Epifaunal Assemblages of the Fan Mussel Atrina fragilis (Mollusca: Bivalvia) in the Sea of Marmara
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Epifauna
2.2. Genetic Analysis
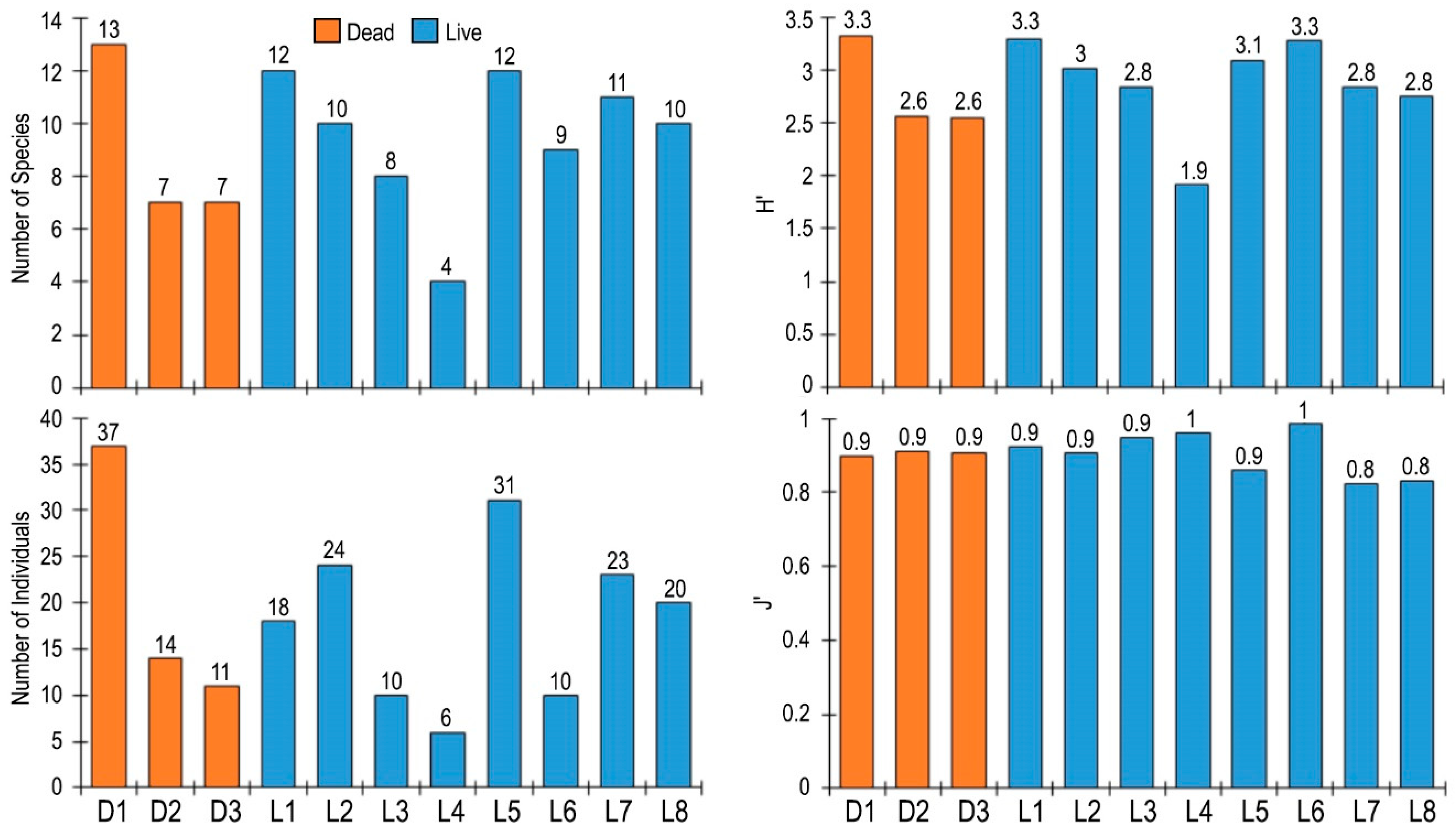
3. Results
3.1. Species Identification
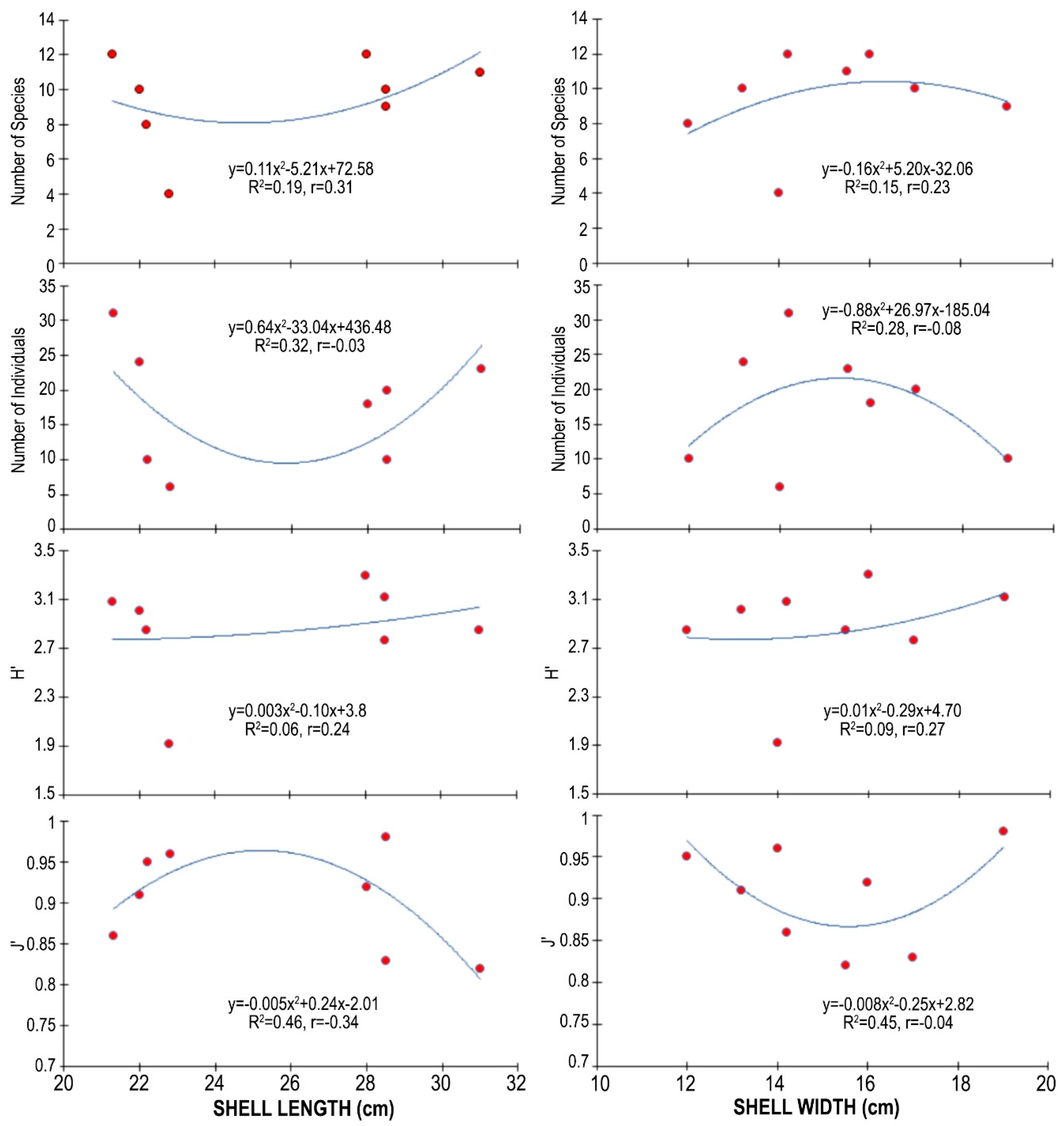
3.2. The Population Features of Atrina fragilis
3.3. Epifauna on Atrina fragilis Shell
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabaoui, L.; Tlih-Zouari, S.; Cosentino, A.; Ben Hassine, O.K. Associated fauna of the fan shell Pinna nobilis (Mollusca: Bivalvia) in the northern and eastern Tunisian coasts. Sci. Mar. 2009, 73, 129–141. [Google Scholar] [CrossRef]
- Rabaoui, L.; Belgacem, W.; Ismail, D.B.; Mansour, L.; Tlig-Zouari, S. Engineering effect of Pinna nobilis shells on benthic communities. Oceanologia 2015, 57, 271–279. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Yokes, M.B.; Güçlüsoy, H. Pinna nobilis in the south Marmara Islands (Sea of Marmara); it still remains uninfected by the epidemic and acts as egg laying substratum for an alien invader. Mediterr. Mar. Sci. 2021, 22, 161–168. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Carella, F.; Çinar, M.E.; Čižmek, H.; Jimenez, C.; Kersting, D.K.; Moreno, D.; Rabaoui, L.; Vicente, N. The fan mussel Pinna nobilis on the brink of extinction in the Mediterranean. In Imperiled: The Encyclopedia of Conservation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 700–709. [Google Scholar]
- Kersting, D.K.; García-March, J.R. Long-term assessment of recruitment, early stages and population dynamics of the endangered Mediterranean fan mussel Pinna nobilis in the Columbretes Islands (NW Mediterranean). Mar. Environ. Res. 2017, 130, 282–292. [Google Scholar] [CrossRef]
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C. Pinna nobilis. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2019; pp. 1–22. [Google Scholar]
- Gvozdenovic, S.; Macic, V.; Pesic, V.; Nikolic, M.; Peras, I.; Mandic, M. Review on Pinna rudis (Linnaeus: Pinnidae) presence in the Mediterranean. Agric. For. 2019, 65, 115–126. [Google Scholar]
- Zotou, M.; Papdakis, O.; Catanese, G.; Stranga, Y.; Ragkousis, M.; Kampouris, T.E.; Naasan Aga-Spyridopoulou, R.; Papadimitriou, E.; Koutsoubas, D.; Katsanevakis, S. New kid in town: Pinna rudis spreads in the eastern Mediterranean. Mediterr. Mar. Sci. 2023, 24, 709–721. [Google Scholar] [CrossRef]
- Gamulin-Brida, H. Biocoenoses benthiques de la mer Adriatique. Inst. Oceanogr. Ribar. 1974, 15, 1–61. [Google Scholar]
- Poutiers, J.M. Bivalves. In Méditerranée et Mer Noire. Zone de pêche 37. Révision 1. Fiches FAO d’Identification des Espèces pour les Besoins de la Pêche; Fischer, W., Scneider, M., Bouchon, M.L., Eds.; FAO: Rome, Italy, 1987; pp. 370–505. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique. Rec. Trav. Stat. Mar. Endoume 1964, 31, 5–137. [Google Scholar]
- Chaillot, L. Echouage d’individus entiers d’Atrina fragilis (Pennant, 1777) (Mollusques Bivalves Pinnidae) sur la côte du pertuis Breton (Département de la Vendée) en Janvier 2014. Ann. Soc. Sci. Nat. Charente-Marit. 2022, 11, 303–328. [Google Scholar]
- Šimunović, A.; Piccinetti, C.; Bartulović, M.; Grubelić, I. Distribution of Atrina fragilis (Pennant, 1777) (Pinnidae, Mollusca, Bivalvia) in the Adriatic Sea. Acta Adriat. 2001, 42, 49–59. [Google Scholar]
- Demir, M. Shells of Mollusca collected from the seas of Turkey. Turk. J. Zool. 2003, 27, 101–140. [Google Scholar]
- Öztürk, B.; Bitlis, B.; Türkçü, N. Diversity of Mollusca along the coasts of Türkiye. Turk. J. Zool. 2024, 48, 531–571. [Google Scholar] [CrossRef]
- Fryganiotis, K.; Antoniadou, C.; Chintiroglou, C. Comparative distribution of the fan mussel Atrina fragilis (Bivalvia, Pinnidae) in protected and trawled areas of the north Aegean Sea (Thermaikos Gulf). Med. Mar. Sci. 2013, 14, 119–124. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Froglia, C.; Atkinson, R.J.A.; Moore, P.G. The impact of Rapido trawling for scallops, Pecten jacobaeus (L.), on the benthos of the Gulf of Venice. ICES J. Mar. Sci. 1999, 56, 111–124. [Google Scholar] [CrossRef]
- Nunn, J. Atrina fragilis—Fan mussel. In Northern Ireland Priority Species and Species of Conservation Concern Reports; National Museums Northern Ireland: Cultra, UK, 2007; Available online: http://www.habitas.org.uk/priority/species.asp?item=40786 (accessed on 15 February 2008).
- MolluscaBase eds. MolluscaBase eds. MolluscaBase. Pinna pernula Chemnitz, 1785. 2021. Available online: https://molluscabase.org/aphia.php?p=taxdetails&id=852331 (accessed on 22 May 2025).
- Cummings, V.J.; Thrush, S.F.; Hewitt, J.E.; Turner, S.J. The influence of the pinnid bivalve Atrina zelandica (Gray) on benthic macroinvertebrate communities in soft-sediment habitats. J. Exp. Mar. Biol. Ecol. 1998, 228, 227–240. [Google Scholar] [CrossRef]
- Nowell, A.R.M.; Jumars, P.A. Flow environments of aquatic benthos. Annu. Rev. Ecol. Syst. 1984, 15, 303–328. [Google Scholar] [CrossRef]
- Commito, J.A.; Boncavage, E.M. Suspension-feeders and coexisting infauna: An enhancement counter example. J. Exp. Mar. Biol. Ecol. 1989, 125, 33–42. [Google Scholar] [CrossRef]
- Keough, M.J. Dynamics of the epifauna of the bivalve Pinna bicolor: Interactions among recruitment, predation, and competition. ESA J. 1984, 65, 677–688. [Google Scholar] [CrossRef]
- Cosentino, A.; Giacobbe, S. Aspects of epizoobiontic mollusc assemblages on Pinna shells. I. Composition and structure. Cah. Biol. Mar. 2007, 48, 187–197. [Google Scholar]
- Addis, P.; Secci, M.; Brundu, G.; Manunza, A.; Corrias, S.; Cau, A. Density, size structure, shell orientation and epibiontic colonization of the fan mussel Pinna nobilis L. 1758 (Mollusca: Bivalvia) in three contrasting habitats in an estuarine area of Sardinia (W Mediterranean). Sci. Mar. 2009, 73, 143–152. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoğlu, M.; Yokeş, M.B.; Güçlüsoy, H. The last fortress fell: Mass mortality of Pinna nobilis in the Sea of Marmara. Mediterr. Mar. Sci. 2021, 22, 669–676. [Google Scholar] [CrossRef]
- Buric, P.; Iveša, N.; Brajković, A.; Žunec, A.; Matulja, I.; Kovacic, I.; Jaklin, A.; Millotti, G.; Paliaga, P.; Pustijanac, E.; et al. Seasonal macrofaunal diversity in the shells of dead Pinna nobilis Linnaeus, 1758 in southern Istria. Oceans 2025, 6, 26. [Google Scholar] [CrossRef]
- Lopes, E.P.; Monteiro, N.; Santos, A.M. Epibiotic assemblages on the pen shell Pinna rudis (Bivalvia, Pinni-dae) at Matiota Beach, São Vicente Island, Cabo Verde. Afr. J. Mar. Sci. 2020, 42, 13–21. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoğlu, M.; Yokeş, M.B.; Güçlüsoy, H. Soft-bottom macrobenthic assemblages around the south Marmara Islands (Sea of Marmara). In Ecological Changes in the Sea of Marmara; İşinibilir-Okyar, M., Kıdeys, A., Malej, A., Eds.; İstanbul Üniversity Press: Istanbul, Turkey, 2024; pp. 693–719. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part 1. Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1998; No. 306.1, Rev. 2. [Google Scholar]
- Torgerson, W.S. Theory and Methods of Scaling; Wiley: New York, NY, USA, 1958. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome C Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Marion, A.F. Notes sur la faune des Dardanelles et du Bosphore. Ann. Mus. Hist. Nat. Marseille Série II 1898, 1, 163–182. [Google Scholar]
- Albayrak, S.; Balkis, H.; Balkis, N. Bivalvia (Mollusca) fauna of the Sea of Marmara. Acta Adriat. 2004, 45, 9–26. [Google Scholar]
- Howson, C.M.; Clark, L.; Mercer, T.S.; James, B. Marine Biological Survey to Establish the Distribution and Status of Fan Mussels Atrina fragilis and Other Marine Protected Areas (MPA) Search Features Within the Sound of Canna, Inner Hebrides; Scotttish Natural Heritage Commissioned Report No. 438; Scottish Natural Heritage: Edinburgh, UK, 2012; 186p. [Google Scholar]
- Hewitt, J.E.; Thrush, S.F.; Legrendre, P.; Cummings, V.J.; Norkko, A. Integrating heterogeneity across spatial scales: Interactions between Atrina zelandica and benthic macrofauna. Mar. Ecol. Prog. Ser. 2002, 239, 115–128. [Google Scholar] [CrossRef]
- Solandt, J.L. The fan shell Atrina fragilis—A species of conservation concern. Br. Wildl. 2003, 14, 423–427. [Google Scholar]
- Chavez-Villalba, J.; Reynaga-Franco, F.D.; Hoyos-Chairez, F. Worldwide overview of reproduction, juvenile collection, spat production and cultivation of pen shells. Rev. Aquac. 2022, 14, 1371–1388. [Google Scholar] [CrossRef]
- Bastari, A.; Beccacece, J.; Ferretti, F.; Micheli, F.; Cerrano, C. Local Ecological Knowledge indicates temporal trends of benthic invertebrate species of the Adriatic Sea. Front. Mar. Sci. 2017, 4, 157. [Google Scholar] [CrossRef]
- Maeno, Y.; Yurimoto, T.; Nasu, H.; Ito, S.; Aishima, N.; Matsuyama, T.; Kamaishi, T.; Oseko, N.; Watanabe, Y. Virus-like particles associated with mass mortalities of the pen shell Atrina pectinata in Japan. Dis. Aquat. Organ. 2006, 71, 169–173. [Google Scholar] [CrossRef]
- Derrien-Courtel, S. Faune et Flore Benthiques du Littoral Breton; Proposition d’especes determinantes pour la realisation des fiches ZNIEFF-Mer et de listes complementaires. Document CSRPN Bretagne; 2010; 61p. [Google Scholar]
- Zengin, M.; Akyol, O. Description of by-catch species from the coastal shrimp beam trawl fishery in Turkey. J. Appl. Ichthyol. 2009, 25, 211–214. [Google Scholar] [CrossRef]
- Banach-Esteve, G.; Vázquez-Luis, M.; Deudero, S. Temporal trends in sessile epibionts of the endemic bi-valve Pinna nobilis: Variability in Lophocladia lallemandii colonization. Thalassas 2015, 31, 19–29. [Google Scholar]











| Stations | Coordinates | Depth | Date | |
|---|---|---|---|---|
| 1 | Start | 40°33.97′ N–27°44.52′ E | 67 | 3 April 2021 |
| End | 40°34.43′ N–27°46.15′ E | 66 | ||
| 3 | Start | 40°26.17′ N–27°37.40′ E | 43 | 5 April 2021 |
| End | 40°25.93′ N–27°35.84′ E | 45 | ||
| 4 | Start | 40°26.19′ N–27°37.31′ E | 42 | 10 September 2021 |
| End | 40°25.99′ N–27°35.75′ E | 45 | ||
| 5 | Start | 40°27.24′ N–27°34.59′ E | 43 | 13 September 2021 |
| End | 40°26.53′ N–27°33.16′ E | 45 | ||
| 6 | Start | 40°31.05′ N–27°38.67′ E | 52 | 12 December 2021 |
| End | 40°31.11′ N–27°40.21′ E | 54 |
| Stations | 4 | 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dead (D)/Live (L) Individual | D | D | D | L | L | L | L | L | L | L | L | L | 6D, 1L |
| PORIFERA | |||||||||||||
| Agelasida | |||||||||||||
| Prosuberites longispinus Topsent, 1893 | 1 | - | - | - | - | - | - | - | 1 | 1 | 1 | - | - |
| CNIDARIA | |||||||||||||
| Hydrozoa | |||||||||||||
| Clytia paulensis (Vanhöffen, 1910) | - | - | 1 | - | - | - | - | - | - | - | - | - | - |
| Anthozoa | |||||||||||||
| Alcyonium palmatum Pallas, 1766 | - | - | - | - | - | 1 | - | - | - | - | - | - | - |
| Epizoanthus arenaceus (Delle Chiaje, 1841) | 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| POLYCHAETA | |||||||||||||
| Polynoidae | |||||||||||||
| Harmothoe sp. | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Euphrosinidae | |||||||||||||
| Euphrosine foliosa A. and M. Edwards, 1833 | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Syllidae | |||||||||||||
| Myrianida sp.1 | - | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| Myrianida sp.2 | 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Syllis hyalina Grube, 1863 | 6 | - | - | 2 | 1 | - | - | - | - | - | 1 | - | 2 |
| Nereididae | |||||||||||||
| Composetia hircinicola (Eisig, 1869) | 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Lumbrineridae | |||||||||||||
| Abyssoninoe hibernica (McIntosh, 1903) | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| Eunicidae | |||||||||||||
| Eunice vittata (Delle Chiaje, 1828) | 1 | 1 | - | 1 | 3 | - | - | - | 1 | - | - | - | 3 |
| Spionidae | |||||||||||||
| Polydora sp. | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Cirratulidae | |||||||||||||
| Aphelochaeta sp. | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Cirratulus sp. | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Cirriformia sp. | - | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Monticellina sp. | - | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Maldanidae | |||||||||||||
| Metasychis gotoi (Izuka, 1902) | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Terebellidae | |||||||||||||
| Amphitrite rubra (Risso, 1828) | - | - | - | - | - | - | 2 | - | - | 1 | 1 | - | 7 |
| Terebella lapidaria Linnaeus 1767 | 7 | - | 1 | - | 4 | 1 | - | 4 | 2 | - | 1 | - | 3 |
| Neoamphitrite figulus (Dalyell, 1853) | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Polycirrus sp. | - | - | 1 | - | - | - | - | - | - | - | - | - | - |
| Sabellidae | |||||||||||||
| Branchiomma bombyx (Dalyell, 1853) | - | - | - | - | 3 | - | - | 1 | - | - | - | - | 1 |
| Pseudopotamilla saxicava (Quatrefages, 1866) | 4 | 3 | - | 1 | 6 | 1 | - | - | 1 | 4 | - | - | - |
| Serpulidae | |||||||||||||
| Hydroides norvegica Gunnerus, 1768 | - | - | - | 1 | - | - | - | - | - | - | - | - | 4 |
| Protula tubularia (Montagu, 1803) | 3 | 1 | 1 | 2 | 1 | - | 1 | 1 | 9 | 1 | - | 21 | |
| Serpula concharum Langerhans, 1880 | 4 | - | - | 5 | 3 | 1 | - | 10 | - | 2 | 7 | - | 1 |
| Serpula vermicularis Linnaeus, 1767 | - | - | - | 1 | - | 1 | - | 4 | - | - | 5 | - | 2 |
| Vermiliopsis infundibulum (Philippi, 1844) | 5 | 4 | - | - | - | - | - | - | - | - | - | - | - |
| CRUSTACEA | |||||||||||||
| Isopoda | |||||||||||||
| Gnathia sp. | 2 | 1 | 4 | - | 1 | - | - | - | 1 | - | 1 | - | 1 |
| Decapoda | |||||||||||||
| Pisa nodipes Leach, 1815 | - | - | - | - | - | 1 | - | - | - | - | - | - | - |
| MOLLUSCA | |||||||||||||
| Gastropoda | |||||||||||||
| Calyptraea chinensis (Linnaeus, 1758) | - | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Bivalvia | |||||||||||||
| Anomia ephippium Linnaeus, 1758 | - | 1 | 2 | - | - | - | - | - | - | 1 | - | - | 2 |
| Musculus subpictus (Cantraine, 1835) | - | - | - | - | - | - | - | 2 | - | - | - | - | - |
| Ostrea edulis Linnaeus, 1758 | - | - | - | - | - | 3 | - | 2 | - | - | - | - | - |
| Pteria hirundo (Linnaeus, 1758) | - | - | - | - | - | - | - | 3 | - | - | - | - | - |
| Striarca lactea (Linnaeus, 1758) | - | - | - | 1 | - | - | - | - | - | - | - | - | 1 |
| BRYOZOA | |||||||||||||
| Cheliostomatida | |||||||||||||
| Arthropoma cecilii (Audouin, 1826) | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Callopora dumerilii (Audouin, 1826) | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Smittoidea reticulata (MacGillivray, 1842) | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Ctenostomatida | |||||||||||||
| Benedenipora sp. | - | - | - | 1 | - | - | 1 | - | - | - | - | - | - |
| ECHINODERMATA | |||||||||||||
| Ophiuroidea | |||||||||||||
| Amphiura chiajei Forbes, 1843 | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| TUNICATA | |||||||||||||
| Ascidiacea | |||||||||||||
| Tunicata sp.1 | 1 | 3 | - | 1 | 1 | - | 2 | 1 | - | - | - | - | 1 |
| Didemnum sp. | - | - | - | 1 | - | - | - | 1 | - | - | 1 | 1 | 1 |
| Ascidiella aspersa (Müller, 1776) | - | - | - | - | - | - | 1 | - | - | - | - | - | 1 |
| Ascidia virginea Müller, 1776 | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - |
| Phallusia mammillata (Cuvier, 1815) | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çinar, M.E.; Yokeş, M.B.; Erdogan-Dereli, D.; Açik, S.; Evcen, A. Epifaunal Assemblages of the Fan Mussel Atrina fragilis (Mollusca: Bivalvia) in the Sea of Marmara. Biology 2025, 14, 945. https://doi.org/10.3390/biology14080945
Çinar ME, Yokeş MB, Erdogan-Dereli D, Açik S, Evcen A. Epifaunal Assemblages of the Fan Mussel Atrina fragilis (Mollusca: Bivalvia) in the Sea of Marmara. Biology. 2025; 14(8):945. https://doi.org/10.3390/biology14080945
Chicago/Turabian StyleÇinar, Melih Ertan, Mehmet Baki Yokeş, Deniz Erdogan-Dereli, Sermin Açik, and Alper Evcen. 2025. "Epifaunal Assemblages of the Fan Mussel Atrina fragilis (Mollusca: Bivalvia) in the Sea of Marmara" Biology 14, no. 8: 945. https://doi.org/10.3390/biology14080945
APA StyleÇinar, M. E., Yokeş, M. B., Erdogan-Dereli, D., Açik, S., & Evcen, A. (2025). Epifaunal Assemblages of the Fan Mussel Atrina fragilis (Mollusca: Bivalvia) in the Sea of Marmara. Biology, 14(8), 945. https://doi.org/10.3390/biology14080945







