The Physiological Cost of Being Hot: High Thermal Stress and Disturbance Decrease Energy Reserves in Dragonflies in the Wild
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
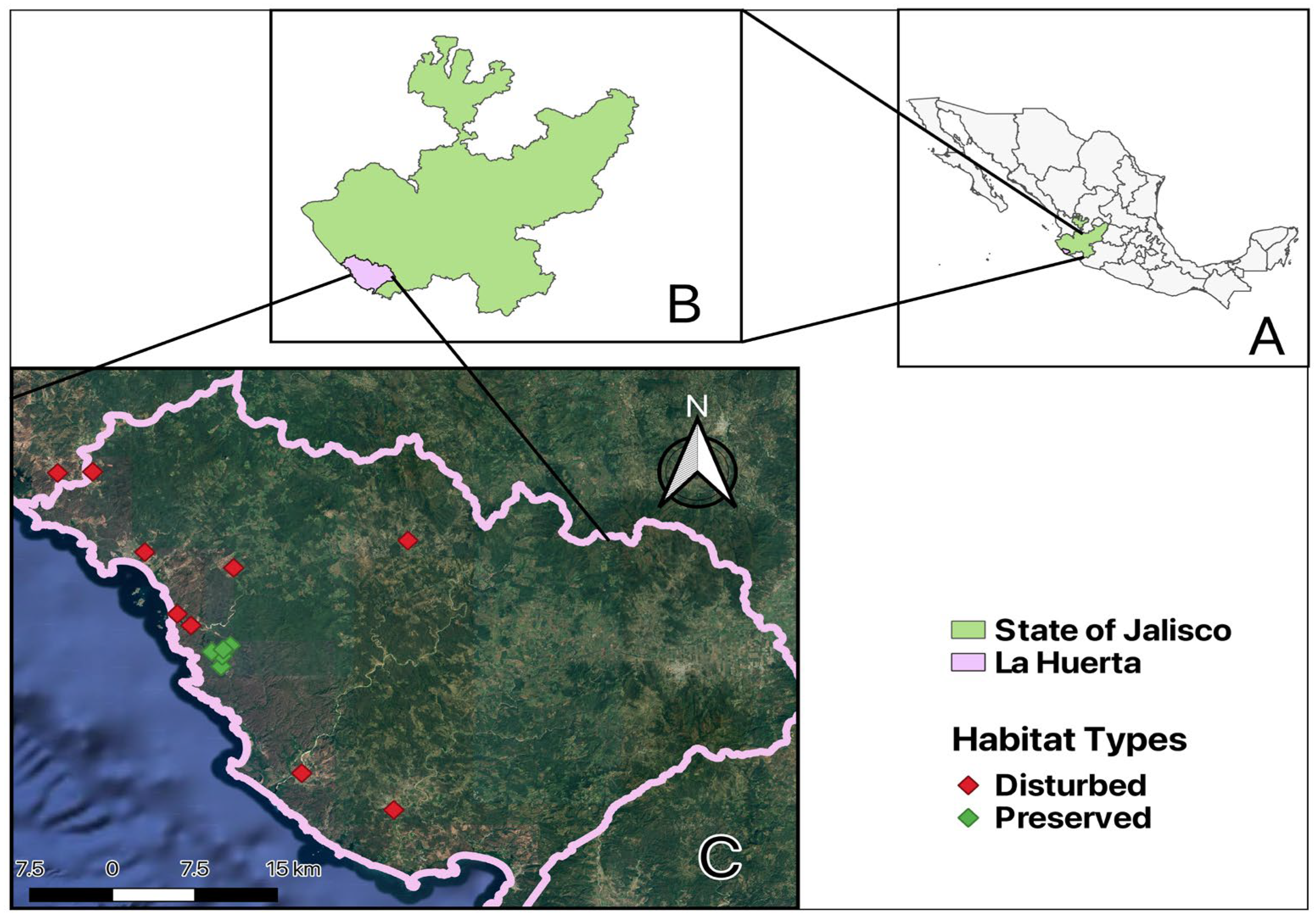
2.1. Study Site
2.2. Environmental and Body Temperature Recording
2.3. Thermographic Image Analysis and Thermal Stress Analysis
2.4. Energy Reserves Calculation
2.5. Statistical Analysis
3. Results
3.1. Environmental Temperatures
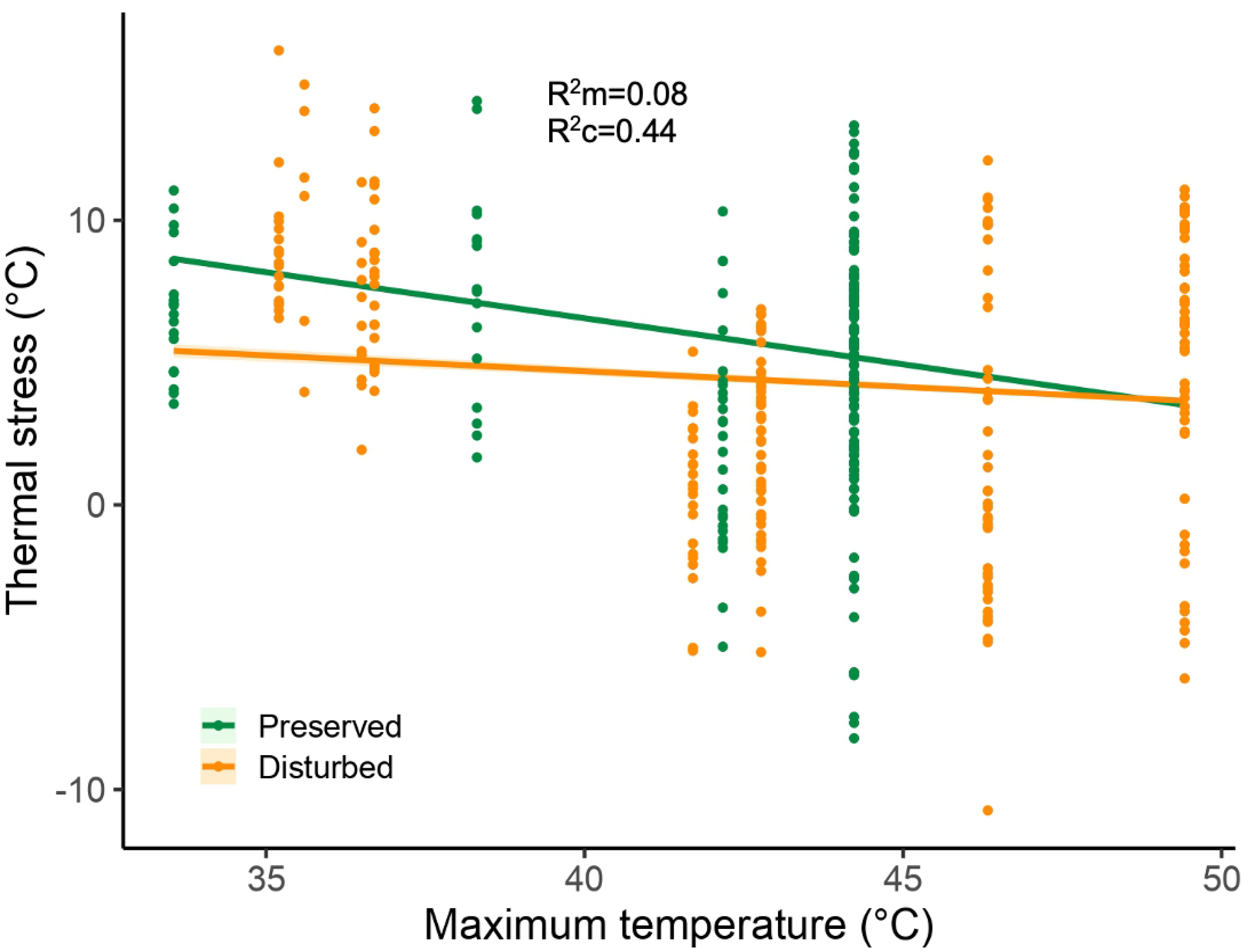
3.2. Thermal Stress
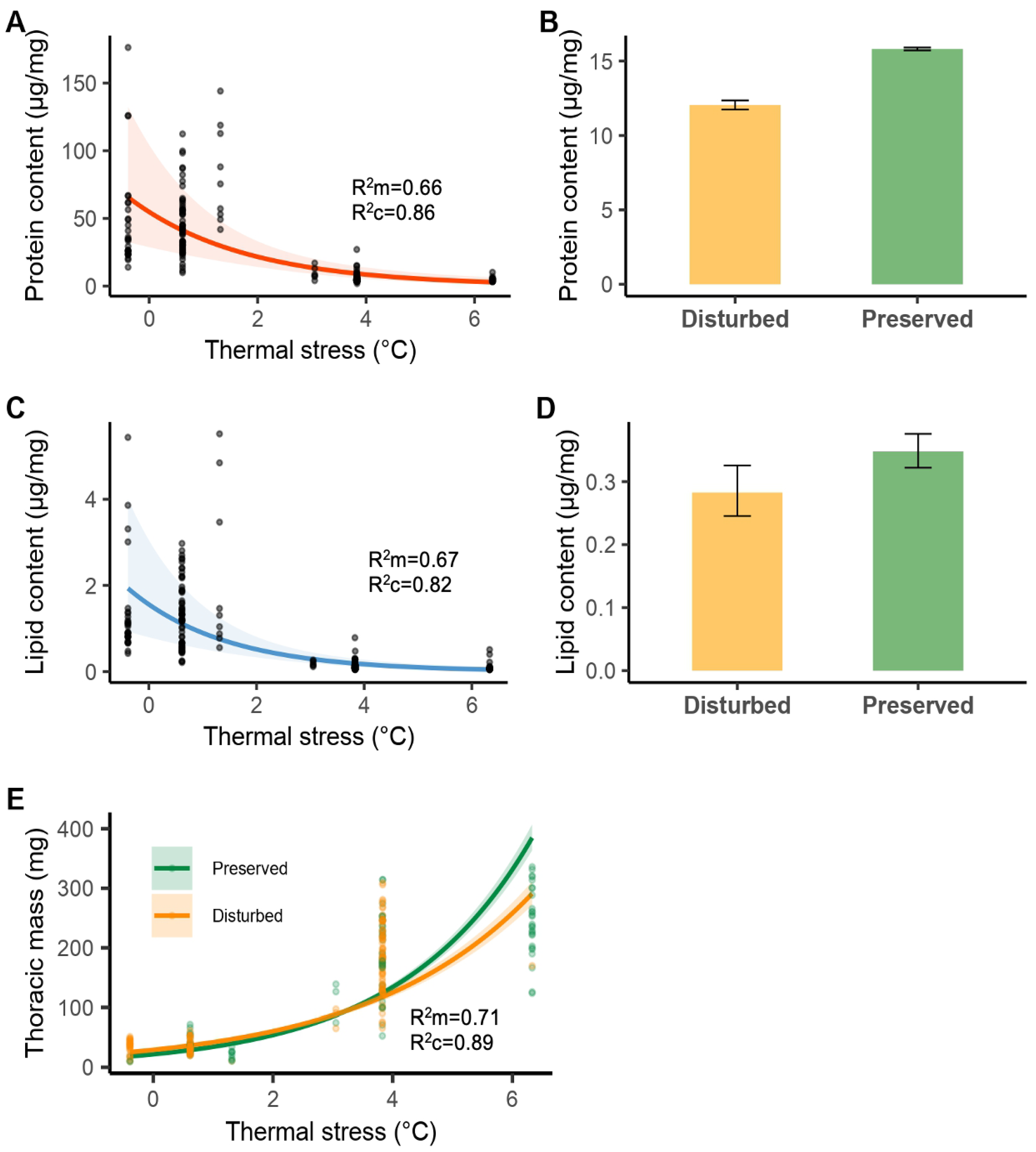
3.3. Energy Reserves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Püttker, T.; Crouzeilles, R.; Almeida-Gomes, M.; Schmoeller, M.; Maurenza, D.; Alves-Pinto, H.; Pardini, R.; Vieira, M.V.; Banks-Leite, C.; Fonseca, C.R.; et al. Indirect Effects of Habitat Loss via Habitat Fragmentation: A Cross-Taxa Analysis of Forest-Dependent Species. Biol. Conserv. 2020, 241, 108368. [Google Scholar] [CrossRef]
- Piessens, K.; Adriaens, D.; Jacquemyn, H.; Honnay, O. Synergistic Effects of an Extreme Weather Event and Habitat Fragmentation on a Specialised Insect Herbivore. Oecologia 2009, 159, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, T.D. Insect Responses to Major Landscape-Level Disturbance. Annu. Rev. Entomol. 2012, 57, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Royer, P.D.; Cobb, N.S.; Clifford, M.J.; Huang, C.-Y.; Breshears, D.D.; Adams, H.D.; Villegas, J.C. Extreme Climatic Event-Triggered Overstorey Vegetation Loss Increases Understorey Solar Input Regionally: Primary and Secondary Ecological Implications. J. Ecol. 2011, 99, 714–723. [Google Scholar] [CrossRef]
- Tuff, K.T.; Tuff, T.; Davies, K.F. A Framework for Integrating Thermal Biology into Fragmentation Research. Ecol. Lett. 2016, 19, 361–374. [Google Scholar] [CrossRef]
- Zlotnick, O.B.; Musselman, K.N.; Levy, O. Deforestation Poses Deleterious Effects to Tree-Climbing Species under Climate Change. Nat. Clim. Change 2024, 14, 289–295. [Google Scholar] [CrossRef]
- Bernaschini, M.L.; Trumper, E.; Valladares, G.; Salvo, A. Are All Edges Equal? Microclimatic Conditions, Geographical Orientation and Biological Implications in a Fragmented Forest. Agric. Ecosyst. Environ. 2019, 280, 142–151. [Google Scholar] [CrossRef]
- Mendonça, F.Z.; Bernardy, J.V.; Oliveira, C.E.K.; Oliveira, P.B.G.; De Marco, P. Temperature Effect on the Development of Tropical Dragonfly Eggs. Neotrop. Entomol. 2018, 47, 484–491. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Zurita, G.A. Physiological Traits Explain the Response of Dung Beetles to Land Use at Local and Regional Scales. Sci. Rep. 2025, 15, 7424. [Google Scholar] [CrossRef]
- Calvão, L.B.; Brito, J.D.S.; Ferreira, D.; Cunha, E.J.; Oliveira-Junior, J.M.B.; Juen, L. Effects of the Loss of Forest Cover on Odonate Communities in Eastern Amazonia. J. Insect Conserv. 2022, 27, 205–218. [Google Scholar] [CrossRef]
- Checa, M.F.; Rodriguez, J.; Willmott, K.R.; Liger, B. Microclimate Variability Significantly Affects the Composition, Abundance and Phenology of Butterfly Communities in a Highly Threatened Neotropical Dry Forest. Fla. Entomol. 2014, 97, 1–13. [Google Scholar] [CrossRef]
- De Marco Júnior, P.; Batista, J.D.; Cabette, H.S.R. Community Assembly of Adult Odonates in Tropical Streams: An Ecophysiological Hypothesis. PLoS ONE 2015, 10, e0123023. [Google Scholar] [CrossRef]
- Worthen, W.B.; Guevara-Mora, M. The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects. Diversity 2024, 16, 557. [Google Scholar] [CrossRef]
- Greiser, C.; von Schmalensee, L.; Lindestad, O.; Gotthard, K.; Lehmann, P. Microclimatic Variation Affects Developmental Phenology, Synchrony and Voltinism in an Insect Population. Funct. Ecol. 2022, 36, 3036–3048. [Google Scholar] [CrossRef]
- Duffy, G.A.; Coetzee, B.W.T.; Janion-Scheepers, C.; Chown, S.L. Microclimate-Based Macrophysiology: Implications for Insects in a Warming World. Curr. Opin. Insect Sci. 2015, 11, 84–89. [Google Scholar] [CrossRef]
- Pincebourde, S.; Woods, H.A. There Is Plenty of Room at the Bottom: Microclimates Drive Insect Vulnerability to Climate Change. Curr. Opin. Insect Sci. 2020, 41, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bota-Sierra, C.A.; García-Robledo, C.; Escobar, F.; Novelo-Gutiérrez, R.; Londoño, G.A. Environment, Taxonomy and Morphology Constrain Insect Thermal Physiology along Tropical Mountains. Funct. Ecol. 2022, 36, 1924–1935. [Google Scholar] [CrossRef]
- Castillo-Pérez, E.U.; Suárez-Tovar, C.M.; González-Tokman, D.; Schondube, J.E.; Córdoba-Aguilar, A. Insect Thermal Limits in Warm and Perturbed Habitats: Dragonflies and Damselflies as Study Cases. J. Therm. Biol. 2022, 103, 103164. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Zurita, G.A. Thermal Niche Helps to Explain the Ability of Dung Beetles to Exploit Disturbed Habitats. Sci. Rep. 2020, 10, 13364. [Google Scholar] [CrossRef]
- Heinrich, B. Insect Thermoregulation. Endeavour 1995, 19, 28–33. [Google Scholar] [CrossRef]
- Verdú, J.R.; Arellano, L.; Numa, C. Thermoregulation in Endothermic Dung Beetles (Coleoptera: Scarabaeidae): Effect of Body Size and Ecophysiological Constraints in Flight. J. Insect Physiol. 2006, 52, 854–860. [Google Scholar] [CrossRef]
- Heinrich, B. Thermoregulation in Endothermic Insects. Science 1974, 185, 747–756. [Google Scholar] [CrossRef]
- Rocha, T.S.; Calvão, L.B.; Juen, L.; Oliveira-Junior, J.M.B. Effect of Environmental Integrity on the Functional Composition of the Odonata (Insecta) Community in Streams in the Eastern Amazon. Front. Ecol. Evol. 2023, 11, 1166057. [Google Scholar] [CrossRef]
- May, M.L. Thermoregulation and Adaptation to Temperature in Dragonflies (Odonata: Anisoptera). Ecol. Monogr. 1976, 46, 1–32. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999; ISBN 0-8014-2592-1. [Google Scholar]
- Castillo-Pérez, E.U.; Rivera-Duarte, J.D.; Abellán, P.; del-Val, E.; González-Tokman, D.; Córdoba-Aguilar, A. Thriving in the Heat: How High Temperatures and Habitat Disturbance Shape Odonate Taxonomic and Functional Diversity in the Tropics. Insect Conserv. Divers. 2024, 18, 343–356. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Castillo-Pérez, E.U.; Sandoval-García, I.A.; Schondube, J.E.; Cano-Santana, Z.; Córdoba-Aguilar, A. Resilient Dragons: Exploring Odonata Communities in an Urbanization Gradient. Ecol. Indic. 2022, 141, 109134. [Google Scholar] [CrossRef]
- Castillo-Pérez, U.; May, M.L.; Córdoba-Aguilar, A. Thermoregulation in Odonata. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Beatty, C., Bried, J., Eds.; Oxford University Press: Oxford, UK, 2022; pp. 101–112. [Google Scholar] [CrossRef]
- Polcyn, D.M. Thermoregulation During Summer Activity in Mojave Desert Dragonflies (Odonata: Anisoptera). Funct. Ecol. 1994, 8, 441–449. [Google Scholar] [CrossRef]
- Heinrich, B.C.; Casey, T.M. Heat Transfer in Dragonflies: ‘Fliers’ and ‘Perchers’. J. Exp. Biol. 1978, 74, 17–36. [Google Scholar] [CrossRef]
- Heinrich, B. Dragonflies Now and Then. In The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation; Heinrich, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 117–142. ISBN 978-3-662-10340-1. [Google Scholar]
- May, M.L. Simultaneous Control of Head and Thoracic Temperature by the Green Darner Dragonfly Anax Junius (Odonata: Aeshnidae). J. Exp. Biol. 1995, 198, 2373–2384. [Google Scholar] [CrossRef]
- Lahondère, C. Recent Advances in Insect Thermoregulation. J. Exp. Biol. 2023, 226, jeb245751. [Google Scholar] [CrossRef]
- May, M. Insect Thermoregulation. Annu. Rev. Entomol. 1979, 24, 313–349. [Google Scholar] [CrossRef]
- Klepsatel, P.; Wildridge, D.; Gáliková, M. Temperature Induces Changes in Drosophila Energy Stores. Sci. Rep. 2019, 9, 5239. [Google Scholar] [CrossRef]
- Corbet, P.S.; May, M.L. Fliers and Perchers among Odonata: Dichotomy or Multidimensional Continuum? A Provisional Reappraisal. Int. J. Odonatol. 2008, 11, 155–171. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C. Sen Effect of Acclimation on Heat-Escape Temperatures of Two Aphid Species: Implications for Estimating Behavioral Response of Insects to Climate Warming. J. Insect Physiol. 2012, 58, 303–309. [Google Scholar] [CrossRef]
- Klepsatel, P.; Gáliková, M.; Xu, Y.; Kühnlein, R.P. Thermal Stress Depletes Energy Reserves in Drosophila. Sci. Rep. 2016, 6, 33667. [Google Scholar] [CrossRef]
- García-Oliva, F.; Camou, A.; Maass, J.M. El Clima de La Región Central de La Costa Del Pacífico Mexicano. In Historia Natural de Chamela; Noguera-Alderte, A.N., Vega-Rivera, J.H., García-Aldrete, A.N., Quesada, M., Eds.; Instituto de Biología, UNAM: Ciudad de Mexico, Mexico, 2002; pp. 3–10. [Google Scholar]
- Portela Salomão, R.; González-Tokman, D.; Dáttilo, W.; López-Acosta, J.C.; Favila, M.E. Landscape Structure and Composition Define the Body Condition of Dung Beetles (Coleoptera: Scarabaeinae) in a Fragmented Tropical Rainforest. Ecol. Indic. 2018, 88, 144–151. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; Cordero-Rivera, A.; Novelo-Gutiérrez, R.; Sánchez-Herrera, M.; Londoño, G.A. Can High Temperatures Affect Body Size in Insects? The Case of Rubyspot Damselflies in the Colombian Western Andes. Diversity 2024, 16, 743. [Google Scholar] [CrossRef]
- O’Donnell, M.J. A Perspective on Insect Water Balance. J. Exp. Biol. 2022, 225, jeb242358. [Google Scholar] [CrossRef] [PubMed]
- Flores-Casas, R.; Ortega-Huerta, M.A. Modelling Land Cover Changes in the Tropical Dry Forest Surrounding the Chamela-Cuixmala Biosphere Reserve, Mexico. Int. J. Remote Sens. 2019, 40, 6948–6974. [Google Scholar] [CrossRef]
- Martínez-Ibarra, J.A.; Martínez-Hernández, F.; Villalobos, G.; Vences-Blanco, M.O.; Salazar-Schettino, P.M. Update on the Distribution of Triatoma Bolivari and Triatoma Brailovskyi (Hemiptera: Reduviidae: Triatominae) in Western Mexico. J. Vector Ecol. 2010, 35, 432–434. [Google Scholar] [CrossRef]
- Takano-Rojas, H.; Murray-Tortarolo, G.; Maass, M.; Castillo, A. Characterization, Variability and Long-Term Trends on Local Climate in a Mexican Tropical Dry Forest. Int. J. Climatol. 2023, 43, 5077–5091. [Google Scholar] [CrossRef]
- IBUNAM. Datos Climáticos Estación Chamela. Available online: http://www.ibiologia.unam.mx/ebchamela/www/clima.html (accessed on 1 November 2019).
- Sánchez-Azofeifa, G.A.; Quesada, M.; Cuevas-Reyes, P.; Castillo, A.; Sánchez-Montoya, G. Land Cover and Conservation in the Area of Influence of the Chamela-Cuixmala Biosphere Reserve, Mexico. Ecol. Manag. 2009, 258, 907–912. [Google Scholar] [CrossRef]
- Villa-Galaviz, E.; Boege, K.; Del-Val, E. Resilience in Plant-Herbivore Networks during Secondary Succession. PLoS ONE 2012, 7, e53009. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Crowley, P.H.; Brodin, T. Sexual Size Dimorphism and Sex Ratios in Dragonflies (Odonata). Biol. J. Linn. Soc. 2005, 86, 507–513. [Google Scholar] [CrossRef]
- Foray, V.; Pelisson, P.-F.; Bel-Venner, M.-C.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D.; Benjamin, R. A Handbook for Uncovering the Complete Energetic Budget in Insects: The van Handel’s Method (1985) Revisited. Physiol. Entomol. 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Marden, J.H. Dragonfly Flight Performance: A Model System for Biomechanics, Physiological Genetics, and Animal Competitive Behaviour. In Dragonflies and Damselflies. Model Organisms for Ecological and Evolutionary Research; Oxford University Press: Oxford, UK, 2008; pp. 249–261. [Google Scholar]
- Li, X.; Zhou, Y.; Wu, K. Biological Characteristics and Energy Metabolism of Migrating Insects. Metabolites 2023, 13, 439. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid Determination of Total Lipids in Mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 9780387953649. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Mazerolle, M.J. AICcmodavg, Version 2.3-4; Model Selection and Multimodel Inference Based on (Q)AIC(c), R package version 2.3-4; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Chown, S.L.; Nicolson, S.W. Thermoregulation. In Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect Responses to Heat: Physiological Mechanisms, Evolution and Ecological Implications in a Warming World. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Candy, D.J.; Becker, A.; Wegener, G. Coordination and Integration of Metabolism in Insect Flight *. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 497–512. [Google Scholar] [CrossRef]
- Janssens, M. Hormonal Control of Flight Metabolism in Odonata? Master’s Thesis, University of Cape Town, Cape Town, South Africa, 1995. [Google Scholar]
- Sacktor, B. Biochemistry of Insect Flight. In Insect Biochemistry and Function; Candy, D.J., Kilby, B.A., Eds.; Springer: Boston, MA, USA, 1975; pp. 1–88. ISBN 978-1-4899-3204-4. [Google Scholar]
- Kallapur, V.L.; George, C.J. Fatty Acid Oxidation by the Flight Muscles of the Dragonfly, Pantala Flavescens. J. Insect Physiol. 1973, 19, 1035–1040. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; Rocha-Ortega, M. Damselfly (Odonata: Calopterygidae) Population Decline in an Urbanizing Watershed. J. Insect Sci. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Tovar, C.M.; Rocha-Ortega, M.; Córdoba-Aguilar, A. Is Body Condition of Mexican Rubyspot (Odonata:Zygoptera) Associated with Urbanization? J. Insect Conserv. 2023, 27, 961–969. [Google Scholar] [CrossRef]
- Salomão, R.P.; Alvarado, F.; Baena-Díaz, F.; Favila, M.E.; Iannuzzi, L.; Liberal, C.N.; Santos, B.A.; Villegas-Guzmán, G.A.; González-Tokman, D. Negative Effects of Urbanisation on the Physical Condition of an Endemic Dung Beetle from a Neotropical Hotspot. Ecol. Entomol. 2020, 45, 886–895. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; González-Tokman, D.M. The Behavioral and Physiological Ecology of Adult Rubyspot Damselflies (Hetaerina, Calopterygidae, Odonata). Adv. Study Behav. 2014, 46, 311–341. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime Consequences of Food Protein-Carbohydrate Content for an Insect Herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Rocha-Ortega, M.; Juen, L.; Córdoba-Aguilar, A. From the Forest to the City: The Persistence of Dragonflies and Damselflies in the Urban Jungle. Biodivers. Conserv. 2024, 33, 91–113. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Rocha-Ortega, M.; González-Voyer, A.; González-Tokman, D.; Córdoba-Aguilar, A. The Larger the Damselfly, the More Likely to Be Threatened: A Sexual Selection Approach. J. Insect Conserv. 2019, 23, 535–545. [Google Scholar] [CrossRef]
- Bernardino, G.V.D.S.; Mesquita, V.P.; Bobrowiec, P.E.D.; Iannuzzi, L.; Salomão, R.P.; Cornelius, C. Habitat Loss Reduces Abundance and Body Size of Forest-Dwelling Dung Beetles in an Amazonian Urban Landscape. Urban. Ecosyst. 2024, 27, 1175–1190. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Juen, L. The Zygoptera/Anisoptera Ratio (Insecta: Odonata): A New Tool for Habitat Alterations Assessment in Amazonian Streams. Neotrop. Entomol. 2019, 48, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Hassall, C. Odonata as Candidate Macroecological Barometers for Global Climate Change. Freshw. Sci. 2015, 34, 1040–1049. [Google Scholar] [CrossRef]
- Šigutová, H.; Šipoš, J.; Dolný, A. A Novel Approach Involving the Use of Odonata as Indicators of Tropical Forest Degradation: When Family Matters. Ecol. Indic. 2019, 104, 229–236. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Spatial and Temporal Effects of Land Use Change as Potential Drivers of Odonate Community Composition but Not Species Richness. Biodivers. Conserv. 2019, 28, 451–466. [Google Scholar] [CrossRef]
- Samways, M.J. Farm Dams as Nature Reserves for Dragonflies (Odonata) at Various Altitudes in the Natal Drakensberg Mountains, South Africa. Biol. Conserv. 1989, 48, 181–187. [Google Scholar] [CrossRef]
- Samways, M.J.; Córdoba-Aguilar, A.; Deacon, C.; Alves-Martins, F.; Baird, I.R.C.; Barmentlo, S.H.; Brasil, L.S.; Bried, J.T.; Clausnitzer, V.; Cordero-Rivera, A.; et al. Scientists’ Warning on the Need for Greater Inclusion of Dragonflies in Global Conservation. Insect Conserv. Divers. 2025, 18, 465–484. [Google Scholar] [CrossRef]
- May, M.L. Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies. Insects 2019, 10, 62. [Google Scholar] [CrossRef]
- Williams, E.B.; Chumchal, M.M.; Drenner, R.W.; Kennedy, J.H. Seasonality of Odonate-mediated Methylmercury Flux from Permanent and Semipermanent Ponds and Potential Risk to Red-winged Blackbirds (Agelaius phoeniceus). Environ. Toxicol. Chem. 2017, 36, 2833–2837. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; San Miguel-Rodríguez, M.; Rocha-Ortega, M.; Lanz-Mendoza, H.; Cime-Castillo, J.; Benelli, G. Adult Damselflies as Possible Regulators of Mosquito Populations in Urban Areas. Pest. Manag. Sci. 2021, 77, 4274–4287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Pérez, E.U.; Ensaldo-Cárdenas, A.S.; Suárez-Tovar, C.M.; Rivera-Duarte, J.D.; González-Tokman, D.; Córdoba-Aguilar, A. The Physiological Cost of Being Hot: High Thermal Stress and Disturbance Decrease Energy Reserves in Dragonflies in the Wild. Biology 2025, 14, 956. https://doi.org/10.3390/biology14080956
Castillo-Pérez EU, Ensaldo-Cárdenas AS, Suárez-Tovar CM, Rivera-Duarte JD, González-Tokman D, Córdoba-Aguilar A. The Physiological Cost of Being Hot: High Thermal Stress and Disturbance Decrease Energy Reserves in Dragonflies in the Wild. Biology. 2025; 14(8):956. https://doi.org/10.3390/biology14080956
Chicago/Turabian StyleCastillo-Pérez, Eduardo Ulises, Angélica S. Ensaldo-Cárdenas, Catalina M. Suárez-Tovar, José D. Rivera-Duarte, Daniel González-Tokman, and Alex Córdoba-Aguilar. 2025. "The Physiological Cost of Being Hot: High Thermal Stress and Disturbance Decrease Energy Reserves in Dragonflies in the Wild" Biology 14, no. 8: 956. https://doi.org/10.3390/biology14080956
APA StyleCastillo-Pérez, E. U., Ensaldo-Cárdenas, A. S., Suárez-Tovar, C. M., Rivera-Duarte, J. D., González-Tokman, D., & Córdoba-Aguilar, A. (2025). The Physiological Cost of Being Hot: High Thermal Stress and Disturbance Decrease Energy Reserves in Dragonflies in the Wild. Biology, 14(8), 956. https://doi.org/10.3390/biology14080956







