High Warming Restricts the Growth and Movement of a Larval Chinese Critically Endangered Relict Newt
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
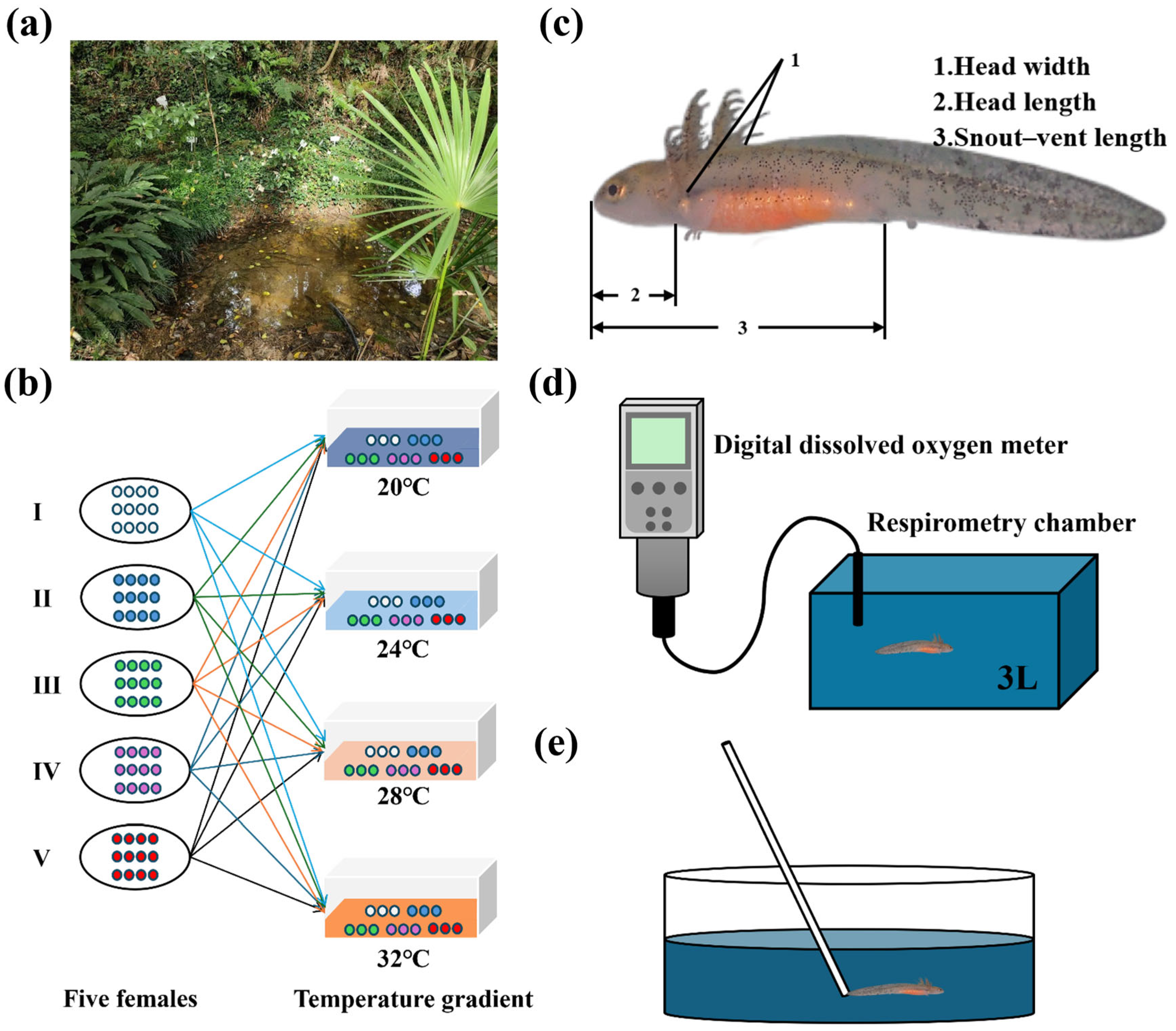
2.1. Study Species and Husbandry
2.2. Experiment Design
2.3. Morphometric Parameters
2.4. Basal Metabolic Rate
2.5. Locomotor Performance
2.6. Data Analysis
3. Results
3.1. Morphometric Parameters
3.2. Basal Metabolic Rate
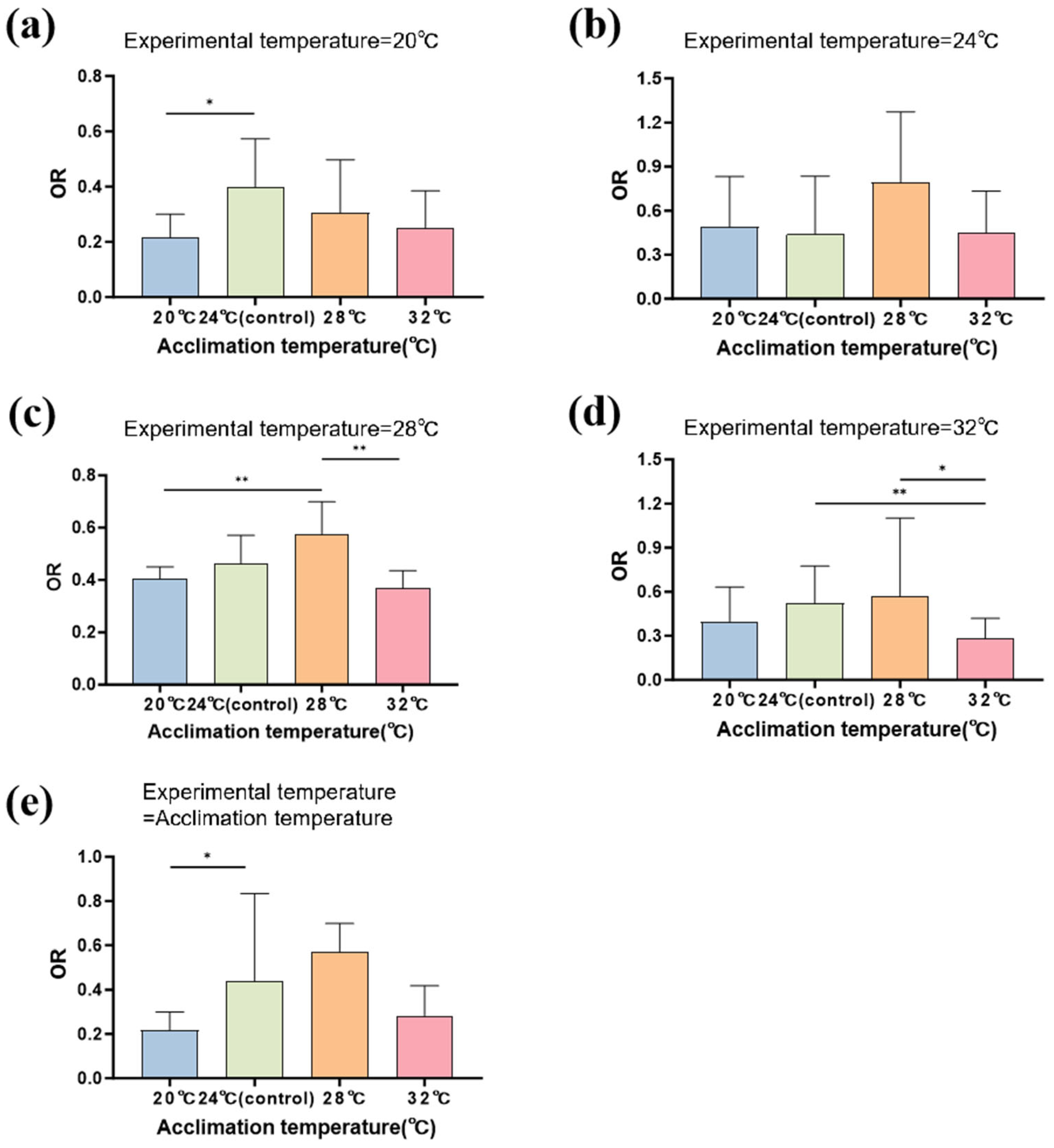
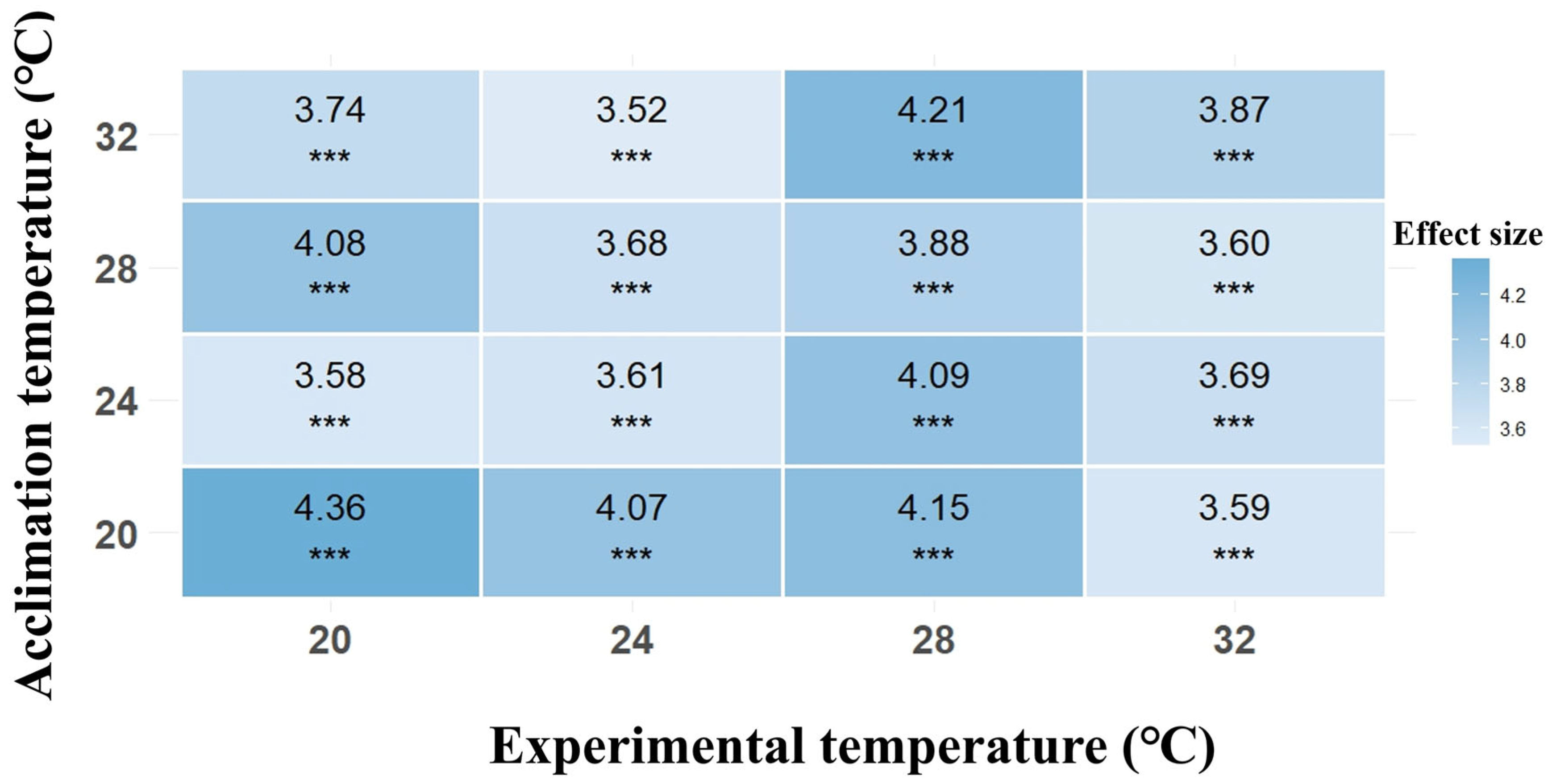
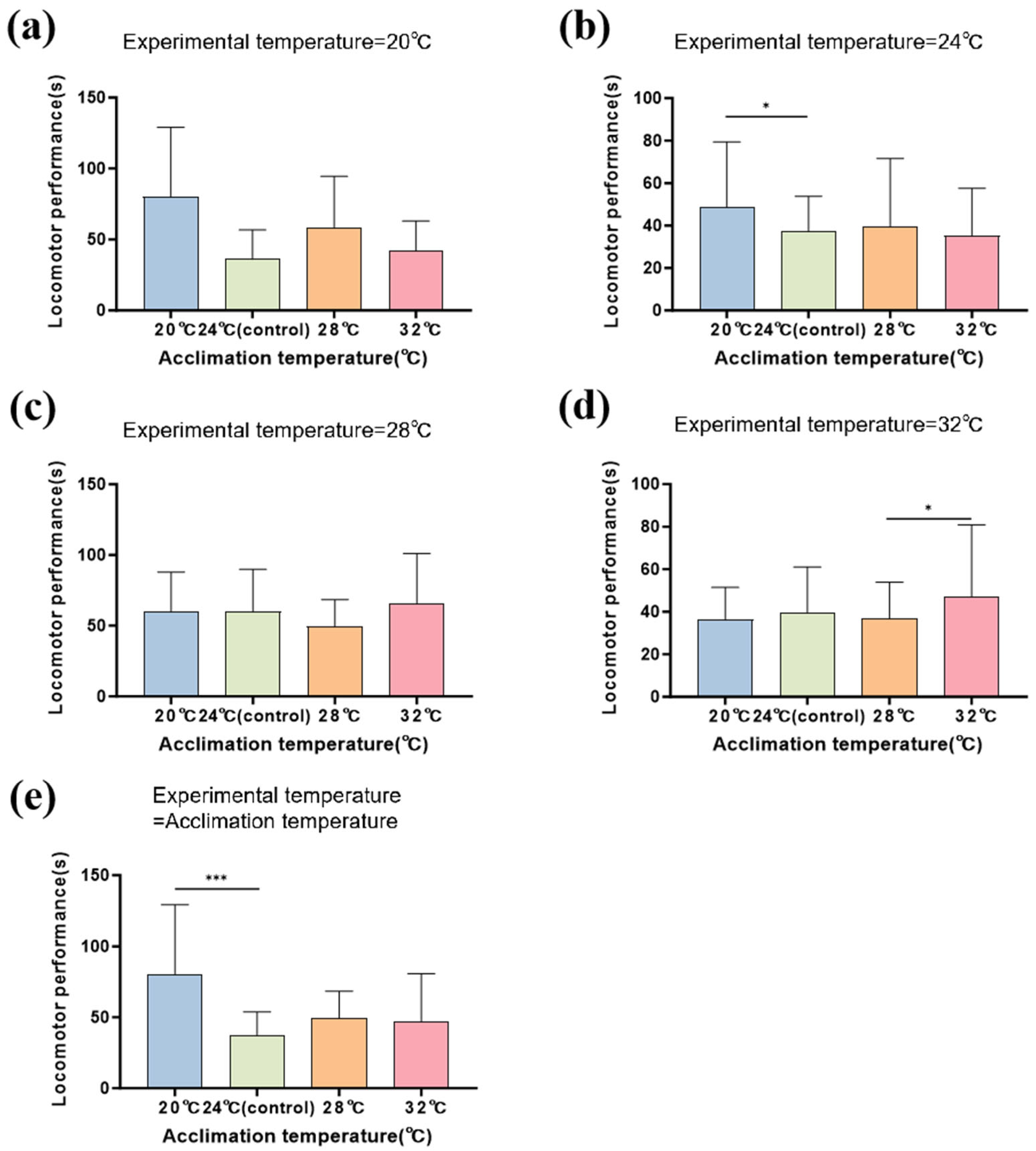
3.3. Locomotor Performance
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faghih-sabzevari, N.; Farashi, A. Identification of biodiversity hotspots for threatened mammal species under future climate. J. Nat. Conserv. 2024, 82, 126741. [Google Scholar] [CrossRef]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [CrossRef]
- Levis, N.A.; Pfennig, D.W. Phenotypic plasticity, canalization, and the origins of novelty: Evidence and mechanisms from amphibians. Semin. Cell Dev. Biol. 2019, 88, 80–90. [Google Scholar] [CrossRef]
- Hutchison, V.H.; Ferrance, M.R. Thermal tolerances of Rana pipiens acclimated to daily temperature cycles. Herpetologica 1970, 28, 1–8. [Google Scholar]
- Liu, L.; Li, C.; Li, N.; Xu, H.; Wang, Y. Effects of water temperature on tadpole phenotypic plasticity in bufo gargariz ans (Anura: bufonidae). Sichuan J. Zool. 2006, 25, 214–217. [Google Scholar]
- Newman, R.A. Ecological constraints on amphibian metamorphosis: Interactions of temperature and larval density with responses to changing food level. Oecologia 1998, 115, 9–16. [Google Scholar] [CrossRef]
- Álvarez, D.; Nicieza, A.G. Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct. Ecol. 2002, 16, 640–648. [Google Scholar] [CrossRef]
- Tejedo, M.; Marangoni, F.; Pertoldi, C.; Richter-Boix, A.; Laurila, A.; Orizaola, G.; Nicieza, A.G.; Álvarez, D.; Gomez-Mestre, I. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim. Res. 2010, 43, 31–39. [Google Scholar] [CrossRef]
- Sinai, N.; Glos, J.; Mohan, A.V.; Lyra, M.L.; Riepe, M.; Thöle, E.; Zummach, C.; Ruthsatz, K. Developmental plasticity in amphibian larvae across the world: Investigating the roles of temperature and latitude. J. Therm. Biol. 2022, 106, 103233. [Google Scholar] [CrossRef]
- Wilson, R.S.; Franklin, C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002, 17, 66–70. [Google Scholar] [CrossRef]
- Hubáček, J.; Gvoždík, L. Terrestrial amphibians respond to rapidly changing temperatures with individual plasticity of exploratory behaviour. J. Therm. Biol. 2024, 119, 103757. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Clifton, I.T.; Vazquez, T.K. Developmental plasticity of thermal ecology traits in reptiles: Trends, potential benefits, and research needs. J. Therm. Biol. 2019, 84, 74–82. [Google Scholar] [CrossRef]
- Helferich, J.N.; King, R.B.; Faust, L.J.; Baker, S.J.; Dreslik, M.J.; Otterbein, K.; Moore, J.A.; Wynn, D.; Bell, T.A.; Bailey, R.L.; et al. Projected climate change effects on individual growth rates and size in a threatened pitviper. Clim. Change Ecol. 2025, 9, 100091. [Google Scholar] [CrossRef]
- Hall, J.M.; Tiatragul, S.; Turner, M.K.; Warner, D.A. Within the optimal thermal range, temperature fluctuations with similar means have little effect on offspring phenotypes: A comparison of two approaches that simulate natural nest conditions. J. Therm. Biol. 2024, 125, 103949. [Google Scholar] [CrossRef]
- Ujszegi, J.; Bertalan, R.; Ujhegyi, N.; Verebélyi, V.; Nemesházi, E.; Mikó, Z.; Kásler, A.; Herczeg, D.; Szederkényi, M.; Vili, N.; et al. “Heat waves” experienced during larval life have species-specific consequences on life-history traits and sexual development in anuran amphibians. Sci. Total Environ. 2022, 835, 155297. [Google Scholar] [CrossRef]
- Tuckett, Q.M.; Hill, J.E.; Everett, K.; Goodman, C.; Wooley, E.S.; Durland Donahou, A.; Lapham, L.; Buckman, K.; Johnson, S.; Romagosa, C. Thermal tolerance for the tropical clawed frog, Xenopus tropicalis with comments on comparative methods for amphibian studies. J. Therm. Biol. 2024, 123, 103890. [Google Scholar] [CrossRef]
- Greenspan, S.E.; Roznik, E.A.; Edwards, L.; Duffy, R.; Berger, L.; Bower, D.S.; Pike, D.A.; Schwarzkopf, L.; Alford, R.A. Constant-temperature predictions underestimate growth of a fungal amphibian pathogen under individual host thermal profiles. J. Therm. Biol. 2023, 111, 103394. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Xia, L.; Zhang, W.; Qu, Y.; Li, H.; Yan, J.; Zhou, K.; Li, P. Life-history traits trade-off in gecko (Gekko japonicus) under the influence of climate warming and spirotetramat: Different adaptations to stressors in female and male. Sci. Total Environ. 2025, 958, 177978. [Google Scholar] [CrossRef]
- Peng, L.Q.; Tang, M.; Liao, J.H.; Liang, S.Y.; Gan, L.T.; Hua, K.J.; Chen, Y.; Li, H.; Chen, W.; Merila, J. Effects of temperature on growth and development of amphibian larvae across an altitudinal gradient in the tibetan plateau. Anim. Biol. 2020, 70, 239–250. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: San Diego, CA, USA, 1994; Volume 25, pp. 1–58. [Google Scholar]
- Ghosh, S.M.; Testa, N.D.; Shingleton, A.W. Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130174. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Xi, X.; Sun, S. Artificial warming increases size at metamorphosis in plateau frogs (Rana kukunoris) in the presence of predators. Aquat. Ecol. 2014, 48, 423–434. [Google Scholar] [CrossRef]
- Fryxell, D.C.; Hoover, A.N.; Alvarez, D.A.; Arnesen, F.J.; Benavente, J.N.; Moffett, E.R.; Kinnison, M.T.; Simon, K.S.; Palkovacs, E.P. Recent warming reduces the reproductive advantage of large size and contributes to evolutionary downsizing in nature. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200608. [Google Scholar] [CrossRef]
- Norin, T.; Metcalfe, N.B. Ecological and evolutionary consequences of metabolic rate plasticity in response to environmental change. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180180. [Google Scholar] [CrossRef]
- Rocha, P.L.; Branco, L.G.S. Seasonal changes in the cardiovascular, respiratory and metabolic responses to temperature and hypoxia in the bullfrog Rana catesbeiana. J. Exp. Biol. 1998, 201, 761–768. [Google Scholar] [CrossRef]
- Padilla, P.; Herrel, A.; Denoël, M. Invading new climates at what cost? ontogenetic differences in the thermal dependence of metabolic rate in an invasive amphibian. J. Therm. Biol. 2024, 121, 103836. [Google Scholar] [CrossRef]
- Cominassi, L.; Ressel, K.N.; Brooking, A.A.; Marbacher, P.; Ransdell-Green, E.C.; O’Brien, K.M. Metabolic rate increases with acclimation temperature and is associated with mitochondrial function in some tissues of threespine stickleback. J. Exp. Biol. 2022, 225, jeb244659. [Google Scholar] [CrossRef]
- Homyack, J.A.; Haas, C.A.; Hopkins, W.A. Influence of temperature and body mass on standard metabolic rate of eastern red-backed salamanders (Plethodon cinereus). J. Therm. Biol. 2010, 35, 143–146. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Drees, C.; Becker, L.I.; Hartmann, L.; Reese, J.; Reinhardt, S.; Robinson, T.; Sabatino, N.M.; Peck, M.A.; et al. Altered thyroid hormone levels affect the capacity for temperature-induced developmental plasticity in larvae of Rana temporaria and Xenopus laevis. J. Therm. Biol. 2020, 90, 102599. [Google Scholar] [CrossRef]
- Ontivero, R.E.; Quiroga, L.B.; Rodríguez, C.Y.; Bustos, D.; Sanabria, E.A. Effect of water stress on locomotor performance in Pleurodema nebulosum (anura: Leptodactylidae) a native frog from the argentina monte desert. J. Arid. Environ. 2024, 223, 105190. [Google Scholar] [CrossRef]
- Navas, C.A.; Gomes, F.R.; Carvalho, J.E. Thermal relationships and exercise physiology in anuran amphibians: Integration and evolutionary implications. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 344–362. [Google Scholar] [CrossRef]
- Brodie, E.D.; Ducey, P.K.; Lemos-Espinal, J. Antipredator behavior of the salamander bolitoglossa rufescens: Effects of temperature and location of stimulus. J. Herpetol. 1991, 25, 99–101. [Google Scholar] [CrossRef]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Araspin, L.; Wagener, C.; Padilla, P.; Herrel, A.; Measey, J. Shifts in the thermal dependence of locomotor performance across an altitudinal gradient in native populations of Xenopus laevis. Physiol. Biochem. Zool. 2023, 96, 272–281. [Google Scholar] [CrossRef]
- He, J.; Tang, X.; Pu, P.; Zhang, T.; Niu, Z.; Meng, F.; Xi, L.; Ma, M.; Wu, J.; Ma, M.; et al. Influence of high temperatures and heat wave on thermal biology, locomotor performance, and antioxidant system of high-altitude frog Nanorana pleskei endemic to qinghai-tibet plateau. Front. Ecol. Evol. 2021, 9, 763191. [Google Scholar] [CrossRef]
- Drakulić, S.; Feldhaar, H.; Lisičić, D.; Mioč, M.; Cizelj, I.; Seiler, M.; Spatz, T.; Rödel, M.-O. Local differences of thermal preferences in european common frog (Rana temporaria linnaeus, 1758) tadpoles. Zool. Anz. 2017, 268, 47–54. [Google Scholar] [CrossRef]
- Xie, F.; Gu, H. Echinotriton chinhaiensis, The IUCN Red List of Threatened Species. 2023, e.T59447A48311626. Available online: https://www.iucnredlist.org/species/59447/48311626 (accessed on 26 February 2025).
- Fei, L. Chinhai spiny newt and endangered status. Chin. J. Zool. 1992, 27, 39–41. [Google Scholar] [CrossRef]
- Li, T.; Zhu, X.; Wu, G.; Song, X.; Xu, A. Spawning ground microhabitat selection by the chinhai spiny newt (Echinotriton chinhaiensis). Biodivers. Sci. 2023, 31, 22293. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Feng, S. Research on Habitat Restoration and Population Expansion Techniques of Chinhai Spiny Newt; China Jiliang University: Hangzhou, China, 2023. [Google Scholar]
- China Jiliang University Approval of Ethics Committee of China Jiliang University for Ethical Review of Laboratory Animals; China Jiliang University: Hangzhou, China, 2023.
- Zhang, J. Study on Molecular Identification Techniques and Early Life History of Echinotriton Chinhaiensis; China Jiliang University: Hangzhou, China, 2021. [Google Scholar]
- Russell, W.M.S.; Burch, R. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 1. [Google Scholar]
- Zhu, W.; Zhao, T.; Zhao, C.; Li, C.; Xie, F.; Liu, J.; Jiang, J. How will warming affect the growth and body size of the largest extant amphibian? More than the temperature–size rule. Sci. Total Environ. 2023, 859 Pt 1, 160105. [Google Scholar] [CrossRef]
- Turriago, J.L.; Tejedo, M.; Hoyos, J.M.; Camacho, A.; Bernal, M.H. The time course of acclimation of critical thermal maxima is modulated by the magnitude of temperature change and thermal daily fluctuations. J. Therm. Biol. 2023, 114, 103545. [Google Scholar] [CrossRef]
- Sun, B.; Lu, H.; Cheng, K.; Liu, W.; Han, X.; Cui, L.; Li, X.; Li, S.; Hao, X.; Li, F.; et al. The semi-natural climate chambers across latitudes: A broadly applicable husbandry and experimental system for terrestrial ectotherms under climate change. Adv. Sci. 2025, 12, e2414185. [Google Scholar] [CrossRef]
- Zhu, X.; Qiu, X.; Li, W.; Feng, S.; Xu, A. Density mediates the predator-induced growth and metamorphic plasticity of chinhai spiny newt larvae. Animals 2024, 14, 1510. [Google Scholar] [CrossRef]
- Labus, N.; Cvijanovic, M.; Vukov, T. Sexual size and shape dimorphism in Salamandra salamandra (Amphibia, Caudata, Salamandridae) from the central balkans. Arch. Biol. Sci. 2013, 65, 969–975. [Google Scholar] [CrossRef]
- Vučić, T.; Ivanović, A.; Nikolić, S.; Jovanović, J.; Cvijanović, M. Reproductive characteristics of two triturus species (Amphibia: Caudata). Arch. Biol. Sci. 2020, 72, 321–328. [Google Scholar] [CrossRef]
- Chabot, D.; McKenzie, D.J.; Craig, J.F. Metabolic rate in fishes: Definitions, methods and significance for conservation physiology. J. Fish Biol. 2016, 88, 1–9. [Google Scholar] [CrossRef]
- Lin, X.; Xie, X.; Ou, Y.; Li, J.; Wu, S. The study of the oxygen consumption rate and asphyxiation point of juvenile Eleutheronema tetradactylum under different temperatures. J. Fish. Res. 2024, 46, 121–128. [Google Scholar] [CrossRef]
- Wijethunga, U.; Greenlees, M.; Shine, R. Moving south: Effects of water temperatures on the larval development of invasive cane toads (Rhinella marina) in cool-temperate australia. Ecol. Evol. 2016, 6, 6993–7003. [Google Scholar] [CrossRef]
- Moore, M.M.; Foster, E.G.; Amer, A.; Fraire, L.; Head, A.; Blanchette, A.; Hankison, S.J.; Gunderson, A.R.; Gangloff, E.J. Urban wall lizards are resilient to high levels of blood lead. Environ. Res. 2025, 264 Pt 1, 120248. [Google Scholar] [CrossRef]
- Zhu, X.; Qiu, X.; Chen, K.; Li, W.; Xu, A. The presence of predators constrains larval development process by influencing critical developmental windows in the endangered chinhai spiny newt. Ecol. Evol. 2024, 14, e11396. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2025. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 11 May 2025).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- McGillycuddy, M.; Popovic, G.; Bolker, B.M.; Warton, D.I. Parsimoniously fitting large multivariate random effects in glmmtmb. J. Stat. Softw. 2025, 112, 1–19. [Google Scholar] [CrossRef]
- Colomer, M.À.; Margalida, A.; Sanuy, I.; Llorente, G.A.; Sanuy, D.; Pujol-Buxó, E. A computational model approach to assess the effect of climate change on the growth and development of tadpoles. Ecol. Modell. 2021, 461, 109763. [Google Scholar] [CrossRef]
- Loisel, A.; Isla, A.; Daufresne, M. Variation of thermal plasticity in growth and reproduction patterns: Importance of ancestral and developmental temperatures. J. Therm. Biol. 2019, 84, 460–468. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2024. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 12 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Mastrandrea, M.D. Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. Proc. Natl. Acad. Sci. USA 2010, 107, 12581–12586. [Google Scholar] [CrossRef]
- Extinction Risk from Climate Change|Nature. Nature 2004, 427, 145–148. [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of amphibians to global warming. Nature 2025, 639, 954–961. [Google Scholar] [CrossRef]
- Guderley, H. Locomotor performance and muscle metabolic capacities: Impact of temperature and energetic status. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 371–382. [Google Scholar] [CrossRef]
- Porter, E.S.; Gamperl, A.K. Cardiorespiratory physiology and swimming capacity of atlantic salmon (Salmo salar) at cold temperatures. J. Exp. Biol. 2023, 226, jeb245990. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Hoff, K.V.S.; Hillyard, S.D. The effect of temperature on development and behaviour of relict leopard frog tadpoles. Conserv. Physiol. 2017, 5, cow075. [Google Scholar] [CrossRef][Green Version]
- Sandrelli, R.M.; Vadboncoeur, É.; George, S.; Ignatz, E.H.; Swanson, A.K.; Gamperl, A.K. Understanding how winter conditions in the north atlantic affect the physiology and behaviour of atlantic salmon in sea-cages. Aquaculture 2025, 596, 741777. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.R.; ALmohammadi, A.G.; Malik, S.; Khalphallah, A.; Soliman, M.M. Changes in clinicomorphometrical findings, lipid profiles, hepatorenal indices and oxidant/antioxidant status as thermoregulatory adaptive mechanisms in poikilothermic dabb lizard (Uromastyx aegyptia). Sci. Rep. 2023, 13, 3409. [Google Scholar] [CrossRef]
- Catenazzi, A. Ecological implications of metabolic compensation at low temperatures in salamanders. PeerJ 2016, 4, e2072. [Google Scholar] [CrossRef]
- Duan, J.; Feng, Y.; Huang, X.; Wang, K.; Geng, Y.; Deng, Y.; Ou, Y.; Chen, D.; Yang, S. A review: Factors affecting outbreaks of saprolegniosis on aquatic animals. Isr. J. Aquac.-Bamidgeh 2018, 70, 1493. [Google Scholar]
- Costa, S.; Lopes, I. Saprolegniosis in amphibians: An integrated overview of a fluffy killer disease. J. Fungi 2022, 8, 537. [Google Scholar] [CrossRef]
- Bundgaard, A.; Qvortrup, K.; Rasmussen, L.J.; Fago, A. Turtles maintain mitochondrial integrity but reduce mitochondrial respiratory capacity in the heart after cold acclimation and anoxia. J. Exp. Biol. 2019, 222, jeb200410. [Google Scholar] [CrossRef]
- Kristín, P.; Gvoždík, L. Influence of respirometry methods on intraspecific variation in standard metabolic rates in newts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 147–151. [Google Scholar] [CrossRef]
- Fangue, N.A.; Hofmeister, M.; Schulte, P.M. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, fundulus heteroclitus. J. Exp. Biol. 2006, 209, 2859–2872. [Google Scholar] [CrossRef]
- Hu, Q.; Tian, H.; Xiao, H. Effects of temperature and sex steroids on sex ratio, growth, and growth-related gene expression in the chinese giant salamander andrias davidianus. Aquat. Biol. 2019, 28, 79–90. [Google Scholar] [CrossRef]
- Bellakhal, M.; Neveu, A.; Fartouna-Bellakhal, M.; Missaoui, H.; Aleya, L. Effects of temperature, density and food quality on larval growth and metamorphosis in the north african green frog Pelophylax saharicus. J. Therm. Biol. 2014, 45, 81–86. [Google Scholar] [CrossRef]
- Laugen, A.T.; Laurila, A.; Merilä, J. Latitudinal and temperature-dependent variation in embryonic development and growth in rana temporaria. Oecologia 2003, 135, 548–554. [Google Scholar] [CrossRef]
- Lau, E.T.C.; Leung, K.M.Y.; Karraker, N.E. Native amphibian larvae exhibit higher upper thermal limits but lower performance than their introduced predator Gambusia affinis. J. Therm. Biol. 2019, 81, 154–161. [Google Scholar] [CrossRef]
- Yang, L. Research on the Physiological and Ecological Responses of the Chinese Alligator to Temperature Chan; Anhui Normal University: Wuhu, China, 2024. [Google Scholar]
- Fang, L.; Fu, C.; Zou, W.; Ren, D.; Xu, J.; Zhao, L.; Fang, S. A preliminary study on behavioral adaptation to the thermal environment of the chinese alligator. J. Zhejiang For. Sci. Technol. 2022, 42, 16–24. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are mountain passes higher in the tropics? janzen’s hypothesis revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef]
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. Biol. Sci. 2000, 267, 739–745. [Google Scholar] [CrossRef]
- Dillon, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Zhao, J.; Lin, Z.; Fan, X. Effect of temperature on the oxygen consumption rate of tadpoles of the black-spotted pond frog (Pelophylax nigromaculatus) at different developmental stages. J. Aquac. 2023, 44, 44–49. [Google Scholar] [CrossRef]
- Wilson, R.S.; James, R.S.; Johnston, I.A. Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog xenopus laevis. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2000, 170, 117–124. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, H.; Wang, Y.; Zhang, L.; Ding, G. Combined effects of thermal environment and gene regulation on metabolic enzyme activities of major metabolic tissues in a winter-breeding amphibian. J. Therm. Biol. 2024, 125, 104000. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef]
- Araspin, L.; Martinez, A.S.; Wagener, C.; Courant, J.; Louppe, V.; Padilla, P.; Measey, J.; Herrel, A. Rapid shifts in the temperature dependence of locomotor performance in an invasive frog, Xenopus laevis, implications for conservation. Integr. Comp. Biol. 2020, 60, 456–466. [Google Scholar] [CrossRef]
- Kern, P.; Cramp, R.L.; Franklin, C.E. Physiological responses of ectotherms to daily temperature variation. J. Exp. Biol. 2015, 218, 3068–3076. [Google Scholar] [CrossRef]
- Lu, H.; Geng, J.; Xu, W.; Ping, J.; Zhang, Y. Physiological response and changes in swimming performance after thermal acclimation in juvenile chinese fire-belly newts, Cynops orientalis. Acta Ecol. Sin. 2017, 37, 1603–1610. [Google Scholar] [CrossRef][Green Version]
- Peng, M.; Huang, Y.; Xia, J. Recent progress on the beneficial acclimation hypothesis. Chin. J. Ecol. 2020, 39, 678–683. [Google Scholar] [CrossRef]
- Sun, B.; Ma, L.; Wang, Y.; Mi, C.; Buckley, L.B.; Levy, O.; Lu, H.; Du, W. Latitudinal embryonic thermal tolerance and plasticity shape the vulnerability of oviparous species to climate change. Ecol. Monogr. 2021, 91, e01468. [Google Scholar] [CrossRef]
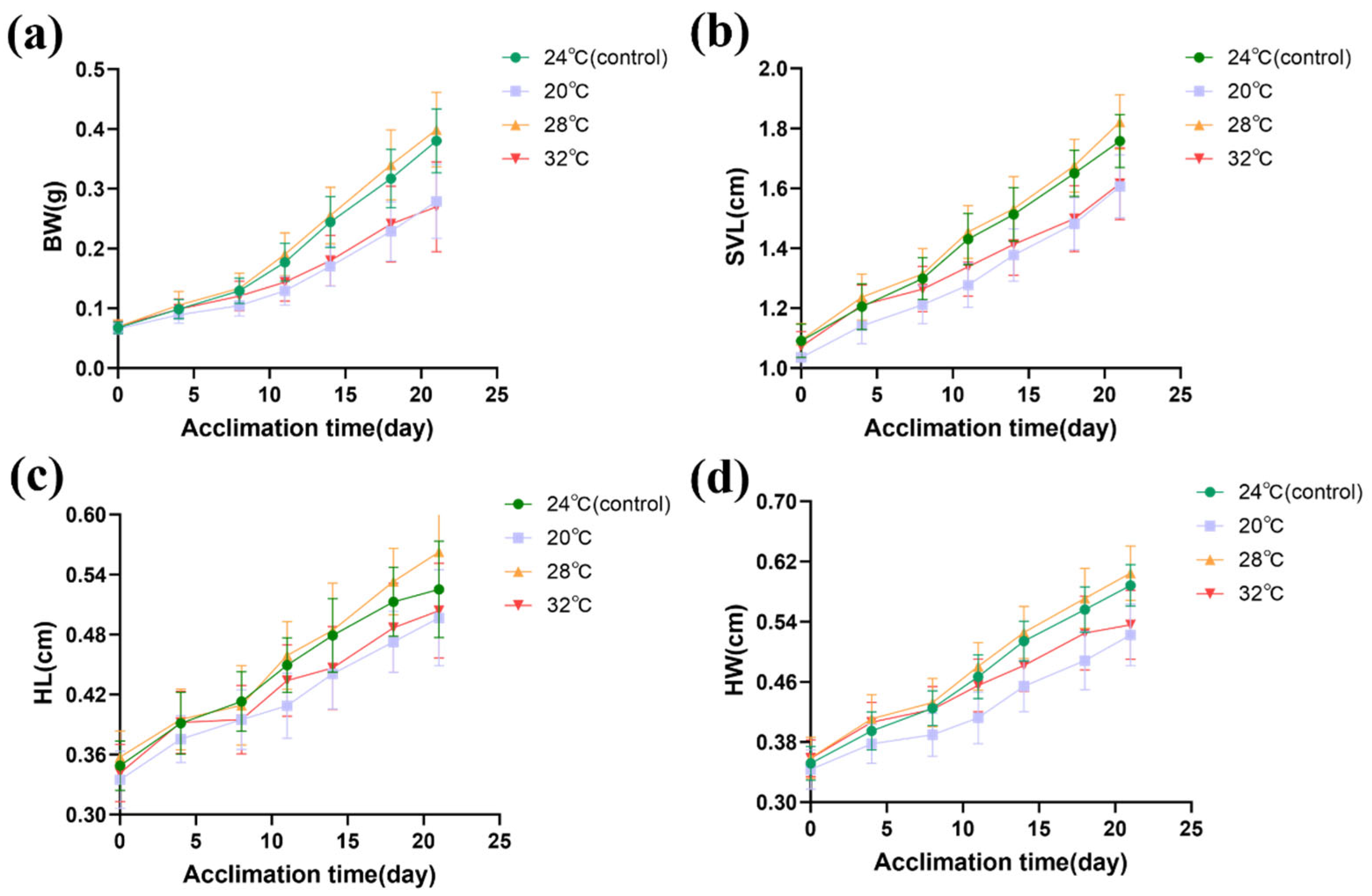
| Morphometric Parameters | Factor | Num df | Den df | F Value | p |
|---|---|---|---|---|---|
| Body weight | Acclimation temperature | 3 | 18.510 | 69.642 | <0.001 |
| (g) | Acclimation time | 6 | 1435.250 | 1526.547 | <0.001 |
| Acclimation temperature × Acclimation time | 18 | 1434.320 | 14.345 | <0.001 | |
| Snout–vent length | Acclimation temperature | 3 | 23.800 | 104.452 | <0.001 |
| (cm) | Acclimation time | 6 | 1435.000 | 1447.040 | <0.001 |
| Acclimation temperature × Acclimation time | 18 | 1434.500 | 10.778 | <0.001 | |
| Head length | Acclimation temperature | 3 | 15.670 | 43.573 | <0.001 |
| (cm) | Acclimation time | 6 | 1432.560 | 647.221 | <0.001 |
| Acclimation temperature × Acclimation time | 18 | 1430.880 | 4.868 | <0.001 | |
| Head width | Acclimation temperature | 3 | 12.890 | 88.056 | <0.001 |
| (cm) | Acclimation time | 6 | 1427.210 | 1105.180 | <0.001 |
| Acclimation temperature × Acclimation time | 18 | 1425.790 | 11.370 | <0.001 |
| Basal Metabolic Rate | X2 | df | p |
|---|---|---|---|
| Acclimation temperature | 4.955 | 3 | 0.175 |
| Experimental temperature | 8.650 | 3 | 0.034 |
| Acclimation temperature × Experimental temperature | 5.919 | 9 | 0.748 |
| Locomotor Performance | Chisq | df | p |
|---|---|---|---|
| Acclimation temperature | 42.312 | 3 | <0.001 |
| Experimental temperature | 31.683 | 3 | <0.001 |
| Acclimation temperature × Experimental temperature | 44.660 | 9 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Feng, S.; Zhao, S.; An, D.; Mao, J.; Song, X.; Zhang, W.; Xu, A. High Warming Restricts the Growth and Movement of a Larval Chinese Critically Endangered Relict Newt. Biology 2025, 14, 942. https://doi.org/10.3390/biology14080942
Li W, Feng S, Zhao S, An D, Mao J, Song X, Zhang W, Xu A. High Warming Restricts the Growth and Movement of a Larval Chinese Critically Endangered Relict Newt. Biology. 2025; 14(8):942. https://doi.org/10.3390/biology14080942
Chicago/Turabian StyleLi, Wei, Shiyan Feng, Shanshan Zhao, Di An, Jindi Mao, Xiao Song, Wei Zhang, and Aichun Xu. 2025. "High Warming Restricts the Growth and Movement of a Larval Chinese Critically Endangered Relict Newt" Biology 14, no. 8: 942. https://doi.org/10.3390/biology14080942
APA StyleLi, W., Feng, S., Zhao, S., An, D., Mao, J., Song, X., Zhang, W., & Xu, A. (2025). High Warming Restricts the Growth and Movement of a Larval Chinese Critically Endangered Relict Newt. Biology, 14(8), 942. https://doi.org/10.3390/biology14080942








