Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Soil Physicochemical Analysis
2.4. Soil DNA Extraction, High-Throughput Sequencing, and Bioinformatics Analysis
2.5. Data Processing
3. Results and Analysis
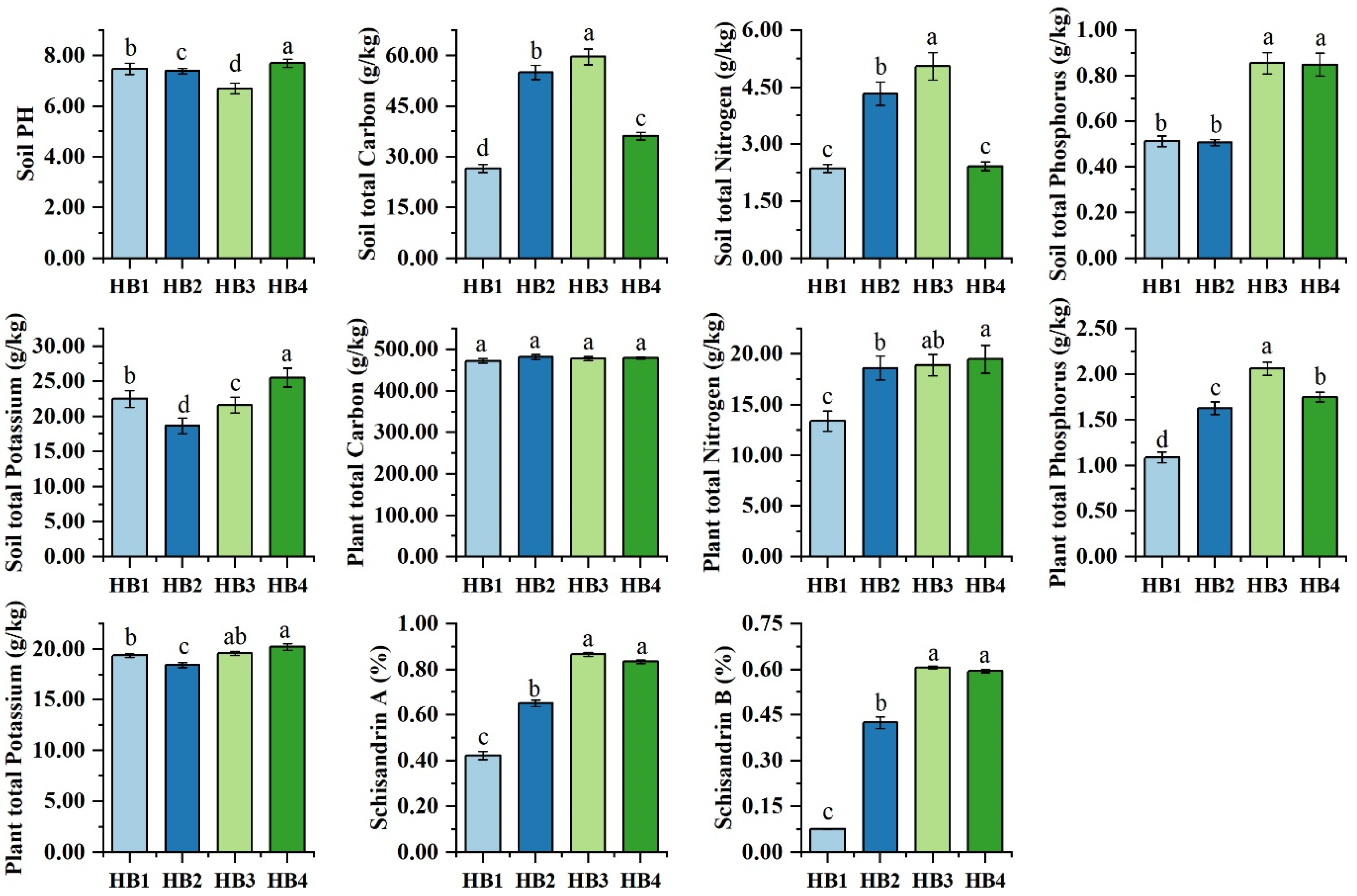
3.1. Soil Physicochemical Properties
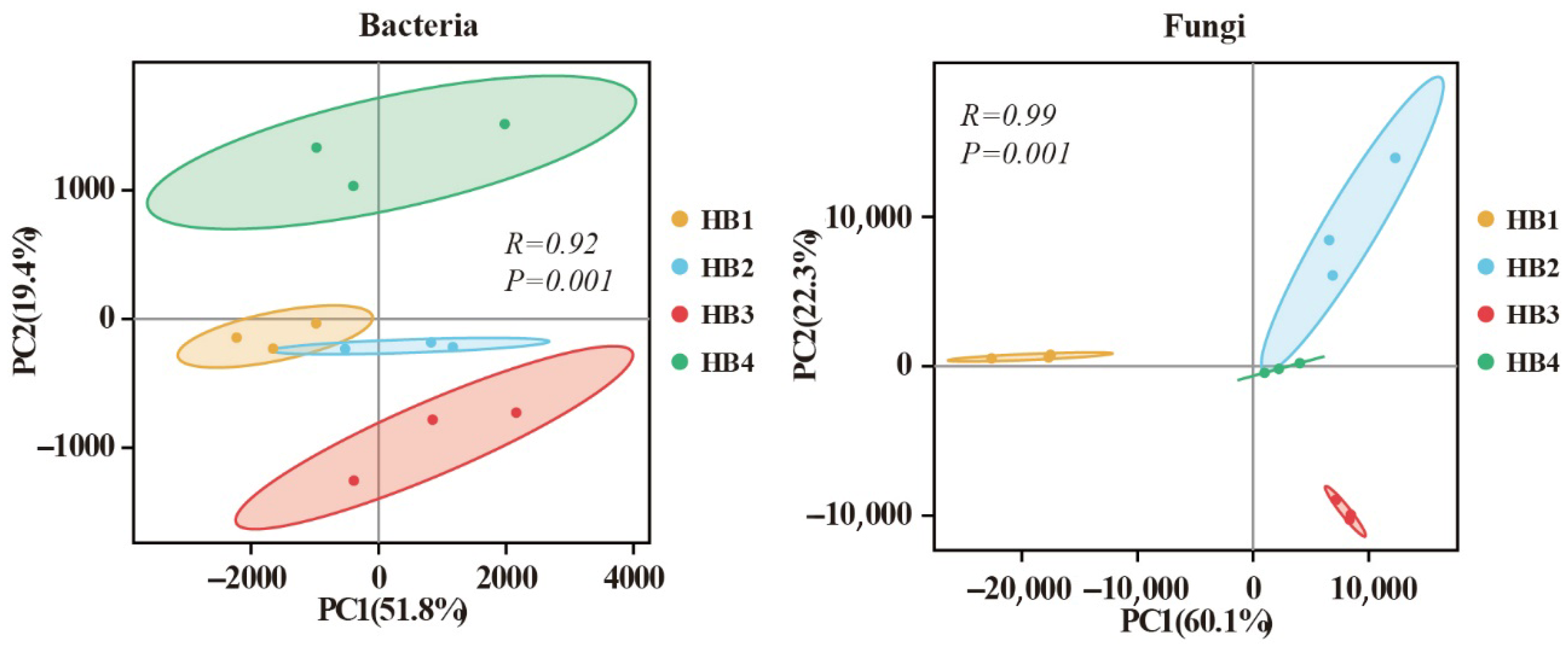
3.2. Soil Microbial Community Composition
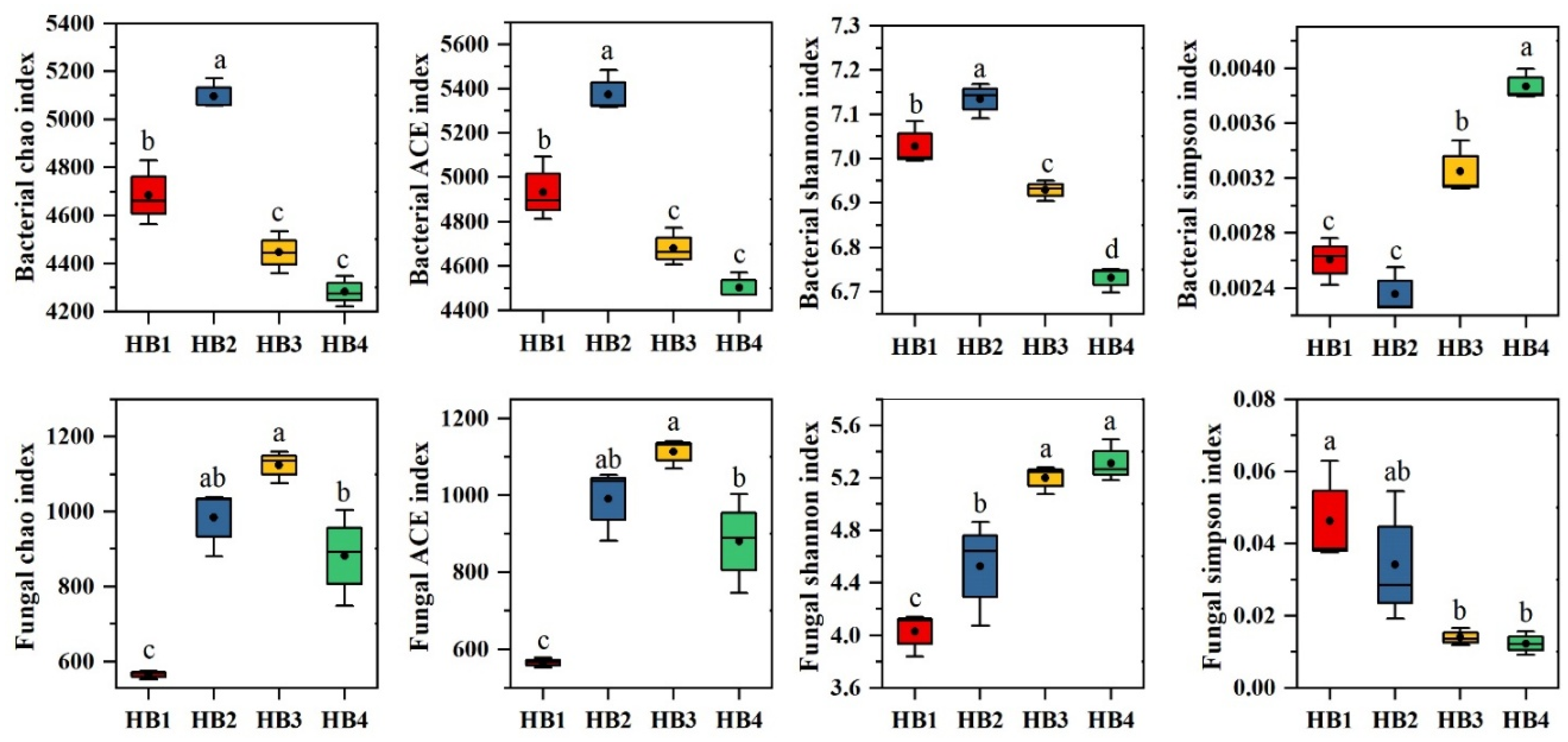
3.3. Soil Microbial Community Diversity
3.4. Soil Microbial Community Assembly Mechanisms
3.5. Co-Occurrence Network Characteristics of Soil Microbial Communities
3.6. Relationships Between Soil Microbial Communities and Physicochemical Factors
4. Discussion
4.1. Effects of Altitude on Soil Microbial Community Characteristics in Schisandra sphenanthera
4.2. Effects of Altitude on Soil Carbon Sequestration in Schisandra sphenanthera
4.3. Effects of Altitude on Soil Microbial Interactions in Schisandra sphenanthera
4.4. Effects of Altitude on Soil Microbial Assembly Mechanisms in Schisandra sphenanthera
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Cao, Y.; Li, Y.; Sun, S.; Zhang, J.; Zhang, G. Schisandrin B attenuates CCl4-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des. Dev. Ther. 2017, 11, 2179–2191. [Google Scholar] [CrossRef]
- Deng, Z.; Dong, Y.; Zhou, X.; Lu, J.-H.; Yue, Z. Pharmacological modulation of autophagy for Alzheimer’s disease therapy: Opportunities and obstacles. Acta Pharm. Sin. B 2022, 12, 1688–1706. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, M.; Jin, Y.-C.; Li, M.; Yu, K.-X.; Yu, H.-B. Schisandrin B, a dual positive allosteric modulator of GABAA and glycine receptors, alleviates seizures in multiple mouse models. Acta Pharmacol. Sin. 2024, 45, 465–479. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.; Wang, X.; Wu, Z.; Zhou, J.; Wang, C.; Chen, R.; Qin, X.; Yang, H.; Wei, H. The phenotypic diversity of Schisandra sphenanthera fruit and SVR model for phenotype forecasting. Ind. Crops Prod. 2022, 186, 115162. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Li, W.; Wang, C.; Li, H.; Ju, W.; Chen, J.; Sun, J. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem. Biodivers. 2018, 15, e1800030. [Google Scholar] [CrossRef]
- Sheng, Y.; Dong, J.; Wang, R.; Wang, Y.; Huang, X.; Chen, C. The influence of storage conditions on the quality of Schisandra chinensis fruits: A integrated investigation of constituent and antioxidant activity. Heliyon 2024, 10, e32194. [Google Scholar] [CrossRef]
- Ge, J.; Chen, J.; Zi, F.; Song, T.; Hu, L.; He, Z.; Wu, L.; Ding, Y.; Li, H. Seasonal Variations in Macrobenthos Communities and Their Relationship with Environmental Factors in the Alpine Yuqu River. Biology 2025, 14, 120. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Liu, Y.; Xu, Q.; Zhu, C.; Ling, N.; Guo, J.; Li, R.; Huang, W.; Guo, S. Relative importance of altitude shifts with plant and microbial diversity to soil multifunctionality in grasslands of north-western China. Plant Soil 2024, 504, 545–560. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, 10-1128. [Google Scholar] [CrossRef]
- Jiao, S.; Peng, Z.; Qi, J.; Gao, J.; Wei, G. Linking bacterial-fungal relationships to microbial diversity and soil nutrient cycling. Msystems 2021, 6, 10-1128. [Google Scholar] [CrossRef]
- Murugan, R.; Djukic, I.; Keiblinger, K.; Zehetner, F.; Bierbaumer, M.; Zechmeister-Bolternstern, S.; Joergernsen, R.G. Spatial distribution of microbial biomass and residues across soil aggregate fractions at different elevations in the Central Austrian Alps. Geoderma 2019, 339, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, S.; Hayat, K.; Liao, X.; Chen, J.; Zhang, L.; Xie, Y. Investigating the Effects of Elevation on Microbial Communities and Soil Properties at Fanjing Mountain, China. Forests 2024, 15, 1980. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, W.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610, 750–758. [Google Scholar] [CrossRef]
- Yu, W.; Sheng, L.; Wang, X.; Tang, X.; Yuan, J.; Luo, W. Soil Microbial Carbon Use Efficiency in Natural Terrestrial Ecosystems. Biology 2025, 14, 348. [Google Scholar] [CrossRef]
- Rimé, D.; Nazaret, S.; Gourbière, F.; Cadet, P.; Moënne-Loccoz, Y. Comparison of sandy soils suppressive or conducive to ectoparasitic nematode damage on sugarcane. Phytopathology 2003, 93, 1437–1444. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 539–579. [Google Scholar] [CrossRef]
- Bremner, J. Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Brealey, L. The determination of potassium in fertilisers by flame photometry. Analyst 1951, 76, 340–343. [Google Scholar] [CrossRef]
- Yu, B.; Sheng, D.; Tan, Q. Determination of schisandrin A and schisandrin B in traditional Chinese medicine preparation huganpian tablet by RP-HPLC. Chem. Pharm. Bull. 2019, 67, 713–716. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, P.; Xu, L.; Qu, L.; Han, Y.; Zheng, L.; Gong, X. Disproportionate responses between free-living and particle-attached bacteria during the transition to oxygen-deficient zones in the Bohai Seawater. Sci. Total Environ. 2021, 791, 148097. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil organic carbon and total nitrogen stocks as affected by vegetation types and altitude across the mountainous regions in the Yunnan Province, south-western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Cai, Q.-L.; Jia, G.-K.; Wei, C.; Zhong, N.; Lv, L.-L.; Li, J.; Pang, H.; Yang, W. Response of microbial structure characteristics and enzyme activity to different altitude. Front. Microbiol. 2025, 16, 1588591. [Google Scholar] [CrossRef]
- Ye, Y.-L.; Ma, K.-J.; Fu, Y.-H.; Wu, Z.-C.; Fu, G.-Y.; Sun, C.; Xu, X.-W. The heterogeneity of microbial diversity and its drivers in two types of sediments from tidal flats in Beibu Gulf, China. Front. Mar. Sci. 2023, 10, 1256393. [Google Scholar] [CrossRef]
- Akwakwa, G.H.; Daryl, K.S.P.; Riaz, A.; Xiaoyan, W. Varying nitrogen fertilization and soil bacterial community dynamics at three growth phases of winter wheat production. Plant Growth Regul. 2024, 104, 1383–1397. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Ran, Y.; Li, W.; Shao, M.; Zhang, Y.; Ji, Q.; Ding, X. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- Jain, V.; Arya, P.; Yagnik, S.M.; Raval, V.H.; Singh, N.A. Microbial diversity of cold-water reservoirs and their prospective applications. In Current Status of Fresh Water Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 49–75. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, X.; Li, P.; Si, Z.; Gao, Y.; Li, J. Composition and diversity of soil microbial communities in walnut orchards at different altitudes in southeastern Tibet. Land 2023, 12, 1419. [Google Scholar] [CrossRef]
- Ahmad, R.; Gao, J.; Li, W.; Zhang, Y.; Gao, Z.; Khan, A.; Ali, I.; Ullah, S.; Fahad, S. Response of soil nutrients, enzyme activities, and fungal communities to biochar availability in the rhizosphere of mountainous apple trees. Plant Soil 2023, 489, 277–293. [Google Scholar] [CrossRef]
- James, T.Y.; Letcher, P.M.; Longcore, J.E.; Mozley-Standridge, S.E.; Porter, D.; Powell, M.J.; Griffith, G.W.; Vilgalys, R. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 2006, 98, 860–871. [Google Scholar] [CrossRef]
- Chen, W.-M.; Moulin, L.; Bontemps, C.; Vandamme, P.; Béna, G.; Boivin-Masson, C. Legume symbiotic nitrogen fixation byβ-Proteobacteria is widespread inNature. J. Bacteriol. 2003, 185, 7266–7272. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, K.; Nie, L.; Liu, L.; Zhou, J.; Wu, K.; Ye, H.; Wu, Z. Effects of biological agents on rhizosphere microecological environment and nutrient availability for rice. Front. Microbiol. 2025, 15, 1447527. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Fornasier, F.; Paletto, A.; Ceotto, E.; De Meo, I. Ascomycota and Basidiomycota fungal phyla as indicators of land use efficiency for soil organic carbon accrual with woody plantations. Ecol. Indic. 2024, 160, 111796. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, K.; Zhang, N.; Wang, S.; Zhou, Z.; Sun, J. Response of cbbL Carbon-Sequestering Microorganisms to Simulated Warming in the River Source Wetland of the Wayan Mountains. Biology 2025, 14, 708. [Google Scholar] [CrossRef]
- Marañón, E.; Lorenzo, M.P.; Cermeño, P.; Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates. ISME J. 2018, 12, 1836–1845. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Saurabh Kumar, S.K.; Suyal, D.; Amit Yadav, A.Y.; Yogesh Shouche, Y.S.; Reeta Goel, R.G. Microbial diversity and soil physiochemical characteristic of higher altitude. PLoS ONE 2019, 14, e0213844. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Eloka-Eboka, A.C.; Bwapwa, J.K.; Maroa, S. Biomass for CO2 sequestration. Encycl. Renew. Sustain. Mater. 2020, 3, 277–290. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bolan, N.; Farrell, M.; Sarkar, B.; Sarker, J.R.; Kirkham, M.B.; Hossain, M.Z.; Kim, G.-H. Role of cultural and nutrient management practices in carbon sequestration in agricultural soil. Adv. Agron. 2021, 166, 131–196. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Lal, R.; Singh Yadav, G.; Mitran, T.; Meena, B.L.; Dotaniya, M.L.; EL-Sabagh, A. Role of legumes in soil carbon sequestration. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 109–138. [Google Scholar] [CrossRef]
- Khatoon, H.; Solanki, P.; Narayan, M.; Tewari, L.; Rai, J.; Hina Khatoon, C. Role of microbes in organic carbon decomposition and maintenance of soil ecosystem. Int. J. Chem. Stud. 2017, 5, 1648–1656. [Google Scholar]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Carr, A.; Diener, C.; Baliga, N.S.; Gibbons, S.M. Use and abuse of correlation analyses in microbial ecology. ISME J. 2019, 13, 2647–2655. [Google Scholar] [CrossRef]
- Rosbakh, S.; Leingärtner, A.; Hoiss, B.; Krauss, J.; Steffan-Dewenter, I.; Poschlod, P. Contrasting effects of extreme drought and snowmelt patterns on mountain plants along an elevation gradient. Front. Plant Sci. 2017, 8, 1478. [Google Scholar] [CrossRef]
- Eheneden, I.; Wang, R.; Zhao, J. Antibiotic removal by microalgae-bacteria consortium: Metabolic pathways and microbial responses. Sci. Total Environ. 2023, 891, 164489. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Cavaliere, M.; Feng, S.; Soyer, O.S.; Jiménez, J.I. Cooperation in microbial communities and their biotechnological applications. Environ. Microbiol. 2017, 19, 2949–2963. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Dantas de Paula, M.; Forrest, M.; Langan, L.; Bendix, J.; Homeier, J.; Velescu, A.; Wilcke, W.; Hickler, T. Nutrient cycling drives plant community trait assembly and ecosystem functioning in a tropical mountain biodiversity hotspot. New Phytol. 2021, 232, 551–566. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wu, J.H.; Sun, Y.X.; Zhang, Y.Y.; Zhao, Y.F.; Huang, Z.; Duan, W.H. Climate and geochemistry at different altitudes influence soil fungal community aggregation patterns in alpine grasslands. Sci. Total Environ. 2023, 881, 163375. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Pan, Y.-P.; Liu, Y.; Li, M. High-level diversity of basal fungal lineages and the control of fungal community assembly by stochastic processes in mangrove sediments. Appl. Environ. Microbiol. 2021, 87, e00928-21. [Google Scholar] [CrossRef]
- Yang, L.; Ning, D.; Yang, Y.; He, N.; Li, X.; Cornell, C.R.; Bates, C.T.; Filimonenko, E.; Kuzyakov, Y.; Zhou, J. Precipitation balances deterministic and stochastic processes of bacterial community assembly in grassland soils. Soil Biol. Biochem. 2022, 168, 108635. [Google Scholar] [CrossRef]
- Pham, P.; Shi, Y.; Khan, I.; Sumarah, M.; Renaud, J.; Sunohara, M.; Craiovan, E.; Lapen, D.; Aris-Brosou, S.; Chen, W. The functions and factors governing fungal communities and diversity in agricultural waters: Insights into the ecosystem services aquatic mycobiota provide. Front. Microbiol. 2024, 15, 1460330. [Google Scholar] [CrossRef]



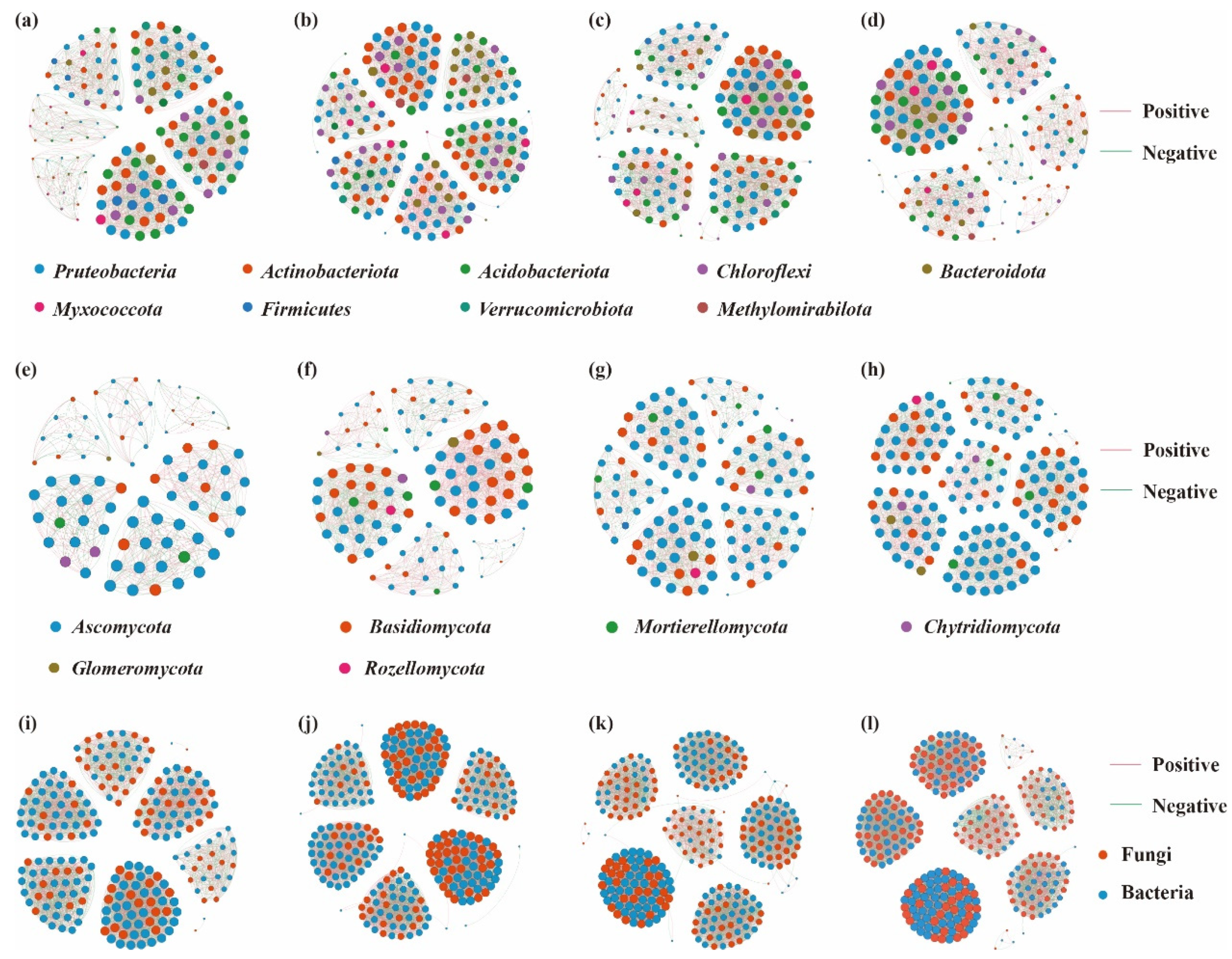

| Treatments | Nodes | Links | Positive Edges% | Negative Edges% | Average Degree | Graph Density | Modularity | |
|---|---|---|---|---|---|---|---|---|
| Bacteria | HB1 | 150 | 1978 | 51.40 | 48.60 | 26.37 | 0.783 | 0.177 |
| HB2 | 185 | 2591 | 56.10 | 43.90 | 28.01 | 0.825 | 0.152 | |
| HB3 | 164 | 2363 | 52.90 | 47.10 | 28.82 | 0.748 | 0.177 | |
| HB4 | 147 | 2017 | 56.30 | 43.70 | 27.44 | 0.669 | 0.188 | |
| Fungi | HB1 | 79 | 527 | 57.70 | 42.30 | 13.34 | 0.78 | 0.141 |
| HB2 | 112 | 1198 | 65.40 | 34.60 | 21.39 | 0.722 | 0.193 | |
| HB3 | 130 | 1322 | 59.30 | 40.70 | 20.34 | 0.808 | 0.158 | |
| HB4 | 160 | 1917 | 55.30 | 44.70 | 23.96 | 0.825 | 0.151 | |
| Bacteria × Fungi | HB1 | 233 | 4445 | 52.10 | 47.90 | 38.16 | 0.807 | 0.164 |
| HB2 | 297 | 7067 | 53.80 | 46.20 | 47.59 | 0.814 | 0.161 | |
| HB3 | 298 | 7001 | 52.50 | 47.50 | 46.99 | 0.79 | 0.158 | |
| HB4 | 307 | 7659 | 52.50 | 47.50 | 49.9 | 0.775 | 0.163 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yang, L.; Cong, X.; Mao, Z.; Zhou, Y. Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients. Biology 2025, 14, 944. https://doi.org/10.3390/biology14080944
Li W, Yang L, Cong X, Mao Z, Zhou Y. Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients. Biology. 2025; 14(8):944. https://doi.org/10.3390/biology14080944
Chicago/Turabian StyleLi, Weimin, Luyao Yang, Xiaofeng Cong, Zhuxin Mao, and Yafu Zhou. 2025. "Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients" Biology 14, no. 8: 944. https://doi.org/10.3390/biology14080944
APA StyleLi, W., Yang, L., Cong, X., Mao, Z., & Zhou, Y. (2025). Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients. Biology, 14(8), 944. https://doi.org/10.3390/biology14080944






