The Ghrelin Analog GHRP-6, Delivered Through Aquafeeds, Modulates the Endocrine and Immune Responses of Sparus aurata Following IFA Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance and Diets
2.2. Experimental Design and Sampling Procedure
2.3. Plasma Analyses
2.4. Gene Expression
2.5. Histomorphological and Histochemical Analysis
2.6. Morphometric Analyses
2.7. Statistical Analyses
3. Results
3.1. Plasma Biochemistry
3.2. Immunoglobulin and Cortisol in Plasma
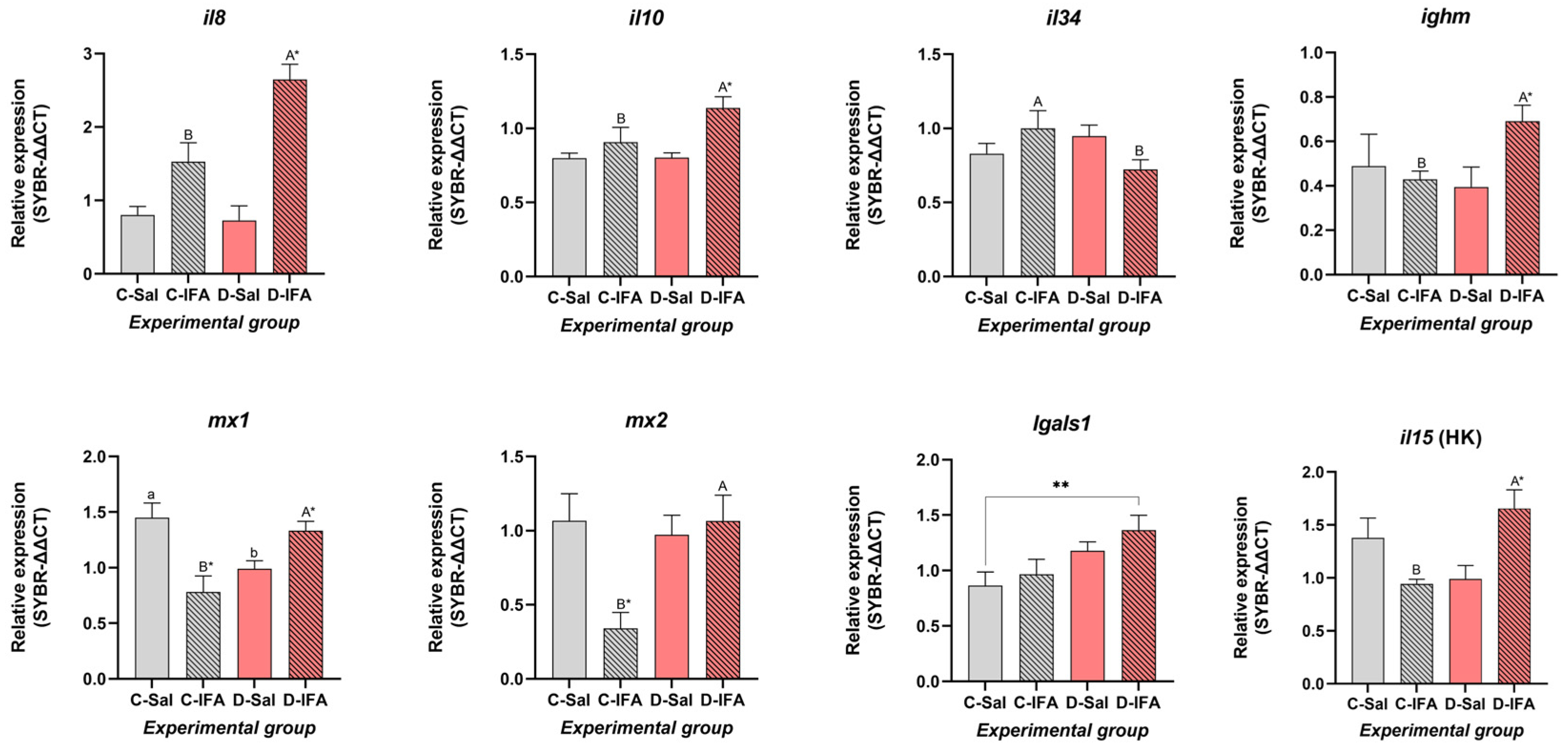
3.3. Gene Expression
3.3.1. Anterior Intestine
3.3.2. Posterior Intestine
3.3.3. Spleen and Head Kidney
3.4. Multivariate Analysis
3.5. Histomorphological and Histochemical Analysis of the Intestine and Spleen
4. Discussion
4.1. Plasma Biochemistry
4.2. Gene Expression
4.3. Histomorphological and Histochemical Analysis
4.4. Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Mai, K.; Øverland, M.; Robb, D.; Roubach, R.; Schrama, J.; Small, B.; et al. Harvesting the benefits of nutritional research to address global challenges in the 21st century. J. World Aquacult. Soc. 2023, 54, 343–363. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Huang, J.; Huang, B.; Li, H. The exploitation of probiotics, prebiotics and symbiotic in aquaculture: Present study, limitations and future directions.: A review. Aquac. Inter. 2020, 28, 1017–1041. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef]
- Barrows, F.T.; Hardy, R.W. Feed additives. In Encyclopedia of Aquaculture; Stickney, R.R., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 335–340. [Google Scholar]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, A.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; Piazzon, M.C.; de las Heras, V.; Calduch-Giner, J.A.; Puyalto, M.; Tinsley, J.; Makol, A.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Dietary sodium heptanoate helps to improve feed efficiency, growth hormone status and swimming performance in gilthead sea bream (Sparus aurata). Aquacult. Nutr. 2018, 24, 1638–1651. [Google Scholar] [CrossRef]
- Perera, E.; Sánchez-Ruiz, D.; Sáez, M.I.; Galafat, A.; Barany, A.; Fernández-Castro, M.; Vizcaíno, A.J.; Fuentes, J.; Martínez, T.F.; Mancera, J.M.; et al. Low dietary inclusion of nutraceuticals from microalgae improves feed efficiency and modifies intermediary metabolisms in gilthead sea bream (Sparus aurata). Sci. Rep. 2020, 10, 18676. [Google Scholar] [CrossRef]
- Molina-Roque, L.; Bárany, A.; Sáez, M.I.; Alarcón, F.J.; Tapia, S.T.; Fuentes, J.; Mancera, J.M.; Perera, E.; Martos-Sitcha, J.A. Biotechnological treatment of microalgae enhances growth performance, hepatic carbohydrate metabolism and intestinal physiology in gilthead seabream (Sparus aurata) juveniles close to commercial size. Aquac. Rep. 2022, 25, 101248. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquacult. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Martín, D.; Ordás, M.C.; Carvalho, I.; Díaz-Rosales, P.; Nuñez-Ortiz, N.; Vicente-Gil, S.; Arrogante, A.; Zarza, C.; Machado, M.; Costas, B.; et al. L-methionine supplementation modulates IgM+ B cell responses in rainbow trout. Front. Immunol. 2023, 14, 1264228. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, S.; Dutta, J.; Ahmad, I.; Rather, M.A.; Badroo, I.A.; Bhat, T.A.; Ahmad, I.; Amin, A.; Shah, A.; Qadri, T.; et al. Biogenic silver nanoparticles: Synthesis, applications and challenges in food sector with special emphasis on aquaculture. Food Chem. X 2023, 20, 101051. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Beneficial roles of nutrients as immunostimulants in aquaculture: A review. Aquac. Fish. 2024, 9, 707–720. [Google Scholar] [CrossRef]
- Momany, F.A.; Bowers, C.Y.; Reynolds, G.A.; Chang, D.; Hong, A.; Newlander, K. Design, synthesis, and biological activity of peptides which release growth hormone in vitro. Endocrinology 1981, 108, 31–39. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdoun, K.A. Safety and concerns of hormonal application in farm animal production: A review. J. Appl. Anim. Res. 2022, 50, 426–439. [Google Scholar] [CrossRef]
- Yahashi, S.; Kang, K.S.; Kaiya, H.; Matsuda, K. GHRP-6 mimics ghrelin-induced stimulation of food intake and suppression of locomotor activity in goldfish. Peptides 2012, 34, 324–328. [Google Scholar] [CrossRef]
- Reyes, D.; Estrada, M.P.; Martínez, R. Ghrelin as a Promising Immunostimulant in Aquaculture: Mechanisms and Therapeutic Potential. Bionatura 2025, 2, 6. [Google Scholar] [CrossRef]
- Martínez, R.; Fernández-Trujillo, M.A.; Hernández, L.; Page, A.; Béjar, J.; Estrada, M.P. Growth hormone secretagogue peptide A233 upregulates Mx expression in teleost fish in vitro and in vivo. Arch. Virol. 2022, 167, 2041–2047. [Google Scholar] [CrossRef]
- Martinez, R.; Ubieta, K.; Herrera, F.; Forellat, A.; Morales, R.; de la Nuez, A.; Rodriguez, R.; Reyes, O.; Oliva, A.; Estrada, M.P. A novel GH secretagogue, A233, exhibits enhanced growth activity and innate immune system stimulation in teleosts fish. J. Endocrinol. 2012, 214, 409. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Morales, C.; Arenal, A.; Morales, A.; Herrera, F.; González, V.; Estrada, M.P. Growth Hormone Secretagogue (A233) Improves Growth and Changes the Tissue Fatty Acid Profile in Juvenile Tilapia (Oreochromis niloticus). Lipids 2018, 53, 429–436. [Google Scholar] [CrossRef]
- Bowers, C.Y.; Momany, F.A.; Reynolds, G.A.; Hong, A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 1984, 114, 1537–1545. [Google Scholar] [CrossRef]
- Lugo, J.M.; Rodriguez, A.; Helguera, Y.; Morales, R.; Gonzalez, O.; Acosta, J.; Besada, V.; Sanchez, A.; Estrada, M.P. Recombinant novel pituitary adenylate cyclase-activating polypeptide from African catfish (Clarias gariepinus) authenticates its biological function as a growth-promoting factor in low vertebrates. J. Endocrinol. 2008, 197, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Carpio, Y.; Morales, A.; Lugo, J.M.; Herrera, F.; Zaldívar, C.; Carrillo, O.; Arenal, A.; Pimentel, E.; Estrada, M.P. Oral administration of the growth hormone secretagogue-6 (GHRP-6) enhances growth and non-specific immune responses in tilapia (Oreochromis sp.). Aquaculture 2016, 452, 304–310. [Google Scholar] [CrossRef]
- Lugo, J.M.; Oliva, A.; Morales, A.; Reyes, O.; Garay, H.E.; Herrera, F.; Cabrales, A.; Pérez, E.; Estrada, M.P. The biological role of pituitary adenylate cyclase-activating polypeptide (PACAP) in growth and feeding behavior in juvenile fish. J. Pept. Sci. 2010, 16, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Hernández, L.; Gil, L.; Carpio, Y.; Morales, A.; Herrera, F.; Rodríguez-Mallón, A.; Leal, Y.; Blanco, A.; Estrada, M.P. Growth hormone releasing peptide-6 enhanced antibody titers against subunit antigens in mice (BALB/c), tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Vaccine 2017, 35, 5722–5728. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.; Camacho, H.; Nuñez-Robainas, A.; Palenzuela, D.O.; Morales, A.; Basabe, L.; Herrera, F.; Rodrigo, O.; Rodriguez-Gabilondo, A.; Velázquez, J.; et al. Growth hormone secretagogue peptide-6 enhances oreochromicins transcription and antimicrobial activity in tilapia (Oreochromis sp.). Fish Shellfish Immunol. 2021, 119, 508–515. [Google Scholar] [CrossRef]
- Álvarez-Torres, D.; Martínez, R.; Moreno, P.; Alonso, M.C.; García-Rosado, E.; Estrada, M.P.; Béjar, J. Anti-nervous necrosis virus activity of the growth hormone releasing peptide-6, GHRP-6. Aquac. Inter. 2025, 33, 306. [Google Scholar] [CrossRef]
- Shepherd, B.S.; Johnson, J.K.; Silverstein, J.T.; Parhar, I.S.; Vijayan, M.M.; McGuire, A.; Weber, G.M. Endocrine and orexigenic actions of growth hormone secretagogues in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 390–399. [Google Scholar] [CrossRef]
- Rodríguez-Viera, L.; Martí, I.; Martínez, R.; Perera, E.; Estrada, M.P.; Mancera, J.M.; Martos-Sitcha, J.A. Feed supplementation with the GHRP-6 peptide, a ghrelin analog, improves feed intake, growth performance and aerobic metabolism in the gilthead sea bream Sparus aurata. Fishes 2022, 7, 31. [Google Scholar] [CrossRef]
- Adelmann, M.; Kollner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.J.; Fichtner, D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef]
- Panigrahi, A.; Kiron, V.; Puangkaew, J.; Kobayashi, T.; Satoh, S.; Sugita, H. The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 2005, 243, 241–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaff, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A. The Haematoxylins. In The Theory and Practice of Histological Techniques, 2nd ed.; John, B., Alan, S., Eds.; Longman Group Limited: London, UK, 1982; p. 109. [Google Scholar]
- Martoja, R.; Martoja-Pierson, M.; Mocanut, E.; Coll, D.I. Técnicas de Histología Animal; Toray Masson: Barcelona, Spain, 1970; p. 350. [Google Scholar]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Medale, F.; Kaushik, S.; Pérez-Sánchez, J. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef]
- Li, R.X.; Chen, L.Y.; Limbu, S.M.; Qian, Y.C.; Zhou, W.H.; Chen, L.Q.; Luo, Y.; Qiao, F.; Zhang, M.L.; Du, Z.Y. High cholesterol intake remodels cholesterol turnover and energy homeostasis in Nile tilapia (Oreochromis niloticus). Mar. Life Sci. Technol. 2023, 5, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicators of fish stress? Pan-Am. J. Aquat. Sci. 2010, 5, 158–178. [Google Scholar]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after dietary administration of functional feeds in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 205, 20–28. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Huesa-Cerdán, R.; Hidalgo-Pérez, J.A.; Simó-Mirabet, P.; Rodríguez-Viera, L.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Perera, E. One-carbon nutrients and genistein as nutritional programming effectors in juvenile gilthead seabream (Sparus aurata): Contrasting effects on phenotypic traits. Aquaculture. 2025, 598, 742063. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.L.; Stolte, H.H.; Metz, J.R.; Chadzinska, M.K. Neuroendocrine-immune interactions in teleost fish. In Fish Neuroendocrinology; Academic Press: Cambridge, UK, 2009; pp. 313–364. [Google Scholar] [CrossRef]
- Mancera, J.M.; Smolenaars, M.; Laiz-Carrión, R.; del Río, M.D.P.; Bonga, S.W.; Flik, G. 17β-estradiol affects osmoregulation in Fundulus heteroclitus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 183–191. [Google Scholar] [CrossRef]
- Simó-Mirabet, P.; Caderno, A.; Flores-Llano, M.J.; da Silva, E.G.; Schoenau, W.; Baldisserotto, B.; Martínez-Rodríguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Evaluation of the Sedative Effect of Limonene to Reduce Stress During Transportation of Gilthead Seabream (Sparus aurata). Biology 2025, 14, 115. [Google Scholar] [CrossRef]
- Semple, S.L.; Heath, G.; Rodríguez-Ramos, T.; Betancourt, J.L.; Dixon, B. The Immune System of Bony Fish. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 9780128096338. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef]
- Esteban, M.Á. An overview of the immunological defenses in fish skin. Int. Sch. Res. Notices. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Costas, B.; Couto, A.; Azeredo, R.; Machado, M.; Krogdahl, Å.; Oliva-Teles, A. Gilthead seabream (Sparus aurata) immune responses are modulated after feeding with purified antinutrients. Fish Shellfish Immunol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Campos-Sánchez, J.C.; Mayor-Lafuente, J.; González-Silvera, D.; Guardiola, F.A.; Esteban, M.Á. Acute inflammatory response in the skin of gilthead seabream (Sparus aurata) caused by carrageenin. Fish Shellfish Immunol. 2021, 119, 623–634. [Google Scholar] [CrossRef]
- Esteban, M.Á.; Cerezuela, R. Fish mucosal immunity: Skin. In Mucosal Health in Aquaculture; Academic Press: Cambridge, UK, 2015; pp. 67–92. [Google Scholar]
- Reyes-López, F.E.; Ibarz, A.; Ordóñez-Grande, B.; Vallejos-Vidal, E.; Andree, K.B.; Balasch, J.C.; Fernández-Alacid, L.; Sanahuja, I.; Sánchez-Nuño, S.; Firmino, J.P.; et al. Skin multi-omics-based interactome analysis: Integrating the tissue and mucus exuded layer for a comprehensive understanding of the teleost mucosa functionality as model of study. Front. Immunol. 2021, 11, 613824. [Google Scholar] [CrossRef]
- Buonocore, F.; Randelli, E.; Casani, D.; Marozzi, C.; Scapigliati, G. Cloning of Interferon in Sea Bream (Sparus aurata): An Important Marker for Immune Response after Viral Infection. Aquac. II. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2011, 5, 41–48. [Google Scholar]
- Liu, S.; Hu, G.; Sun, C.; Zhang, S. Anti-viral activity of galectin-1 from flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2013, 34, 1463–1469. [Google Scholar] [CrossRef]
- Zhu, D.; Fu, P.; Huang, R.; Xiong, L.; Wang, Y.; He, L.; Liao, L.; Li, Y.; Zhu, Z.; Wang, Y. Molecular characterization, tissue distribution and functional analysis of galectin 1-like 2 in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019, 94, 455–463. [Google Scholar] [CrossRef]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Immunonutrition: Facilitating mucosal immune response in teleost intestine with amino acids through oxidant-antioxidant balance. Front. Immunol. 2023, 14, 1241615. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Liu, E.; Zhang, H.; Shi, H.; Qiu, G.; Lu, S.; Han, S.; Jiang, H.; Liu, H. Dietary protein optimization for growth and immune enhancement in juvenile hybrid sturgeon (Acipenser baerii× A. schrenckii): Balancing growth performance, serum biochemistry, and expression of immune-related genes. Biology 2024, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Simó-Mirabet, P.; Shin, H.S.; Rosell-Moll, E.; Naya-Catalá, F.; de las Heras, V.; Martos-Sitcha, J.A.; Karalazos, V.; Armero, E.; Arizcun, M.; et al. Selection for growth is associated in gilthead sea bream (Sparus aurata) with diet flexibility, changes in growth patterns and higher intestine plasticity. Aquaculture 2019, 507, 349–360. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquacult. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Refstie, S.; Sahlström, S.; Bråthen, E.; Baeverfjord, G.; Krogedal, P. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 2005, 246, 331–345. [Google Scholar] [CrossRef]
- Mutoloki, S.; Alexandersen, S.; Gravningen, K.; Evensen, Ø. Time-course study of injection site inflammatory reactions following intraperitoneal injection of Atlantic cod (Gadus morhua L.) with oil-adjuvanted vaccines. Fish Shellfish Immunol. 2008, 24, 386–393. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A sequential study of incomplete Freund’s adjuvant-induced peritonitis in Atlantic cod. Fish Shellfish Immunol. 2012, 32, 141–150. [Google Scholar] [CrossRef]
- Mutoloki, S.; Cooper, G.A.; Marjara, I.S.; Koop, B.F.; Evensen, Ø. High gene expression of inflammatory markers and IL-17A correlates with severity of injection site reactions of Atlantic salmon vaccinated with oil-adjuvanted vaccines. BMC Genom. 2010, 11, 336. [Google Scholar] [CrossRef]
- Noia, M.; Domínguez, B.; Leiro, J.; Blanco-Méndez, J.; Luzardo-Álvarez, A.; Lamas, J. Inflammatory responses and side effects generated by several adjuvant-containing vaccines in turbot. Fish Shellfish Immunol. 2014, 38, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, M.P.; Alcaraz-Perez, F.; Lopez-Munoz, A.; Roca, F.J.; Meseguer, J.; Cayuela, M.L.; Mulero, V. Evolution of lipopolysaccharide (LPS) recognition and signaling: Fish TLR4 does not recognize LPS and negatively regulates NF-κB activation. J. Immunol. 2009, 182, 1836–1845. [Google Scholar] [CrossRef] [PubMed]



| Gene | Name | Accession Number |
|---|---|---|
| Reference genes | ||
| actb1 | Actin beta 1 | XM_030406939.1 |
| eef1a | Eukaryotic elongation factor 1 alpha | AF184170.1 |
| Immunological response/inflammatory status | ||
| il8 | Interleukin-8 | JX976619.1 |
| il10 | Interleukin-10 | XM_030418889.1 |
| il15 | Interleukin-15 | JX976625.1 |
| il34 | Interleukin-34 | XM_030427145.1 |
| casp1 | Caspase 1 | XM_030438153.1 |
| lgals1 | Galectin-1 | KF862003.1 |
| lgals8 | Galectin-8 | KF862004.1 |
| ccr3 | c-c chemokine receptor type 3 | XM_030401704.1 |
| ccr9 | c-c chemokine receptor type 9 | XM_030397691.1 |
| mx1 | Interferon-induced GTP-binding protein Mx1 | FJ490556.1 |
| mx2 | Interferon-induced GTP-binding protein Mx2 | FJ490555.1 |
| ighm | Immunoglobulin m | XM_030408618.1 |
| Mucus production and goblet cell differentiation | ||
| muc2 | Mucin 2 | JQ277710.1 |
| muc3b | Mucin 3b | XM_030418634.1 |
| klf4 | Krueppel-like factor 4 | XM_030435936.1 |
| Metabolites | Experimental Groups | ||||||
|---|---|---|---|---|---|---|---|
| C-Sal | C-IFA | D-Sal | D-IFA | p-Diet | p-IFA | p-Interaction | |
| Glucose (mM) | 3.57 ± 0.16 | 4.01 ± 0.18 | 3.72 ± 0.09 | 3.72 ± 0.13 | 0.613 | 0.140 | 0.135 |
| Protein (mg ml−1) | 34.29 ± 1.15 | 33.58 ± 1.76 | 38.86 ± 1.03 | 33.74 ± 1.92 | 0.148 | 0.078 | 0.175 |
| Lactate (mM) | 1.44 ± 0.17 | 2.00 ± 0.12 A* | 1.52 ± 0.08 | 1.43 ± 0.11 B | 0.079 | 0.098 | 0.027 |
| Triglyceride (mM) | 2.55 ± 0.19 | 1.31 ± 0.41 B* | 2.67 ± 0.25 | 2.64 ± 0.36 A | 0.030 | 0.054 | 0.068 |
| Cholesterol (mM) | 6.02 ± 0.35 b | 7.37 ± 0.67 | 8.54 ± 0.42 a | 7.25 ± 0.55 * | 0.006 | 0.304 | 0.008 |
| Hormones and antibodies | |||||||
| Cortisol (ng ml−1) | 6.79 ± 0.78 a | 34.05 ± 6.41 A* | 42.0 ± 4.48 b | 17.27 ± 1.94 B* | ≥0.001 | 0.095 | <0.0001 |
| Immunoglobulin (mg ml−1) | 1.64 ± 0.50 | 2.03 ± 0.41 B | 3.03 ± 0.72 | 4.67 ± 0.65 A | 0.004 | 0.1077 | 0.3042 |
| Osmolality (mOsm/L−1) | 289.6 ± 1.70 | 291.6 ± 5.41 | 298.76 ± 3.13 | 279.0 ± 4.74 * | 0.687 | 0.051 | 0.019 |
| Genes Relative Expression (SYBR-ΔΔCT) | Experimental Groups | ||||||
|---|---|---|---|---|---|---|---|
| C-Sal | C-IFA | D-Sal | D-IFA | p-Diet | p-IFA | p-Interaction | |
| Intestine anterior | |||||||
| il8 | 0.88 ± 0.08 | 1.00 ± 0.13 | 1.26 ± 0.17 | 1.00 ± 0.19 | 0.192 | 0.663 | 0.204 |
| mx2 | 0.55 ± 0.12 | 0.55 ± 0.10 | 0.72 ± 0.09 | 0.77 ± 0.22 | 0.159 | 0.868 | 0.851 |
| lgals8 | 0.88 ± 0.06 | 0.84 ± 0.09 | 1.07 ± 0.09 | 0.88 ± 0.11 | 0.216 | 0.235 | 0.416 |
| ccr3 | 0.85 ± 0.05 | 1.10 ± 0.15 * | 0.82 ± 0.06 | 1.03 ± 0.13 * | 0.650 | 0.031 | 0.844 |
| muc2 | 0.81 ± 0.16 | 0.73 ± 0.09 | 1.19 ± 0.18 | 0.84 ± 0.09 | 0.077 | 0.123 | 0.311 |
| ighm | 1.00 ± 0.19 | 0.94 ± 0.21 | 1.28 ± 0.17 | 1.39 ± 0.17 | 0.194 | 0.609 | 1.000 |
| klf4 | 0.88 ± 0.16 | 0.83 ± 0.11 | 0.55 ± 0.07 | 0.73 ± 0.13 | 0.105 | 0.604 | 0.356 |
| Intestine posterior | |||||||
| mx2 | 0.73 ± 0.15 | 0.91 ± 0.33 | 0.50 ± 0.06 | 0.83 ± 0.23 | 0.526 | 0.299 | 0.769 |
| ccr9 | 1.02 ± 0.07 | 0.99 ± 0.09 | 1.10 ± 0.15 | 0.95 ± 0.11 | 0.882 | 0.419 | 0.575 |
| il8 | 1.05 ± 0.11 | 1.11 ± 0.15 | 1.11 ± 0.14 | 1.05 ± 0.14 | 0.987 | 0.974 | 0.627 |
| il15 | 1.10 ± 0.08 | 1.08 ± 0.12 | 1.12 ± 0.15 | 1.19 ± 0.12 | 0.587 | 0.809 | 0.730 |
| il34 | 0.93 ± 0.06 | 0.84 ± 0.07 | 0.86 ± 0.13 | 0.94 ± 0.07 | 0.880 | 0.940 | 0.290 |
| klf4 | 0.77 ± 0.13 | 0.89 ± 0.04 | 0.76 ± 0.15 | 0.74 ± 0.08 | 0.469 | 0.642 | 0.498 |
| lgals1 | 0.79 ± 0.14 | 1.10 ± 0.10 | 1.14 ± 0.19 | 1.10 ± 0.15 | 0.230 | 0.382 | 0.234 |
| lgals8 | 0.63 ± 0.14 | 1.09 ± 0.14 | 0.58 ± 0.20 | 0.70 ± 0.17 | 0.505 | 0.293 | 0.355 |
| Spleen | |||||||
| casp1 | 0.84 ± 0.14 | 0.84 ± 0.13 | 0.63 ± 0.08 | 0.68 ± 0.09 | 0.113 | 0.828 | 0.821 |
| Head kidney | |||||||
| il10 | 1.12 ± 0.14 | 1.25 ± 0.15 | 1.22 ± 0.12 | 1.63 ± 0.28 | 0.281 | 0.173 | 0.464 |
| Variable | LD1 | LD2 | LD3 |
|---|---|---|---|
| Anterior intestine | |||
| il8 | −0.53 | 0.01 | −0.01 |
| il10 | 0.21 | −0.61 | −0.66 |
| il15 | −1.40 | 0.36 | 0.69 |
| il6 | 0.58 | −0.07 | −0.11 |
| mx1 | −0.20 | −0.31 | 0.11 |
| mx2 | −0.25 | −0.28 | 0.67 |
| lgals1 | 0.10 | −0.03 | −0.98 |
| lgals8 | 0.11 | 0.19 | 0.05 |
| ccr3 | 0.52 | 0.17 | 0.31 |
| ccr9 | 0.58 | −0.20 | −0.47 |
| muc2 | −0.06 | −0.24 | −0.20 |
| ighm | −0.55 | 0.02 | 0.21 |
| klf4 | −0.18 | 0.18 | 0.44 |
| Posterior intestine | |||
| il8 | −0.79 | −0.24 | 0.20 |
| il15 | 0.17 | 0.26 | 0.11 |
| il34 | −0.72 | −0.42 | −0.04 |
| mx1 | 1.38 | 0.51 | −0.94 |
| mx2 | −0.85 | 0.73 | −0.24 |
| lgals1 | 0.30 | −0.17 | −0.32 |
| lgals8 | 0.59 | 0.13 | −0.05 |
| ccr3 | −0.84 | −1.01 | 0.24 |
| ccr9 | −0.21 | 0.61 | 0.29 |
| muc2 | −0.48 | 0.62 | 0.23 |
| muc3b | 0.18 | −0.94 | 0.67 |
| klf4 | 0.49 | 0.20 | −0.01 |
| Head kidney | |||
| il10 | 1.01 | −0.02 | −0.30 |
| il15 | 0.07 | 0.49 | 0.47 |
| Spleen | |||
| il8 | 1.74 | 0.34 | 1.16 |
| il10 | 0.42 | 0.26 | 0.24 |
| il34 | −0.76 | 0.58 | 0.21 |
| mx1 | 1.48 | −0.34 | −0.74 |
| mx2 | 0.60 | −0.42 | −0.37 |
| casp1 | −0.30 | −0.64 | −0.52 |
| lgals1 | −0.38 | −0.30 | 0.25 |
| ighm | −0.07 | 0.15 | 0.45 |
| Plasma | |||
| Ig | 0.07 | −0.33 | −0.63 |
| Morphometric Parameters and Tissue | Experimental Groups | |||
|---|---|---|---|---|
| C-SAL | C-IFA | D-SAL | D-IFA | |
| MSR-AI | 7.36 ± 0.78 | 7.66 ± 0.50 | 6.69 ± 1.21 | 8.20 ± 0.80 |
| MSR-PI | 5.53 ± 0.57 | 5.59 ± 0.29 | 6.08 ± 0.67 | 5.53 ± 0.29 |
| GCs-AI | 834.80 ± 43.14 | 719.98 ± 39.78 | 885.45 ± 80.40 | 802.17 ± 47.30 |
| GCs-PI | 1539.97 ± 92.01 | 1405.24 ± 140.15 | 1235.43 ± 117.68 | 1436.18 ± 66.13 |
| MMCs-S | 4.53 ± 0.52 | 5.68 ± 1.36 | 6.21 ± 1.16 | 4.03 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Viera, L.; Caderno, A.; Martinez, R.; Martinez-Rodríguez, G.; Oliva, M.; Perera, E.; Mancera, J.M.; Martos-Sitcha, J.A. The Ghrelin Analog GHRP-6, Delivered Through Aquafeeds, Modulates the Endocrine and Immune Responses of Sparus aurata Following IFA Treatment. Biology 2025, 14, 941. https://doi.org/10.3390/biology14080941
Rodríguez-Viera L, Caderno A, Martinez R, Martinez-Rodríguez G, Oliva M, Perera E, Mancera JM, Martos-Sitcha JA. The Ghrelin Analog GHRP-6, Delivered Through Aquafeeds, Modulates the Endocrine and Immune Responses of Sparus aurata Following IFA Treatment. Biology. 2025; 14(8):941. https://doi.org/10.3390/biology14080941
Chicago/Turabian StyleRodríguez-Viera, Leandro, Anyell Caderno, Rebeca Martinez, Gonzalo Martinez-Rodríguez, Milagrosa Oliva, Erick Perera, Juan Miguel Mancera, and Juan Antonio Martos-Sitcha. 2025. "The Ghrelin Analog GHRP-6, Delivered Through Aquafeeds, Modulates the Endocrine and Immune Responses of Sparus aurata Following IFA Treatment" Biology 14, no. 8: 941. https://doi.org/10.3390/biology14080941
APA StyleRodríguez-Viera, L., Caderno, A., Martinez, R., Martinez-Rodríguez, G., Oliva, M., Perera, E., Mancera, J. M., & Martos-Sitcha, J. A. (2025). The Ghrelin Analog GHRP-6, Delivered Through Aquafeeds, Modulates the Endocrine and Immune Responses of Sparus aurata Following IFA Treatment. Biology, 14(8), 941. https://doi.org/10.3390/biology14080941







