Seasonal Spatial Distribution Patterns of the Sand Crab Ovalipes punctatus (De Haan 1833) in the Southern Yellow and East China Seas and Predictions from Various Climate Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Survey Procedures
2.2. Ensemble Model
3. Results and Discussion
3.1. Seasonal Spatial Distribution Characteristics and Patterns
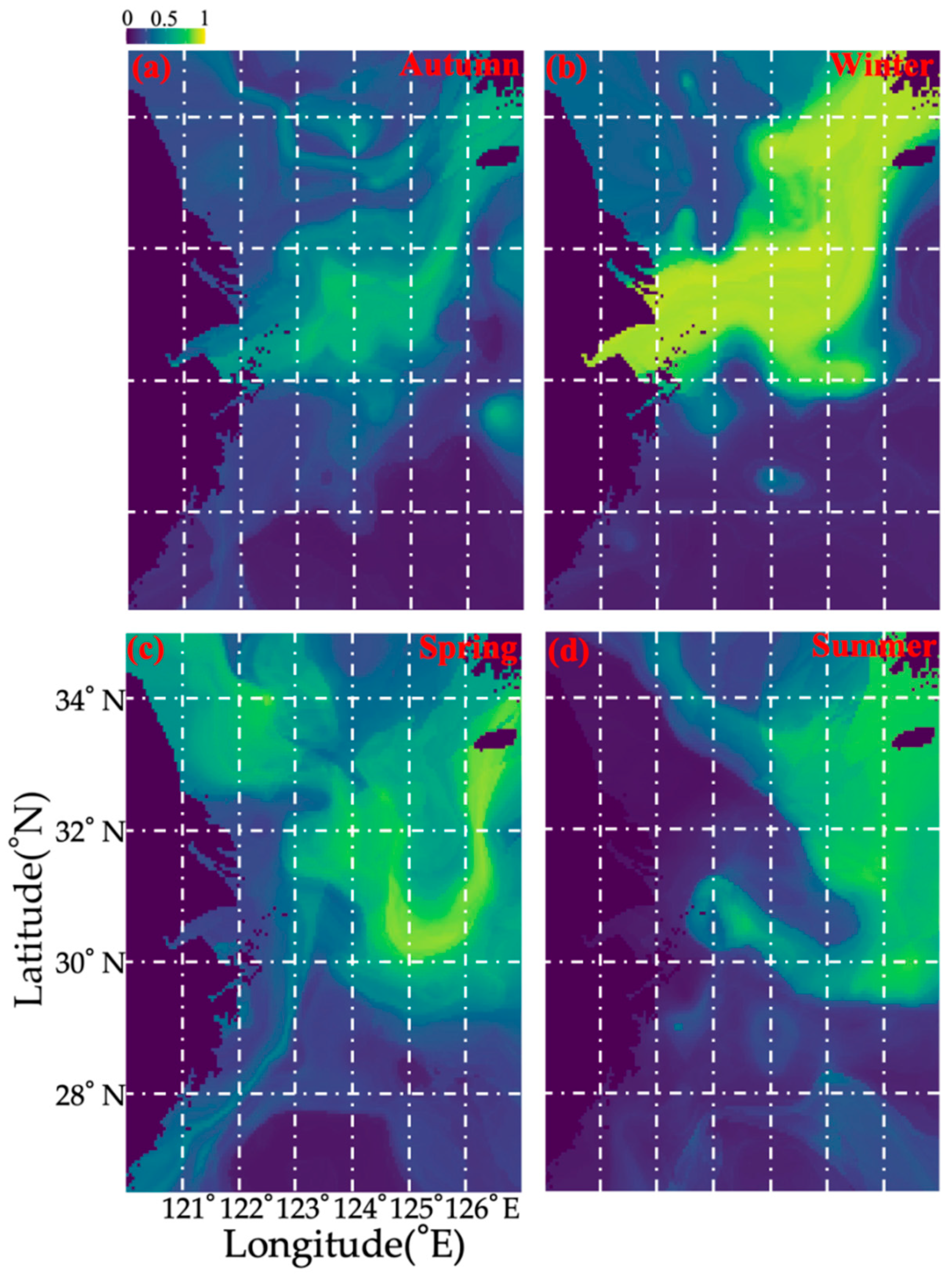
3.2. Seasonal Variations of Environmental Variables
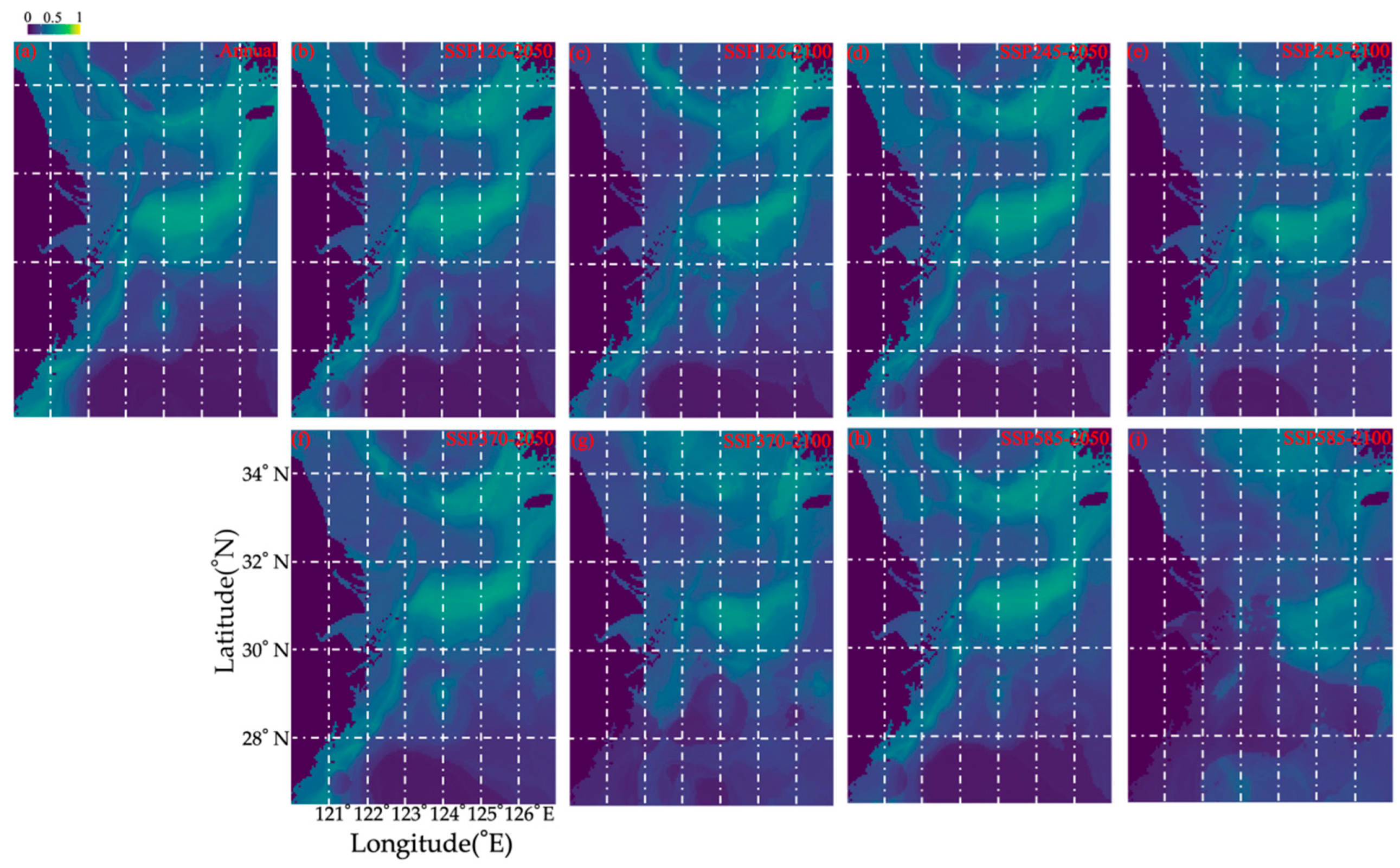
3.3. Habitat Predictions Under Different Climate Projections
3.4. Implications for Fisheries Management, Underlying Benefits, and Future Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, C.G.; Song, H.T.; Yao, G.Z. Study on distribution of Ovalipes punctatus (crab) in the East China Sea. J. Fish. China 2005, 29, 198–204. (In Chinese) [Google Scholar]
- Wu, G.F.; Xie, Q.J. Biological traits and seasonal spatial distribution pattern of main economic crab species in Mindongbei fishing ground. J. Fujian Fish. 2002, 1, 10–14. (In Chinese) [Google Scholar]
- Brown, A.C.; McLachlan, A. Ecology of Sandy Shores; Elsevier Press: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Jiang, X.Q.; Yu, C.G. Reproductive biology of Ovalipes punctatus in the East China Sea. J. Zhejiang Ocean. Univ. Nat. Sci. Ed. 2012, 31, 285–289. (In Chinese) [Google Scholar]
- Ye, Q.T.; Zhang, Z.L.; Ye, S.Z. The biomass distribution and biological characteristic of Ovalipes Punctatus in the Eastern Fujian Outer Sea. J. Fujian Fish. 2004, 4, 47–51. (In Chinese) [Google Scholar]
- Lee, H.; Jeong, J.; Choi, Y. Sex-specific differences in the growth and population characteristics of sand crab Ovalipes punctatus (De Haan, 1833) in coastal waters of Korea. Sci. Rep. 2024, 14, 20137. [Google Scholar] [CrossRef]
- Zheng, W.; Han, Z.; Chen, G.; Yu, C.; Gao, T. Mitochondrial DNA variation in the East China Sea and Yellow Sea populations of swimming crab Ovalipes punctatus. Mitochondrial DNA 2015, 26, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, C.G.; Zheng, J.; Chen, X.Q.; Ning, P. Biological characteristics and seasonal variations of Ovalipes punctatus in the Zhoushan fishing ground. Oceanol. Et Limnol. Sin. 2011, 42, 274–278. (In Chinese) [Google Scholar]
- Li, J.; Chen, X.; Fang, X.; Yu, H. Analysis on flesh rate and muscle nutritional value in Ovalipes punctatus. Food Ind. 2012, 33, 93–96. (In Chinese) [Google Scholar]
- Fisheries Administration Bureau; MARA; PRC. China Fishery Statistics Yearbooks; China Agriculture Press: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Hoegh-Guldberg, O. Coral reef sustainability through adaptation: Glimmer of hope or persistent mirage? Curr. Opin. Environ. Sustain. 2014, 7, 127–133. [Google Scholar] [CrossRef]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef]
- Xu, S.C.; Zhang, Y.; Zhou, Y.; Xu, S.; Yue, S.D.; Liu, M.J.; Zhang, X. Warming northward shifting southern limits of the iconic temperate seagrass (Zostera marina). iScience 2022, 25, 104755. [Google Scholar] [CrossRef]
- Koepper, S.; Revie, C.W.; Stryhn, H.; Clark, K.F.; Scott-Tibbetts, S.; Thakur, K.K. Spatial and temporal patterns in the sex ratio of American lobsters (Homarus americanus) in southwestern Nova Scotia, Canada. Sci. Rep. 2021, 11, 24100. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wei, H.; Song, G.S.; Xie, C. IPCC-CMIP5 Based projection and analysis of future sea surface temperature changes in coastal Seas East of China. Oceanol. Et Limnol. Sin. 2020, 51, 1288–1300, (In Chinese with English abstract). [Google Scholar]
- IPCC (Intergov. Panel Clim. Change). Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1–30. [Google Scholar]
- Ning, X.R.; Lin, C.L.; Su, J.L.; Liu, C.G.; Hao, Q.; Le, F.F.; Tang, Q. Long-term environmental changes and the responses of the ecosystems in the Bohai Sea during 1960–1996. Deep Sea Res. Part II 2010, 57, 1079–1091. [Google Scholar] [CrossRef]
- Yu, C.; Song, H.; Yao, G. Biological characteristics of Ovalipes punctatus in the East China Sea. J. Fish. China 2004, 28, 657–662. (In Chinese) [Google Scholar]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II 2023, 207, 105227. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Xing, Q.; Li, Y.; Tian, H.; Luo, Y.; Ito, S.; Tian, Y. Climate change drives fish communities: Changing multiple facets of fish biodiversity in the Northwest Pacific Ocean. Sci. Total Environ. 2024, 955, 176854. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Yu, C.; Song, H.; Yao, G.; Lu, H. Composition and distribution of economic crab species in the East China Sea. Oceanol. Et Limnol. Sin. 2006, 37, 53–60. (In Chinese) [Google Scholar]
- Preez, H.H.D.; Mclachlan, A. Distribution of the portunid crab Ovalipes punctatus (De hann) in Algoa Bay and salinity and temperature tolerances of its zoeae. Afr. Zool. 1984, 19, 302–304. [Google Scholar]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Li, Z.L.; Jin, X.S.; Zhang, B.; Zhou, Z.P.; Shan, X.J.; Dai, F.Q. Interannual variations in the population characteristics of the Pacific cod Gadus macrocephalus in the Yellow Sea. Oceanol. Et Limnol. Sin. 2012, 43, 924–931. (In Chinese) [Google Scholar]
- Merino, G.; Barange, M.; Blanchard, J.L.; Harle, J.; Holmes, R.; Allen, I.; Allison, E.H.; Badjeck, M.C.; Dulvy, N.K.; Holt, J.; et al. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate? Glob. Environ. Change 2012, 22, 795–806. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Han, Z. Effects of climate change on the potential habitat distribution of swimming crab Portunus trituberculatus under the species distribution model. J. Oceanol. Linmlogy 2022, 40, 1556–1565. [Google Scholar] [CrossRef]
- Rilov, G. Multi-species collapses at the warm edge of a warming sea. Sci. Rep. 2016, 6, 36897. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawaguchi, K. Nocturnal occurrence of the swimming crab Ovalipes punctatus in the swash zone of a sandy beach in northeastern Japan. Fish. Bull. Natl. Ocean. Atmos. Adm. 2001, 99, 510–515. [Google Scholar]
- Yu, C.G.; Han, Z.Q.; Zheng, J.; Xue, L.J.; Gao, T.X. Genetic population structure of Ovalipes punctatus revealed by AFLP markers. Sci. Res. Essays 2010, 5, 1649–1654. [Google Scholar]
- Torrejón-Magallanes, J.; Ángeles-González, L.E.; Csirke, J.; Bouchon, M.; Morales-Bojórquez, E.; Arreguín-Sánchez, F. Modeling the Pacific chub mackerel (Scomber japonicus) ecological niche and future scenarios in the northern Peruvian Current System. Prog. Oceanogr. 2021, 197, 102672. [Google Scholar] [CrossRef]

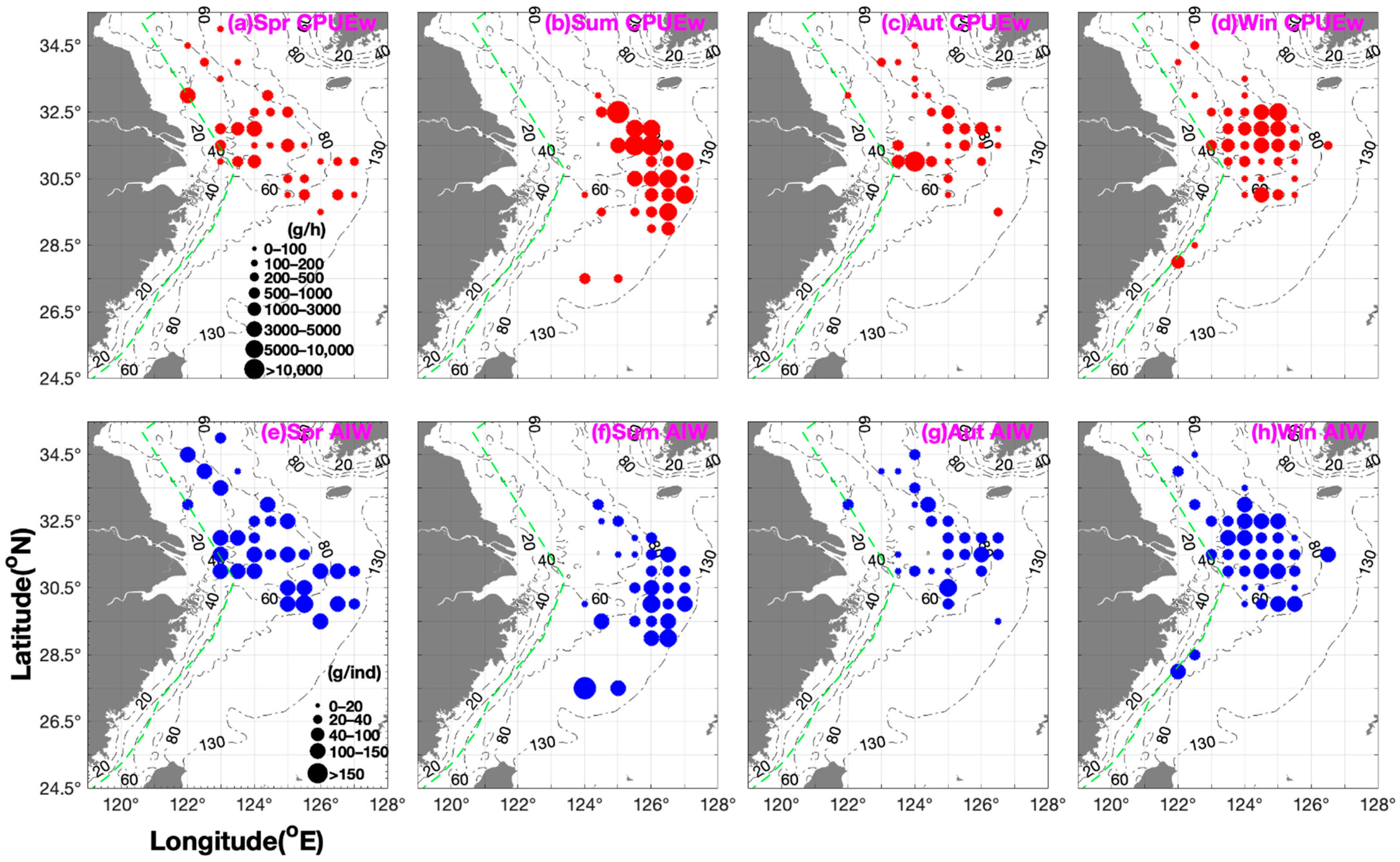

| Mean Value | Total Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | B% | N | N% | AIW | B | B% | N | N% | AIW | AIW% | |
| Spring | |||||||||||
| (2) | 70.3 | 5.9% | 1.5 | 5.5% | 38.2 | 281.4 | 3.3% | 6 | 3% | 152.7 | 9.6% |
| (5) | 509 | 42.5% | 14 | 51.2% | 41.4 | 4581.3 | 53.8% | 126.2 | 63% | 372.8 | 23.3% |
| (7) | 233.5 | 19.5% | 4.2 | 15.3% | 53.2 | 1401.3 | 16.5% | 25.2 | 12.6% | 319.4 | 20% |
| (8) | 225.1 | 18.8% | 5.4 | 19.6% | 42.5 | 1125.5 | 13.2% | 26.9 | 13.4% | 212.3 | 13.3% |
| (10) | 159.9 | 13.3% | 2.3 | 8.4% | 77.2 | 1119.3 | 13.2% | 16 | 8% | 540.1 | 33.8% |
| Summer | |||||||||||
| (5) | 262.1 | 2.3% | 15.2 | 3.1% | 18.9 | 524.2 | 0.9% | 30.5 | 1.4% | 37.7 | 2.7% |
| (6) | 7155.8 | 61.8% | 320.8 | 65.9% | 21.8 | 21,467.4 | 37.4% | 962.4 | 43.8% | 65.5 | 4.7% |
| (8) | 2434.8 | 21% | 117.8 | 24.2% | 31.4 | 17,043.6 | 29.7% | 824.8 | 37.6% | 219.5 | 15.7% |
| (10) | 1440 | 12.4% | 30.8 | 6.3% | 45 | 17,279.8 | 30.1% | 369.7 | 16.8% | 539.8 | 38.7% |
| (14)&(16) | 278 | 2.4% | 2.1 | 0.4% | 133.3 | 1113.8 | 1.9% | 8.5 | 0.4% | 533.4 | 38.2% |
| Autumn | |||||||||||
| (2) | 62.3 | 2.2% | 3.8 | 3.1% | 16.3 | 187 | 1.6% | 11.5 | 2.3% | 48.8 | 7.8% |
| (5) | 60.1 | 2.1% | 2.1 | 1.7% | 32.8 | 300.3 | 2.5% | 10.5 | 2.1% | 164.2 | 26.3% |
| (6) | 443.6 | 15.6% | 16.6 | 13.6% | 26 | 2218 | 18.7% | 82.8 | 16.4% | 130.2 | 20.9% |
| (7) | 2009.2 | 70.7% | 87.5 | 71.7% | 18.5 | 8036.8 | 67.7% | 349.8 | 69.5% | 74.2 | 11.9% |
| (8) | 113.5 | 4% | 4.1 | 3.4% | 29.2 | 680.8 | 5.7% | 24.9 | 4.9% | 175.3 | 28.1% |
| (10) | 151.5 | 5.3% | 7.9 | 6.5% | 15.4 | 454.4 | 3.8% | 23.8 | 4.7% | 30.8 | 4.9% |
| Winter | |||||||||||
| (2) | 92.8 | 4.6% | 5 | 8.5% | 25.4 | 185.6 | 1% | 10 | 1.7% | 50.8 | 4.1% |
| (5) | 820 | 40.5% | 21.2 | 36.1% | 36.8 | 10,659.8 | 55% | 276 | 48.3% | 478.5 | 38.2% |
| (7) | 478.4 | 23.6% | 15.6 | 26.5% | 36.2 | 5740.3 | 29.6% | 187 | 32.7% | 434.5 | 34.7% |
| (9) | 309.1 | 15.3% | 13 | 22.1% | 23 | 2163.7 | 11.2% | 90.8 | 15.9% | 161.2 | 12.9% |
| (11) | 324.7 | 16% | 4 | 6.8% | 63.5 | 649.4 | 3.3% | 8 | 1.4% | 127.1 | 10.1% |
| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Mean CPUEw at collection stations (g·h−1) | 274.48 | 2051.03 | 456.82 | 538.86 |
| Value range of CPUEw (g·h−1) | 11.84–1286.24 | 18.9–13120 | 10.95–6896.94 | 16.06–3450.67 |
| Mean CPUEn at collection stations (ind·h−1) | 6.46 | 78.43 | 19.36 | 15.88 |
| Value range of CPUEn (ind·h−1) | 1–44.75 | 1.09–568 | 1–282.35 | 0.97–84 |
| Mean AIW (g·ind−1) | 51.53 | 49.85 | 28.66 | 34.78 |
| Value range of AIW (g·ind−1) | 8.97–104.33 | 3.5–203.17 | 7.3–121.62 | 6.7–87.06 |
| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| SST (°C) | 12.61–18.02 | 26.15–29.61 | 16.97–22.69 | 9.14–18.41 |
| SSS (‰) | 30.58–33.95 | 27.73–33.97 | 30.49–34.02 | 31.6–34.34 |
| SBT (°C) | 10.1–18.03 | 12.55–28 | 9.04–22.7 | 9.26–18.52 |
| SBS (‰) | 30.58–34.31 | 31.69–34.65 | 31.37–34.77 | 31.69–34.48 |
| Depth (m) | 20–105 | 43–120 | 21–102 | 19–90 |
| Case. | Loss% | Gain% | Gain% − Loss% |
|---|---|---|---|
| SSP126–2050 | −25.76% | 7.77% | −18.00% |
| SSP126–2100 | −38.55% | 10.95% | −27.61% |
| SSP245–2050 | −22.22% | 9.96% | −12.26% |
| SSP245–2100 | −43.92% | 24.11% | −19.81% |
| SSP370–2050 | −31.50% | 9.41% | −22.10% |
| SSP370–2100 | −60.09% | 28.30% | −31.79% |
| SSP585–2050 | −34.24% | 9.62% | −24.62% |
| SSP585–2100 | −78.49% | 24.72% | −53.77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Ling, J.; Zheng, H.; Song, X.; Liu, Z.; Li, H. Seasonal Spatial Distribution Patterns of the Sand Crab Ovalipes punctatus (De Haan 1833) in the Southern Yellow and East China Seas and Predictions from Various Climate Scenarios. Biology 2025, 14, 947. https://doi.org/10.3390/biology14080947
Xu M, Ling J, Zheng H, Song X, Liu Z, Li H. Seasonal Spatial Distribution Patterns of the Sand Crab Ovalipes punctatus (De Haan 1833) in the Southern Yellow and East China Seas and Predictions from Various Climate Scenarios. Biology. 2025; 14(8):947. https://doi.org/10.3390/biology14080947
Chicago/Turabian StyleXu, Min, Jianzhong Ling, Haisu Zheng, Xiaojing Song, Zunlei Liu, and Huiyu Li. 2025. "Seasonal Spatial Distribution Patterns of the Sand Crab Ovalipes punctatus (De Haan 1833) in the Southern Yellow and East China Seas and Predictions from Various Climate Scenarios" Biology 14, no. 8: 947. https://doi.org/10.3390/biology14080947
APA StyleXu, M., Ling, J., Zheng, H., Song, X., Liu, Z., & Li, H. (2025). Seasonal Spatial Distribution Patterns of the Sand Crab Ovalipes punctatus (De Haan 1833) in the Southern Yellow and East China Seas and Predictions from Various Climate Scenarios. Biology, 14(8), 947. https://doi.org/10.3390/biology14080947







