [Google Scholar] [CrossRef]
- Yatsu, A. Review of population dynamics and management of small pelagic fishes around the Japanese Archipelago. Fish. Sci. 2019, 85, 611–639. [Google Scholar] [CrossRef]
- Kamimura, Y.; Taga, M.; Yukami, R.; Watanabe, C.; Furuichi, S. Intra-and inter-specific density dependence of body condition, growth, and habitat temperature in chub mackerel (Scomber japonicus). ICES J. Mar. Sci. 2021, 78, 3254–3264. [Google Scholar] [CrossRef]
- Wang, L.M.; Ma, S.Y.; Liu, Y.; Li, J.C.; Liu, S.G.; Lin, L.S.; Tian, Y.J. Fluctuations in the abundance of chub mackerel in relation to climatic/oceanic regime shifts in the northwest Pacific Ocean since the 1970s. J. Mar. Syst. 2022, 218, 103541. [Google Scholar] [CrossRef]
- Nishikawa, H.; Itoh, S.; Yasuda, I.; Komatsu, K. Overlap between suitable nursery grounds for Japanese anchovy (Engraulis japonicus) and Japanese sardine (Sardinops melanostictus) larvae. Aquaculture, Fish Fish. 2022, 2, 179–188. [Google Scholar] [CrossRef]
- Kuroda, H.; Saito, T.; Kaga, T.; Takasuka, A.; Kamimura, Y.; Furuichi, S.; Nakanowatari, T. Unconventional sea surface temperature regime around Japan in the 2000s–2010s: Potential influences on major fisheries resources. Front. Mar. Sci. 2020, 7, 574904. [Google Scholar] [CrossRef]
- Sarr, O.; Kindong, R.; Tian, S. Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846). J. Mar. Sci. Eng. 2021, 9, 1403. [Google Scholar] [CrossRef]
- Hong, J.B.; Kim, D.Y.; Kim, D.H. Stock Assessment of Chub Mackerel (Scomber japonicus) in the Northwest Pacific Ocean Based on Catch and Resilience Data. Sustainability 2022, 15, 358. [Google Scholar] [CrossRef]
- Tang, F.H.; Dai, S.W.; Fan, W.; Cui, X.S.; Zhang, H. Study on stomach composition and feeding level of Chub Mackerel in the Northwest Pacific. J. Agric. Sci. Technol. 2020, 22, 138–1148. [Google Scholar]
- Tzeng, T.D.; Haung, H.L.; Wang, D.; Yeh, S.Y. Genetic diversity and population expansion of the common mackerel (Scomber japonicus) off Taiwan. J. Fish. Soc. Taiwan 2007, 34, 237–245. [Google Scholar]
- Xue, Y.; Jin, X.S.; Zhang, B.; Lang, Z.L. Feeding habits of three sciaenid fishes in the Southern Yellow Sea. J. Fish. China 2005, 29, 178–187. [Google Scholar]
- Zhang, B. Feeding and varies with the change of body length of Cleisthenes herzensteini in the central of the Yellow Sea. Chin. J. Appl. Ecol. 2007, 18, 1849–1854. [Google Scholar]
- Bascompte, J. Mutualistic networks. Front. Ecol. Environ. 2009, 7, 429–436. [Google Scholar] [CrossRef]
- Schweiger, O.; Heikkinen, R.K.; Harpke, A.; Hickler, T.; Klotz, S.; Kudrna, O.; Kühn, L.; Pöyry, J.; Settele, J. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob. Ecol. Biogeogr. 2012, 21, 88–99. [Google Scholar] [CrossRef]
- Li, Y.S.; Pan, L.Z.; Chen, X.J. Effect of spawning ground location on the transport and growth of chub mackerel (Scomber japonicus) eggs and larvae in the East China Sea. Acta Oceanol. Sin. 2014, 34, 92–97. [Google Scholar] [CrossRef]
- Levine, J.M.; Bascompte, J.; Adler, P.B.; Allesina, S. Beyond pairwise mechanisms of species coexistence in complex communities. Nature 2017, 546, 56–64. [Google Scholar] [CrossRef]
- Bower, J.R.; Ichii, T. The red flying squid (Ommastrephes bartramii): A review of recent research and the fishery in Japan. Fish. Res. 2005, 76, 39–55. [Google Scholar] [CrossRef]
- Laird, R.A.; Schamp, B.S. Competitive intransitivity promotes species coexistence. Am. Nat. 2006, 168, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Allesina, S.; Levine, J.M. A competitive network theory of species diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 5638–5642. [Google Scholar] [CrossRef] [PubMed]
- Gallien, L.; Zimmermann, N.E.; Levine, J.M.; Adler, P.B. The effects of intransitive competition on coexistence. Ecol. Lett. 2017, 20, 791–800. [Google Scholar] [CrossRef] [PubMed]




| Years | Species | Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | November | Total | ||
| 2017 | S. melanostictus | 164 | 177 | 172 | 157 | 150 | 132 | 952 |
| S. japonicus | 124 | 136 | 106 | 87 | 88 | 78 | 619 | |
| Overlap | 112 | 124 | 84 | 87 | 85 | 72 | 564 | |
| 2018 | S. melanostictus | 65 | 67 | 32 | 32 | 28 | 24 | 248 |
| S. japonicus | 75 | 77 | 40 | 41 | 33 | 88 | 354 | |
| Overlap | 65 | 67 | 30 | 31 | 28 | 24 | 245 | |
| 2019 | S. melanostictus | 43 | 27 | 30 | 13 | 13 | 25 | 151 |
| S. japonicus | 42 | 27 | 32 | 13 | 13 | 26 | 153 | |
| Overlap | 42 | 27 | 29 | 9 | 13 | 25 | 145 | |
| 2020 | S. melanostictus | 50 | 51 | 43 | 48 | 41 | 48 | 281 |
| S. japonicus | 37 | 45 | 34 | 31 | 37 | 48 | 232 | |
| Overlap | 32 | 45 | 34 | 30 | 37 | 46 | 224 | |
| 2021 | S. melanostictus | 110 | 131 | 113 | 125 | 91 | 83 | 653 |
| S. japonicus | 70 | 52 | 32 | 29 | 42 | 47 | 272 | |
| Overlap | 70 | 52 | 30 | 29 | 40 | 45 | 266 | |
| Variable (unit) | Name | Time Frame | Spatial Resolution | Source | Data Acquisition |
|---|---|---|---|---|---|
| T_0 m (°C) | Sea surface temperature | June to November 2017–2021 | 0.25° | APDRC | http://apdrc.soest.hawaii.edu/las_ofes/v6/dataset?catitem=71 (accessed on 9 October 2023) |
| T_50 m (°C) | Sea 50 m depth temperature | ||||
| T_100 m (°C) | Sea 100 m depth temperature | ||||
| T_150 m (°C) | Sea 150 m depth temperature | ||||
| T_200 m (°C) | Sea 200 m depth temperature | ||||
| SSH (m) | Sea surface height | ||||
| SSS (‰) | Sea surface salinity | CMEMS | https://marine.copernicus.eu (accessed on 10 October 2023) | ||
| Chl.a (mg m−3) | Chlorophyll a | ||||
| U (m s−1) | X-direction | ||||
| V (m s−1) | Y-direction | ||||
| EKE (m s−1) | Eddy kinetic energy | ||||
| SSTA | Sea surface temperature anomaly | NOAA | https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php (accessed on 10 October 2023) | ||
| ONI | Ocean Nino index |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhu, H.; Wang, J.; Zhang, F.; Zhang, S.; Zhang, H. Effects of Interspecific Competition on Habitat Shifts of Sardinops melanostictus (Temminck et Schlegel, 1846) and Scomber japonicus (Houttuyn, 1782) in the Northwest Pacific. Biology 2025, 14, 968. https://doi.org/10.3390/biology14080968
Liu S, Zhu H, Wang J, Zhang F, Zhang S, Zhang H. Effects of Interspecific Competition on Habitat Shifts of Sardinops melanostictus (Temminck et Schlegel, 1846) and Scomber japonicus (Houttuyn, 1782) in the Northwest Pacific. Biology. 2025; 14(8):968. https://doi.org/10.3390/biology14080968
Chicago/Turabian StyleLiu, Siyuan, Hanji Zhu, Jianhua Wang, Famou Zhang, Shengmao Zhang, and Heng Zhang. 2025. "Effects of Interspecific Competition on Habitat Shifts of Sardinops melanostictus (Temminck et Schlegel, 1846) and Scomber japonicus (Houttuyn, 1782) in the Northwest Pacific" Biology 14, no. 8: 968. https://doi.org/10.3390/biology14080968
APA StyleLiu, S., Zhu, H., Wang, J., Zhang, F., Zhang, S., & Zhang, H. (2025). Effects of Interspecific Competition on Habitat Shifts of Sardinops melanostictus (Temminck et Schlegel, 1846) and Scomber japonicus (Houttuyn, 1782) in the Northwest Pacific. Biology, 14(8), 968. https://doi.org/10.3390/biology14080968








