Simple Summary
The rapid growth of the global population and the consequent increase in food demand have made enhancing crop yields a primary focus of agricultural research. Although traditional breeding techniques have improved crop photosynthetic efficiency, their potential appears to be approaching its limit. Consequently, future research should prioritize innovative strategies to further enhance photosynthetic efficiency and stress tolerance, thereby promoting sustainable agriculture. In this study, the HaGLK gene was cloned from sunflower and subsequently overexpressed in transgenic rice through genetic transformation. Evaluation of photosynthetic capacity, agronomic traits, and stress tolerance revealed that heterologous expression of HaGLK enhances photosynthetic efficiency, crop yields, and stress resilience in rice. These results provide valuable genetic resources and references for breeding new rice varieties with salt tolerance, drought resistance, and high yield and also establish a theoretical foundation for further exploration of the HaGLK gene’s function in sunflower.
Abstract
GOLDEN2-LIKEs (GLKs) are important transcription factors for the chloroplast development influencing photosynthesis, nutrition, senescence, and stress response in plants. Sunflower (Helianthus annuus) is a highly photosynthetic plant; here, a GLK-homologues gene HaGLK was identified from the sunflower genome by bioinformatics. To analyze the bio-function of HaGLK, transgenic rice plants overexpressing HaGLK (HaGLK-OE) were constructed and characterized via phenotype. Compared to the wild-type control rice variety Zhonghua 11 (ZH11), the HaGLK-OE lines exhibited increased photosynthetic pigment contents, higher net photosynthetic rates, and enlarged chloroplast area; meanwhile, genes involved in both photosynthesis and chlorophyll biosynthesis were also significantly up-regulated. Significantly, the HaGLK-OE plants showed a 12–13% increase in yield per plant. Additionally, the HaGLK-OE plants were demonstrated to have improved salt and drought tolerance compared to the control ZH11. Our results indicated that the HaGLK gene could play multiple roles in photosynthesis and stress response in rice, underscoring its potential value for improving crop productivity and environmental adaptability in breeding.
1. Introduction
Photosynthesis is a process in plants using sunlight, carbon dioxide, and water to produce oxygen and glucose. Photosynthesis can directly affect crop yield by converting the glucose into biomass, including grains, fruits, and other harvestable parts. A chloroplast is an organelle in plant cells where photosynthesis occurs, containing chlorophyll and other pigments to transform solar energy into chemical energy. The structure and function of a chloroplast are fundamental to sustaining photosynthesis. Evidence has shown that the accumulation of chlorophyll content in leaves was positively correlated with the plant’s light-absorption capacity, photosynthetic efficiency, and crop yield [1]. The transcription factors GOLDEN2-LIKEs (GLKs) are key regulators for chlorophyll biosynthesis and photosynthesis in plants by regulating photosynthesis-associated nuclear genes (PhANGs) [2].
GLKs belong to the GARP superfamily, containing a DNA-binding domain (DBD), a proline-rich domain, and a GLK/C-terminal (GCT) box [3]. As of now, it has been discovered that diploid plants typically contain two homologous GLK genes [4]. Interestingly, the function of the two GLKs is mostly redundancy in C3 plants with only one type of chloroplast in mesophyll cells, such as with Arabidopsis, rice, and tomato [5]. Nevertheless, the chloroplasts in mesophyll cells and bundle-sheath cells are different in C4 plants, and the GLKs display unredundancy to determine the differentiation of chloroplasts, such as with maize [6]. Since C4 plants exhibit higher photosynthetic efficiency than that of C3 plants, ectopic expression of the maize GLK genes in rice successfully improved photosynthetic capacity and increased yield of the transgenic plants [7]. In addition, GLKs also participate in the stress response of plants. Overexpression of GLK genes could enhance plant tolerance to biotic and abiotic stresses, such as drought [8], low temperature [9], or ozone [10]. GLKs can interact with lesion-simulating disease 1 (LSD1) to regulate reactive oxygen species (ROS) production, which leads to programmed cell death (PCD) and immunity to disease [11,12].
Sunflower (Helianthus annuus) is a C3 plant, which exhibits distinctive photosynthetic efficiency comparable to that of C4 plants [13]. Sunflower is also known for its drought and salt tolerance and its ability to grow in barren and arid regions [14,15,16]. Notably, we found that only one GLK gene (HaGLK) in sunflower is different from the other diploid plants [17]. We hypothesize that overexpression of the HaGLK gene in rice may enhance its tolerance to salt stress, drought resistance, and photosynthetic capacity. To test this hypothesis, we constructed the overexpression of HaGLK rice transgenic plants and analyzed their photosynthetic performance, agronomic traits, and stress resistance. Here, we show that under the same growth conditions, overexpression of HaGLK not only improved the photosynthetic capacity and per-plant yield of rice but also enhanced its tolerance to salt stress and drought resistance.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The variety of sunflower (Helianthus annuus) used is ‘Hong yun’. Rice (Oryza sativa) japonica variety Zhonghua 11 (ZH11) was used as the recipient for genetic transformation of the HaGLK gene. For measuring photosynthetic capacity and agronomic traits, rice plants were planted in the field of an experimental farm with a planting density of 20 cm × 20 cm in summer in South China. Water and fertilizer management was conducted according to conventional planting practices. For salt and drought treatment, rice seeds were soaked in water under 37.0 °C for 2 days, and the germinated seeds were transferred into 96-well plates with holes at the bottom and cultured using the Coolaber nutrient solution [18] in a growth chamber under 28 °C (16 h light/8 h dark).
2.2. Bioinformatic Analysis
Amino acid sequences of AtGLKs from Arabidopsis (Arabidopsis thaliana) were used to search the homology in the genome of sunflower by BLAST in the National Center for Biological Information (NCBI) database (https://www.ncbi.nlm.nih.gov/, accessed on 10 May 2025). Sequence alignments were carried by MEGA5.0 and visualized by GeneDoc2.7, and the conserved motifs of GLK proteins were analyzed using MEME5.4.0 (https://meme-suite.org/meme/ accessed on 10 May 2025).
2.3. PCR Cloning of HaGLK
Total RNA of sunflower leaves was extracted using TRIzol reagent (Thermo, San Jose, CA, USA) and reverse transcribed to full-length cDNA by the PrimeScript RT Master Mix reverse transcription kit (TaKaRa, Hangzhou, China). PCR primers were designed according to the coding sequence (CDS) of HaGLK (LOC110940494). The sequences of the primer pair are 5′-ATGTTAGCTATTGTGTCACCA-3′ and 5′-TCAAATGCATGTTGGTGGTAT-3′. The KOD FX (TOBOYO, Guangzhou, China) was used for PCR with the 20 µL volume solution the included 0.4 µL each of forward and reverse primer (100 ng μL−1), 2 µL cDNA, 1.2 µL DNA polymerase KOD, 2 µL dNTP (2 mM), 2 µL 10× buffer, and 12 µL ddH2O. The PCR program was processed as: 94 °C for 2 min; 94 °C for 30 s; 58 °C for 1 min; 72 °C for 30 s, 30 cycles; and 72 °C for 10 min.
2.4. Vector Construction and Rice Genetic Transformation
Primer pair, 5′-ggtgttacttaagcttATGTTAGCTATTGTGTCACCAT-3′ and 5′-tgtagtccatggtaccTCAAATGCATGTTGGTGGTAT-3′ (adaptors are underlined), was used to amplify the CDS of HaGLK by KOD FX PCR kit (TOBOYO, Guangzhou, China) according to the manufacturer’s instructions. Vector pCAMBLA1300 with a maize (Zea mays) ubiquitin (Ubi) promoter in the multiple clone site (MCS) was selected. The PCR products and the vector were digested by the restriction enzymes Hind III and Kpn I (TaKaRa, Hangzhou, China) and recombined by the In-Fusion HD cloning kits (TaKaRa, Hangzhou, China) according to the manufacturer’s instructions. This constructed vector, in which the expression of HaGLK was driven by the Ubi promoter, was genetically transformed into rice variety ZH11, calli-induced from mature seeds through the method of Agrobacterium-mediated transformation. Callus induction, transformant selection, and seedling regeneration were carried out under continuous light at 32 °C [19]. To identify the positive transgenic plants, the genomic DNA of T0 plants was extracted by the CTAB method [20] and tested by PCR mix (CWBIO, Beijing, China) using the primer pair 5′-ATGTTAGCTATTGTGTCACCA-3′ and 5′-TCAAATGCATGTTGGTGGTAT-3′. The thermal cycling protocol comprised an initial denaturation at 95 °C for 60 s, followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s, and an extension at 72 °C for 30 s, with a final extension step at 72 °C for 5 min.
2.5. Gene Expression Analysis
Total RNA of leaves from the positive transgenic plants was extracted using TRIzol reagent (Thermo, San Jose, CA, USA) and reverse transcribed to full-length cDNA by the PrimeScript RT Master Mix reverse transcription kit (TaKaRa, Hangzhou, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed using the SYBR Green Master Mix kit (TOYOBO, Guangzhou, China). The PCR process was as follows: 95 °C for 60 s, 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 15 s, for a total of 40 cycles. The housekeeping gene Actin was used for the standardization control. The primers used for qPCR are listed in Table 1. Each experiment included three biological replicates. The data from the qPCR were analyzed by the 2−△△Ct method [21].

Table 1.
Sequences of primer used for qPCR.
2.6. Content Measurement of Photosynthetic Pigments
At the tillering stage, the flag leaves were selected for extraction of photosynthetic pigments. Leaves were cut into 4 mm fragments, weighed at 0.1 g, and incubated with 3 mL 80% acetone (v/v) in a centrifugal tube under dark conditions for 48 h. Then, the contents of pigments were measured using a spectrophotometer by the method described by Porra et al. [22]. Three biologic replicates were carried out for each sample.
2.7. Photosynthetic Assay
At the tillering stage, the net photosynthetic rate (Pn) of the flag leaves was measured on sunny day using a LI-COR 6800 photosynthesizer (LI-COR, Lincoln, NE, USA). The light-response curve was measured at CO2 concentration of 400 μmol mol−1, using the built-in light-response program with varying photon density ranging from 0 to 2800 μmol m−2s−1.
2.8. Ultrastructural Analysis of Chloroplasts
At the heading stage of the rice, the fresh flag leaf samples were collected from the same leaf position for experimental analysis. Subsequently, the protocol described by Feder et al. [23] was employed, utilizing chemical fixation and resin embedding to prepare plant specimens for observation of chloroplast ultrastructure. Three biologic replicates were carried out for each sample. The sizes of chloroplasts were quantified by ImageJ2.1.0.
2.9. Statistical Measurement of Rice Agronomic Traits
At the mature stage of the rice, 20 plants from each sample were randomly selected to measure agriculture traits, including plant height, flag-leaf length and width, effective tiller number, panicle length, grains per panicle, seed-setting rate, grain length and width, and thousand-grain weight and yield per plant. The data were statistically analyzed by GraphPad Prism9.5.
2.10. Stress Treatments
Germinated rice seeds were germinated with 100 mM NaCl or 15% PEG6000 for 7 days, followed by 3 days recovery in the Coolaber nutrient solution; then, the stem and root lengths were measured. Six biologic replicates were carried out for each sample.
Two-week-old seedlings (n = 48) were transplanted into the 150 mM NaCl or 20% PEG6000 for 7 days in hydroponic systems, followed by 3 days recovery in the Coolaber nutrient solution. Stress tolerance was evaluated by measuring post-recovery survival rates. Survival rate = number of surviving plants/total number of plants × 100%.
3. Results
3.1. Bioinformatic Identification of HaGLK Gene
Amino acid (aa) sequences of ZmGLK1 and ZmGLK2 from maize, as well as AtGLK1 and AtGLK2 from Arabidopsis, were respectively used for NCBI BLASTN1.30 in the genome of sunflower, and only one putative GLK-like gene (GenBank: LOC110940494) has been found. HaGLK is located on chromosome 5 of sunflower, containing six exons. The CDS is 1252 bp in length, encoding a 417 aa protein. By NCBI BLASTP1.30, it showed that the consistencies of protein sequences of HaGLK with ZmGLK1 and ZmGLK2 were 41.79% and 41.28%; HaGLK with AtGLK1 and AtGLK2 were 47.67% and 43.55%, respectively. Sequence alignment with AtGLK and ZmGLK proteins showed that HaGLK has a Myb-type DBD-conserved domain, which recognizes and binds to specific DNA sequences in the promoter region of target genes [24], and a GLK-specific C-terminal GCT box (Figure 1).
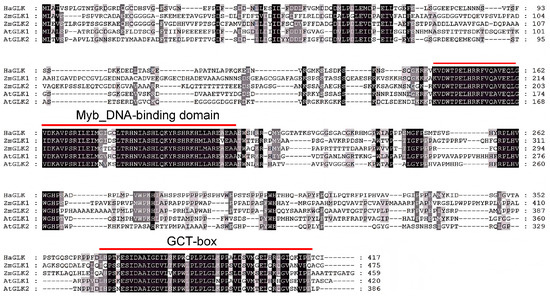
Figure 1.
Comparative alignment of HaGLK, ZmGLK, and AtGLK amino acid sequences.
3.2. Generation and Identification of HaGLK Transgenic Rice
The full-length cDNA of the HaGLK gene was amplified by PCR and transformed into rice variety ZH11, in which HaGLK was driven by the Ubi promoter. From twenty-one T0 plants, eight positive ones were firstly identified by PCR using the primers of a hygromycin-resistant gene. Then, the eight transgenic plants were further confirmed by the PCR using HaGLK and OsGLK1 gene-specific primers. Results show that the OsGLK gene can be amplified from ZH11 and the transgenic plants, while the HaGLK gene can only be amplified from the transgenic plants (Figure 2A and Figure S1). Subsequently, the HaGLK gene transcription levels were tested by qPCR. Results show that the expression of HaGLK vary in different transgenic lines, and two lines, HaGLK-OE1 and HaGLK-OE2, exhibited higher expression levels than those of other lines (Figure 2B), which were selected for further characterization.
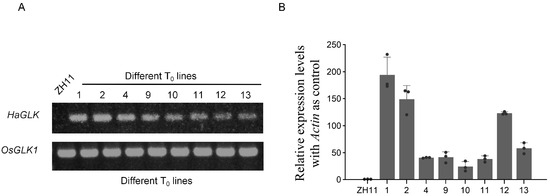
Figure 2.
Molecular identification of HaGLK transgenic lines. (A) PCR identification of HaGLK transgenic rice; (B) relative expression of HaGLK by quantitative real-time PCR (qPCR). Each dot represents a biological replicate.
3.3. Photosynthetic Capacity Analysis in HaGLK Transgenic Rice
At the tillering stage, photosynthetic pigment contents in leaves of the HaGLK-OE1 and HaGLK-OE2 transgenic lines were significantly higher than in ZH11 (Figure 3A). The expression levels of photosynthesis and chlorophyll biosynthesis-related genes in HaGLK-OE1 and HaGLK-OE2 transgenic lines were significantly higher than in ZH11 (Figure 3B), as shown by qPCR. Transmission electron microscopy (TEM) analysis revealed that the chloroplast area of transgenic lines HaGLK-OE1 and HaGLK-OE2 increased by 30% and 54.3%, respectively, while the number of thylakoids per chloroplast rose by 38.8% and 36.7%, respectively (Figure 3D–I). At the heading stage, the net photosynthetic ratio (Pn) of flag leaves from the two transgenic lines and ZH11 was measured, and the Pn of transgenic lines was significantly higher than that of ZH11 with the increasing light intensity (Figure 3C).
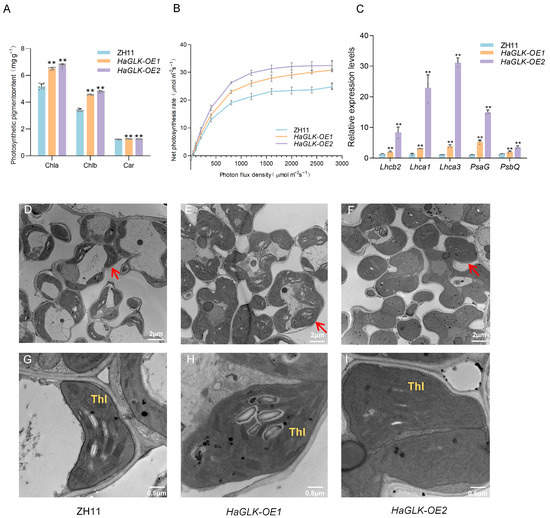
Figure 3.
Photosynthesis-related indicator analysis of ZH11 and HaGLK transgenic lines. (A) Photosynthetic pigment content of flag leaves at the tillering stage (n = 9 biological replicates); (B) net photosynthesis rate curves fitted by the built-in light-response program (LI-COR 6800) with the CO2 concentration of 400 μmol mol−1 (n = 3 biological replicates); (C) relative expression of photosynthesis-related genes by qPCR (n = 3 biological replicates); (D–I) transmission electron micrographs of flag-leaf cross-sections of ZH11 and HaGLK transgenic lines; (D–F) chloroplasts in mesophyll cells, with arrows pointing to representative chloroplasts (bar = 2 μm); (G–I) enlarged micrographs of chloroplasts in mesophyll cells (bar = 0.5 μm). Thl: a stack of granal thylakoids. Data are means ± SD (Student’s t-tests, ** p < 0.01). Each dot represents a biological replicate.
3.4. Agronomic Trait Survey of HaGLK Transgenic Rice
The agronomic traits of HaGLK-OE1 and HaGLK-OE2 transgenic lines were statistically evaluated compared to ZH11. The results show that there were no significant changes in plant height and tiller number (Figure 4A,B). However, the flag-leaf length and flag-leaf width increased in the transgenic lines (Figure 4C,D), which effectively expands the area of photosynthesis of plants. Notably, the panicle length and grain number per panicle of the transgenic lines significantly increased (Figure 4E,F), while the seed-setting ratio and 1000-grain weights of the transgenic lines demonstrated no significant change (Figure 4G,H), which results in enhancement of yields per plant by 13.1% and 12.6% for HaGLK-OE1 and HaGLK-OE2, respectively (Figure 4L). Interestingly, the grain shape of HaGLK-OE transgenic lines has become longer and thinner, because their grain length increased and their grain thickness decreased with no change of grain width (Figure 4I–K).
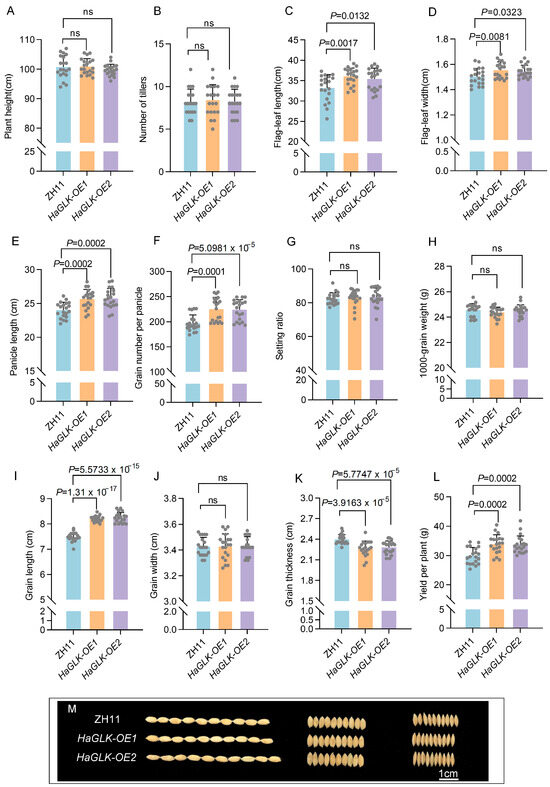
Figure 4.
Agronomic trait analysis of ZH11 and HaGLK transgenic lines. (A) Plant height; (B) number of tillers; (C) flag-leaf length; (D) flag-leaf width; (E) panicle length; (F) grain number per panicle; (G) setting ratio; (H) 1000-grain weight; (I) grain length; (J) grain width; (K) grain thickness; (L) yield per plant; (I–K) ten grains in length (I), width (J), and thickness (K) between ZH11 and HaGLK transgenic lines; (M) comparison of grain shape (bar = 1 cm). Data are means ± SD (Student’s t-tests, ns: non-significance, n = 20 biological replicates). Each dot represents a biological replicate.
3.5. Stress Tolerance Analysis of HaGLK Transgenic Rice
Abiotic stresses such as salt and drought have been shown to severely inhibit the normal growth and development process of rice, which is an important factor limiting rice yield [25]. To further investigate the stress tolerance of HaGLK transgenic rice, stress treatments were performed on the transgenic rice lines and ZH11. Under normal conditions, the growth status of HaGLK-OE1 and HaGLK-OE2 transgenic seedlings showed no phenotypic differences compared to ZH11, indicating that heterologous expression of the HaGLK gene did not affect the growth and development of rice seedlings during the early stage.
When sprouting seeds at the germination stage were treated with a 100 mM NaCl solution for 7 days, measurements showed that the shoot and root lengths of HaGLK-OE1 and HaGLK-OE2 transgenic lines were found to be significantly higher than those of ZH11 (Figure 5A–C). Subsequently, 2-week-old seedlings were subjected to 150 mM NaCl solution for 7 days, followed by 3 days of recovery. ZH11 seedlings exhibited severe symptoms such as leaf desiccation, bending, wilting, and even plant death, whereas HaGLK-OE1 and HaGLK-OE2 transgenic lines showed much milder symptoms of leaf desiccation, bending, and wilting (Figure 5D). Statistical analysis of survival rates revealed that the survival rates of HaGLK-OE1 and HaGLK-OE2 transgenic lines were 82% and 80%, respectively, significantly higher than the 64% survival rate of ZH11 (Figure 5E).
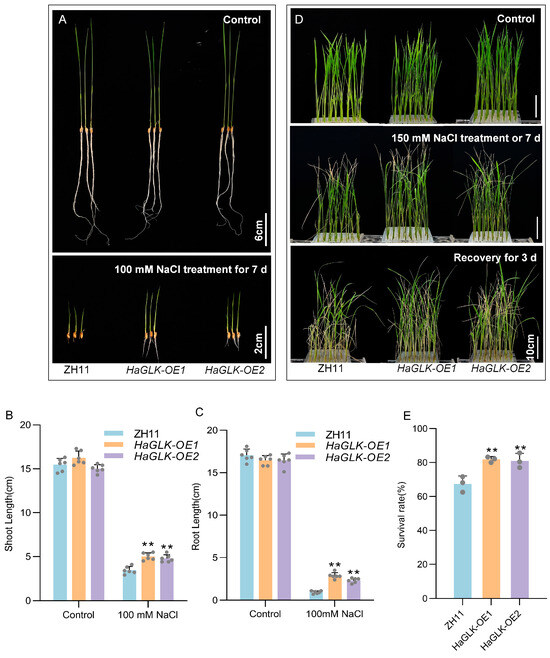
Figure 5.
Phenotypic analysis of the HaGLK transgenic lines under NaCl treatment. (A) Germination stage—sprouting seeds were supplemented with 100 mM NaCl (bar = 2 cm) and control without any additional additives (bar = 6 cm); (B,C) shoot and root length of plants under 100 mM NaCl treatment (n = 6 biological replicates); (D) seedling stage—2-week-old seedlings were supplemented with 150 mM NaCl and control without any additional additives (bar = 10 cm); (E) survival rates of seedlings with 150 mM NaCl (n = 3 biological replicates). Data are means ± SD (Student’s t tests, ** p < 0.01). Each dot represents a biological replicate.
Under drought stress, HaGLK-OE1 and HaGLK-OE2 transgenic lines displayed strong tolerance. At the seed germination stage, after 7 days of treatment with 15% PEG6000, the growth of the ZH11 was significantly inhibited, in which the shoot and root lengths were shorter and had smaller leaves compared to the transgenic lines (Figure 6A–C). When 2-week-old seedlings were subjected to 20% PEG6000 drought stress for 7 days, the HaGLK-OE1 and HaGLK-OE2 transgenic lines showed good tolerance, as most leaves remained normal growth except for slight necrosis and drooping at the leaf tips; on the contrary, the ZH11 seedlings showed significant curling, wilting, and lodging after stress treatment. After 3 days of recovery, most seedlings of HaGLK-OE1 and HaGLK-OE2 transgenic lines resumed normal growth (Figure 6D). The survival rates of HaGLK-OE1 and HaGLK-OE2 transgenic lines were 92% and 89%, respectively, significantly higher than the 75% survival rate of ZH11 (Figure 6E). These results indicate that heterologous expression of HaGLK enhances the salt and drought tolerance of rice plants.
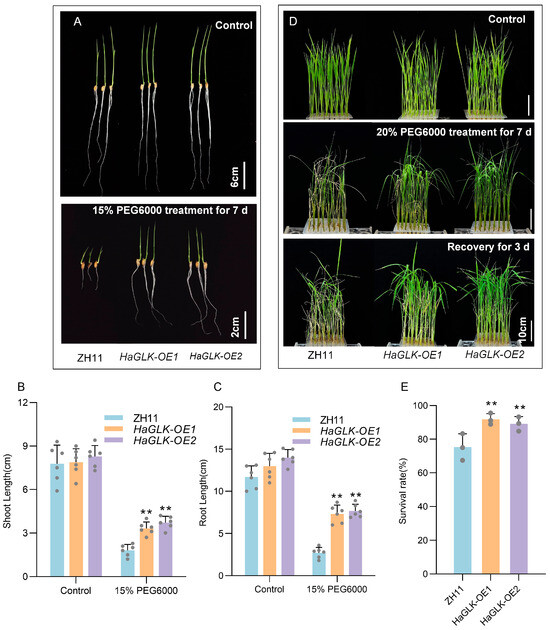
Figure 6.
Phenotypic analysis of the HaGLK transgenic lines under PEG6000 treatment. (A) Germination stage—sprouting seeds were supplemented with 15% PEG6000 (bar = 2 cm) and control without any additional additives (bar = 6 cm); (B,C) shoot and root length of plants under 15% PEG6000 treatment (n = 6 biological replicates); (D) seedling stage—2-week-old seedlings were supplemented with 20% PEG6000 and control without any additional additives (bar = 10 cm); (E) survival rates of seedlings with 20% PEG6000 (n = 3 biological replicates). Data are means ± SD (Student’s t tests, ** p < 0.01). Each dot represents a biological replicate.
4. Discussion
In the face of the dual pressures of global population growth and limited arable land resources, it is imperative to stabilize the yield of rice as one of the major food crops. Research demonstrates that GLKs transcription factors regulate chloroplast biogenesis and function by activating chlorophyll biosynthesis and photosynthesis-related genes [26]. Constitutive GLK expression enhances chloroplast development in photosynthetic tissues [27] and promotes photosynthetic activity in non-green cells [28]. Recent studies reveal that the PsbS subunit protein of photosystem II overexpression in rice improves non-photochemical quenching under fluctuating light, boosting photosynthetic efficiency and yield [29]. Specifically, OsGLK1 and OsGLK2 bind to promoters of light-harvesting Lhca/Lhcb genes to transactivate expression [30], while the transcription factor OsNFYB7 from the nuclear factor Y family inhibits GLK1-mediated transcriptional activation, diminishing chlorophyll buildup in rice embryos [31]. Consistent with these mechanisms, our findings show that the overexpressed HaGLK gene in rice up-regulates the expression of light-harvesting genes and photosystem subunits, significantly increasing photosynthetic pigments, net photosynthetic rate, and chloroplast size in leaves (Figure 3). However, HaGLK overexpression caused neither developmental delays nor enhanced photosynthesis in non-green tissues during whole-plant growth.
Heightened chlorophyll accumulation in foliage is linked to augmented photosynthetic performance and greater agricultural yield [1]. GLKs exhibit broad application prospects in crop breeding, where regulating the expression of photosynthesis-associated GLKs represents an effective strategy for enhancing agricultural productivity. In Arabidopsis, it was observed that high expression of AtGLK1 through leaf- and rachis wall-specific promoters resulted in significant enhancements in photosynthesis and an increase in seed oil content by 2.88% and 10.75%, respectively [32]. In oil-seed rape (Brassica napus), overexpression of BnGLK1a resulted in a 10% increase in the 1000-grain weight of seeds [33]. Furthermore, overexpression of ZmGLK1 and ZmGLK2 in rice led to a 30% to 40% increase in yield, while expression driven by the native promoter can enhance yields by 47% to 70% [34,35]. In this study, we found that heterologous expression of HaGLK in rice and the yield per plant of HaGLK-OE1 and HaGLK-OE2 increased by 13.06% and 12.60%, respectively (Figure 4L). Agronomic trait investigations revealed that the leaf length and width of HaGLK transgenic rice increased significantly. Although the HaGLK gene altered the grain shape of rice, there was no significant difference in the seed-setting rate compared with ZH11 (Figure 4). This indicates that HaGLK transgenic rice increased the total yield by increasing the number of grains per panicle. Meanwhile, HaGLK also affected the increase in panicle length, proving that HaGLK plays an important role in the growth and development of rice. These research results provide a scientific basis for further studies on the functions of GLK and its potential applications in rice breeding.
The growth status of leaves is an important indicator of plants’ response to abiotic stresses [36]. When plants exhibit greater resistance to drought and salt, the chlorophyll content stays higher, and with a stronger membrane stability, it can make better use of light energy [37]. Under salt and drought treatment, leaves are prone to water loss, causing a reduction in turgor pressure and leading to wilting or death. GLKs play a significant role in plant responses to abiotic stress, and genome-wide analyses predict a conserved function of GLKs in the abiotic stress responses of various crops [34,38]. Under drought- and cold-stress conditions, the growth and yield of the cotton (Gossypium hirsutum) ghglk1 mutant are significantly affected, and heterologous expression of GhGLK1 in Arabidopsis can enhance tolerance to drought and cold stress [9]. The chimeric repressor GLKs-SRDX down-regulates K+ channel genes in Arabidopsis to enhance ozone tolerance [10]. The AhGLK1 of peanut (Arachis hypogaea) was transformed into the glk1glk2 mutant of Arabidopsis and affected the morphological development and photosynthesis of Arabidopsis, rescuing the pale green phenotype of the mutant. The survival rate of the mutant during the drought recovery process has been improved [39]. Additionally, GLKs are involved in regulating plant sensitivity to ABA and ion-channel activity. They enhance seedling sensitivity to osmotic stress mediated by the ABA signaling pathway by regulating WRKY40 [40]. Under NaCl and PEG6000 treatment, HaGLK transgenic rice exhibited significantly longer roots and stems during the germination phase, the degree of leaf wilting was significantly lower compared to ZH11, and the survival rate of seedlings was also significantly improved (Figure 5 and Figure 6). It indicates that under stress conditions, HaGLK transgenic rice rapidly adjusts stomatal behavior by closing stomata to minimize water dissipation, thereby upholding cellular turgor pressure, retaining leaf erectness, supporting routine metabolism, and improving resistance to salinity and drought stresses. Sunflowers, as C3 plants, exhibit photosynthetic efficiency comparable to that of C4 plants due to their ability to sustain photosynthesis with lower CO2 concentrations [13]. Thus, even with closed stomata and decreased CO2 absorption, chlorophyll levels in HaGLK transgenic rice increase, allowing photosynthetic efficiency to be preserved. Further exploration of the mechanisms by which heterologous expression of HaGLK affects chloroplast development in rice and the responses to salt and drought stress is important for the stable increase of rice yield and enhancement of its stress resistance.
5. Conclusions
In this study, transgenic rice lines overexpressing HaGLK were generated via genetic transformation. Comprehensive analyses of photosynthetic capacity, agronomic traits, and stress tolerance demonstrated that HaGLK-overexpressed transgenic rice significantly increased photosynthetic pigment content, net photosynthetic rate, chloroplast size, and other photosynthesis-related parameters as well as per-plant yield, while markedly enhancing tolerance to salt stress and drought resistance. Future research should aim to elucidate the mechanisms by which heterologous HaGLK expression modulates photosynthetic efficiency and stress responses in rice. These findings will provide vital genetic resources for breeding salt- and drought-tolerant rice cultivars, while establishing a theoretical foundation for functional studies of the HaGLK gene in sunflower.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14080946/s1. Figure S1: Electrophoretic image of HaGLK transgenic rice by PCR identification.
Author Contributions
Data curation and investigation, J.L., M.Z., J.H. and Y.L.; Writing—original draft, J.L. and M.Z.; Visualization and formal analysis, J.L., M.Z. and Q.G.; Writing—review and editing, Q.G. and X.C.; Project administration and supervision, B.M. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32272096), the Natural Science Foundation of Zhejiang Province (LZ24C130003), the Program of ‘Xinmiao’ (Potential) Talents in Zhejiang Province (2023R404039), and Science and Technology Key Projects of Jinhua City (2024-2-017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this publication are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Shen, S.R.; Yaun, J.J.; Xu, Y.L. Biological function and molecular mechanism of the transcription factor GLKs in plants: A review. Chin. J. Biotechnol. 2022, 38, 2700–2712. [Google Scholar] [CrossRef]
- Wang, H.T.; Xu, F.F. Identification and expression analysis of the GLK gene family in tea plant (Camellia sinensis) and a functional study of CsGLK54 under low-temperature stress. Sci. Rep. 2024, 14, 12465. [Google Scholar] [CrossRef]
- Ali, N.; Chen, H.; Zhang, C.; Khan, S.A.; Gandeka, M.; Xie, D.; Zhuang, W. Ectopic expression of AhGLK1b (GOLDEN2-like transcription factor) in Arabidopsis confers dual resistance to fungal and bacterial pathogens. Genes 2020, 11, 343. [Google Scholar] [CrossRef]
- Botero, K.; Restrepo, S.; Andres, A.P. A genome-scale metabolic model of potato late blight suggests a photosynthesis suppression mechanism. BMC Genom. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Mai, K.K.K.; Lara, M.V.; Chuong, S.D.X.; Jansonius, N.M.; Weber, A.P.M.; Edwards, G.E.; Franceschi, V.R. Electron tomography analysis of thylakoid assembly and fission in chloroplasts of a single-cell C4 plant, Bienertia sinuspersici. Sci. Rep. 2019, 9, 19640. [Google Scholar] [CrossRef]
- Yeh, S.Y.; Lin, H.H.; Chang, Y.M.; Chang, Y.L.; Chang, C.K.; Huang, Y.C.; Ho, Y.W.; Lin, C.Y.; Zheng, J.Z.; Jane, W.N.; et al. Maize Golden2-like transcription factors boost rice chloroplast development, photosynthesis, and grain yield. Plant Physiol. 2022, 188, 442–459. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, J.; Chen, S.; Liu, H.; Zhou, J.; Yang, W. Maize GOLDEN2-LIKE proteins enhance drought tolerance in rice by promoting stomatal closure. Plant Physiol. 2024, 194, 774–786. [Google Scholar] [CrossRef]
- Liu, J.; Mehari, T.G.; Xu, Y.; Peng, R.; Zhou, L.; Zhang, X.; Chen, Y.; Wang, K. GhGLK1 a Key candidate fene from GARP family enhances cold and drought stress tolerance in cotton. Front. Plant Sci. 2021, 12, 759312. [Google Scholar] [CrossRef]
- Nagatoshi, Y.; Mitsuda, N.; Hayashi, M.; Kubo, A.; Yamaguchi, M.; Ito, T.; Matsuda, M.; Nakamura, T.; Ohme-Takagi, M. GOLDEN 2-LIKE transcription factors for chloroplast development affect ozone tolerance through the regulation of stomatal movement. Proc. Natl. Acad. Sci. USA 2016, 113, 4218–4223. [Google Scholar] [CrossRef]
- Lv, R.; Li, Z.; Li, M.; Zhang, Y.; Yang, X.; Wang, Y.; Zhang, X.; Li, F. Uncoupled expression of nuclear and plastid photosynthesis-associated genes contributes to cell death in a lesion mimic mutant. Plant Cell 2019, 31, 210–230. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.P.; Liu, T.; Ma, J.; Zhang, Y.; Lin, J.; Sun, L.; Deng, X.W. Antagonistic modules regulate photosynthesis-associated nuclear genes via GOLDEN2-LIKE transcription factors. Plant Physiol. 2022, 188, 2308–2324. [Google Scholar] [CrossRef]
- Potter, J.R. Maintenance of high photosynthetic rates during the accumulation of high leaf starch levels in sunflower and soybean. Plant Physiol. 1980, 66, 528–531. [Google Scholar] [CrossRef]
- Li, W.; Zeng, Y.; Yin, F.; Zhang, X.; Liu, J.; Chen, H.; Wang, Y.; Yang, X.; Zhou, M.; Liu, Y. Genome-wide identification and comprehensive analysis of the NAC transcription factor family in sunflower during salt and drought stress. Sci. Rep. 2021, 11, 19865. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Wu, Y.; Li, Y.; Wang, J.; Liu, H.; Chen, S.; Yang, W.; Zhou, J. Genome-wide identification and evolution of the SAP gene family in sunflower (Helianthus annuus L.) and expression analysis under salt and drought stress. PeerJ 2024, 12, e17808. [Google Scholar] [CrossRef]
- Niu, S.; Tang, M.; Du, C.; Zhang, Y.; Wang, X.; Li, J.; Chen, L. Molecular bases of rice quality regulation and efects of abiotic stress on rice quality. China Rice 2022, 28, 10–19. [Google Scholar] [CrossRef]
- Wang, P.; Fouracres, J.; Kelly, S.; Karki, S.; Lago, C.; Hudson, D.; Powell, B.; Monson, R.K.; Quick, W.P. Evolution of GOLDEN2-LIKE gene function in C3 and C4 plants. Planta 2013, 237, 481–495. [Google Scholar] [CrossRef]
- Chun, Y.; Fang, J.; Savelieva, E.M.; Hu, X.; Zhou, H.; Wang, Y.; Li, Y.; Wang, J.; Zhang, Y.; Wang, Z.; et al. The cytokinin receptor OHK4/OsHK4 regulates inflorescence architecture in rice via an IDEAL PLANT ARCHITECTURE1/WEALTHY FARMER’S PANICLE-mediated positive feedback circuit. Plant Cell 2023, 36, 40–64. [Google Scholar] [CrossRef]
- Toki, S.; Hara, N.; Ono, K.; Saito, H.; Matsuoka, M.; Tanaka, H.; Ohtomo, S.; Mori, M.; Nakamura, C. Early infection of scutellum tissue with Agrobacterium allows high- speed transformation of rice. Plant J. 2006, 47, 969–976. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Ned, F. Plant Microtechnique: Some principles and new methods. Am. J. Bot. 1968, 55, 123–142. [Google Scholar] [CrossRef]
- Dubos, C.; Strakraba, T.; Schmidt-Eddy, B. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Park, B.; Burke, J.M. Phylogeography and the evolutionary history of sunflower (Helianthus annuus L.): Wild diversity and the dynamics of domestication. Genes 2020, 11, 266. [Google Scholar] [CrossRef]
- Martín, G.; Leivar, P.; Ludevid, D.; Tepperman, J.M.; Quail, P.H.; Monte, E. Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nat. Commun. 2016, 7, 11431. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Vrebalov, J.T.; Gapper, N.E.; Zheng, Y.; Zhong, S.; Fei, Z. Tomato GOLDEN2-LIKE transcription factors reveal molecular gradients that function during fruit development and ripening. Plant Cell 2014, 26, 585–601. [Google Scholar] [CrossRef]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell 2012, 24, 1081–1095. [Google Scholar] [CrossRef]
- Hubbart, S.; Smillie, I.R.A.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1, 22. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, E.Y.; Han, S.H.; Piao, W.; An, G.; Todaka, D.; Yamaguchi-Shinozaki, K.; Peak, N.C. Rice phytochrome-interacting factor-like1 (OsPIL1) is involved in the promotion of chlorophyll biosynthesis through feed-forward regulatory loops. J. Exp. Bot. 2017, 68, 4103–4114. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, T.; Zhiguo, E.; Niu, B.; Chen, C. OsNF-YB7 inactivates OsGLK1 to inhibit chlorophyll biosynthesis in rice embryo. eLife 2024, 13, RP96553. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Kuang, C.; Ma, X.; Zhang, Y.; Wang, J.; Zhou, X.; Liu, S.; Wang, P.; Huang, J. Important photosynthetic contribution of silique wall to seed yield-related traits in Arabidopsis thaliana. Photosynth. Res. 2018, 137, 493–501. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Y.; Zhao, Z.; Li, X.; Yang, J.; Wang, H.; Liu, X.; Zhang, Y.; Li, J.; Liu, F. A Golden2-like transcription factor, BnGLK1a, improves chloroplast development, photosynthesis, and seed weight in rapeseed. J. Integr. Agric. 2024, 23, 1481–1493. [Google Scholar] [CrossRef]
- Alam, I.; Jin, Y.; Zhang, Q.; Yang, Y.; Liu, J.; Liu, Y.; Luo, H.; Zheng, H.; Wang, X.; Yu, F. Identification of GOLDEN2-like transcription factor genes in soybeans and their role in regulating plant development and metal ion stresses. Front. Plant Sci. 2022, 13, 1052659. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Li, J.; Gao, Y.; Liu, M.; Li, Y.; Li, C.; Xia, X.; Xiao, L.; Zhang, J. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef]
- Rucker, K.S.; Kvien, C.K.; Holbrook, C.C.; Hook, J.E.; Bridges, D.C. Identification of peanut genotypes with improved drought avoidance traits 1. Peanut Sci. 1995, 22, 14–18. [Google Scholar] [CrossRef]
- Luo, X.; Sun, M.; Xu, R.; Jia, B.; Zhang, H.; Li, Y.; Yang, J.; Jia, L.; Zhang, Z. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020, 39, 181–194. [Google Scholar] [CrossRef]
- Wu, R.; Guo, L.; Guo, Y.; Wang, H.; Liu, X.; Zhang, J.; Li, Y.; Chen, S. The G2-Like gene family in Populus trichocarpa: Identification, evolution and expression profiles. BMC Genom. Data 2023, 24, 37. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhong, S.; Zhang, H.; Wang, X.; Zhou, B.; Zhang, Q.; Wang, J. AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci. Rep. 2018, 8, 2250. [Google Scholar] [CrossRef]
- Ahmad, R.; Liu, Y.; Wang, T.J.; Meng, X.; Zhang, H.; Pham, B.; Kim, S.H.; Yun, D.J. GOLDEN2-LIKE transcription factors regulate WRKY40 expression in response to abscisic acid. Plant Physiol. 2019, 179, 1844–1860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).