Enhanced Phosphorus Removal by Iron-Carbon in Constructed Wetlands Across Salinity Gradients: Mechanisms and Microbial Insights for Aquaculture Tailwater Treatment
Simple Summary
Abstract
1. Introduction
2. Material and Methods
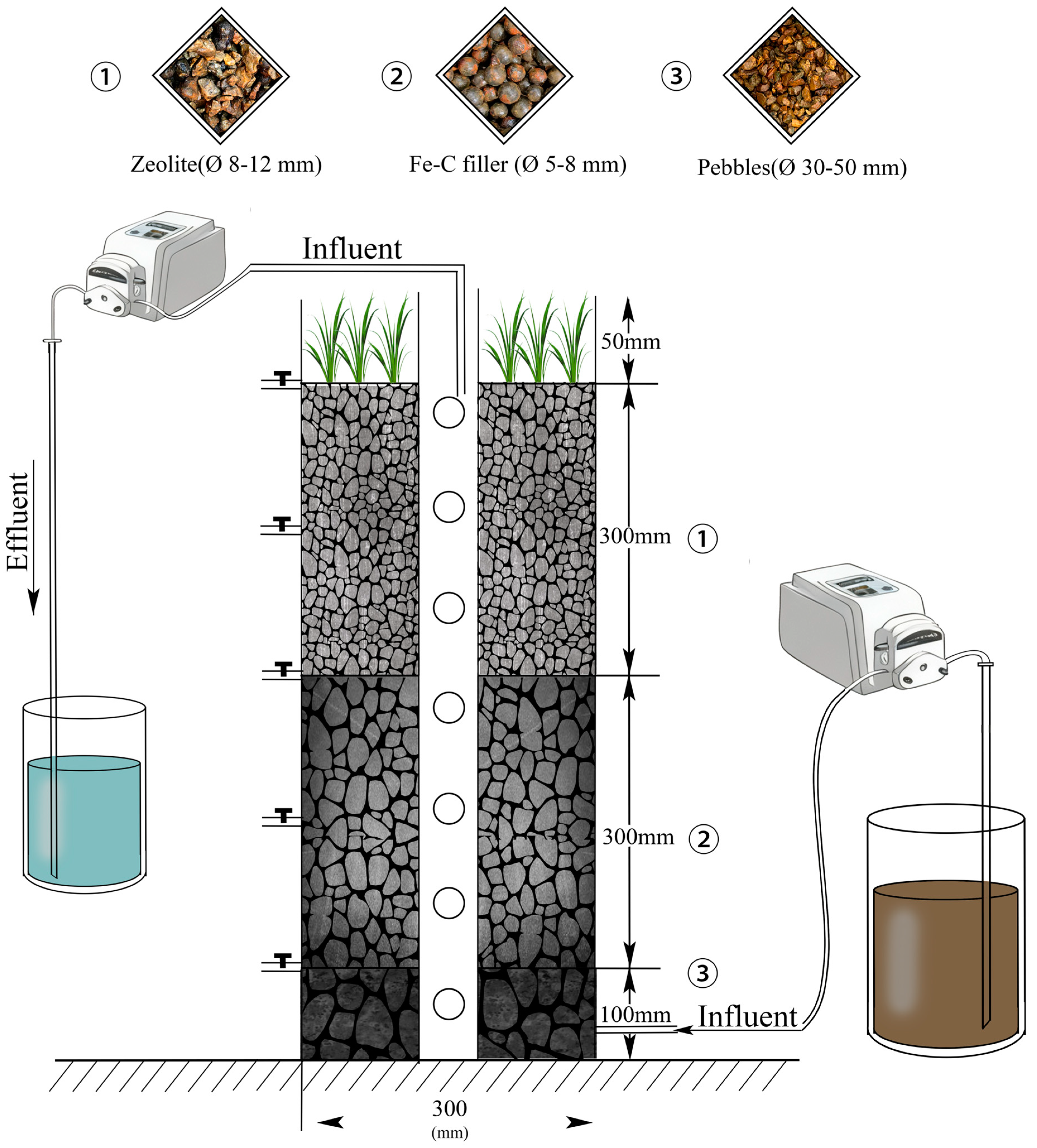
2.1. System Setup
2.2. Experimental Design
2.2.1. Operation of IC-CWs
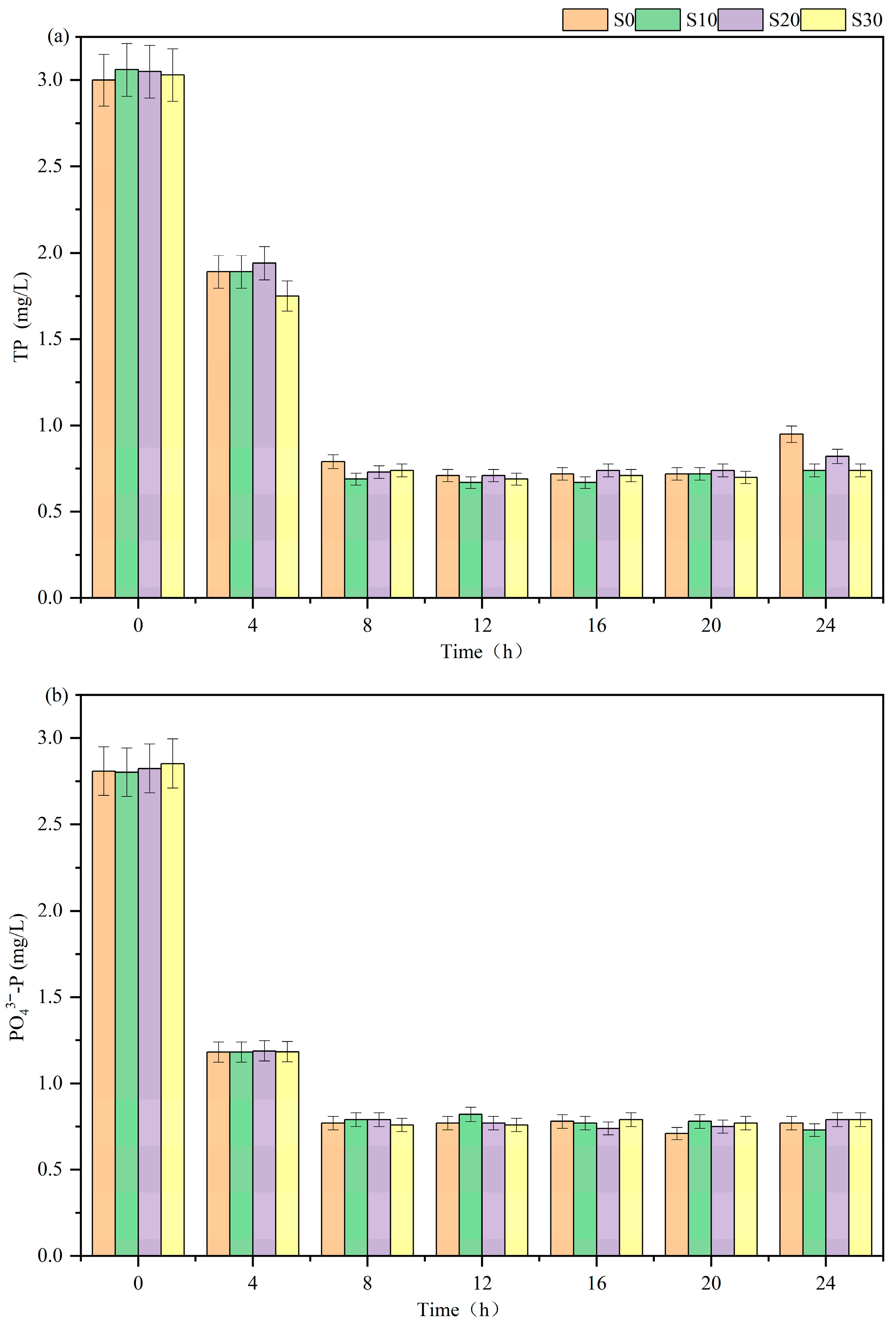
2.2.2. Batch Experiment
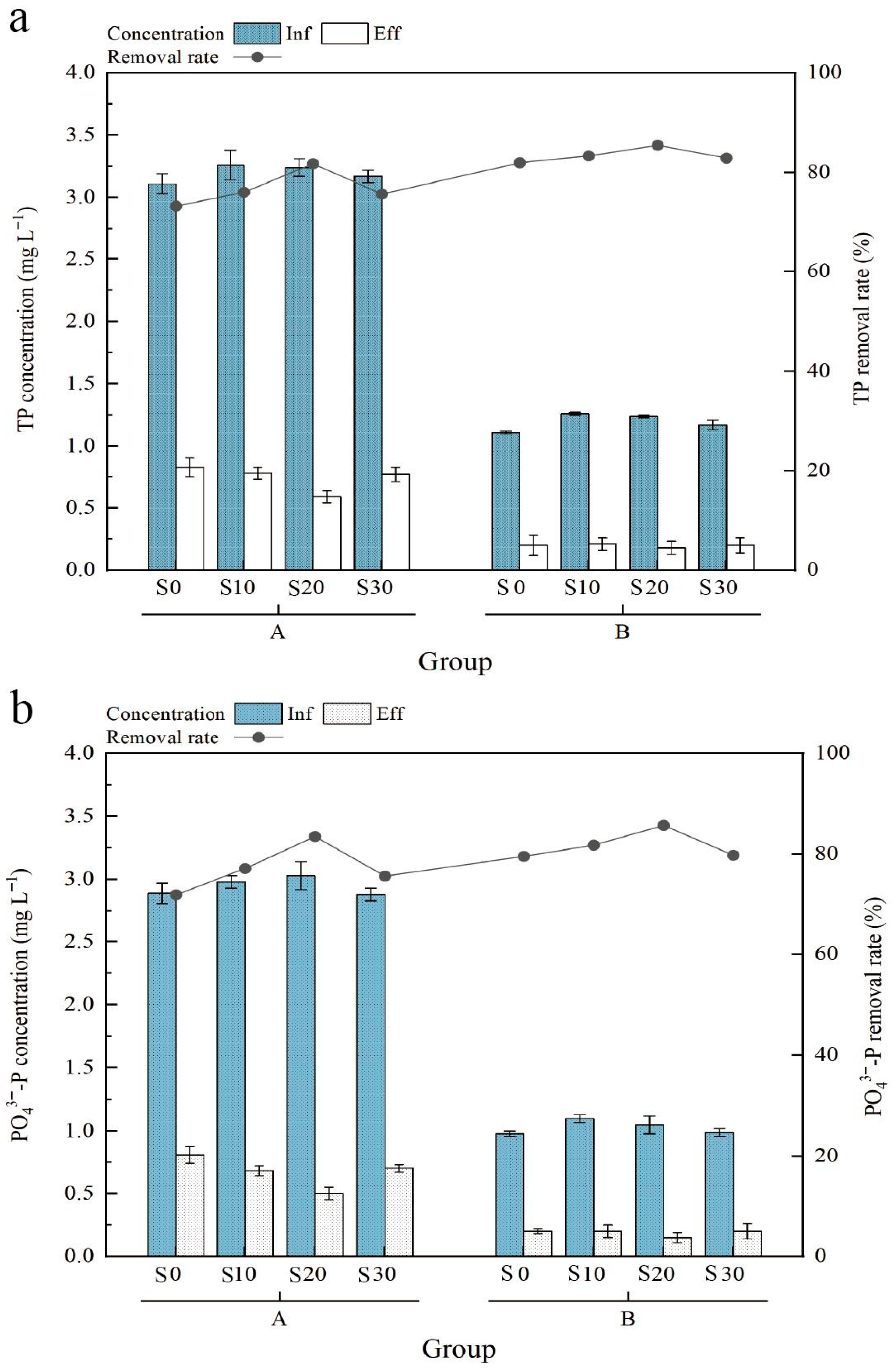
2.2.3. Validation with Exopalaemon carinicauda Culture Effluent
2.3. Substrate Sample Collecting and Analysis
2.3.1. Metagenomic Sequencing Sample Collection and Analytical Methods
2.3.2. Substrate Sample Enzyme Activity Assay Methods
2.4. Statical Analysis
3. Results and Discussion
3.1. Phosphorus Removal Efficiency in IC-CWs Under Varying Salinity Conditions
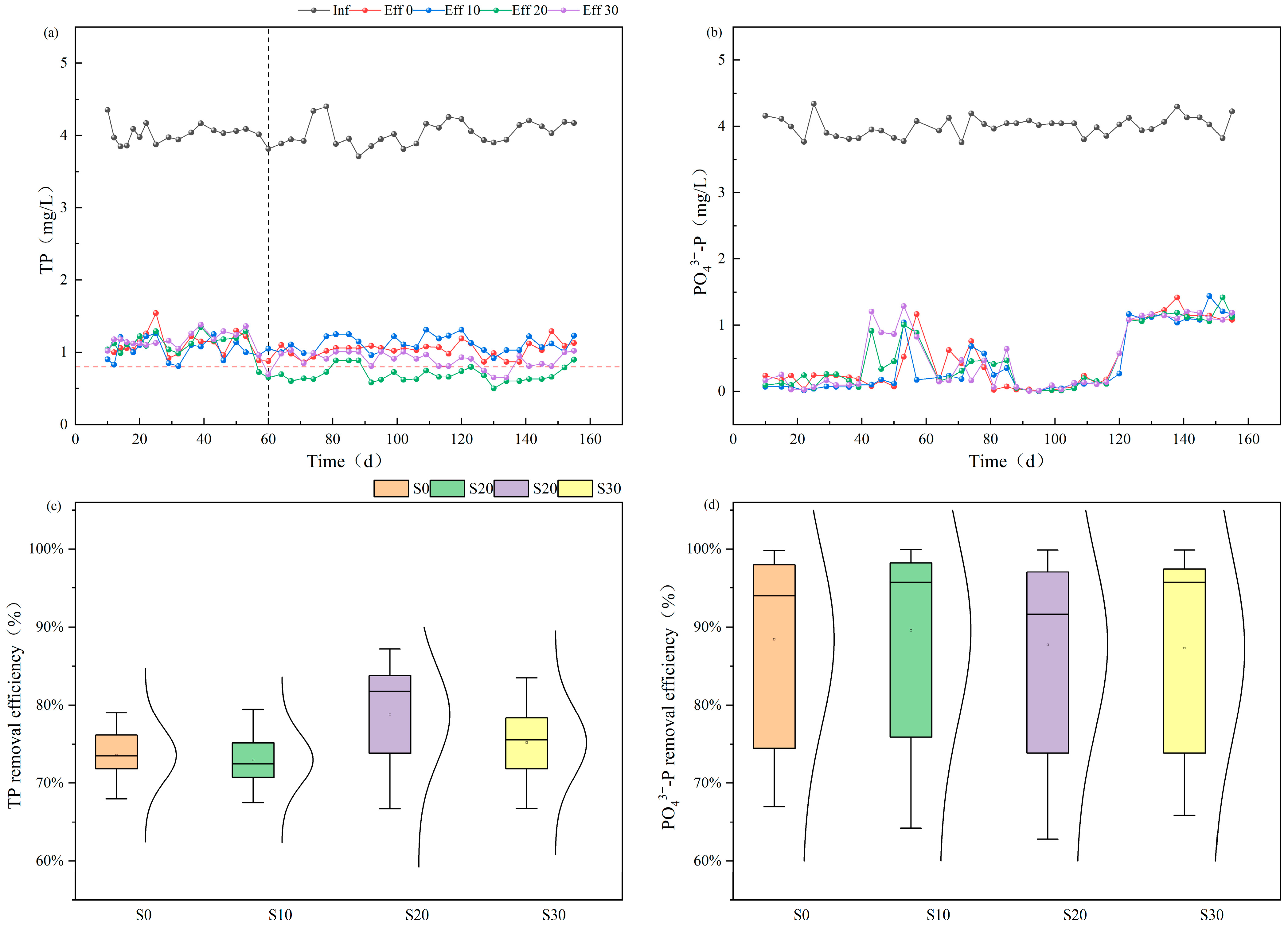
3.1.1. Long-Term Monitoring of Phosphorus Removal Efficiency
3.1.2. Phosphorus Removal Efficiency of Batch Experiment
3.1.3. Phosphorus Removal Efficiency When Treating Aquaculture Tail Water of Exopalaemon carinicauda
3.2. Iron Release Dynamics in IC-CWs Under Different Salinity Conditions
3.3. Microbial Analysis
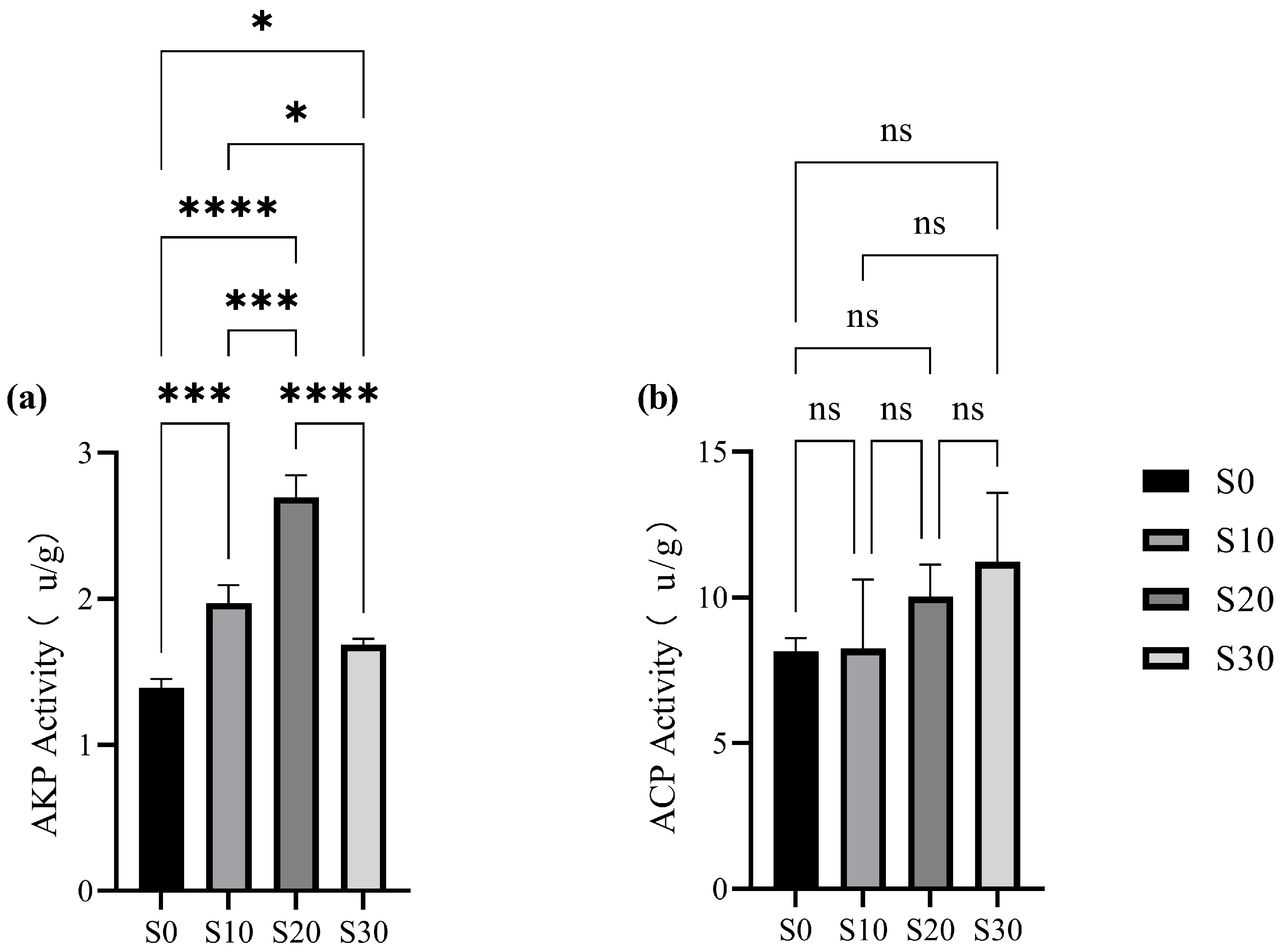
3.3.1. Analysis of Microbial Enzyme Activity in Wetland Substrate
- (1)
- Ionic regulation of protein conformation: Optimal ion concentrations maintain the structural integrity of the AKP protein conformation, ensuring enzymatic functionality;
- (2)
- Charge shielding effects: Saline ions reduce electrostatic repulsion between the enzyme and substrates through charge shielding, thereby improving the binding efficiency of organic phosphorus compounds.
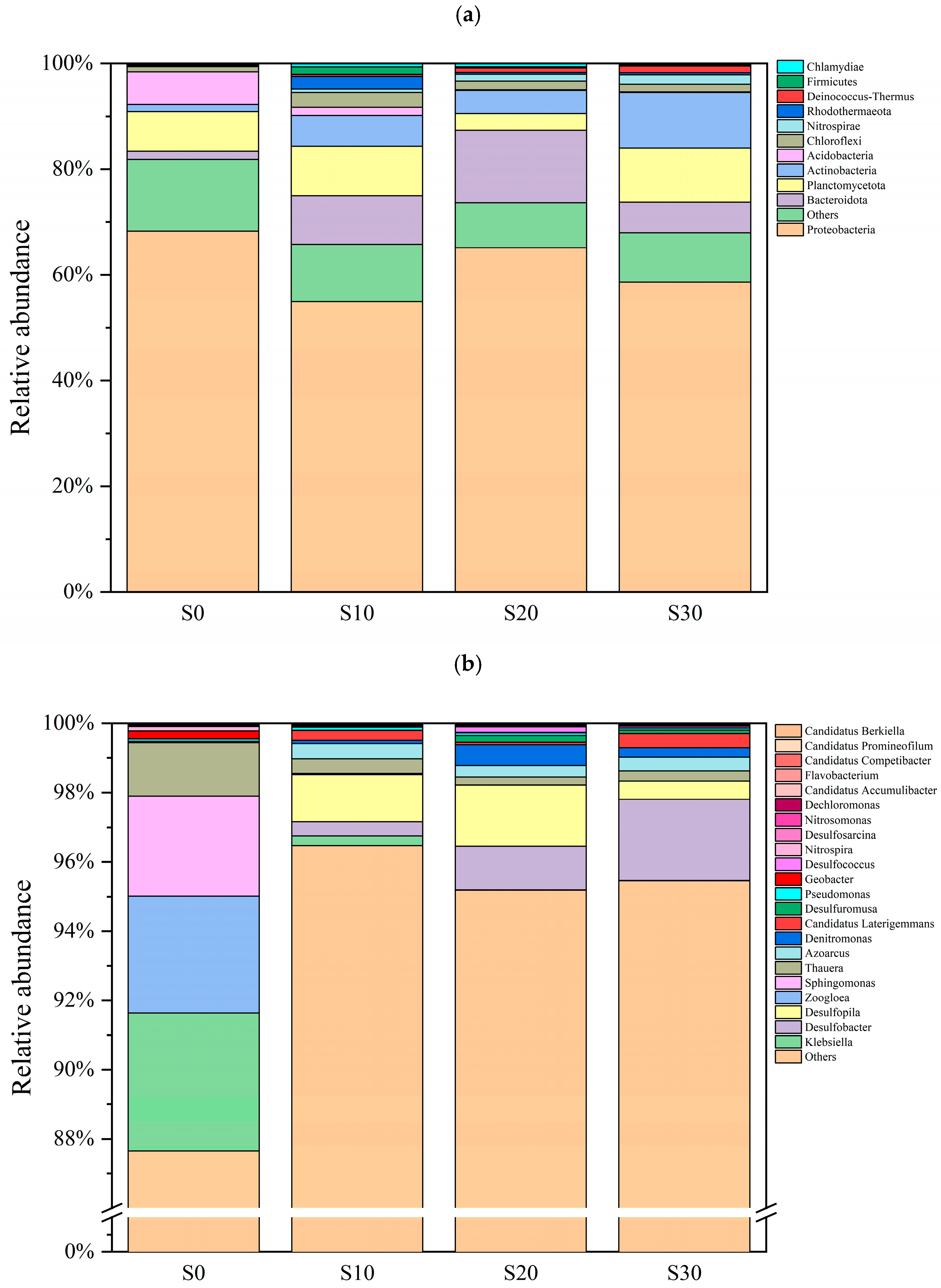
3.3.2. Changes in Phosphorus-Accumulating Microbial Communities
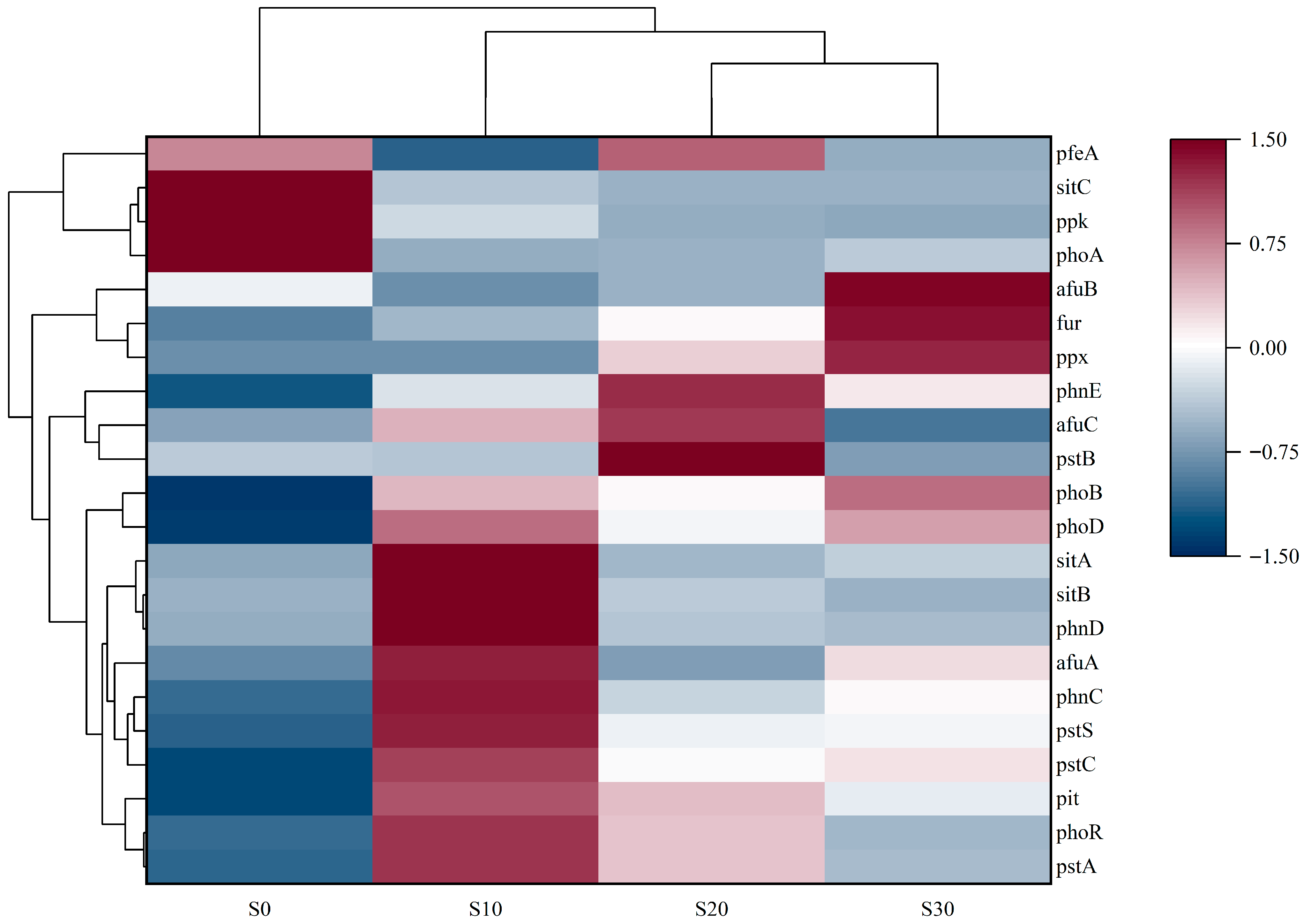
3.3.3. Variations in Functional Gene Abundance of PAOs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hayes, M.A.; Jesse, A.; Tabet, B.; Reef, R.; Keuskamp, J.A.; Lovelock, C.E. The contrasting effects of nutrient enrichment on growth, biomass allocation and decomposition of plant tissue in coastal wetlands. Plant Soil 2017, 416, 193–204. [Google Scholar] [CrossRef]
- Cui, J.; Li, J.; Cui, J.; Wang, W.; Wu, Y.; Xu, B.; Chang, Y.; Liu, X.; Li, H.; Yao, D. Removal effects of a biomass bottom ash composite on tailwater phosphate and its application in a rural sewage treatment plant. Sci. Total Environ. 2022, 812, 152549. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Kamali, N.; Mahjouri, M. Phosphorus removal and recovery: State of the science and challenges. Environ. Sci. Pollut. Res. 2022, 29, 58561–58589. [Google Scholar] [CrossRef]
- Yeoman, S.; Stephenson, T.; Lester, J.N.; Perry, R. The removal of phosphorus during wastewater treatment: A review. Environ. Pollut. 1988, 49, 183–233. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Z.; Wang, Y.; Wei, Q.; Kong, W.; Yu, J.; Mei, H.; Zhang, C.; Zhang, C. The removal and recovery of non-orthophosphate from wastewater: Current practices and future directions. ACS ES&T Eng. 2024, 4, 2587–2606. [Google Scholar] [CrossRef]
- Munir, R.; Muneer, A.; Sadia, B.; Younas, F.; Zahid, M.; Yaseen, M.; Noreen, S. Biochar imparted constructed wetlands (CWs) for enhanced biodegradation of organic and inorganic pollutants along with its limitation. Environ. Monit. Assess. 2024, 196, 425. [Google Scholar] [CrossRef]
- Muduli, M.; Choudharya, M.; Ray, S. A review on constructed wetlands for environmental and emerging contaminants removal from wastewater: Traditional and recent developments. Environ. Dev. Sustain. 2024, 26, 30181–30220. [Google Scholar] [CrossRef]
- Pandiarajan, S.; Sankararajan, V. A review of advanced solutions in constructed wetlands for sustainable wastewater management. Phys. Fluids 2025, 37, 011303. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus immobilization in water and sediment using iron-based materials: A review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef] [PubMed]
- Almeelbi, T.; Bezbaruah, A. Aqueous phosphate removal using nanoscale zero-valent iron. J. Nanoparticle Res. 2012, 14, 900. [Google Scholar] [CrossRef]
- Kao, C.; Zhang, Q.; Li, J.; Gao, R.; Li, W.; Li, X.; Wang, S.; Peng, Y. Simultaneous nitrogen and phosphorus removal from municipal wastewater by Fe(III)/Fe(II) cycling mediated partial-denitrification/anammox. Bioresour. Technol. 2022, 363, 127997. [Google Scholar] [CrossRef]
- Amado, L.; Albuquerque, A.; Santo, A.E. Influence of stormwater infiltration on the treatment capacity of a LECA-based horizontal subsurface flow constructed wetland. Ecol. Eng. 2012, 39, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Guo, X.; Wang, A.; Pan, Y.; Ma, J.; Lu, S.; Liu, D. A comprehensive review on iron–carbon microelectrolysis constructed wetlands: Efficiency, mechanism and prospects. Water Res. 2025, 268, 122648. [Google Scholar] [CrossRef]
- Li, M.; Liang, Z.; Callier, M.D.; Roque D’Orbcastel, E.; Sun, G.; Ma, X.; Li, X.; Wang, S.; Liu, Y.; Song, X. Nutrients removal and substrate enzyme activities in vertical subsurface flow constructed wetlands for mariculture wastewater treatment: Effects of ammonia nitrogen loading rates and salinity levels. Mar. Pollut. Bull. 2018, 131, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Raju, C.S.; Almind-Jørgensen, N.; Laustrup, M.; Reitzel, K.; Nielsen, U.G. Variation in phosphorus speciation of sewage sludge throughout three wastewater treatment plants: Determined by sequential extraction combined with microscopy, NMR spectroscopy, and powder X-ray diffraction. Environ. Sci. Technol. 2022, 56, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hou, Z.; Wu, L.; Liang, Y.; Wei, C. Effects of salinity and nitrogen on cotton growth in arid environment. Plant Soil 2010, 326, 61–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Liu, G.; Liu, T.; Feng, Y. Intensified nitrogen and phosphorus removal and mechanism revelation in constructed wetlands amended with iron-carbon based substrate towards low C/N ratio wastewater. Chem. Eng. J. 2024, 481, 148931. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Qiu, T.; Zhou, L.; Sun, J.; Du, Y. The dissolution of total suspended solids and treatment strategy of tailwater in a Litopenaeus vannamei recirculating aquaculture system. J. Oceanol. Limnol. 2023, 41, 1197–1205. [Google Scholar] [CrossRef]
- GB 17378.4-2007; The Specification for Marine Monitoring—Part 4: Seawater Analysis. Standards Press of China: Beijing, China, 2007.
- HJ/T 345-2007; Water Quality—Determination of Fluoride—Fluoride Ion Selective Electrode Method. China Environmental Science Press: Beijing, China, 2007.
- Shu, H.; Ma, Y.; Lu, H.; Sun, H.; Zhao, J.; Ruan, Z.; Zhou, Z.; Liu, Y.; Liu, F.; Xu, J.; et al. Simultaneous aerobic nitrogen and phosphate removal capability of novel salt-tolerant strain, Pseudomonas mendocina A4: Characterization, mechanism and application potential. Bioresour. Technol. 2024, 393, 130047. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Li, S.; Wang, Z.; Chen, H.; Yi, L.; Chen, X.; Yang, Q.; Xu, W.; Wang, A.; et al. Concerning the stability of seawater electrolysis: A corrosion mechanism study of halide on Ni-based anode. Nat. Commun. 2023, 14, 4822. [Google Scholar] [CrossRef] [PubMed]
- Trazzi, P.A.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and desorption of phosphate on biochars. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Mesquita, C.; Albuquerque, A.; Amaral, L.; Nogueira, R. Effectiveness and temporal variation of a full-scale horizontal constructed wetland in reducing nitrogen and phosphorus from domestic wastewater. ChemEngineering 2018, 2, 3. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, M.; Zhou, X.; Chen, W.; Lu, D.; Zhang, Y.; Shao, X. Enhanced removal mechanism of iron carbon micro-electrolysis constructed wetland on C, N, and P in salty permitted effluent of wastewater treatment plant. Sci. Total Environ. 2019, 649, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate adsorption on synthetic goethite and akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, C.; Wu, F.; Xu, H.; Zhao, Z.; Han, Z.; Zhang, H.; Yang, S. Enhanced removal of organic, nutrients, and PFCs in the iron-carbon micro-electrolysis constructed wetlands: Mechanism and iron cycle. Chem. Eng. J. 2023, 457, 141174. [Google Scholar] [CrossRef]
- Caravelli, A.H.; Contreras, E.M.; Zaritzky, N.E. Phosphorous removal in batch systems using ferric chloride in the presence of activated sludges. J. Hazard. Mater. 2010, 177, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, K.; Jin, J.; Yu, X.; Qiu, L.; Ding, S.; Lu, H.; Cai, J. Effects of Fe(II)/P ratio and pH on phosphorus removal by ferrous salt and approach to mechanisms. Sep. Purif. Technol. 2013, 118, 801–805. [Google Scholar] [CrossRef]
- Smith, F.; Brownlie, F.; Hodgkiess, T.; Toumpis, A.; Pearson, A.; Galloway, A.M. Effect of salinity on the corrosive wear behaviour of engineering steels in aqueous solutions. Wear 2020, 462–463, 203515. [Google Scholar] [CrossRef]
- Chohan, I.M.; Ahmad, A.; Sallih, N.; Bheel, N.; Salilew, W.M.; Almaliki, A.H. Effect of seawater salinity, pH, and temperature on external corrosion behavior and microhardness of offshore oil and gas pipeline: RSM modelling and optimization. Sci. Rep. 2024, 14, 16543. [Google Scholar] [CrossRef]
- Mao, Y.; Ninh Pham, A.; Xin, Y.; David, W.T. Effects of pH, floc age and organic compounds on the removal of phosphate by pre-polymerized hydrous ferric oxides. Sep. Purif. Technol. 2012, 91, 38–45. [Google Scholar] [CrossRef]
- Gamshadzehi, E.; Nassiri, M.; Ershadifar, H. One-pot synthesis of microporous Fe2O3/g-C3N4 and its application for efficient removal of phosphate from sewage and polluted seawater. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 7–15. [Google Scholar] [CrossRef]
- Gupta, T.T.; Gupta, N.K.; Pestrak, M.J.; Dusane, D.H.; Harro, J.M.; Horswill, A.R.; Stoodley, P. Staphylococcus aureus Aggregates on Orthopedic Materials under Varying Levels of Shear Stress. Appl. Environ. Microbiol. 2020, 86, e1220–e1234. [Google Scholar] [CrossRef]
- Rezaei, Z.; Amoozegar, M.A.; Moghimi, H. Innovative approaches in bioremediation: The role of halophilic microorganisms in mitigating hydrocarbons, toxic metals, and microplastics in hypersaline environments. Microb. Cell Fact. 2025, 24, 184. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Huang, R.; Diaz, J.M.; Tang, Y. Rethinking the biotic and abiotic remineralization of complex phosphate molecules in soils and sediments. Sci. Total Environ. 2022, 833, 155187. [Google Scholar] [CrossRef] [PubMed]
- Auton, M.; Bolen, D.W. Predicting the energetics of osmolyte-induced protein folding/unfolding. Proc. Natl. Acad. Sci. USA 2005, 102, 15065–15068. [Google Scholar] [CrossRef]
- Janicka, E.; Kanclerz, J.; Agaj, T. Ecological Importance of Alkaline Phosphatase Activity and Acid Phosphatase Activity in Lakes with Different Catchment Use Structures. Appl. Sci. 2024, 14, 497. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628–629, 53–63. [Google Scholar] [CrossRef]
- Cook, L.J.; Francoeur, S.N. Effects of simulated short-term road salt exposure on lotic periphyton function. J. Freshw. Ecol. 2012, 28, 211–223. [Google Scholar] [CrossRef]
- Labidi, N.; Snoussi, S.; Ammari, M.; Metoui, W.; Yousfi, N.B.; Hamrouni, L.; Abdelly, C. Enhancement of Acid Phosphatase Secretion and PI Acquisition in Suaeda Fruticosa on Calcareous Soil by High Saline Level. Biol. Futur. 2010, 61, 470–485. [Google Scholar] [CrossRef]
- Xu, X.; Du, Z.; Mou, W.; Deng, R.; Gu, L. Role of humic substances and alkaline in phosphorus release from sludge pre-treated by (alkali-) hydrothermal. Sci. Total Environ. 2023, 859, 160099. [Google Scholar] [CrossRef]
- Ni, M.; Chen, Y.; Pan, Y.; Huang, Y.; Li, D.; Li, L.; Huang, B.; Song, Z. Study on community structure and metabolic mechanism of dominant polyphosphate-accumulating organisms (PAOs) and glycogen-accumulating organisms (GAOs) in suspended biofilm based on phosphate recovery. Sci. Total Environ. 2022, 815, 152678. [Google Scholar] [CrossRef]
- Stasi, R.; Neves, H.I.; Spira, B. Phosphate uptake by the phosphonate transport system PhnCDE. BMC Microbiol. 2019, 19, 79. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Chai, Y.; Chen, R.; Zhao, Y.; Borriss, R.; Ding, X.; Wu, X.; Ye, J.; Hao, D.; et al. A phosphate starvation induced small RNA promotes Bacillus biofilm formation. Npj Biofilms Microbiomes 2024, 10, 115. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.; Xie, H.; Hao, D.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q.; Wang, B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J. Clean. Prod. 2021, 288, 125119. [Google Scholar] [CrossRef]
- Kragelund, C.; Caterina, L.; Borger, A.; Thelen, K.; Eikelboom, D.; Tandoi, V.; Kong, Y.; Waarde, J.V.D.; Krooneman, J.; Rossetti, S.; et al. Identity, abundance and ecophysiology of filamentous Chloroflexi species present in activated sludge treatment plants. FEMS Microbiol. Ecol. 2007, 59, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Rock phosphate-solubilizing Actinomycetes: Screening for plant growth-promoting activities. World J. Microbiol. Biotechnol. 2008, 24, 2565–2575. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Alves, V.M.C.; Marriel, I.E.; Gomes, E.A.; Scotti, M.R.; Carneiro, N.P.; Guimarães, C.T.; Schaffert, R.E.; Sá, N.M.H. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol. Biochem. 2009, 41, 1782–1787. [Google Scholar] [CrossRef]
- Abdoli, S.; Asgari Lajayer, B.; Dehghanian, Z.; Bagheri, N.; Vafaei, A.H.; Chamani, M.; Rani, S.; Lin, Z.; Shu, W.; Price, G.W. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water 2024, 16, 2507. [Google Scholar] [CrossRef]
- Liang, N.; Qaisar, M.; Zhang, K.; Zhu, X.; Cai, J.; Zheng, P. Phosphorus removal performance of Sulfide-Based autotrophic denitrification process. Chem. Eng. J. 2024, 501, 157217. [Google Scholar] [CrossRef]
- Nishijima, T.; Furuhashi, M.; Sakaoka, S.; Morikami, A.; Tsukagoshi, H. Ectopic expression of Mesembryanthemum crystallinum sodium transporter McHKT2 provides salt stress tolerance in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2017, 81, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Mahmood, A.; Alawadi, H.F.N.; Adiba, A.; Javaid, M.M.; Khan, B.A.; Wahid, A.; Abdullah, F.; Seleiman, M.F. Impact of boron on Glycine max L. to mitigate salt stress by modulating the morpho-physiological and biochemical responses. BMC Plant Biol. 2025, 25, 286. [Google Scholar] [CrossRef]
- Rangarajan, E.S.; Nadeau, G.; Li, Y.; Wagner, J.; Hung, M.; Schrag, J.D.; Cygler, M.; Matte, A. The Structure of the Exopolyphosphatase (PPX) from Escherichia coli O157:H7 Suggests a Binding Mode for Long Polyphosphate Chains. J. Mol. Biol. 2006, 359, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Liu, Y.; Zhang, L. A novel constructed wetland based on iron carbon substrates: Performance optimization and mechanisms of simultaneous removal of nitrogen and phosphorus. Environ. Sci. Pollut. Res. 2023, 30, 23035–23046. [Google Scholar] [CrossRef] [PubMed]
- Runyen-Janecky, L.J.; Reeves, S.A.; Gonzales, E.G.; Payne, S.M. Contribution of the Shigella flexneri Sit, Iuc, and Feo Iron Acquisition Systems to Iron Acquisition In Vitro and in Cultured Cells. Infect. Immun. 2003, 71, 1919–1928. [Google Scholar] [CrossRef] [PubMed][Green Version]
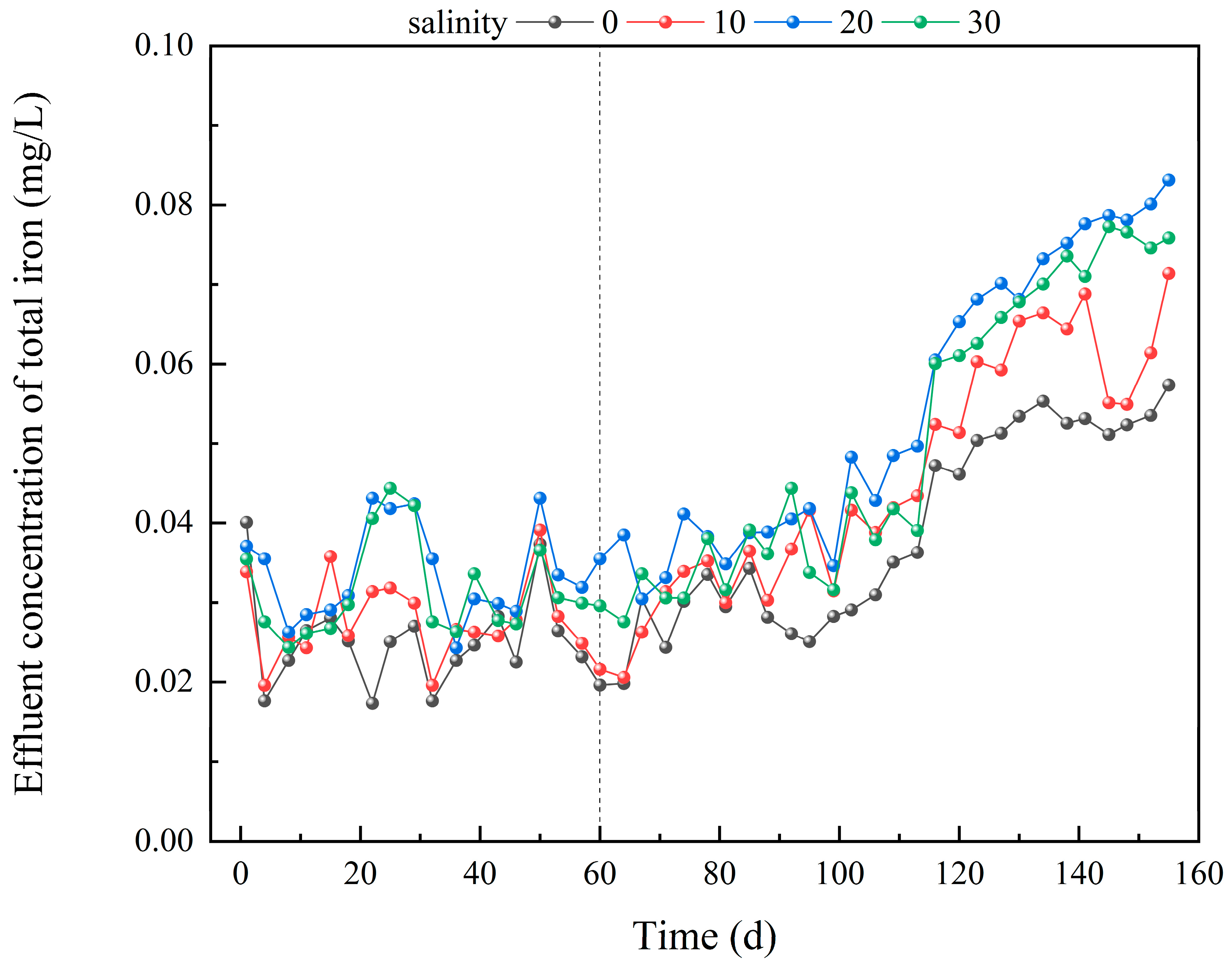
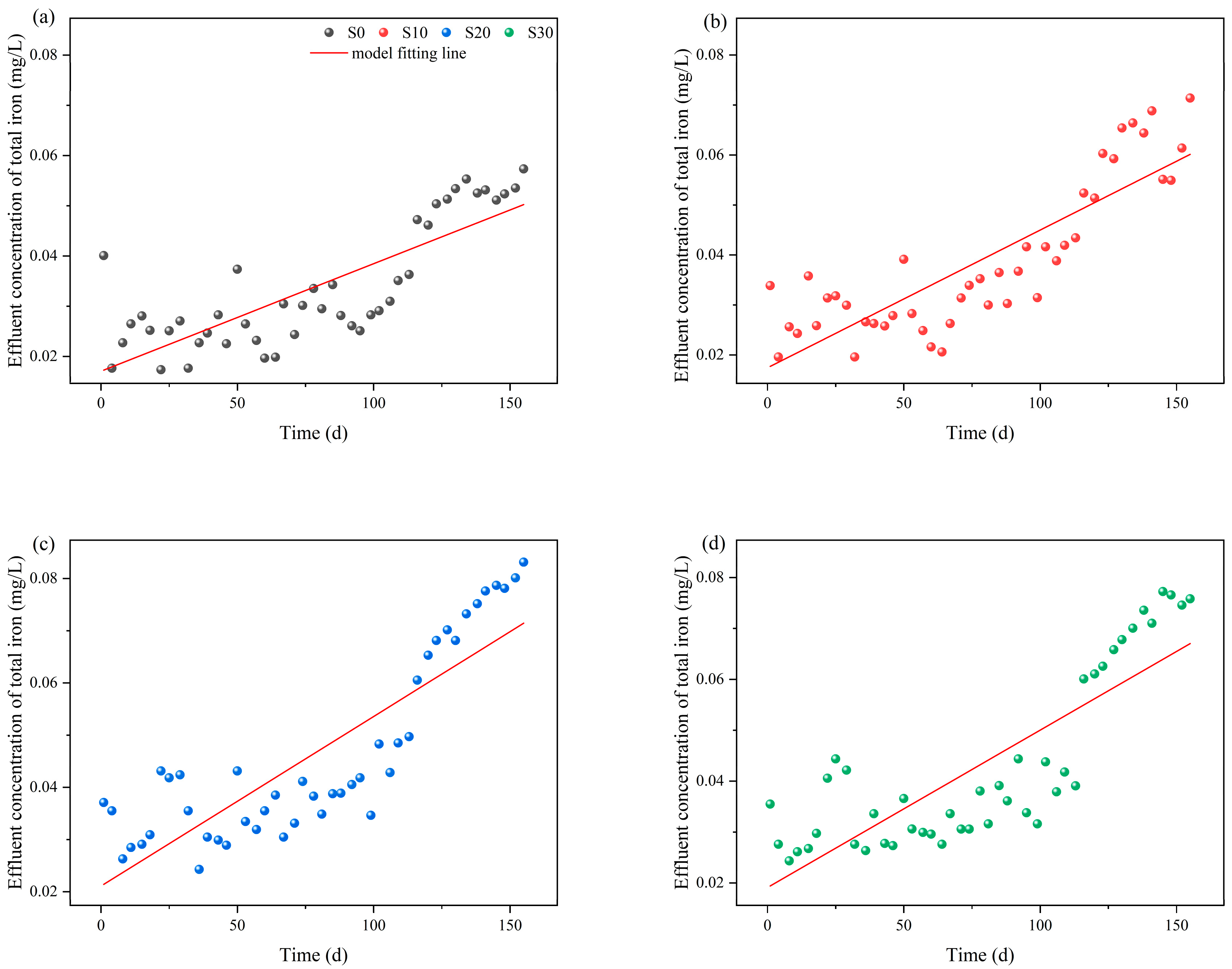
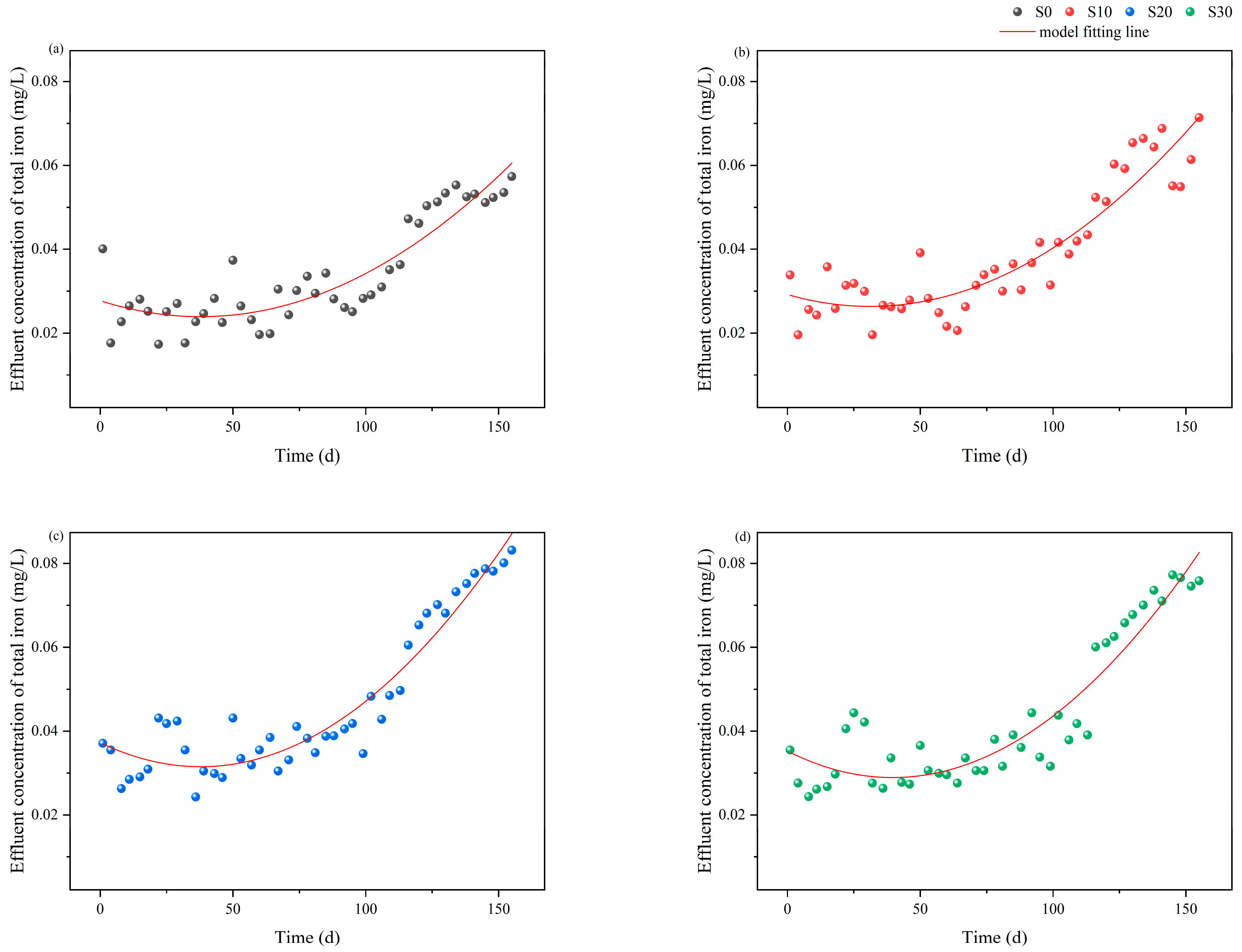
| Salinity Groups | Slope of the Linear Model (b) | Linear Term Coefficient (B1) | Quadratic Term Coefficient (B2) | R12 | R22 | ΔR2 |
|---|---|---|---|---|---|---|
| S0 | 2.14 × 10−4 a | −2.02 × 10−4 a | 2.67 × 10−4 a | 0.65 | 0.81 | 0.17 |
| S10 | 2.76 × 10−4 ab | −1.83 × 10−4 ab | 2.94 × 10−4 ab | 0.72 | 0.85 | 0.14 |
| S20 | 3.25 × 10−4 b | −3.06 × 10−4 b | 4.05 × 10−4 b | 0.72 | 0.91 | 0.19 |
| S30 | 3.09 × 10−4 b | −3.19 × 10−4 b | 4.03 × 10−4 b | 0.68 | 0.88 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, R.; Chen, B.; He, X.; Cai, C.; Zhai, T.; Shi, H.; Li, N.; Ma, X. Enhanced Phosphorus Removal by Iron-Carbon in Constructed Wetlands Across Salinity Gradients: Mechanisms and Microbial Insights for Aquaculture Tailwater Treatment. Biology 2025, 14, 1459. https://doi.org/10.3390/biology14101459
Yin R, Chen B, He X, Cai C, Zhai T, Shi H, Li N, Ma X. Enhanced Phosphorus Removal by Iron-Carbon in Constructed Wetlands Across Salinity Gradients: Mechanisms and Microbial Insights for Aquaculture Tailwater Treatment. Biology. 2025; 14(10):1459. https://doi.org/10.3390/biology14101459
Chicago/Turabian StyleYin, Rui, Boan Chen, Xinyu He, Chen Cai, Tangfang Zhai, Haoyang Shi, Na Li, and Xiaona Ma. 2025. "Enhanced Phosphorus Removal by Iron-Carbon in Constructed Wetlands Across Salinity Gradients: Mechanisms and Microbial Insights for Aquaculture Tailwater Treatment" Biology 14, no. 10: 1459. https://doi.org/10.3390/biology14101459
APA StyleYin, R., Chen, B., He, X., Cai, C., Zhai, T., Shi, H., Li, N., & Ma, X. (2025). Enhanced Phosphorus Removal by Iron-Carbon in Constructed Wetlands Across Salinity Gradients: Mechanisms and Microbial Insights for Aquaculture Tailwater Treatment. Biology, 14(10), 1459. https://doi.org/10.3390/biology14101459





