Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management
Simple Summary
Abstract
1. Introduction
2. Microbiome Diversity in Lepidoptera
3. Endosymbionts
4. Gut Microbiome Dynamics
5. Key Factors Influencing the Composition and Function of the Insect Gut Microbiome
5.1. Diet and Environmental Influences on the Gut Microbiome in Lepidoptera
5.2. Influence of Host Phylogeny
5.3. Impact of Developmental Transitions on Microbiome Composition
5.4. Captivity and Rearing Practices: Unveiling Their Impact on Microbiome Dynamics
5.5. Unraveling Symbiotic Alliances: Lepidoptera and Their Gut Microbiota
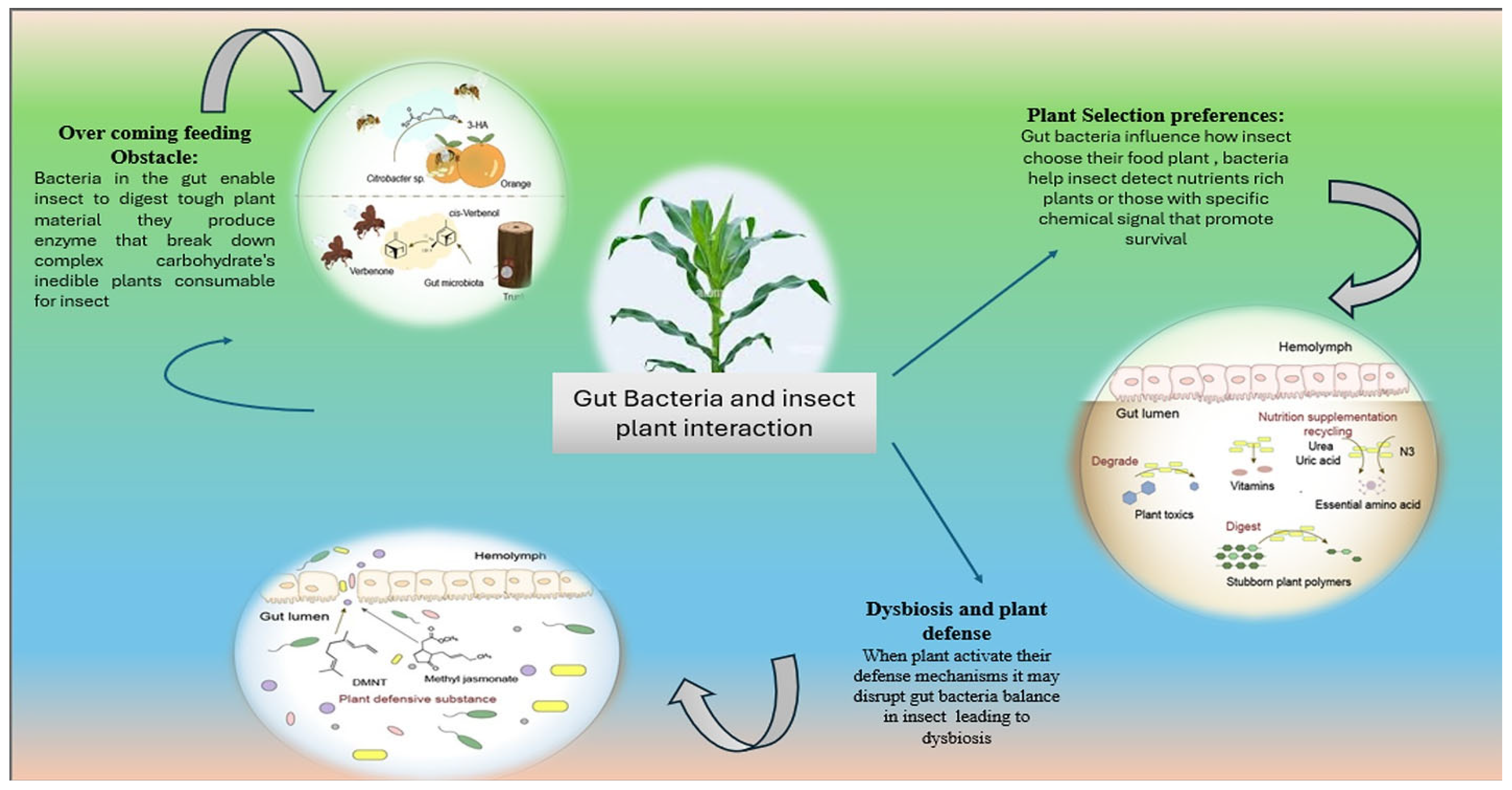
6. Gut Bacteria as Key Modulators of Insect–Plant Interactions
7. Unraveling the Role of Gut Microbiota in Shaping Insect Host Plant Selection
8. Harnessing Insect Gut Microbes: How Symbiotic Bacteria Break Down Plant Polymers and Supply Essential Nutrients:
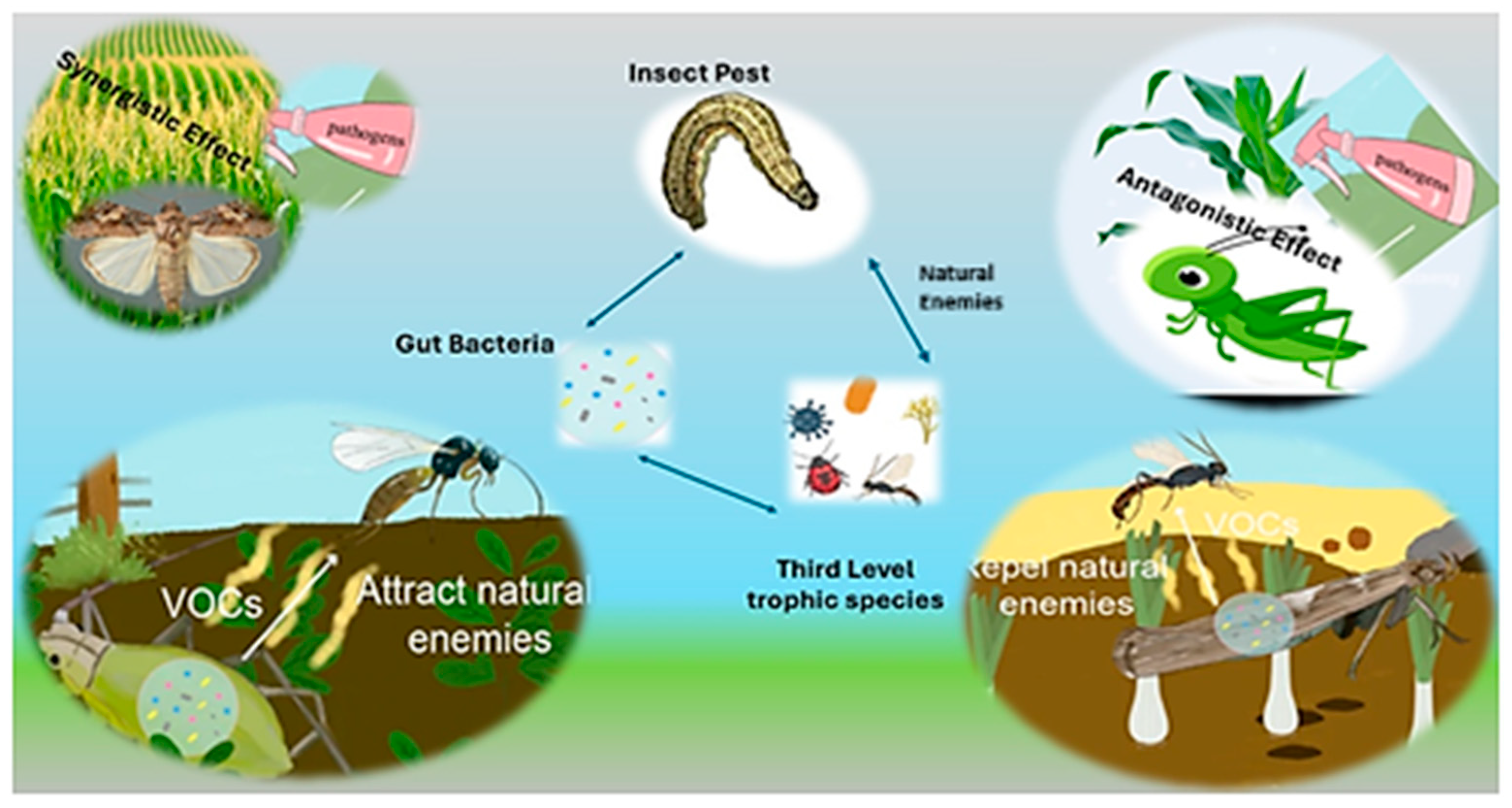
9. How Gut Bacteria Modulate Third-Trophic-Level Dynamics to Influence Insect–Plant Relationships?
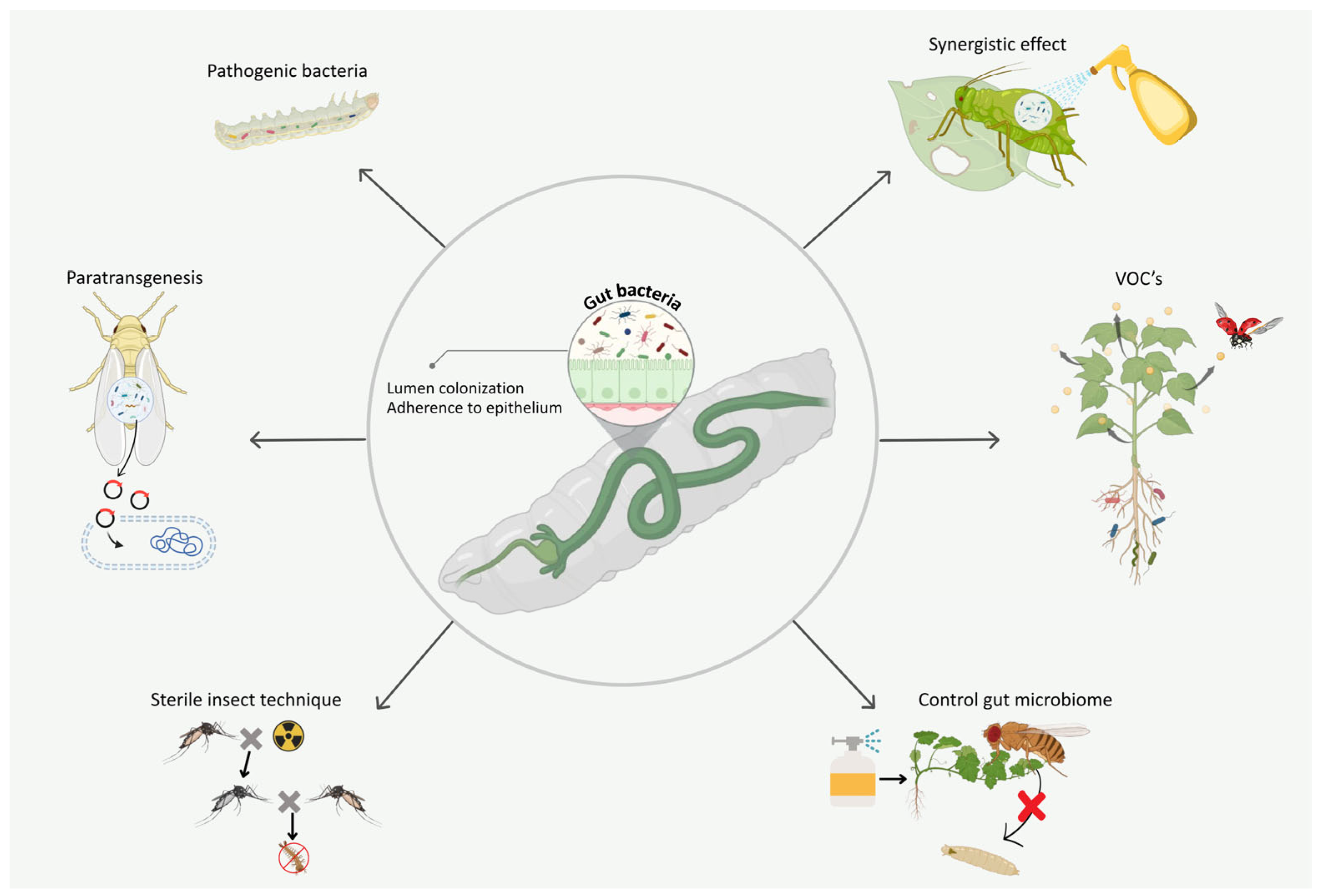
10. Gut Bacteria Modulate the Efficacy of Microbial Insecticides
11. Gut Bacteria Produce VOCs to Attract or Repel Natural Enemies of the Host
12. The Dual Insecticidal Power of Gut Bacteria: Direct Impacts and Untapped Potential
13. Fall Armyworm
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavichiolli de Oliveira, N.; Cônsoli, F.L. Beyond Host Regulation: Changes in Gut Microbiome of Permissive and Non-Permissive Hosts Following Parasitization by the Wasp Cotesia Flavipes. FEMS Microbiol. Ecol. 2020, 96, fiz206. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover Effects of Larval Exposure to Different Environmental Bacteria Drive Adult Trait Variation in a Mosquito Vector. Sci. Adv. 2017, 3, e1700585. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Liu, C.; Shi, Z.; Pang, L.; Chen, C.; Chen, Y.; Pan, R.; Zhou, W.; Chen, X.; et al. HGT Is Widespread in Insects and Contributes to Male Courtship in Lepidopterans. Cell 2022, 185, 2975–2987.e10. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Ray, S.; Shikano, I.; Peiffer, M.; Jones, A.G.; Luthe, D.S.; Hoover, K.; Felton, G.W. Plant Defenses Interact with Insect Enteric Bacteria by Initiating a Leaky Gut Syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 15991–15996. [Google Scholar] [CrossRef] [PubMed]
- Ravenscraft, A.; Berry, M.; Hammer, T.; Peay, K.; Boggs, C. Structure and Function of the Bacterial and Fungal Gut Microbiota of Neotropical Butterflies. Ecol. Monogr. 2019, 89, e01346. [Google Scholar] [CrossRef]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L.; Shao, Y. Gut Bacterial and Fungal Communities of the Domesticated Silkworm (Bombyx mori) and Wild Mulberry-Feeding Relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Marín-Miret, J.; González-Serrano, F.; Rosas, T.; Baixeras, J.; Latorre, A.; Pérez-Cobas, A.E.; Moya, A. Temporal Variations Shape the Gut Microbiome Ecology of the Moth Brithys Crini. Environ. Microbiol. 2022, 24, 3939–3953. [Google Scholar] [CrossRef] [PubMed]
- Windfelder, A.G.; Müller, F.H.H.; Mc Larney, B.; Hentschel, M.; Böhringer, A.C.; Von Bredow, C.-R.; Leinberger, F.H.; Kampschulte, M.; Maier, L.; von Bredow, Y.M.; et al. High-Throughput Screening of Caterpillars as a Platform to Study Host–Microbe Interactions and Enteric Immunity. Nat. Commun. 2022, 13, 7216. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.; Dara, S.K. Microbial Biopesticides for Invertebrate Pests and Their Markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bulla Jr, L.A.; Rhodes, R.A.; St. Julian, G. Bacteria as Insect Pathogens. Annu. Rev. Microbiol. 1975, 29, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Cory, J.S. Ecology and Evolution of Pathogens in Natural Populations of Lepidoptera. Evol. Appl. 2016, 9, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Enzyme Producing Insect Gut Microbes: An Unexplored Biotechnological Aspect. Crit. Rev. Biotechnol. 2022, 42, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.S.; Laviad-Shitrit, S.; Lalzar, M.; Halpern, M.; Inbar, M. Cascading Effects on Bacterial Communities: Cattle Grazing Causes a Shift in the Microbiome of a Herbivorous Caterpillar. ISME J. 2018, 12, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, L.M.; Servin-Garciduenas, L.E.; Martinez-Romero, E. Arthropod–Spiroplasma Relationship in the Genomic Era. FEMS Microbiol. Ecol. 2015, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, N.; Martens, R.; Tebbe, C.C. Origin and Diversity of Metabolically Active Gut Bacteria from Laboratory-Bred Larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl. Environ. Microbiol. 2008, 74, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.S.d.; Barcelos, R.M.; Mendes, T.A.d.O.; Mafra, C. Molecular Evidence of Rickettsia Felis in Phereoeca sp. Rev. Bras. Parasitol. Vet. 2021, 30, e015620. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the Bacterial Community of the Gypsy Moth Larval Midgut by Using Culturing and Culture-Independent Methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Mason, C.J.; Peiffer, M.; Zhang, D.; Shao, Y.; Felton, G.W. Enterococcal Symbionts of Caterpillars Facilitate the Utilization of a Suboptimal Diet. J. Insect Physiol. 2022, 138, 104369. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, C.; Liang, X.; Lu, X.; Gao, Q.; Alonso-Pernas, P.; Teh, B.-S.; Novoselov, A.L.; Boland, W.; Shao, Y. Draft Genome Sequence of Enterococcus Mundtii SL 16, an Indigenous Gut Bacterium of the Polyphagous Pest Spodoptera littoralis. Front. Microbiol. 2016, 7, 1676. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The Incidence of Bacterial Endosymbionts in Terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150249. [Google Scholar] [CrossRef] [PubMed]
- Duplouy, A.; Hornett, E.A. Uncovering the Hidden Players in Lepidoptera Biology: The Heritable Microbial Endosymbionts. PeerJ 2018, 6, e4629. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; St. Clair, A.; Peiffer, M.; Gomez, E.; Jones, A.G.; Felton, G.W.; Hoover, K. Diet Influences Proliferation and Stability of Gut Bacterial Populations in Herbivorous Lepidopteran Larvae. PLoS ONE 2020, 15, e0229848. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, C.; Liu, T. Metagenomic Profiling of Gut Microbiota in Fall Armyworm (Spodoptera frugiperda) Larvae Fed on Different Host Plants. BMC Microbiol. 2024, 24, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, H.; He, J.; Liang, X.; Zhang, N.; Shao, Y.; Zhang, F.; Lu, X. The Gut Commensal Bacterium Enterococcus faecalis LX10 Contributes to Defending against Nosema Bombycis Infection in Bombyx Mori. Pest Manag. Sci. 2022, 78, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.F.; Kielak, A.M.; Hannula, S.E.; Heinen, R.; Jongen, R.; Keesmaat, I.; De Long, J.R.; Bezemer, T.M. Microbiomes of a Specialist Caterpillar Are Consistent across Different Habitats but Also Resemble the Local Soil Microbial Communities. Anim. Microbiome 2020, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghanavi, H.R.; Twort, V.G.; Duplouy, A. Exploring Bycatch Diversity of Organisms in Whole Genome Sequencing of Erebidae moths (Lepidoptera). Sci. Rep. 2021, 11, 24499. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Vuong, T.; Guo, J.; Shi, J.; Shi, Z.; Zhang, G.; Zhang, J. Diel Pattern in the Structure and Function of the Gut Microbial Community in Lymantria Dispar Asiatica (Lepidoptera: Lymantriidae) Larvae. Arch. Insect Biochem. Physiol. 2020, 104, e21691. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hong, Y.; Mai, Z.; Zhu, Q.; Guo, L. Internal and External Microbial Community of the Thitarodes Moth, the Host of Ophiocordyceps Sinensis. Microorganisms 2019, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; Teh, B.S.; Murali, A.; Schmidt-Heck, W.; Schlenker, Y.; Vogel, H.; Boland, W. Transcriptomics Reveal the Survival Strategies of Enterococcus mundtii in the Gut of Spodoptera Littoralis. J. Chem. Ecol. 2021, 47, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.M.F.F.; Wallau, G.L.; Loreto, E.L.S. Multiple Long-Range Host Shifts of Major Wolbachia Supergroups Infecting Arthropods. Sci. Rep. 2022, 12, 8131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, D.; Zhao, S.; Wu, K. Susceptibilities of the Invasive Fall Armyworm (Spodoptera frugiperda) to the Insecticidal Proteins of Bt Maize in China. Toxins 2022, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in Butterflies and Moths: Geographic Structure in Infection Frequency. Front. Zool. 2015, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Breinholt, J.W.; Kawahara, A.Y. Evidence for Common Horizontal Transmission of Wolbachia among Butterflies and Moths. BMC Evol. Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-O.; Noda, H.; Blackwell, M. Insect Symbiosis: Derivation of Yeast-like Endosymbionts within an Entomopathogenic Filamentous Lineage. Mol. Biol. Evol. 2001, 18, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Longdon, B.; Jiggins, F.M. Vertically Transmitted Viral Endosymbionts of Insects: Do Sigma Viruses Walk Alone? Proc. R. Soc. B Biol. Sci. 2012, 279, 3889–3898. [Google Scholar] [CrossRef] [PubMed]
- Longdon, B.; Day, J.P.; Schulz, N.; Leftwich, P.T.; De Jong, M.A.; Breuker, C.J.; Gibbs, M.; Obbard, D.J.; Wilfert, L.; Smith, S.C.L.; et al. Vertically Transmitted Rhabdoviruses Are Found across Three Insect Families and Have Dynamic Interactions with Their Hosts. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162381. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Hoshino, M.; Nakai, M.; Kunimi, Y. Novel RNA Sequences Associated with Late Male Killing in Homona magnanima. Proc. R. Soc. B Biol. Sci. 2008, 275, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, Y.; Graham, R.I.; Wilson, K.; Wu, K. Densovirus Is a Mutualistic Symbiont of a Global Crop Pest (Helicoverpa armigera) and Protects against a Baculovirus and Bt Biopesticide. PLoS Pathog. 2014, 10, e1004490. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Prem Anand, A.A.; Vennison, S.J.; Sankar, S.G.; Gilwax Prabhu, D.I.; Vasan, P.T.; Raghuraman, T.; Jerome Geoffrey, C.; Vendan, S.E. Isolation and Characterization of Bacteria from the Gut of Bombyx Mori That Degrade Cellulose, Xylan, Pectin and Starch and Their Impact on Digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar]
- Takatsuka, J.; Kunimi, Y. Intestinal Bacteria Affect Growth of Bacillus Thuringiensis in Larvae of the Oriental Tea Tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae). J. Invertebr. Pathol. 2000, 76, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars Lack a Resident Gut Microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, C.; Baixeras, J.; Latorre, A.; Porcar, M. The Generalist inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp. Front. Microbiol. 2016, 7, 1005. [Google Scholar] [CrossRef] [PubMed]
- Hannula, S.E.; Zhu, F.; Heinen, R.; Bezemer, T.M. Foliar-Feeding Insects Acquire Microbiomes from the Soil Rather than the Host Plant. Nat. Commun. 2019, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Allonsius, C.N.; Van Beeck, W.; De Boeck, I.; Wittouck, S.; Lebeer, S. The Microbiome of the Invertebrate Model Host Galleria Mellonella Is Dominated by Enterococcus. Anim. Microbiome 2019, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gohl, P.; LeMoine, C.M.R.; Cassone, B.J. Diet and Ontogeny Drastically Alter the Larval Microbiome of the Invertebrate Model Galleria Mellonella. Can. J. Microbiol. 2022, 68, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.F.; Omoto, C.; Cônsoli, F.L. Gut Bacteria of Field-Collected Larvae of Spodoptera Frugiperda Undergo Selection and Are More Diverse and Active in Metabolizing Multiple Insecticides than Laboratory-Selected Resistant Strains. J. Pest Sci. 2020, 93, 833–851. [Google Scholar] [CrossRef]
- González-Serrano, F.; Pérez-Cobas, A.E.; Rosas, T.; Baixeras, J.; Latorre, A.; Moya, A. The Gut Microbiota Composition of the Moth Brithys Crini Reflects Insect Metamorphosis. Microb. Ecol. 2020, 79, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Higuita Palacio, M.F.; Montoya, O.I.; Saldamando, C.I.; García-Bonilla, E.; Junca, H.; Cadavid-Restrepo, G.E.; Moreno-Herrera, C.X. Dry and Rainy Seasons Significantly Alter the Gut Microbiome Composition and Reveal a Key Enterococcus sp.(Lactobacillales: Enterococcaceae) Core Component in Spodoptera frugiperda (Lepidoptera: Noctuidae) Corn Strain from Northwestern Colombia. J. Insect Sci. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Lowe-Power, T.M.; Rubert-Nason, K.F.; Lindroth, R.L.; Raffa, K.F. Interactions between Bacteria and Aspen Defense Chemicals at the Phyllosphere–Herbivore Interface. J. Chem. Ecol. 2016, 42, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mereghetti, V.; Chouaia, B.; Limonta, L.; Locatelli, D.P.; Montagna, M. Evidence for a Conserved Microbiota across the Different Developmental Stages of Plodia interpunctella. Insect Sci. 2019, 26, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.; Mereghetti, V.; Gargari, G.; Guglielmetti, S.; Faoro, F.; Lozzia, G.; Locatelli, D.; Limonta, L. Evidence of a Bacterial Core in the Stored Products Pest Plodia Interpunctella: The Influence of Different Diets. Environ. Microbiol. 2016, 18, 4961–4973. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Serteyn, L.; Sare, A.R.; Massart, S.; Delvigne, F.; Francis, F. Co-Diet Supplementation of Low Density Polyethylene and Honeybee Wax Did Not Influence the Core Gut Bacteria and Associated Enzymes of Galleria mellonella Larvae (Lepidoptera: Pyralidae). Int. Microbiol. 2023, 26, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Tomás, A.A.; Sittenfeld, A.; Uribe-Lorío, L.; Chavarría, F.; Mora, M.; Janzen, D.H.; Goodman, R.M.; Simon, H.M. Comparison of Midgut Bacterial Diversity in Tropical caterpillars (Lepidoptera: Saturniidae) Fed on Different Diets. Environ. Entomol. 2011, 40, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Dickerson, J.C.; McMillan, W.O.; Fierer, N. Heliconius Butterflies Host Characteristic and Phylogenetically Structured Adult-Stage Microbiomes. Appl. Environ. Microbiol. 2020, 86, e02007-20. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. Insect-Microbe Mutualism without Vertical Transmission: A Stinkbug Acquires a Beneficial Gut Symbiont from the Environment Every Generation. Appl. Environ. Microbiol. 2007, 73, 4308–4316. [Google Scholar] [CrossRef] [PubMed]
- Martinson, V.G.; Carpinteyro-Ponce, J.; Moran, N.A.; Markow, T.A. A Distinctive and Host-Restricted Gut Microbiota in Populations of a Cactophilic Drosophila Species. Appl. Environ. Microbiol. 2017, 83, e01551-17. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, H.C.; Barelli, C. Conserve the Germs: The Gut Microbiota and Adaptive Potential. Conserv. Genet. 2019, 20, 19–27. [Google Scholar] [CrossRef]
- Koga, R.; Moriyama, M.; Onodera-Tanifuji, N.; Ishii, Y.; Takai, H.; Mizutani, M.; Oguchi, K.; Okura, R.; Suzuki, S.; Gotoh, Y.; et al. Single Mutation Makes Escherichia Coli an Insect Mutualist. Nat. Microbiol. 2022, 7, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Freitak, D.; Schmidtberg, H.; Dickel, F.; Lochnit, G.; Vogel, H.; Vilcinskas, A. The Maternal Transfer of Bacteria Can Mediate Trans-Generational Immune Priming in Insects. Virulence 2014, 5, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cui, Y.; Chu, X.; Li, G.; Yang, M.; Wang, R.; Liang, G.; Wu, S.; Tigabu, M.; Zhang, F.; et al. Gut Bacterial Communities of Lymantria Xylina and Their Associations with Host Development and Diet. Microorganisms 2021, 9, 1860. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.-S.; Apel, J.; Shao, Y.; Boland, W. Colonization of the Intestinal Tract of the Polyphagous Pest Spodoptera Littoralis with the GFP-Tagged Indigenous Gut Bacterium Enterococcus mundtii. Front. Microbiol. 2016, 7, 928. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Bowers, M.D. Gut Microbes May Facilitate Insect Herbivory of Chemically Defended Plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Juottonen, H.; Moghadam, N.N.; Murphy, L.; Mappes, J.; Galarza, J.A. Host’s Genetic Background Determines the Outcome of Reciprocal Faecal Transplantation on Life-History Traits and Microbiome Composition. Anim. Microbiome 2022, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.G.; Urruty, D.M.; Forister, M.L. An Exploration of the Fungal Assemblage in Each Life History Stage of the Butterfly, Lycaeides melissa (Lycaenidae), as Well as Its Host Plant Astragalus canadensis (Fabaceae). Fungal Ecol. 2016, 22, 10–16. [Google Scholar] [CrossRef]
- Rizvi, S.Z.M.; Raman, A. Epiphyas Postvittana (Lepidoptera: Tortricidae)—Botrytis Cinerea (Helotiales: Sclerotiniaceae)—Vitis Vinifera (Vitales: Vitaceae) Interaction: The Role of B. Cinerea on the Development of E. Postvittana in Synthetic Nutritional Media. J. Econ. Entomol. 2015, 108, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera Littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, V.V. Persistence of Intestinal Bacteria in the Developmental Stages of the Monarch Butterfly (Danaus plexippus). J. Invertebr. Pathol. 1972, 20, 51–58. [Google Scholar] [CrossRef]
- Ma, D.; Leulier, F. The Importance of Being Persistent: The First True Resident Gut Symbiont in Drosophila. PLoS Biol. 2018, 16, e2006945. [Google Scholar] [CrossRef] [PubMed]
- Pilon, F.M.; Silva, C.d.R.; Visôtto, L.E.; Barros, R.d.A.; da Silva Júnior, N.R.; Campos, W.G.; de Almeida Oliveira, M.G. Purification and Characterization of Trypsin Produced by Gut Bacteria from Anticarsia Gemmatalis. Arch. Insect Biochem. Physiol. 2017, 96, e21407. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.L.; dos Santos Malta, V.R.; Machado, S.S.; Junior, G.A.L.; da Silva, A.P.V.; Almeida, R.M.R.G.; da Luz, J.M.R. Bacterial Cellulase from the Intestinal Tract of the Sugarcane Borer. Int. J. Biol. Macromol. 2020, 161, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic Sequencing of Diamondback Moth Gut Microbiome Unveils Key Holobiont Adaptations for Herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Couture, J.J.; Raffa, K.F. Plant-Associated Bacteria Degrade Defense Chemicals and Reduce Their Adverse Effects on an Insect Defoliator. Oecologia 2014, 175, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Peiffer, M.; Felton, G.W.; Hoover, K. Host-Specific Larval Lepidopteran Mortality to Pathogenic Serratia Mediated by Poor Diet. J. Invertebr. Pathol. 2022, 194, 107818. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tokuda, G. Cellulolytic Systems in Insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Liu, F.; Lan, Y.; Wang, J.; Wang, L.; Li, J.; Liu, X.; Fan, Z.; Guo, T.; Yue, B. Transcriptomics and Metagenomics of Common Cutworm (Spodoptera litura) and Fall Armyworm (Spodoptera frugiperda) Demonstrate Differences in Detoxification and Development. BMC Genom. 2022, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Z.; Xu, C.; Wang, J.; Mallik, A.U.; Gu, C.; Chen, D.; Lu, L.; Zeng, R.; Song, Y. High Nitrogen in Maize Enriches Gut Microbiota Conferring Insecticide Tolerance in Lepidopteran Pest Spodoptera Litura. iScience 2022, 25, 103726. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-Derived Antimicrobials Contribute to the Control of the Lepidopteran Gut Microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Stepien, T.A.; Blum, J.E.; Holt, J.F.; Labbe, N.H.; Rush, J.S.; Raffa, K.F.; Handelsman, J. From Commensal to Pathogen: Translocation of Enterococcus faecalis from the Midgut to the Hemocoel of Manduca Sexta. mBio 2011, 2, e00065-11. [Google Scholar] [CrossRef] [PubMed]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial Symbionts of Insects as a Source of New Antimicrobials: A Review. Crit. Rev. Microbiol. 2021, 47, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Clarke, A.R.; Bradley, J. Microbial Mediation of Fruit Fly–Host Plant Interactions: Is the Host Plant the “Centre of Activity”? Oikos 2002, 97, 319–328. [Google Scholar] [CrossRef]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect Symbionts as Hidden Players in Insect–Plant Interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Moran, N.A. The Impact of Microbial Symbionts on Host Plant Utilization by Herbivorous Insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef] [PubMed]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, J.; Wang, H.; Bidochka, M.J.; Duan, M.; Xu, W.; Sui, L.; Ren, B.; Li, Q.; Zhang, Z. Fitness Consequences of Oviposition Choice by an Herbivorous Insect on a Host Plant Colonized by an Endophytic Entomopathogenic Fungus. J. Pest Sci. 2023, 96, 745–758. [Google Scholar] [CrossRef]
- Tack, A.J.M.; Dicke, M. Plant Pathogens Structure Arthropod Communities across Multiple Spatial and Temporal Scales. Funct. Ecol. 2013, 27, 633–645. [Google Scholar] [CrossRef]
- De Vries, E.J.; Vos, R.A.; Jacobs, G.; Breeuwer, H.A.J. Western Flower Thrips (Thysanoptera: Thripidae) Preference for Thrips-Damaged Leaves over Fresh Leaves Enables Uptake of Symbiotic Gut Bacteria. Eur. J. Entomol. 2006, 103, 779–786. [Google Scholar] [CrossRef]
- He, M.; Chen, H.; Yang, X.; Gao, Y.; Lu, Y.; Cheng, D. Gut Bacteria Induce Oviposition Preference through Ovipositor Recognition in Fruit Fly. Commun. Biol. 2022, 5, 973. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, M.; Xu, D.; Chen, L.; Sun, J. Sexual Variation of Bacterial Microbiota of Dendroctonus Valens Guts and Frass in Relation to Verbenone Production. J. Insect Physiol. 2016, 95, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hulcr, J.; Sun, J. The Role of Symbiotic Microbes in Insect Invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Vacher, C.; Castagneyrol, B.; Jousselin, E.; Schimann, H. Trees and Insects Have Microbiomes: Consequences for Forest Health and Management. Curr. For. Rep. 2021, 7, 81–96. [Google Scholar] [CrossRef]
- Adams, A.S.; Jordan, M.S.; Adams, S.M.; Suen, G.; Goodwin, L.A.; Davenport, K.W.; Currie, C.R.; Raffa, K.F. Cellulose-Degrading Bacteria Associated with the Invasive Woodwasp Sirex Noctilio. ISME J. 2011, 5, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Arfah, R.A.; Natsir, H.; Atifah, N.; Zarkoni, T.R.; Mahmud, M. Isolation and Characterization of Soil Termites (Macrotermes gilvus) Cellulolytic Bacteria and Activity Determination of Cellulase Enzyme on Newsprint Substrates. In Journal of Physics: Conference Series, Proceedings of the 3rd International Conference on Science, Makassar, India, 26–27 July 2019; IOP Publishing: Bristol, UK, 2019; Volume 1341, p. 32037. [Google Scholar]
- Dantur, K.I.; Enrique, R.; Welin, B.; Castagnaro, A.P. Isolation of Cellulolytic Bacteria from the Intestine of Diatraea Saccharalis Larvae and Evaluation of Their Capacity to Degrade Sugarcane Biomass. AMB Express 2015, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, M.; Ahmad, A.; La Nafie, N.; Zenta, F. Cellulase Enzyme Activity of Bacillus Circulans from Larvae Cossus Cossus in Lignocellulosic Substrat. Am. J. Biomed. Life Sci. 2016, 4, 21. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Karaoz, U.; Bill, M.; Hao, Z.; White III, R.A.; Arellano, A.; Ramanculova, L.; Filley, T.R.; Berry, T.D.; Conrad, M.E.; et al. Gut Anatomical Properties and Microbial Functional Assembly Promote Lignocellulose Deconstruction and Colony Subsistence of a Wood-Feeding Beetle. Nat. Microbiol. 2019, 4, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Okamura, Y.; Haeger, W.; Vogel, H.; Kunert, G.; Pauchet, Y. Metabolic Novelty Originating from Horizontal Gene Transfer Is Essential for Leaf Beetle Survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2205857119. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Kirsch, R.; Pauchet, Y.; Berasategui, A.; Fukumori, K.; Moriyama, M.; Cripps, M.; Windsor, D.; Fukatsu, T.; Gerardo, N.M. Symbiont Digestive Range Reflects Host Plant Breadth in Herbivorous Beetles. Curr. Biol. 2020, 30, 2875–2886.e4. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Zhang, G. Formation Pattern and Regulatory Mechanisms of Pollen Wall in Arabidopsis. J. Plant Physiol. 2021, 260, 153388. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen Wall Development in Flowering Plants. New Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional Diversity within the Simple Gut Microbiota of the Honey Bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guo, W.; Xu, B.; Teng, Z.; Tao, D.; Lou, Y.; Gao, Y. Screening and Identification of Lignin-Degrading Bacteria in Termite Gut and the Construction of LiP-Expressing Recombinant Lactococcus Lactis. Microb. Pathog. 2017, 112, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.K.; Dhawaria, M.; Tripathi, D.; Raturi, V.; Adhikari, D.K.; Kanaujia, P.K. Investigation of Lignin Biodegradation by Trabulsiella Sp. Isolated from Termite Gut. Int. Biodeterior. Biodegradation 2016, 112, 12–17. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Isolation and Characterization of Novel Lignolytic, Cellulolytic, and Hemicellulolytic Bacteria from Wood-Feeding Termite Cryptotermes Brevis. Int. Microbiol. 2019, 22, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional Genomics and Microbiome Profiling of the Asian Longhorned Beetle (Anoplophora glabripennis) Reveal Insights into the Digestive Physiology and Nutritional Ecology of Wood Feeding Beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.D.; Geib, S.M.; Mason, C.J.; Carlson, J.E.; Tien, M.; Chen, H.-Y.; Harding, S.; Tsai, C.-J.; Hoover, K. Host-Plant Induced Changes in Microbial Community Structure and Midgut Gene Expression in an Invasive Polyphage (Anoplophora glabripennis). Sci. Rep. 2018, 8, 9620. [Google Scholar] [CrossRef] [PubMed]
- Mattson, W.J. Herbivory in Relation to Plant Nitrogen Content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Jha, B.; Singh, N.P.; Mishra, A. Proteome Profiling of Seed Storage Proteins Reveals the Nutritional Potential of Salicornia Brachiata Roxb., an Extreme Halophyte. J. Agric. Food Chem. 2012, 60, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Yuval, B.; Jurkevitch, E. Enterobacteria-mediated Nitrogen Fixation in Natural Populations of the Fruit Fly Ceratitis Capitata. Mol. Ecol. 2005, 14, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Nguyen, N.H.; Karaoz, U.; Gross, S.R.; Herman, D.J.; Andersen, G.L.; Bruns, T.D.; Pett-Ridge, J.; Blackwell, M.; Brodie, E.L. Compartmentalized Microbial Composition, Oxygen Gradients and Nitrogen Fixation in the Gut of Odontotaenius Disjunctus. ISME J. 2014, 8, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Cao, S.; Akami, M.; Mansour, A.; Yang, Y.; Jiang, N.; Wang, H.; Zhang, G.; Qi, X.; Xu, P.; et al. Gut Symbiotic Bacteria Are Involved in Nitrogen Recycling in the Tephritid Fruit Fly Bactrocera Dorsalis. BMC Biol. 2022, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sanders, J.G.; Łukasik, P.; D’Amelio, C.L.; Millar, J.S.; Vann, D.R.; Lan, Y.; Newton, J.A.; Schotanus, M.; Kronauer, D.J.C.; et al. Herbivorous Turtle Ants Obtain Essential Nutrients from a Conserved Nitrogen-Recycling Gut Microbiome. Nat. Commun. 2018, 9, 964. [Google Scholar] [CrossRef] [PubMed]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of Gut Symbionts of Insect Pests: A Novel Target for Insect-Pest Control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, T.; Futahashi, R.; Terashima, M.; Barrière, Q.; Lamouche, F.; Takeshita, K.; Meng, X.-Y.; Mitani, Y.; Sone, T.; Shigenobu, S.; et al. Comparative Cytology, Physiology and Transcriptomics of Burkholderia Insecticola in Symbiosis with the Bean Bug Riptortus Pedestris and in Culture. ISME J. 2019, 13, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Bisch, G.; Neuvonen, M.-M.; Pierce, N.E.; Russell, J.A.; Koga, R.; Sanders, J.G.; Łukasik, P.; Andersson, S.G.E. Genome Evolution of Bartonellaceae Symbionts of Ants at the Opposite Ends of the Trophic Scale. Genome Biol. Evol. 2018, 10, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Du, Y.; Yu, J.; Guo, J. MicroRNAs as Potential Biomarkers of Insecticide Exposure: A Review. Chem. Res. Toxicol. 2019, 32, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Deng, J.; Zhou, F.; Cheng, C.; Zhang, L.; Zhang, J.; Lu, M. Gut Microbiota in an Invasive Bark Beetle Infected by a Pathogenic Fungus Accelerates Beetle Mortality. J. Pest Sci. 2019, 92, 343–351. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; De Mandal, S.; Shakeel, M.; Hua, Y.; Shoukat, R.F.; Fu, D.; Jin, F. Gut Microbiota Mediate Plutella Xylostella Susceptibility to Bt Cry1Ac Protoxin Is Associated with Host Immune Response. Environ. Pollut. 2021, 271, 116271. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xu, Z.; Li, L.; Zhang, Y.; Diao, J.; Cao, J.; Xu, L.; Ma, L. Gut Bacterial Microbiota of Lymantria Dispar Asiatica and Its Involvement in Beauveria Bassiana Infection. J. Invertebr. Pathol. 2023, 197, 107897. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, Y.J.; Klepzig, K.D.; Raffa, K.F. Bacteria in Oral Secretions of an Endophytic Insect Inhibit Antagonistic Fungi. Ecol. Entomol. 2006, 31, 636–645. [Google Scholar] [CrossRef]
- Dillon, R.J.; Charnley, A.K. Inhibition of Metarhizium Anisopliae by the Gut Bacterial Flora of the Desert Locust: Characterisation of Antifungal Toxins. Can. J. Microbiol. 1988, 34, 1075–1082. [Google Scholar] [CrossRef]
- Shan, Y.; Shu, C.; Crickmore, N.; Liu, C.; Xiang, W.; Song, F.; Zhang, J. Cultivable Gut Bacteria of Scarabs (Coleoptera: Scarabaeidae) Inhibit Bacillus Thuringiensis Multiplication. Environ. Entomol. 2014, 43, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Wright, D.J.; Bruce, T.J.A.; Staley, J.T. Drought and Root Herbivory Interact to Alter the Response of Above-Ground Parasitoids to Aphid Infested Plants and Associated Plant Volatile Signals. PLoS ONE 2013, 8, e69013. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Y.; Zhu, J.-L.; Zhou, W.-W.; Liu, S.; Khairul, Q.M.; Ansari, N.A.; Zhu, Z.-R. Identification and Expression Analysis of Putative Chemoreception Genes from Cyrtorhinus Lividipennis (Hemiptera: Miridae) Antennal Transcriptome. Sci. Rep. 2018, 8, 12981. [Google Scholar] [CrossRef] [PubMed]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; de Boer, J.G.; Delvigne, F.; Francis, F.; Wäckers, F.; Rediers, H.; Verstrepen, K.J.; Wenseleers, T.; et al. Volatiles of Bacteria Associated with Parasitoid Habitats Elicit Distinct Olfactory Responses in an Aphid Parasitoid and Its Hyperparasitoid. Funct. Ecol. 2020, 34, 507–520. [Google Scholar] [CrossRef]
- Thibout, E.; Guillot, J.F.; Ferary, S.; Limouzin, P.; Auger, J. Origin and Identification of Bacteria Which Produce Kairomones in the Frass of Acrolepiopsis assectella (Lep., Hyponomeutoidea). Experientia 1995, 51, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Lin, S.; Chen, S.; Lin, G.; Song, Q.; Bai, J.; You, M.; Xie, M. Profiling of MicroRNAs in Midguts of Plutella Xylostella Provides Novel Insights into the Bacillus Thuringiensis Resistance. Front. Genet. 2021, 12, 739849. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.R.; Gulzar, A.; Ferré, J.; Wright, D.J. Bacillus Thuringiensis Vip3Aa Toxin Resistance in Heliothis virescens (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 2017, 83, e03506-16. [Google Scholar] [CrossRef] [PubMed]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of Resistance to Insecticidal Proteins from Bacillus Thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Di Lelio, I.; Forni, G.; Magoga, G.; Brunetti, M.; Bruno, D.; Becchimanzi, A.; De Luca, M.G.; Sinno, M.; Barra, E.; Bonelli, M.; et al. A Soil Fungus Confers Plant Resistance against a Phytophagous Insect by Disrupting the Symbiotic Role of Its Gut Microbiota. Proc. Natl. Acad. Sci. USA 2023, 120, e2216922120. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, S.; Sun, L.; Zhang, Y.; Luo, J.; Bock, R.; Zhang, J. Synergistic Action of the Gut Microbiota in Environmental RNA Interference in a Leaf Beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Tu, C.; Luo, J.; Lu, M.; Zhang, S.; Xu, L. Metabolic and Immunological Effects of Gut Microbiota in Leaf Beetles at the Local and Systemic Levels. Integr. Zool. 2021, 16, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sina Rahme, B.; Lestradet, M.; Di Venanzio, G.; Ayyaz, A.; Yamba, M.W.; Lazzaro, M.; Liégeois, S.; Garcia Véscovi, E.; Ferrandon, D. The FliR Gene Contributes to the Virulence of S. Marcescens in a Drosophila Intestinal Infection Model. Sci. Rep. 2022, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Dhammi, P.; Saini, H.S.; Kaur, S. Pathogenicity of Bacteria Isolated from Gut of Spodoptera litura (Lepidoptera: Noctuidae) and Fitness Costs of Insect Associated with Consumption of Bacteria. J. Invertebr. Pathol. 2015, 127, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Osborn, F.; Berlioz, L.; Vitelli-Flores, J.; Monsalve, W.; Dorta, B.; Lemoine, V.R. Pathogenic Effects of Bacteria Isolated from Larvae of Hylesia Metabus Crammer (Lepidoptera: Saturniidae). J. Invertebr. Pathol. 2002, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fezza, T.J.; Follett, P.A.; Shelly, T.E. Effect of the Timing of Pupal Irradiation on the Quality and Sterility of Oriental Fruit Flies (Diptera: Tephritidae) for Use in Sterile Insect Technique. Appl. Entomol. Zool. 2021, 56, 443–450. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Ordoñez, A.; Carrasco, M.; Montoya, P.; Hernández, E. Mass Rearing, Quality Parameters, and Bioconversion in Drosophila suzukii (Diptera: Drosophilidae) for Sterile Insect Technique Purposes. J. Econ. Entomol. 2020, 113, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, Y.; Ito, K.; Himuro, C.; Honma, A. Sterile Males and Females Can Synergistically Suppress Wild Pests Targeted by Sterile Insect Technique. J. Theor. Biol. 2021, 530, 110878. [Google Scholar] [CrossRef] [PubMed]
- Ami, E.B.; Yuval, B.; Jurkevitch, E. Manipulation of the Microbiota of Mass-Reared Mediterranean Fruit Flies Ceratitis capitata (Diptera: Tephritidae) Improves Sterile Male Sexual Performance. ISME J. 2010, 4, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.N.; Lowe, E.C.; Rempoulakis, P.; Herberstein, M.E. Effect of Natural Predators on Queensland Fruit Fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) Control by Sterile Insect Technique (SIT). Pest Manag. Sci. 2019, 75, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cao, L.; Han, R. Plant Quercetin Degradation by Gut Bacterium Raoultella Terrigena of Ghost Moth Thitarodes Xiaojinensis. Front. Microbiol. 2022, 13, 1079550. [Google Scholar] [CrossRef] [PubMed]
- Gandotra, S.; Bhuyan, P.M.; Gogoi, D.K.; Kumar, A.; Subramanian, S. Screening of Nutritionally Important Gut Bacteria from the Lepidopteran Insects through Qualitative Enzyme Assays. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 329–337. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zhao, J.; Dong, Z.; Shao, T. Gut Microbiota of Ostrinia Nubilalis Larvae Degrade Maize Cellulose. Front. Microbiol. 2022, 13, 816954. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, A.; Karimi Dorcheh, E.; Li, R.; Rameshkumar, N.; Baldwin, I.T. Antimicrobial Peptide Expression in a Wild Tobacco Plant Reveals the Limits of Host-Microbe-Manipulations in the Field. eLife 2018, 7, e28715. [Google Scholar] [CrossRef] [PubMed]
- Leroy, P.D.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.J.; Francis, F.; Brostaux, Y.; Felton, G.W.; Haubruge, E. Microorganisms from Aphid Honeydew Attract and Enhance the Efficacy of Natural Enemies. Nat. Commun. 2011, 2, 348. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lou, Q.; Cheng, C.; Lu, M.; Sun, J. Gut-Associated Bacteria of Dendroctonus Valens and Their Involvement in Verbenone Production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Gillette, N.E.; Stein, J.D.; Owen, D.R.; Webster, J.N.; Fiddler, G.O.; Mori, S.R.; Wood, D.L. Verbenone-releasing Flakes Protect Individual Pinus Contorta Trees from Attack by Dendroctonus ponderosae and Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae). Agric. For. Entomol. 2006, 8, 243–251. [Google Scholar] [CrossRef]
- Dyson, P.; Figueiredo, M.; Andongma, A.A.; Whitten, M.M.A. Symbiont-Mediated RNA Interference (SMR): Using Symbiotic Bacteria as Vectors for Delivering RNAi to Insects. RNAi Strateg. Pest Manag. Methods Protoc. 2022, 2360, 295–306. [Google Scholar]
- Li, T.; Wei, Y.; Zhao, C.; Li, S.; Gao, S.; Zhang, Y.; Wu, Y.; Lu, C. Facultative Symbionts Are Potential Agents of Symbiont-Mediated RNAi in Aphids. Front. Microbiol. 2022, 13, 1020461. [Google Scholar] [CrossRef] [PubMed]
- Lariviere, P.J.; Leonard, S.P.; Horak, R.D.; Powell, J.E.; Barrick, J.E. Honey Bee Functional Genomics Using Symbiont-Mediated RNAi. Nat. Protoc. 2023, 18, 902–928. [Google Scholar] [CrossRef] [PubMed]
- Taracena, M.L.; Oliveira, P.L.; Almendares, O.; Umana, C.; Lowenberger, C.; Dotson, E.M.; Paiva-Silva, G.O.; Pennington, P.M. Genetically Modifying the Insect Gut Microbiota to Control Chagas Disease Vectors through Systemic RNAi. PLoS Negl. Trop. Dis. 2015, 9, e0003358. [Google Scholar] [CrossRef] [PubMed]

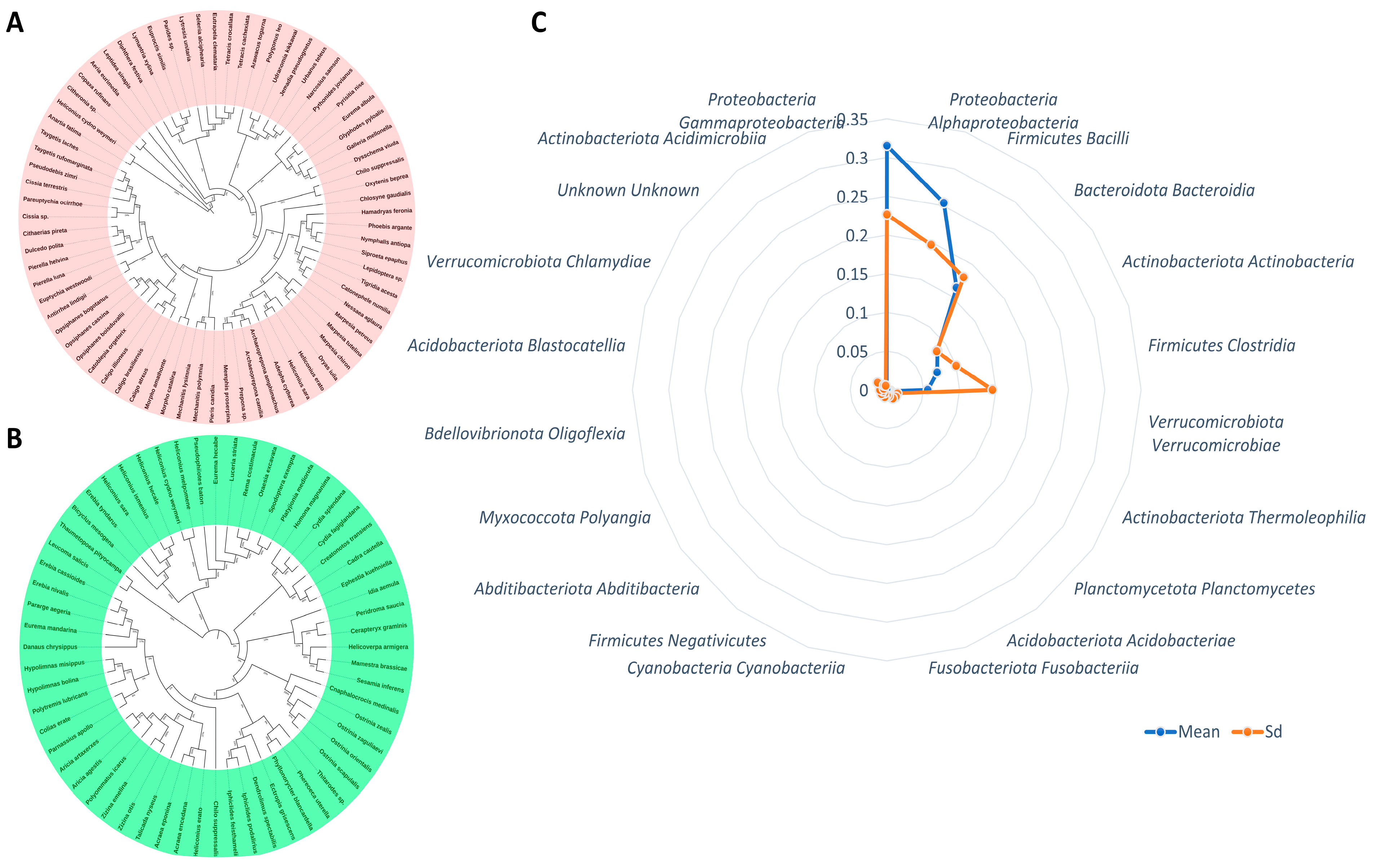

| Microbial Family | Lepidoptera Family and Species | References |
|---|---|---|
| Bacteroidetes | Noctuidae (Spodoptera frugiperda), Pieridae (Pieris canidia) | [19,23,24] |
| Firmicutes | Noctuidae (Spodoptera litura), Lymantriidae (Lymantria dispar) | [4,6,25] |
| Proteobacteria | Saturniidae (Bombyx mori), Pieridae (Pieris rapae) | [3,20] |
| Actinobacteria | Pieridae (Danaus plexippus), Noctuidae (Plutella xylostella) | [3,13] |
| Ascomycota | Saturniidae (Bombyx mori), Tortricidae (Cydia pomonella) | [4,6] |
| Basidiomycota | Noctuidae (Spodoptera frugiperda), Erebidae (Lonomia obliqua) | [23,26] |
| Entomophthorales | Saturniidae (Bombyx mori), Sphingidae (Hylesia metabus) | [19,25] |
| Zygomycota | Noctuidae (Helicoverpa armigera), Saturniidae (Actias luna) | [19,24] |
| Archaea (Methanogens) | Noctuidae (Spodoptera frugiperda), Pieridae (Pieris canidia) | [27] |
| Fusobacteria | Lymantriidae (Lymantria dispar), Noctuidae (Spodoptera litura) | [19,28] |
| Euglenozoa | Pieridae (Danaus plexippus), Noctuidae (Plutella xylostella) | [13,29] |
| Cercozoa | Noctuidae (Spodoptera frugiperda), Tortricidae (Cydia pomonella) | [3,29] |
| Glomeromycota | Saturniidae (Bombyx mori), Lymantriidae (Lymantria dispar) | [30,31] |
| Chytridiomycota | Pieridae (Pieris rapae), Saturniidae (Actias luna) | [32] |
| Chlorophyta | Noctuidae (Helicoverpa armigera), Tortricidae (Cydia pomonella) | [24] |
| Malawimonadida (Protists) | Noctuidae (Spodoptera frugiperda), Saturniidae (Bombyx mori) | [19,27] |
| Stramenopiles | Lymantriidae (Lymantria dispar), Noctuidae (Spodoptera litura) | [28] |
| Category | Factor | Effect on Gut Microbiota |
|---|---|---|
| Host-related Factors | Diet Composition | The types of food and nutrients available to the host influence microbial communities. |
| Host-related Factors | Developmental Stage | Microbial composition varies across different life stages (e.g., larvae, pupae, adults). |
| Host-related Factors | Immune System | The host immune response can regulate microbial colonization and diversity. |
| Environmental Factors | Habitat | The physical environment, such as vegetation type, affects microbial diversity. |
| Environmental Factors | Temperature | Temperature can influence microbial growth and community structure. |
| Environmental Factors | Humidity | Humidity levels can affect the survival and activity of gut microbes. |
| Microbial Interactions | Symbiotic Relationships | Beneficial microbes in mutualistic relationships can shape gut microbiota. |
| Microbial Interactions | Antagonism | Antagonistic interactions between microbes can affect microbial diversity and function. |
| Phylum | Class | Mean | SD |
|---|---|---|---|
| Bacteroidota | Bacteroidia | 0.085900266 | 0.084621476 |
| Proteobacteria | Alphaproteobacteria | 0.253684521 | 0.196852416 |
| Proteobacteria | Gammaproteobacteria | 0.315196589 | 0.226446002 |
| Firmicutes | Bacilli | 0.162957572 | 0.179644224 |
| Actinobacteriota | Actinobacteria | 0.072742687 | 0.100153701 |
| Actinobacteriota | Thermoleophilia | 0.00494136 | 0.015191977 |
| Firmicutes | Clostridia | 0.056172865 | 0.145360754 |
| Verrucomicrobiota | Verrucomicrobiae | 0.005398711 | 0.015210373 |
| Firmicutes | Negativicutes | 0.001961527 | 0.005447803 |
| Planctomycetota | Planctomycetes | 0.004907338 | 0.013982851 |
| Cyanobacteria | Cyanobacteriia | 0.002196259 | 0.009989121 |
| Acidobacteriota | Acidobacteriae | 0.003262416 | 0.008807503 |
| Abditibacteriota | Abditibacteria | 0.001797995 | 0.009358712 |
| Fusobacteriota | Fusobacteriia | 0.002613247 | 0.007239766 |
| Actinobacteriota | Acidimicrobiia | 0.001258584 | 0.005626321 |
| Myxococcota | Polyangia | 0.001640708 | 0.005979046 |
| Verrucomicrobiota | Chlamydiae | 0.001354805 | 0.015833571 |
| Bdellovibrionota | Oligoflexia | 0.001410108 | 0.009619666 |
| Unknown | Unknown | 0.001346226 | 0.006998769 |
| Acidobacteriota | Blastocatellia | 0.001360653 | 0.004650909 |
| Insect | Order | Gut Bacteria | Function of Bacteria |
|---|---|---|---|
| Cossus cossus | Lepidoptera | Bacillus circulans | Production of cellulase |
| Trichoplusia ni | Lepidoptera | Agrobacterium sp. | Degradation of alkaloids |
| Thitarodes xiaojinensis | Lepidoptera | Raoultella terrigena | Degradation of quercetin |
| Plutella xylostella | Lepidoptera | Midgut microbiota | Assisting plant toxins to kill insects |
| Helicoverpa zea | Lepidoptera | Serratia marcescens | Killing insects in synergy with plant defense |
| Spodoptera frugiperda | Lepidoptera | Enterococcus sp. | Interacting with plant defenses to kill insects |
| Spodoptera littoralis | Lepidoptera | Gut microbiota | Dysbiosis of gut microbiota is detrimental to larval survival |
| Diatraea saccharalis | Lepidoptera | Bacillus pumilus | Production of cellulase |
| Dendroctonus valens | Coleoptera | Gutmicrobiota | Assisting beetles to determine the suitability of the pine tree for colonization |
| Cyrtotrachelus buqueti | Coleoptera | Bacillusvelezensis | Production of cellulase |
| Holotrichia paralella | Coleoptera | Pseudomonas sp. | Production of cellulase |
| Osphranteria coerulescens | Coleoptera | Bacillussafensis | Production of cellulase |
| Cassida rubiginosa | Coleoptera | Stammera | Production of pectinase |
| Hypomeces squamosus | Coleoptera | Enterobacter hormaechei | For feeding and females for egg laying |
| Cassida rubiginosa | Coleoptera | Stammera | Production of pectinase |
| Hypomeces squamosus | Coleoptera | Enterobacter hormaechei | For feeding and females for egg laying |
| Sphenophorus levis | Coleoptera | Gut microbiota | Production of pectinase |
| Odontotaenius disjunctus | Coleoptera | Bacteroidetes sp. | Biological nitrogen fixation |
| Melolontha hippocastani | Coleoptera | Burkholderia sp. | Recycling of nitrogen in larvae |
| Hylobius abietis | Coleoptera | Enterobacteriaceae sp. | Degradation of terpenoids |
| Curculio chinensis | Coleoptera | Acinetobacter sp. | Degradation of tea saponin |
| Hypothenemus hampei | Coleoptera | Pseudomonas fulva | Degradation of caffeine |
| Dendroctonus valens | Coleoptera | Novosphingobium sp. | Degradation of phenolic naringenin |
| Psylliodes chrysocephala | Coleoptera | Pantoea sp. | Degradation of isothiocyanates |
| Acrobasis nuxvorella | Coleoptera | Bacillus pumilus | Degradation of Carya illinoinensis tannins |
| Macrotermes gilvus | Isoptera | Provedencia sp. | Production of cellulase |
| Cryptotermes brevis | Isoptera | Bacillus sp. | Production of ligninase |
| Odontotermes obesu | Isoptera | Trabulsiella sp. | Production of ligninase |
| Reticulitermes chinensis | Soptera | Enterobacter hormaechei | Production of ligninase |
| Bactrocera dorsalis | Diptera | Citrobacter sp. | Attracting female flies to lay eggs on the host fruit by VOCs |
| Ceratitis capitata | Diptera | Enterobacteriaceae sp. | Biological nitrogen fixation |
| Bactrocera dorsalis | Diptera | Morganella morganii | Hydrolyzing nitrogenous waste and providing metabolizable nitrogen |
| Riptortus pedestris | Hemiptera | Burkholderia sp. | Supplementation of essential amino acids and B vitamins |
| Dysdercus fasciatus | Hemiptera | Coriobacterium glomerans | Supplementation of B vitamins |
| Sirex noctilio | Hymenoptera | Streptomyces | Production of cellulase |
| Frankliniella occidentalis | Thysanoptera | Erwinia sp. | Attracting larvae |
| Insect Family | Larval Microbiome | Microbiome Communities |
|---|---|---|
| Pieridae | Enterococcus, Bacillus, Pseudomonas | Enterococcus, Pseudomonas |
| Noctuidae | Enterococcus, Serratia, Bacillus | Enterococcus, Serratia |
| Saturniidae | Enterococcus, Acinetobacter | Enterococcus, Bacillus |
| Tortricidae | Bacillus, Enterococcus, Pantoea | Bacillus, Enterococcus, Pantoea |
| Lymantriidae | Enterococcus, Bacillus, Lactobacillus | Enterococcus, Bacillus, Lactobacillus |
| Nymphalidae | Spiroplasma, Enterococcus | Spiroplasma, Enterococcus |
| Hesperiidae | Enterococcus, Bacillus | Enterococcus, Bacillus |
| Papilionidae | Bacillus, Enterococcus | Bacillus, Enterococcus |
| Lycaenidae | Pseudomonas, Enterococcus, Bacillus | Pseudomonas, Enterococcus |
| Pierinae | Bacillus, Enterococcus | Bacillus, Enterococcus |
| Erebidae | Acinetobacter, Serratia | Acinetobacter, Serratia |
| Crambidae | Bacillus, Enterococcus | Bacillus, Enterococcus |
| Geometridae | Bacillus, Enterococcus | Bacillus, Enterococcus |
| Sphingidae | Acinetobacter, Enterococcus | Acinetobacter, Enterococcus |
| Cossidae | Bacillus, Streptomyces, Pseudomonas | Bacillus, Streptomyces, Pseudomonas |
| Arctiidae | Enterococcus, Bacillus, Pseudomonas | Enterococcus, Bacillus, Pseudomonas |
| Sattleridae | Bacillus, Enterococcus, Pseudomonas | Bacillus, Enterococcus, Pseudomonas |
| Pyralidae | Enterococcus, Lactobacillus | Enterococcus, Lactobacillus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, A.; Haq, I.U.; Hyder, M.; Humza, M.; Younas, M.; Akhtar, M.R.; Ghafar, M.A.; Liu, T.-X.; Hou, Y. Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management. Biology 2025, 14, 937. https://doi.org/10.3390/biology14080937
Basit A, Haq IU, Hyder M, Humza M, Younas M, Akhtar MR, Ghafar MA, Liu T-X, Hou Y. Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management. Biology. 2025; 14(8):937. https://doi.org/10.3390/biology14080937
Chicago/Turabian StyleBasit, Abdul, Inzamam Ul Haq, Moazam Hyder, Muhammad Humza, Muhammad Younas, Muhammad Rehan Akhtar, Muhammad Adeel Ghafar, Tong-Xian Liu, and Youming Hou. 2025. "Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management" Biology 14, no. 8: 937. https://doi.org/10.3390/biology14080937
APA StyleBasit, A., Haq, I. U., Hyder, M., Humza, M., Younas, M., Akhtar, M. R., Ghafar, M. A., Liu, T.-X., & Hou, Y. (2025). Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management. Biology, 14(8), 937. https://doi.org/10.3390/biology14080937











