A Synergistic Role of Photosynthetic Bacteria and Fungal Community in Pollutant Removal in an Integrated Aquaculture Wastewater Bioremediation System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
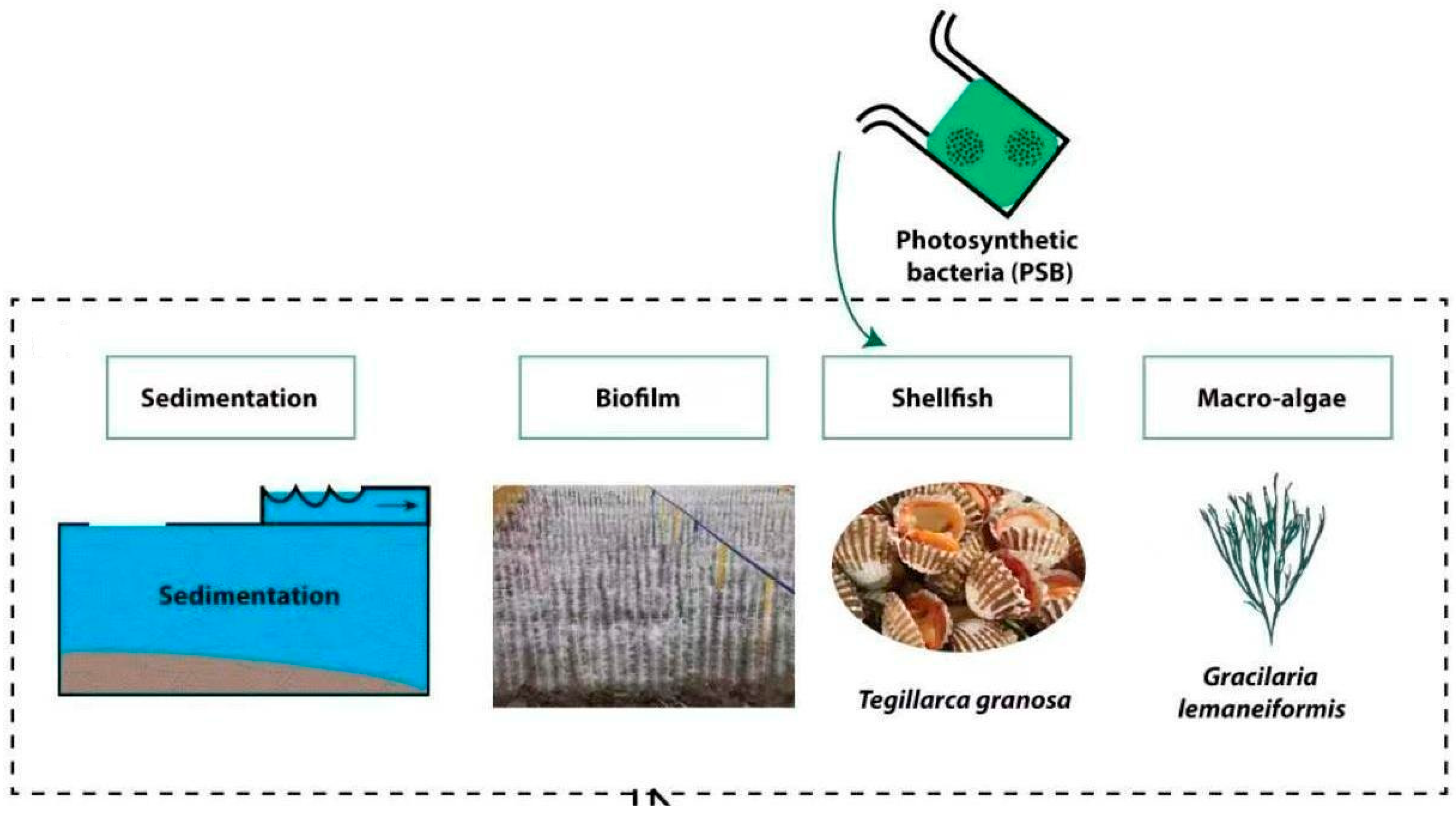
2.1. Experimental Setup
2.2. Sample Collection and Measurement Method
2.3. Internal Transcribed Spacer Sequencing and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
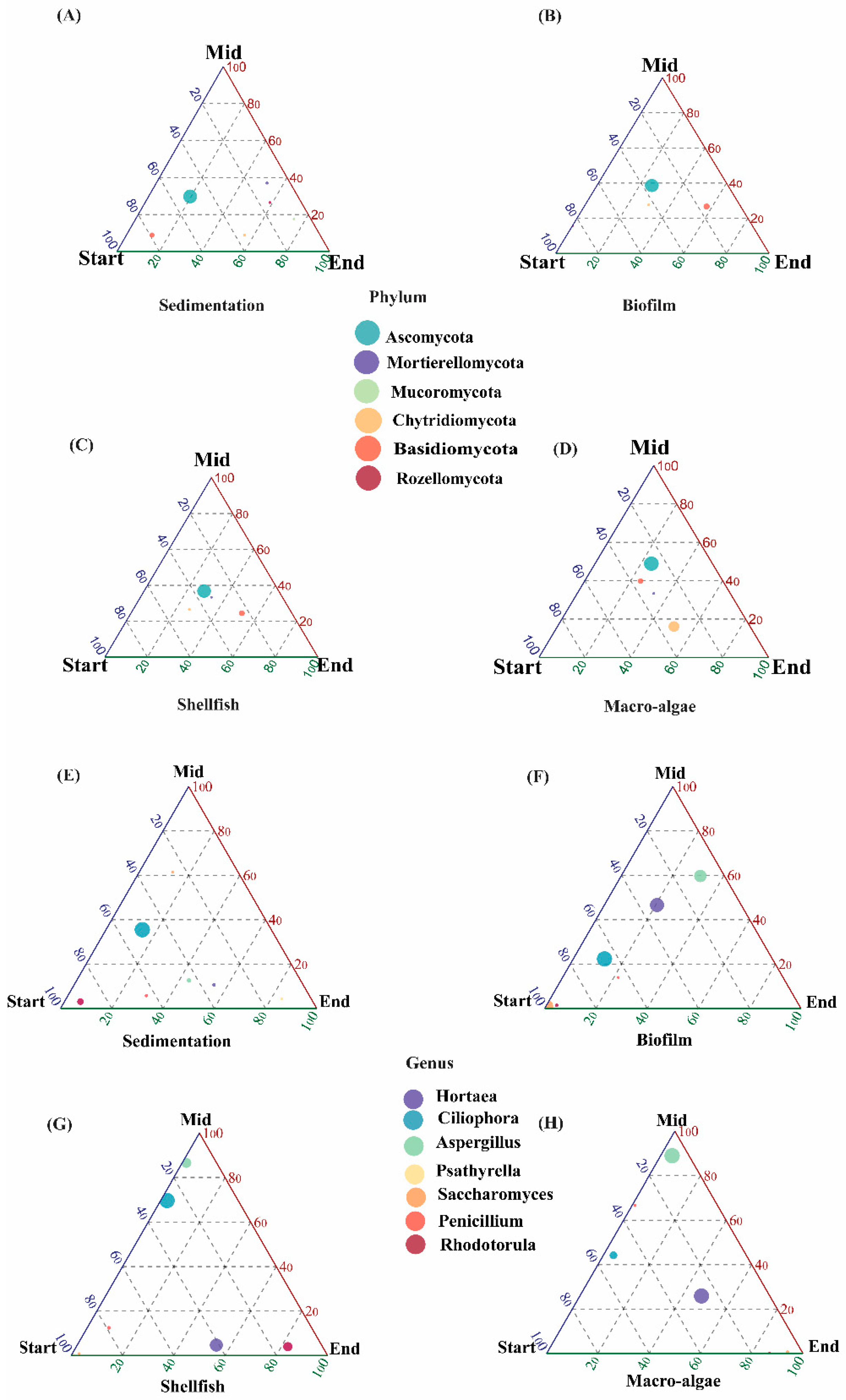
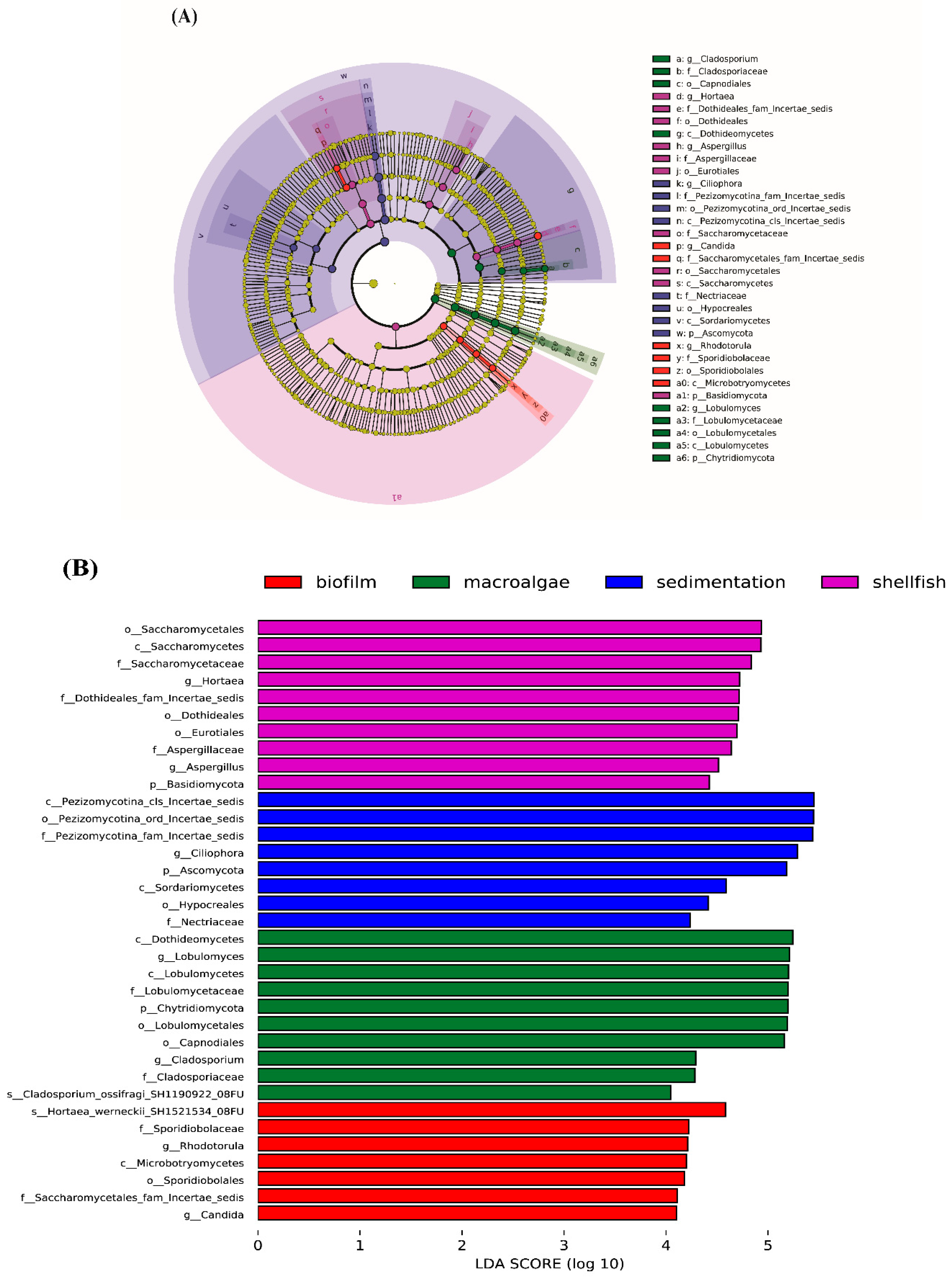
3.1. Fungal Community Composition
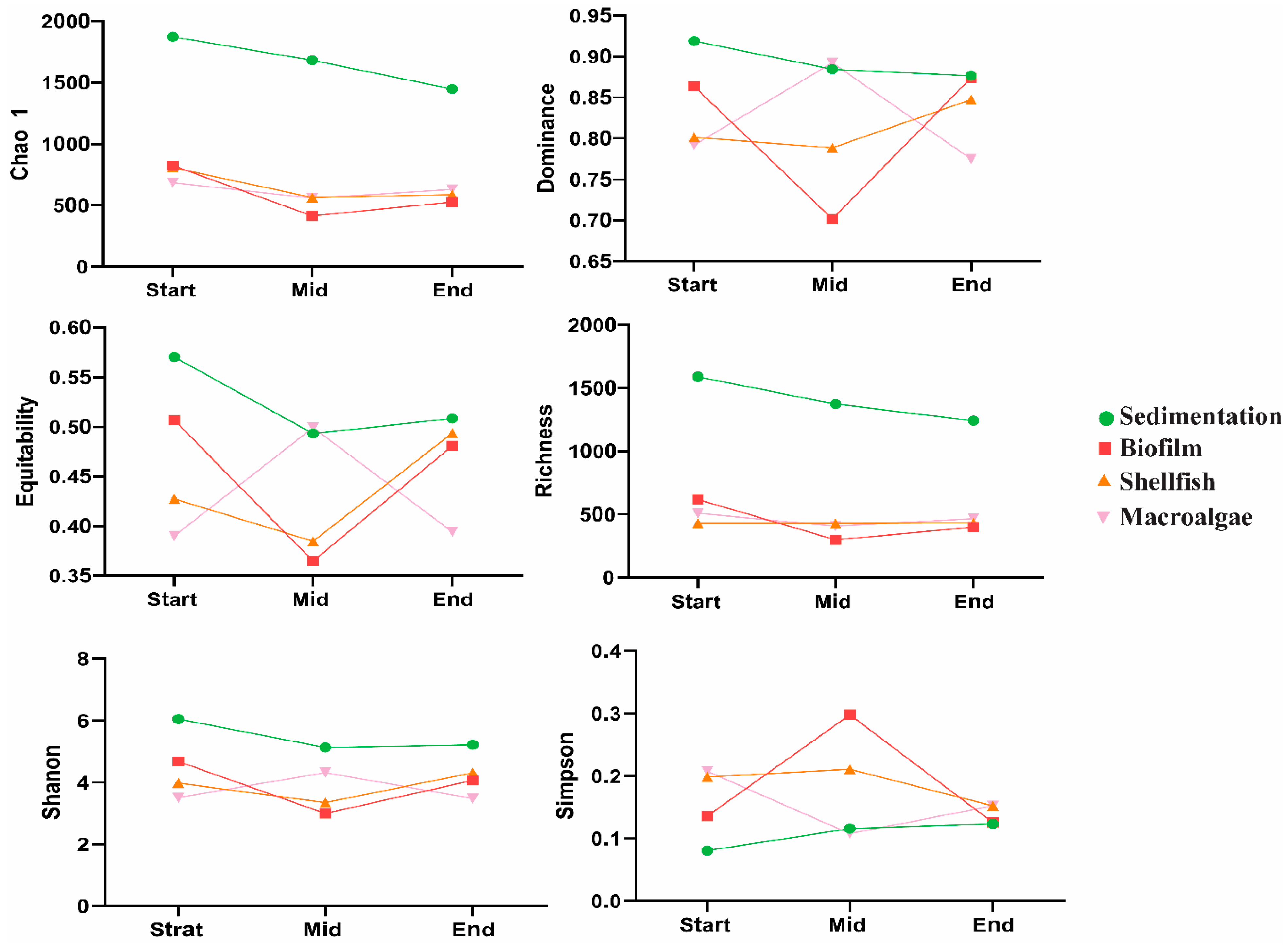
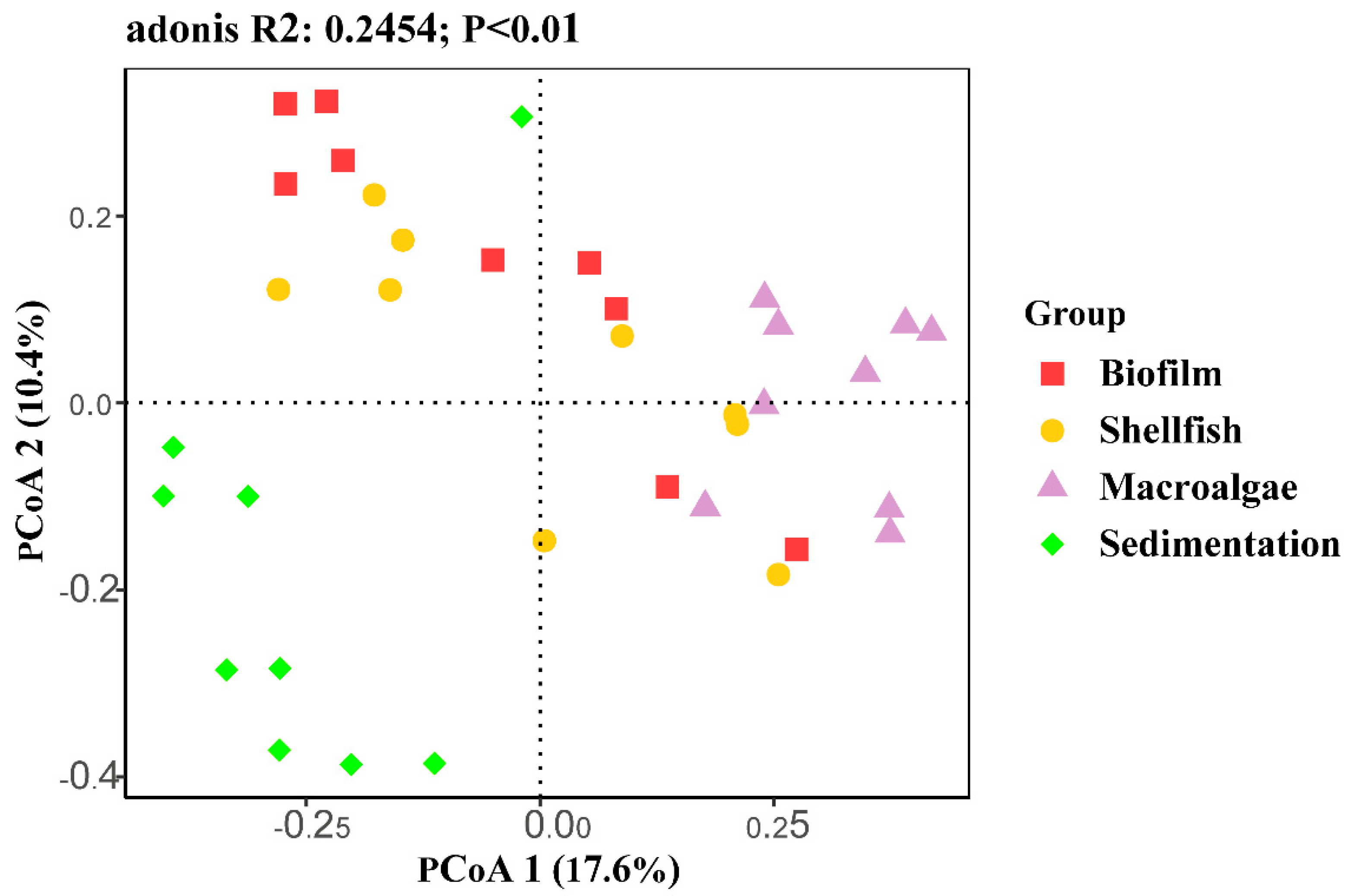
3.2. Dynamics of the Fungal Community
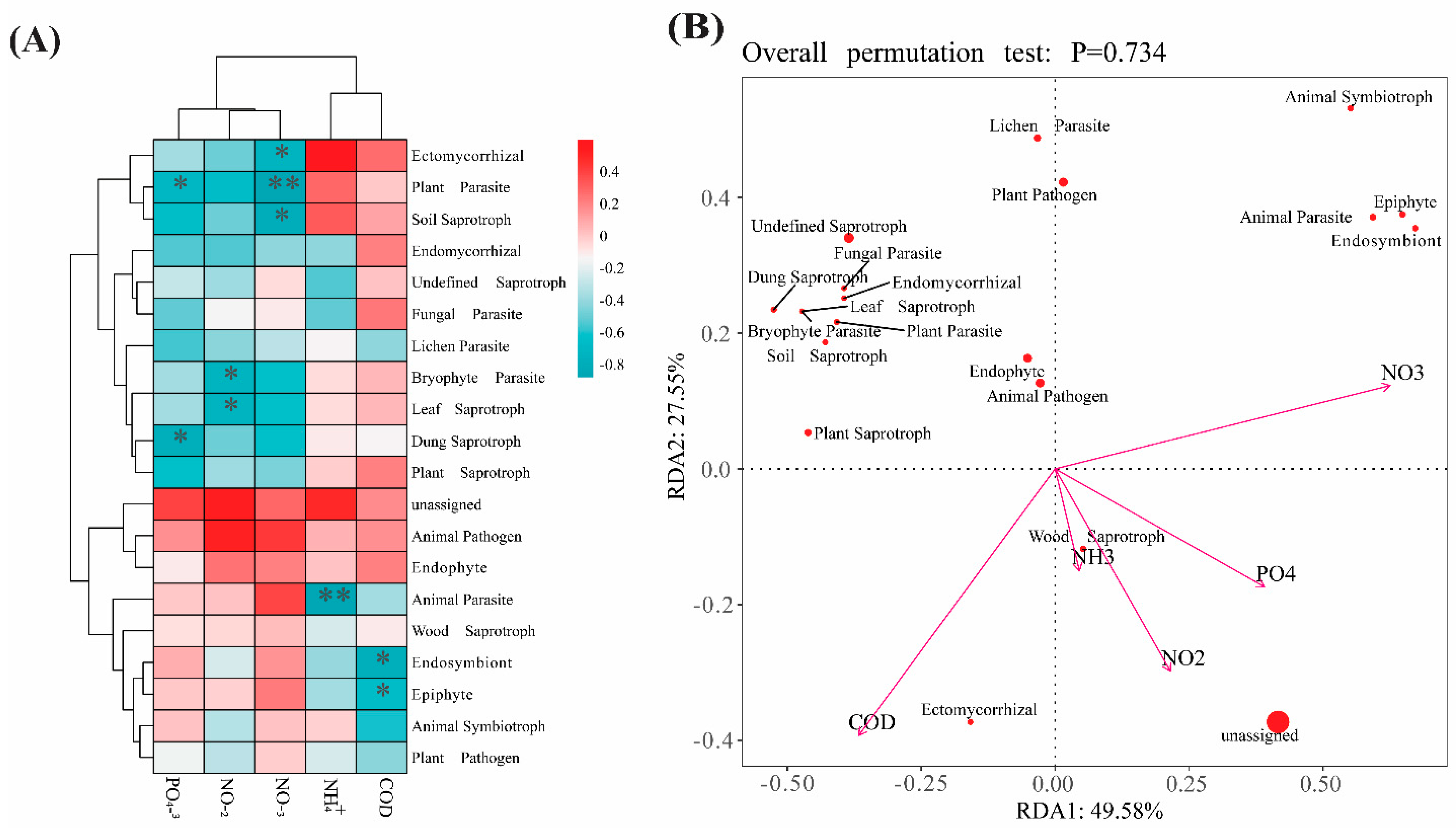
3.3. Correlation Between Nutrients and the Fungal Community
4. Discussion
4.1. Community Composition
4.2. Diversity Indices and Community Composition Shifts
4.3. Fungi Relationships with Nutrients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tucciarone, I.; Secci, G.; Contiero, B.; Parisi, G. Sustainable aquaculture over the last 30 years: An analysis of the scientific literature by the Text Mining approach. Rev. Aquac. 2024, 16, 2064–2076. [Google Scholar] [CrossRef]
- John, E.M.; Krishnapriya, K.; Sankar, T. Treatment of ammonia and nitrite in aquaculture wastewater by an assembled bacterial consortium. Aquaculture 2020, 526, 735390. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008. [Google Scholar]
- Rossi, S.; Pizzera, A.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Piggery wastewater treatment with algae-bacteria consortia: Pilot-scale validation and techno-economic evaluation at farm level. Bioresour. Technol. 2022, 351, 127051. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Muñoz, C.A.; Torres-Franco, A.F.; de Godos, I.; Muñoz, R. Exploring the metabolic capabilities of purple phototrophic bacteria during piggery wastewater treatment. J. Water Process. Eng. 2022, 50, 103317. [Google Scholar] [CrossRef]
- Sun, P.; Liu, C.; Li, A.; Ji, B. Using carbon dioxide-added microalgal-bacterial granular sludge for carbon-neutral municipal wastewater treatment under outdoor conditions: Performance, granule characteristics and environmental sustainability. Sci. Total Environ. 2022, 848, 157657. [Google Scholar] [CrossRef]
- Sun, X.; Xie, T.; Zhang, C.; Zhang, Y.; Zhou, X. Advances in the resource recovery treatment of livestock and poultry wastewater using photosynthetic microorganism. Ind. Water Treat. 2024, 44, 40. [Google Scholar]
- Morrison, H.M.; Bose, A. Purple non-sulfur bacteria for biotechnological applications. J. Ind. Microbiol. Biotechnol. 2025, 52, kuae052. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; He, S.; Zhao, R.; Zhu, D. Purple non-sulfur bacteria technology: A promising and potential approach for wastewater treatment and bioresources recovery. World J. Microbiol. Biotechnol. 2021, 37, 161. [Google Scholar] [CrossRef]
- Puyol, D.; Barry, E.; Hülsen, T.; Batstone, D. A mechanistic model for anaerobic phototrophs in domestic wastewater applications: Photo-anaerobic model (PAnM). Water Res. 2017, 116, 241–253. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, G.; Zhang, J.; Li, X.; Li, J. Performance, 5-aminolevulinic acid (ALA) yield and microbial population dynamics in a photobioreactor system treating soybean wastewater: Effect of hydraulic retention time (HRT) and organic loading rate (OLR). Bioresour. Technol. 2016, 210, 146–152. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Dai, H.; Zhao, Y.; Li, D.; Duan, W.; Guo, Y. Microplastics affect the ammonia oxidation performance of aerobic granular sludge and enrich the intracellular and extracellular antibiotic resistance genes. J. Hazard. Mater. 2021, 409, 124981. [Google Scholar] [CrossRef]
- Tarabas, O.V.; Hnatush, S.O.; Moroz, O.M.; Kovalchuk, M.M. Wastewater bioremediation with using of phototrophic non-sulfur bacteria Rhodopseudomonas yavorovii IMV B-7620. Ecol. Noospherol. 2019, 30, 63–67. [Google Scholar] [CrossRef]
- Xu, C.B.; Sun, X.K.; Li, Y.Y.; Wang, Y.G.; Meng, X.L. Study on optimization of the culture conditions for four Rhodopseudomonas spp. Adv. Mater. Res. 2012, 393, 976–979. [Google Scholar]
- Ivshina, I.; Bazhutin, G.; Tyumina, E. Rhodococcus strains as a good biotool for neutralizing pharmaceutical pollutants and obtaining therapeutically valuable products: Through the past into the future. Front. Microbiol. 2022, 13, 967127. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Azadi, D.; Absalan, A. Bioremediation of phenol, sulfate sodium, and polycyclic aromatic hydrocarbons by Rhodococcus sp. first time isolated and molecular characterized from aquatic and terrestrial ecosystems. Water Environ. J. 2023, 37, 594–603. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Saez, J.M.; Raimondo, E.E.; Benimeli, C.S.; Polti, M.A. Comparative study of single and mixed cultures of actinobacteria for the bioremediation of co-contaminated matrices. J. Environ. Chem. Eng. 2018, 6, 2310–2318. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Nicholaus, R.; Yang, W.; Zheng, Z. Nitrogen and phosphorus-related functional genes enhance nutrient removal in the integrated aquaculture wastewater bioremediation system in the presence of photosynthetic bacteria. Aquac. Int. 2025, 33, 131. [Google Scholar] [CrossRef]
- Khatua, S.; Simal-Gandara, J.; Acharya, K. Myco-remediation of plastic pollution: Current knowledge and future prospects. Biodegradation 2024, 35, 249–279. [Google Scholar] [CrossRef]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ntushelo, K.; Mamba, B.B.; Msagati, T.A.M. Diversity, co-occurrence and implications of fungal communities in wastewater treatment plants. Sci. Rep. 2019, 9, 14056. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, W.; Lu, Q.; Wang, S. Carbon/nitrogen flows and associated microbial communities in full-scale foodwaste treatment plants. Bioresour. Technol. 2023, 388, 129775. [Google Scholar] [CrossRef]
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Nicholaus, R.; Lukwambe, B.; Zhao, L.; Yang, W.; Zhu, J.; Zheng, Z. Bioturbation of blood clam Tegillarca granosa on benthic nutrient fluxes and microbial community in an aquaculture wastewater treatment system. Int. Biodeterior. Biodegrad. 2019, 142, 73–82. [Google Scholar] [CrossRef]
- Ramzan, M.N.; Shen, D.; Wei, Y.; Emmanuel, A.; Yang, W.; Zhu, J.; Wang, Y.; Zheng, Z. Performance and Microbial Community Analysis of Integrated Bioremediation Systems with Photosynthetic Bacteria in Treating Mariculture Tailwater. J. Ocean Univ. China 2025, 24, 515–524. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, D.; Yang, W.; Zheng, Z. Dynamics of fungi and the connection with bacteria in removing nutrients from mariculture wastewater in the integrated bioremediation systems. Int. Biodeterior. Biodegrad. 2024, 194, 105877. [Google Scholar] [CrossRef]
- Emmanuel, A.; Wei, Y.; Ramzan, M.N.; Yang, W.; Zheng, Z. Dynamics of Bacterial Communities and Their Relationship with Nutrients in a Full-Scale Shrimp Recirculating Aquaculture System in Brackish Water. Animals 2025, 15, 1400. [Google Scholar] [CrossRef] [PubMed]
- Raza, B.; Ke, J.; Chen, L.; Shi, Y.; Zhu, J.; Shao, Z.; Zheng, Z.; Lu, K.; Yang, W. Adding glucose combined with microalgae to water improves the benefits of the fungal community on the whiteleg shrimp (Litopenaeus vannamei) culture. Aquac. Rep. 2025, 40, 102580. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, C.; Zhang, N.; Huang, X.; Tong, Z.; Lu, M.; Zhang, C.; Ma, Y. Succession of fungal communities and fungal-bacterial interactions in biofilm samples within a multistage bio-contact oxidation reactor during the treatment of low-COD and high-salinity produced water. Environ. Eng. Res. 2023, 28, 220765. [Google Scholar] [CrossRef]
- Bokade, P.; Bajaj, A. Molecular advances in mycoremediation of polycyclic aromatic hydrocarbons: Exploring fungal bacterial interactions. J. Basic Microbiol. 2023, 63, 239–256. [Google Scholar] [CrossRef]
- Singh, A.K.; Negi, V.; Verma, H. Bioremediation of Recalcitrant Xenobiotic Compounds: Advancements and Challenges. Microsphere 2023, 2, 191–203. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, H.; Huang, Y.; Xie, Z.; Zhang, M.; Wei, Y.; Li, J.; Ma, Y.; Luo, M.; Ding, W.; et al. Revealing the full biosphere structure and versatile metabolic functions in the deepest ocean sediment of the Challenger Deep. Genome Biol. 2021, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Vilchez-Vargas, R.; Kerckhof, F.; Aranda, E.; González-López, J.; Rodelas, B. Community structure, population dynamics and diversity of fungi in a full-scale membrane bioreactor (MBR) for urban wastewater treatment. Water Res. 2016, 105, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Menon, N.G.; Mohapatra, G.; Pisharody, L.; Pattnaik, A.; Bhukya, P.L.; Srivastava, M.; Singh, M.; Barman, M.K.; Gin, K.Y.-H.; et al. The novel SARS-CoV-2 pandemic: Possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci. Total Environ. 2021, 765, 142746. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Pan, Z.; Burgaud, G.; Ma, H.; Zheng, Y.; Wang, M.; Cai, L. Seasonal and spatial dynamics of fungal diversity and communities in the intertidal zones of Qingdao, China. J. Fungi 2023, 9, 1015. [Google Scholar] [CrossRef]
- Chen, C.; Gan, Z.; Xu, R.; Meng, F. Cellulose-induced shifts in microbial communities and microbial interactions in an anoxic/aerobic membrane bioreactor. J. Water Process. Eng. 2021, 42, 102106. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Chen, S.; Li, B.; Sekar, R.; Zhao, Z.; Jia, J.; Wang, Y.; Kang, P. Disentangling the drivers of diversity and distribution of fungal community composition in wastewater treatment plants across spatial scales. Front. Microbiol. 2018, 9, 1291. [Google Scholar] [CrossRef]
- Kasan, N.A.; Dagang, A.N.; Abdullah, M.I. Application of Biofloc Technology (BFT) in Shrimp Aquaculture Industry; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Zhang, Y.; Yao, P.; Sun, C.; Li, S.; Shi, X.; Zhang, X.H.; Liu, J. Vertical diversity and association pattern of total, abundant and rare microbial communities in deep-sea sediments. Mol. Ecol. 2021, 30, 2800–2816. [Google Scholar] [CrossRef]
- Jasim, A.M.; Jasim, B.H.; Kraiem, H.; Flah, A. A multi-objective demand/generation scheduling model-based microgrid energy management system. Sustainability 2022, 14, 10158. [Google Scholar] [CrossRef]
- Nzila, A.; Musa, M.M.; Sankara, S.; Al-Momani, M.; Xiang, L.; Li, Q.X.; Arora, P.K. Degradation of benzo[a]pyrene by halophilic bacterial strain Staphylococcus haemoliticus strain 10SBZ1A. PLoS ONE 2021, 16, e0247723. [Google Scholar] [CrossRef]
- Fayed, B. Nanoparticles in the battle against Candida auris biofilms: Current advances and future prospects. Drug Deliv. Transl. Res. 2025, 15, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.M.; Khatana, K.; Vats, V.; Dhanker, R.; Kumar, R.; Dahms, H.-U.; Hwang, J.-S. Biological approaches integrating algae and bacteria for the degradation of wastewater contaminants—A review. Front. Microbiol. 2022, 12, 801051. [Google Scholar] [CrossRef]
- Selvarajan, R.; Sibanda, T.; Venkatachalam, S.; Kamika, I.; Nel, W.A.J. Industrial wastewaters harbor a unique diversity of bacterial communities revealed by high-throughput amplicon analysis. Ann. Microbiol. 2018, 68, 445–458. [Google Scholar] [CrossRef]
- Aira, M.; Gómez-Roel, A.; Domínguez, J. Earthworms Significantly Alter the Composition, Diversity, Abundance and Pathogen Load of Fungal Communities in Sewage Sludge from Different Urban Wastewater Treatment Plants. Pathogens 2025, 14, 409. [Google Scholar] [CrossRef]
- Dai, H.-H.; Gao, J.-F.; Wang, Z.-Q.; Zhao, Y.-F.; Zhang, D. Behavior of nitrogen, phosphorus and antibiotic resistance genes under polyvinyl chloride microplastics pressures in an aerobic granular sludge system. J. Clean. Prod. 2020, 256, 120402. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Rodriguez-Sanchez, A.; Muñoz-Palazon, B.; Gonzalez-Lopez, J.; Gonzalez-Martinez, A. Microbial community structure and function of activated sludge from a wastewater treatment plant at the Polar Arctic Circle as revealed by metatranscriptomic and next-generation sequencing. J. Environ. Chem. Eng. 2022, 10, 108393. [Google Scholar] [CrossRef]
- Xing, R.; Gao, Q.-B.; Zhang, F.-Q.; Wang, J.-L.; Chen, S.-L. Environmental filtering affects fungal communities more than dispersal limitation in a high-elevation hyperarid basin on Qinghai–Tibet Plateau. FEMS Microbiol. Lett. 2021, 368, fnab033. [Google Scholar] [CrossRef]
- Cameron, M.D.; Timofeevski, S.; Aust, S.D. Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl. Microbiol. Biotechnol. 2000, 54, 751–758. [Google Scholar] [CrossRef]
- Pundir, A.; Thakur, M.S.; Prakash, S.; Kumari, N.; Sharma, N.; Parameswari, E.; He, Z.; Nam, S.; Thakur, M.; Puri, S.; et al. Fungi as versatile biocatalytic tool for treatment of textile wastewater effluents. Environ. Sci. Eur. 2024, 36, 185. [Google Scholar] [CrossRef]
- Cai, W.; Hu, P.; Li, Z.; Kang, Q.; Chen, H.; Zhang, J.; Zhu, S. Effect of high ammonia on granular stability and phosphorus recovery of algal-bacterial granules in treatment of synthetic biogas slurry. Heliyon 2022, 8, e09844. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Xiong, J.; Zhou, Q.-C.; Yuan, Y.; Wang, X.-X.; Sun, P. Dietary yeast hydrolysate and brewer’s yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2018, 82, 121–129. [Google Scholar] [CrossRef]
- Xiong, J.; Jin, M.; Yuan, Y.; Luo, J.X.; Lu, Y.; Zhou, Q.C.; Liang, C.; Tan, Z.L. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1425–1435. [Google Scholar] [CrossRef]
- Uzoh, I.M.; Okebalama, C.B.; Igwe, C.A.; Babalola, O.O. Management of soil-microorganism: Interphase for sustainable soil fertility management and enhanced food security. In Food Security and Safety: African Perspectives; Springer: Cham, Switzerland, 2021; pp. 475–494. [Google Scholar]
- Singh, M.; Singh, D.; Rai, P.K.; Suyal, D.C.; Saurabh, S.; Soni, R.; Giri, K.; Yadav, A.N. Fungi in remediation of hazardous wastes: Current status and future outlook. In Recent Trends in Mycological Research: Volume 2: Environmental and Industrial Perspective; Springer: Cham, Switzerland, 2021; pp. 195–224. [Google Scholar]
- Ye, J.; Yin, H.; Mai, B.; Peng, H.; Qin, H.; He, B.; Zhang, N. Biosorption of chromium from aqueous solution and electroplating wastewater using mixture of Candida lipolytica and dewatered sewage sludge. Bioresour. Technol. 2010, 101, 3893–3902. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Tian, L.; Chen, M.; Jiang, X.D.; Gou, G.; Chen, Y. Optimization of Naphthol Green B adsorption and degradation by nZVI/CS/APT using Plackett-Burman designs and response surface methodology. Desalination Water Treat. 2021, 223, 434–446. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Jiang, H.; Yuan, L.; Zhang, L.; Ma, J.-E.; Zhang, X.; Cheng, M.; Chen, J. Fecal bacteriome and mycobiome in bats with diverse diets in South China. Curr. Microbiol. 2018, 75, 1352–1361. [Google Scholar] [CrossRef]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; An, X.; Xiao, X.; Li, N.; Xie, D.; Lai, F.; Zhang, Q. Treatment of thiocyanate-containing wastewater: A critical review of thiocyanate destruction in industrial effluents. World J. Microbiol. Biotechnol. 2023, 39, 35. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.-W.; Huang, M.-Q.; Yan, H.-J.; Li, D. Static magnetic field increases aerobic nitrogen removal from hypersaline wastewater in activated sludge with coexistence of fungi and bacteria. Bioresour. Technol. 2023, 382, 129194. [Google Scholar] [CrossRef] [PubMed]
- Guest, R.K.; Smith, D.W. A potential new role for fungi in a wastewater MBR biological nitrogen reduction system. J. Environ. Eng. Sci. 2002, 1, 433–437. [Google Scholar] [CrossRef]
- Rosa, F.M.; Mota, T.F.M.; Busso, C.; de Arruda, P.V.; Brito, P.E.M.; Miranda, J.P.M.; Trentin, A.B.; Dekker, R.F.H.; da Cunha, M.A.A. Filamentous fungi as bioremediation agents of industrial effluents: A systematic review. Fermentation 2024, 10, 143. [Google Scholar] [CrossRef]
- Raza, B.; Zheng, Z.; Yang, W. A review on biofloc system technology, history, types, and future economical perceptions in aquaculture. Animals 2024, 14, 1489. [Google Scholar] [CrossRef] [PubMed]
- Raza, B.; Ramzan, M.N.; Yang, W. A review: Improving aquaculture rearing water quality by removal of nutrients using microalgae, challenges and future prospects. Aquaculture 2025, 598, 741959. [Google Scholar] [CrossRef]
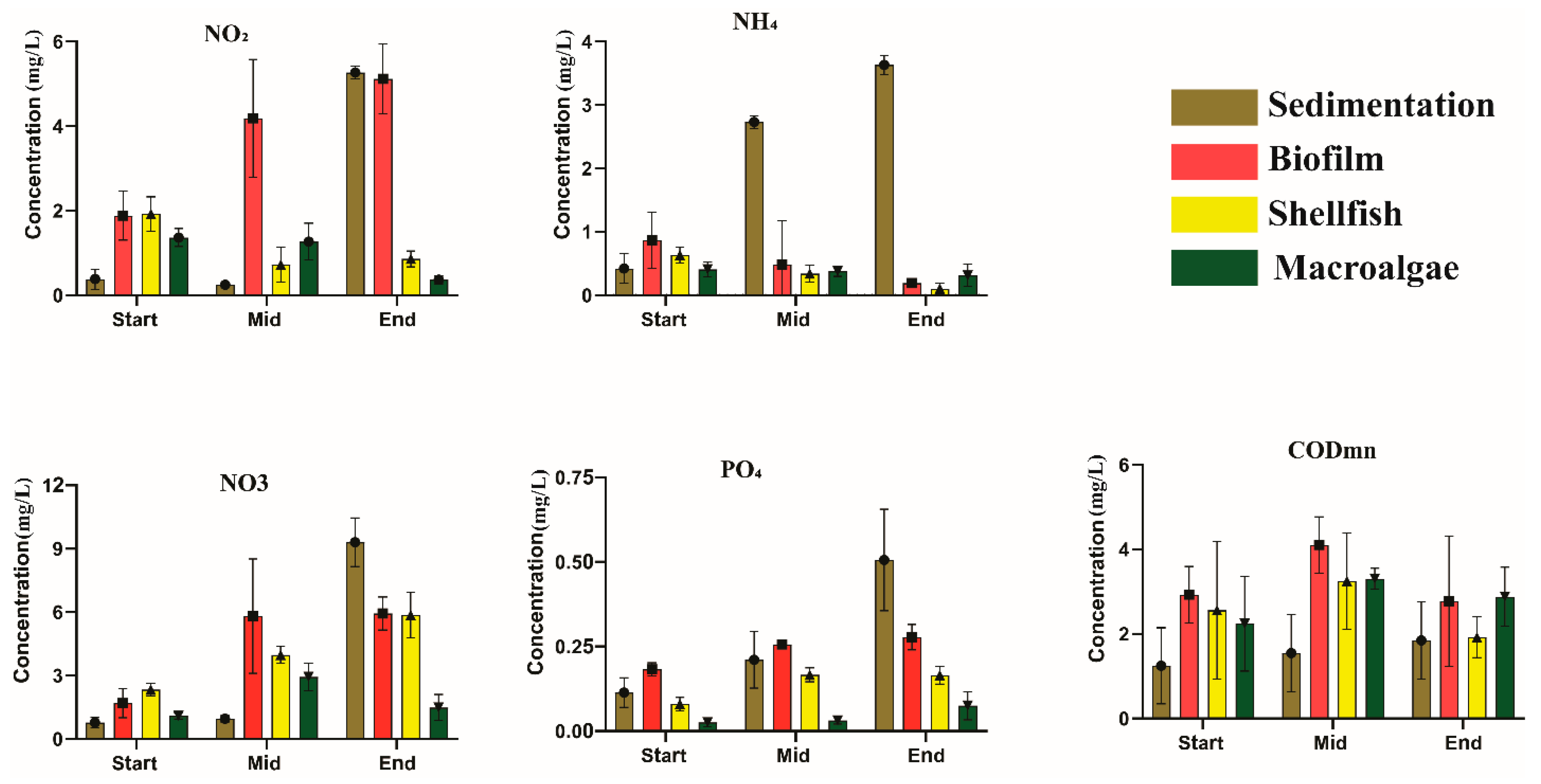
| Parameter (mg L−1) | Sedimentation | Biofilm | Shellfish | Macroalgae | Removal Efficiency (%) |
|---|---|---|---|---|---|
| NO2−-N | 0.422 ± 0.23 b | 0.076 ± 0.0534 a | 0.464 ± 0.0566 b | 0.054 ± 0.0534 a | 87.20 |
| NO3−-N | 13.302 ± 1.15 c | 5.935 ± 0.778 b | 5.865 ± 1.077 b | 1.489 ± 0.6111 a | 88.80 |
| PO43−-P | 0.906 ± 0.15 c | 0.278 ± 0.0378 b | 0.165 ± 0.0260 b | 0.075 ± 0.0141 a | 91.72 |
| NH4+-N | 3.630 ± 0.15 b | 0.191 ± 0.0567 a | 0.106 ± 0.0898 a | 0.313 ± 0.1759 a | 91.37 |
| CODMn | 4.48 ± 0.277 b | 3.36 ± 0.666133 b | 1.28 ± 0.48 a | 2.56 ± 0.2444 ab | 71.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramzan, M.N.; Shen, D.; Wei, Y.; Raza, B.; Yuan, H.; Emmanuel, A.; Mushtaq, Z.; Zheng, Z. A Synergistic Role of Photosynthetic Bacteria and Fungal Community in Pollutant Removal in an Integrated Aquaculture Wastewater Bioremediation System. Biology 2025, 14, 959. https://doi.org/10.3390/biology14080959
Ramzan MN, Shen D, Wei Y, Raza B, Yuan H, Emmanuel A, Mushtaq Z, Zheng Z. A Synergistic Role of Photosynthetic Bacteria and Fungal Community in Pollutant Removal in an Integrated Aquaculture Wastewater Bioremediation System. Biology. 2025; 14(8):959. https://doi.org/10.3390/biology14080959
Chicago/Turabian StyleRamzan, Muhammad Naeem, Ding Shen, Yingzhen Wei, Bilal Raza, Hongmei Yuan, Arslan Emmanuel, Zulqarnain Mushtaq, and Zhongming Zheng. 2025. "A Synergistic Role of Photosynthetic Bacteria and Fungal Community in Pollutant Removal in an Integrated Aquaculture Wastewater Bioremediation System" Biology 14, no. 8: 959. https://doi.org/10.3390/biology14080959
APA StyleRamzan, M. N., Shen, D., Wei, Y., Raza, B., Yuan, H., Emmanuel, A., Mushtaq, Z., & Zheng, Z. (2025). A Synergistic Role of Photosynthetic Bacteria and Fungal Community in Pollutant Removal in an Integrated Aquaculture Wastewater Bioremediation System. Biology, 14(8), 959. https://doi.org/10.3390/biology14080959








