Exercise Ameliorates Dopaminergic Neurodegeneration in Parkinson’s Disease Mice by Suppressing Microglia-Regulated Neuroinflammation Through Irisin/AMPK/Sirt1 Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
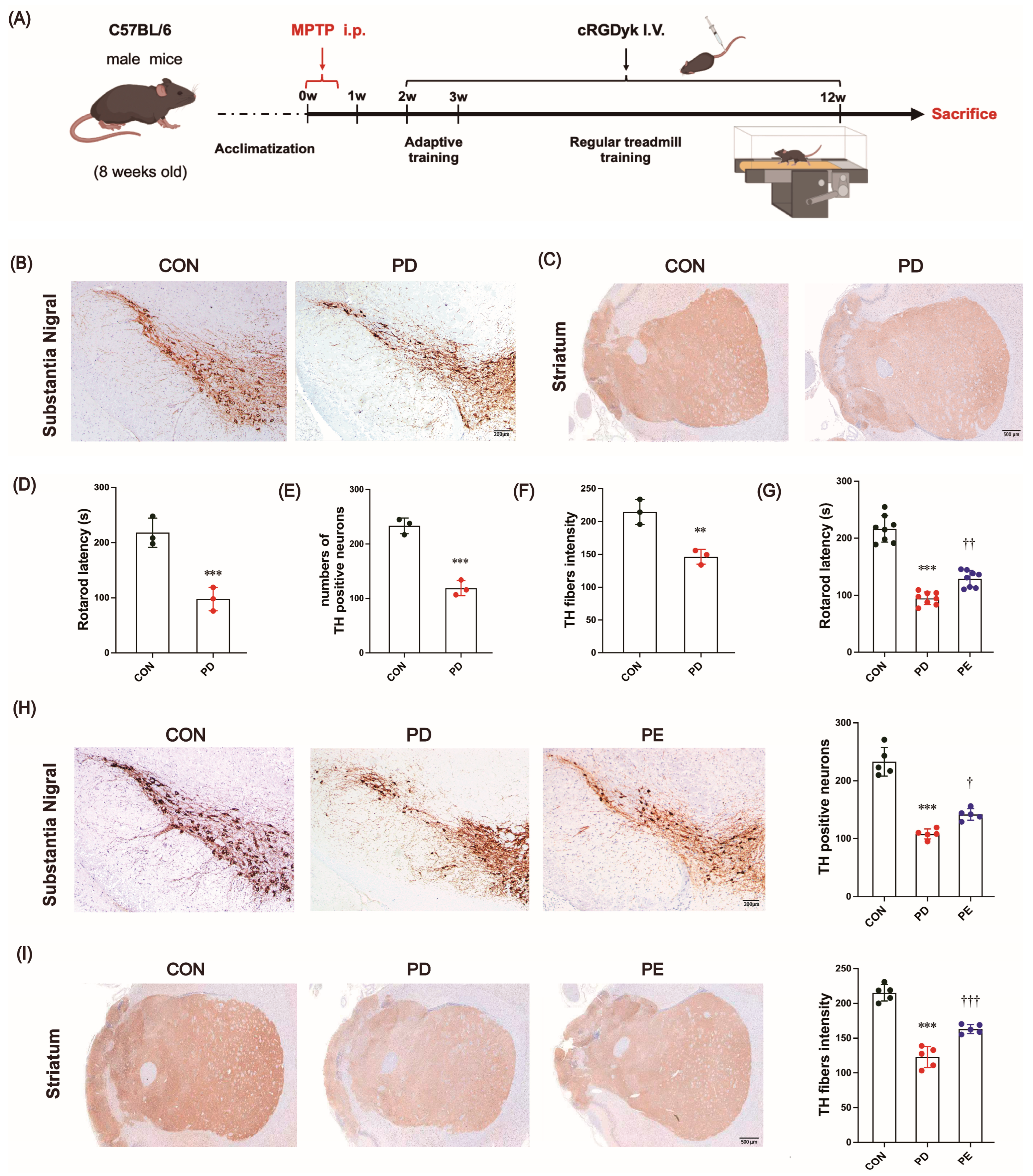
2.1. Animals and Protocols
2.2. PD Model Development and Drug Treatment
2.3. Treadmill Running Protocol
2.4. Rotarod Test
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blot Analysis
2.7. Immunohistochemistry and Immunofluorescence Staining
2.8. Real-Time Quantitative Polymerase Chain Reaction (RT q-PCR)
2.9. Statistical Analysis
3. Results
3.1. MPTP-Induced Locomotor Impairment and the Loss of Dopaminergic Neurons Are Improved by Exercise Intervention
3.2. Exercise Reduces Excessive Apoptosis in Nigrostriatal Neurons of PD Mice
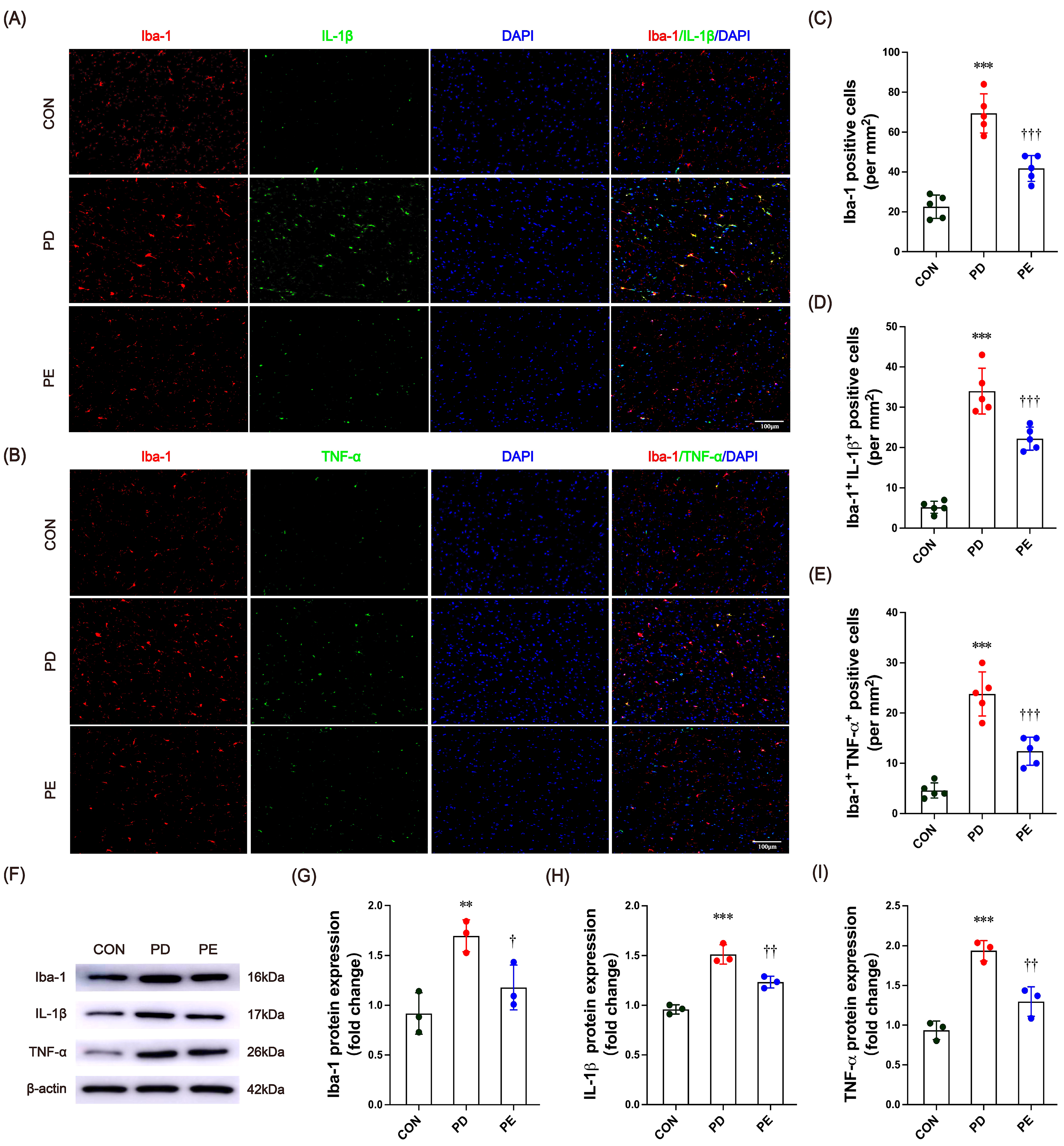
3.3. Exercise Inhibits Microglia Activation and Inflammatory Factor Expression in the Substantia Nigra of PD Mice
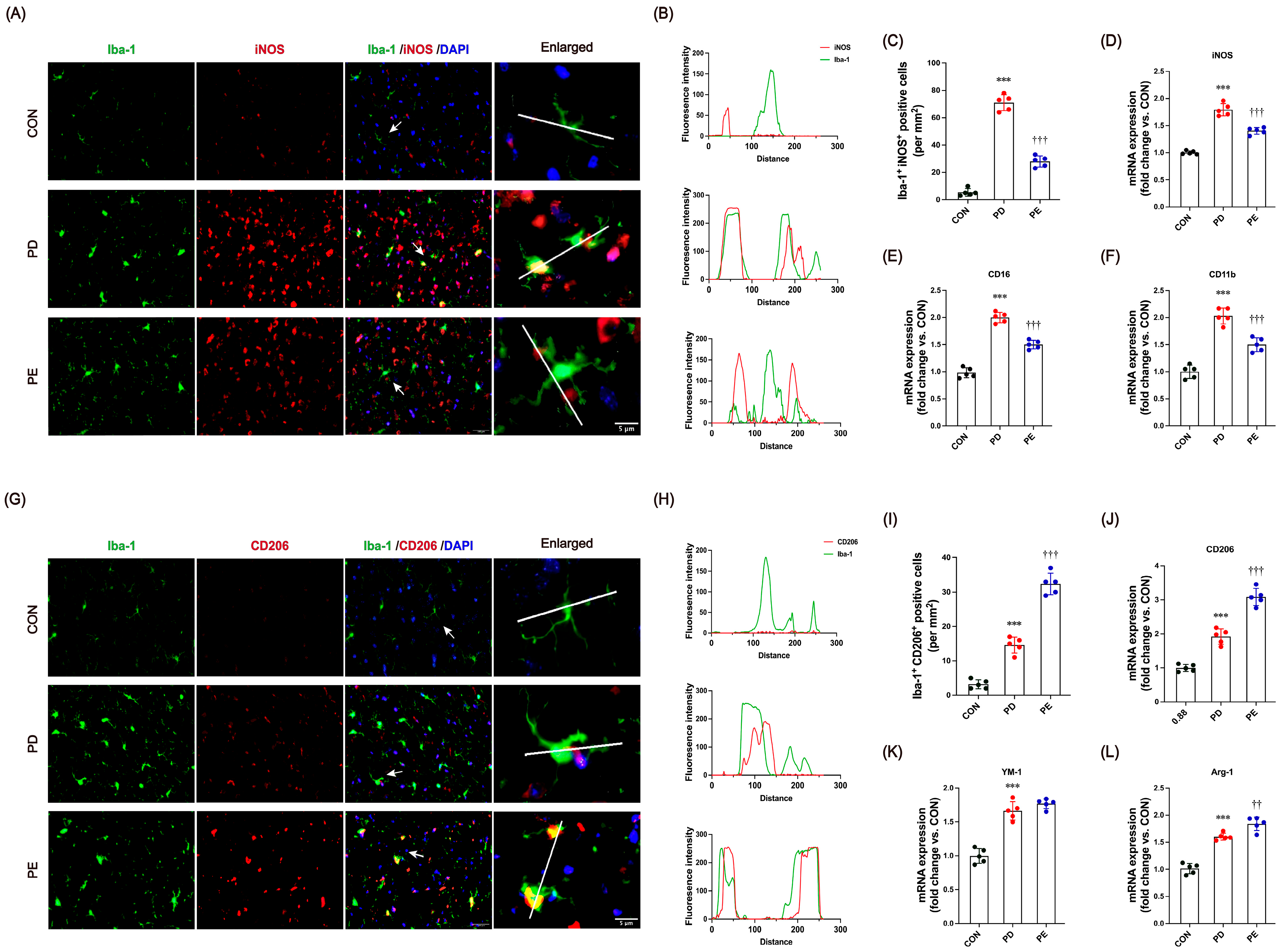
3.4. Exercise Modulates Microglial Transition Toward Anti-Inflammatory Functional States
3.5. Exercise Promotes AMPK/Sirt1 Signaling, but This Is Blocked by the Irisin Receptor Inhibitor
3.6. Blocking Irisin Pathways Could Diminish the Exercise-Induced Neuroprotective Effects on PD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Li, X.; Gao, Z.; Yu, H.; Gu, Y.; Yang, G. Effect of Long-term Exercise Therapy on Motor Symptoms in Parkinson Disease Patients: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Phys. Med. Rehabil. 2022, 101, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Iba, M.; Kim, C.; Sallin, M.; Kwon, S.; Verma, A.; Overk, C.; Rissman, R.A.; Sen, R.; Sen, J.M.; Masliah, E. Neuroinflammation is associated with infiltration of T cells in Lewy body disease and alpha-synuclein transgenic models. J. Neuroinflammation 2020, 17, 214. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, S.; Ruan, S.; Wei, J.; Wei, S.; Chen, W.; Hu, H.; Qin, W.; Li, Y.; Yuan, H.; et al. Neuroprotective effects of cordycepin on MPTP-induced Parkinson’s disease mice via suppressing PI3K/AKT/mTOR and MAPK-mediated neuroinflammation. Free Radic. Biol. Med. 2024, 216, 60–77. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; Zanier, E.R.; Carlino, E.; Panini, N.; Erba, E.; De Simoni, M.G. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol. Dis. 2016, 96, 284–293. [Google Scholar] [CrossRef]
- Li, H.; Wei, J.; Zhang, Z.; Li, J.; Ma, Y.; Zhang, P.; Lin, J. Menstrual blood-derived endometrial stem cells alleviate neuroinflammation by modulating M1/M2 polarization in cell and rat Parkinson’s disease models. Stem Cell Res. Ther. 2023, 14, 85. [Google Scholar] [CrossRef]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef]
- Zhao, R. Exercise mimetics: A novel strategy to combat neuroinflammation and Alzheimer’s disease. J. Neuroinflammation 2024, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R. Can exercise benefits be harnessed with drugs? A new way to combat neurodegenerative diseases by boosting neurogenesis. Transl. Neurodegener. 2024, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, Z.; Gao, J.; Liu, M.; Wang, Y.; Tang, C.; Xiang, J. Treadmill exercise alleviates neuronal damage by suppressing NLRP3 inflammasome and microglial activation in the MPTP mouse model of Parkinson’s disease. Brain Res. Bull. 2021, 174, 349–358. [Google Scholar] [CrossRef]
- Liu, W.; Fu, R.; Wang, Z.; Liu, S.; Tang, C.; Li, L.; Yin, D. Regular Aerobic Exercise-Alleviated Dysregulation of CAMKIIalpha Carbonylation to Mitigate Parkinsonism via Homeostasis of Apoptosis With Autophagy. J. Neuropathol. Exp. Neurol. 2020, 79, 46–61. [Google Scholar] [CrossRef]
- Sung, Y.H.; Kim, S.C.; Hong, H.P.; Park, C.Y.; Shin, M.S.; Kim, C.J.; Seo, J.H.; Kim, D.Y.; Kim, D.J.; Cho, H.J. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012, 91, 1309–1316. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the crossroads of inter-organ communications: Challenge and implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025, 24, e70061. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Kim, O.; Song, J. The Role of Irisin in Alzheimer’s Disease. J. Clin. Med. 2018, 7, 407. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin alphaVbeta5/AMPK signaling pathway after intracerebral hemorrhage in mice. J. Neuroinflammation 2022, 19, 82. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 585821. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, C.; Wang, Y.; Tian, X.; Lin, J.; Zhu, B.; Zhou, Y.; Zhang, X.; Li, N.; Sun, Y.; et al. Exercise ameliorating myocardial injury in type 2 diabetic rats by inhibiting excessive mitochondrial fission involving increased irisin expression and AMP-activated protein kinase phosphorylation. J. Diabetes 2024, 16, e13475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qian, L.; Chen, S.H.; Chu, C.H.; Wilson, B.; Oyarzabal, E.; Ali, S.; Robinson, B.; Rao, D.; Hong, J.S. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson’s disease models. Brain 2015, 138, 1247–1262. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Kou, L.; Chi, X.; Sun, Y.; Han, C.; Wan, F.; Hu, J.; Yin, S.; Wu, J.; Li, Y.; Zhou, Q.; et al. The circadian clock protein Rev-erbalpha provides neuroprotection and attenuates neuroinflammation against Parkinson’s disease via the microglial NLRP3 inflammasome. J. Neuroinflammation 2022, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Wysocka, A.; Stepniewska, A.; Niewiadomska, G. Exercise-Induced Neuroprotection and Recovery of Motor Function in Animal Models of Parkinson’s Disease. Front. Neurol. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Huang, Z.; Fan, X.; Wang, X.; Chen, X.; Guo, H.; Liu, H.; Li, S.; Yu, S.; et al. Dopaminergic system and neurons: Role in multiple neurological diseases. Neuropharmacology 2024, 260, 110133. [Google Scholar] [CrossRef]
- Mochizuki, H.; Goto, K.; Mori, H.; Mizuno, Y. Histochemical detection of apoptosis in Parkinson’s disease. J. Neurol. Sci. 1996, 137, 120–123. [Google Scholar] [CrossRef]
- Wullner, U.; Kornhuber, J.; Weller, M.; Schulz, J.B.; Loschmann, P.A.; Riederer, P.; Klockgether, T. Cell death and apoptosis regulating proteins in Parkinson’s disease—A cautionary note. Acta Neuropathol. 1999, 97, 408–412. [Google Scholar] [CrossRef]
- Erekat, N.S. Apoptosis and its Role in Parkinson’s Disease. In Parkinson’s Disease: Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; eXon: Brisbane, Australia, 2018. [Google Scholar] [CrossRef]
- Perier, C.; Bove, J.; Vila, M. Mitochondria and programmed cell death in Parkinson’s disease: Apoptosis and beyond. Antioxid. Redox Signal. 2012, 16, 883–895. [Google Scholar] [CrossRef]
- Yuan, J.; Yankner, B.A. Apoptosis in the nervous system. Nature 2000, 407, 802–809. [Google Scholar] [CrossRef]
- Phani, S.; Loike, J.D.; Przedborski, S. Neurodegeneration and inflammation in Parkinson’s disease. Park. Relat. Disord. 2012, 18 (Suppl. S1), S207–S209. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.; Rainey-Smith, S.R. Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review. Ageing Res. Rev. 2019, 50, 9–18. [Google Scholar] [CrossRef]
- Choi, H.Y.; Cho, K.H.; Jin, C.; Lee, J.; Kim, T.H.; Jung, W.S.; Moon, S.K.; Ko, C.N.; Cho, S.Y.; Jeon, C.Y.; et al. Exercise Therapies for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Park. Dis. 2020, 2020, 2565320. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.Y.; Wong-Yu, I.S.K. Exercise for Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 1–44. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Tsukita, K.; Sakamaki-Tsukita, H.; Takahashi, R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology 2022, 98, e859–e871. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; Van Leeuwen, J.E.; Vukovic, M.; Akopian, G.; Meshul, C.K.; Holschneider, D.P.; Nacca, A.; Walsh, J.P.; Jakowec, M.W. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov. Disord. 2010, 25, S141–S145. [Google Scholar] [CrossRef]
- Ferreira, A.F.F.; Binda, K.H.; Real, C.C. The effects of treadmill exercise in animal models of Parkinson’s disease: A systematic review. Neurosci. Biobehav. Rev. 2021, 131, 1056–1075. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflammation 2022, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Liu, T.; Mao, Y.; Peng, B. Correction: Novel Microglia-based Therapeutic Approaches to Neurodegenerative Disorders. Neurosci. Bull. 2023, 39, 557. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, T.; Li, J.; Yang, J.; Liu, H.; Zhang, X.J.; Le, W. Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson’s disease. Cell Death Differ. 2014, 21, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Wang, X.; Xia, Y.; Huang, J.; Wang, T.; Lin, Z.; Xiong, N. Melatonin ameliorates Parkinson’s disease via regulating microglia polarization in a RORalpha-dependent pathway. NPJ Park. Dis. 2022, 8, 90. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, J.; Wang, P.; Liu, J.; Zhao, Y.; Jiang, F.; Lou, H. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav. Immun. 2021, 91, 324–338. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Safari, M.; Aldaghi, M.R.; Sameni, H.R.; Ghahari, L.; Khaleghi Lagmouj, Y.; Rahimi Jaberi, K.; Parsaie, H. Irisin protects the substantia nigra dopaminergic neurons in the rat model of Parkinson’s disease. Iran. J. Basic Med. Sci. 2019, 22, 722–728. [Google Scholar] [CrossRef]
- Tang, C.; Liu, M.; Zhou, Z.; Li, H.; Yang, C.; Yang, L.; Xiang, J. Treadmill Exercise Alleviates Cognition Disorder by Activating the FNDC5: Dual Role of Integrin alphaV/beta5 in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 7830. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Hu, Y.; Liu, Q.; Liu, C.; Chai, H.; Luo, Y.; Jin, L.; Li, S. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Welser-Alves, J.V.; Boroujerdi, A.; Tigges, U.; Milner, R. Microglia use multiple mechanisms to mediate interactions with vitronectin; non-essential roles for the highly-expressed alphavbeta3 and alphavbeta5 integrins. J. Neuroinflammation 2011, 8, 157. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Deng, X.; Huang, W.; Yu, J.-H.; Wang, J.-X.; Wang, J.-P.; Yang, S.-B.; Liu, X.; Wang, L.; Zhang, Y.; et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 2017, 91, 185–194. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.-H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Hong, H.; Shi, Y.; Zhou, Y.; Qiao, C.M.; Zhao, W.J.; Zhao, L.P.; Wu, J.; Quan, W.; Niu, G.Y.; et al. Vancomycin Pretreatment on MPTP-Induced Parkinson’s Disease Mice Exerts Neuroprotection by Suppressing Inflammation Both in Brain and Gut. J. Neuroimmune Pharmacol. 2023, 18, 72–89. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Sequence |
|---|---|---|
| iNOS | Forward | 5′-CTGCCCCCCTGCTCACTC-3′ |
| Reverse | 5′-TGGGAGGGGTCGTAATGTCC-3′ | |
| CD16 | Forward | 5′-TTTGGACACCCAGATGTTTCAG-3′ |
| Reverse | 5′-GTCTTCCTTTGAGCACCTGGATC-3′ | |
| CD11b | Forward | 5′-GAGCAGCACTGAGATCCTGTTTAA-3′ |
| Reverse | 5′-ATACGACTCCTGCCCTGGAA-3′ | |
| Arg1 | Forward | 5′-GAACACGGCAGTGGCTTTAAC-3′ |
| Reverse | 5′-TGCTTAGCTCTGTCTGCTTTGC-3′ | |
| YM-1 | Forward | 5′-AGGAAGCCCTCCTAAGGACAAACA-3′ |
| Reverse | 5′-ATGCCCATATGCTGGAAATCCCAC-3′ | |
| CD206 | Forward | 5′-AAGGAAGGTTGGCATTTGT-3′ |
| Reverse | 5′-CCTTTCAATCCTATGCAAGC-3′ | |
| GAPDH | Forward | 5′-TTCAACGGCACAGTCAAGGC-3′ |
| Reverse | 5′-GACTCCACGACATACTCAGCACC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, N.; Wang, Y.; Tian, X.; Lin, J.; Zhang, X.; Xu, H.; Sun, Y.; Zhao, R. Exercise Ameliorates Dopaminergic Neurodegeneration in Parkinson’s Disease Mice by Suppressing Microglia-Regulated Neuroinflammation Through Irisin/AMPK/Sirt1 Pathway. Biology 2025, 14, 955. https://doi.org/10.3390/biology14080955
Wang B, Li N, Wang Y, Tian X, Lin J, Zhang X, Xu H, Sun Y, Zhao R. Exercise Ameliorates Dopaminergic Neurodegeneration in Parkinson’s Disease Mice by Suppressing Microglia-Regulated Neuroinflammation Through Irisin/AMPK/Sirt1 Pathway. Biology. 2025; 14(8):955. https://doi.org/10.3390/biology14080955
Chicago/Turabian StyleWang, Bin, Nan Li, Yuanxin Wang, Xin Tian, Junjie Lin, Xin Zhang, Haocheng Xu, Yu Sun, and Renqing Zhao. 2025. "Exercise Ameliorates Dopaminergic Neurodegeneration in Parkinson’s Disease Mice by Suppressing Microglia-Regulated Neuroinflammation Through Irisin/AMPK/Sirt1 Pathway" Biology 14, no. 8: 955. https://doi.org/10.3390/biology14080955
APA StyleWang, B., Li, N., Wang, Y., Tian, X., Lin, J., Zhang, X., Xu, H., Sun, Y., & Zhao, R. (2025). Exercise Ameliorates Dopaminergic Neurodegeneration in Parkinson’s Disease Mice by Suppressing Microglia-Regulated Neuroinflammation Through Irisin/AMPK/Sirt1 Pathway. Biology, 14(8), 955. https://doi.org/10.3390/biology14080955







