Simple Summary
The usefulness of Wolbachia-based control methods for several insect orders is investigated in this synthesis, with a particular emphasis on sterile insect technique (SIT) and incompatible insect technique (IIT). Strong control tactics and financing are required, as demonstrated by the integration of SIT into the management of Drosophila suzukii and the evaluation of a new SIT/IIT combination against Aedes mosquitoes. The potential of Wolbachia includes biological vector control in agriculture, particularly defence against pests that affect rice. Examining host-shifting dynamics and phenotypic impacts, especially in scale insects, highlights the ecological connectivity that is essential to comprehending the complexity of Wolbachia. The summary discusses the worldwide distribution of Wolbachia, focusing on genetics, medicinal uses, and mutualistic and parasitic adaptations. With developments in gene functional assays and multiomics, Wolbachia research is changing and becoming a paradigm for microbial symbiosis, with profound effects on education and translational research.
Abstract
Effective in a variety of insect orders, including dipteran, lepidopteran, and hemipteran, Wolbachia-based control tactics are investigated, noting the importance of sterile and incompatible insect techniques. Encouraging approaches for controlling Aedes mosquitoes are necessary, as demonstrated by the evaluation of a new SIT/IIT combination and the incorporation of SIT into Drosophila suzukii management. For example, Wolbachia may protect plants from rice pests, demonstrating its potential for agricultural biological vector management. Maternal transmission and cytoplasmic incompatibility dynamics are explored, while Wolbachia phenotypic impacts on mosquito and rice pest management are examined. The importance of host evolutionary distance is emphasised in recent scale insect research that addresses host-shifting. Using greater information, a suggested method for comprehending Wolbachia host variations in various contexts emphasises ecological connectivity. Endosymbionts passed on maternally in nematodes and arthropods, Wolbachia are widely distributed around the world and have evolved both mutualistic and parasitic traits. Wolbachia is positioned as a paradigm for microbial symbiosis due to advancements in multiomics, gene functional assays, and its effect on human health. The challenges and opportunities facing Wolbachia research include scale issues, ecological implications, ethical conundrums, and the possibility of customising strains through genetic engineering. It is thought that cooperative efforts are required to include Wolbachia-based therapies into pest management techniques while ensuring responsible and sustainable ways.
1. Introduction
The vital symbiotic role of microbes in the eukaryote cell origin, their species conformation, ecological inter-relationship, and plant and animal activities around the biosphere are progressively evident [1,2]. The most widespread microbe in the animal kingdom, in which rules of engagement within species and obligations for research may shape, is the alpha-proteobacterium Wolbachia pipientis, which was recorded as a Rickettsia-like organism some hundred years ago in the gonads of numerous insects such as Culex pipiens [3]. The most prevalent intracellular bacterial species is Wolbachia, which can change the reproduction of host insects. Production of female infected progenies is enhanced through parthenogenesis, male killing, feminization, or cytoplasmic incongruity. These changes are considered adjusted for Wolbachia and promote their spread and multiplication [4,5]. Arboviruses such as Yellow fever virus, Zika and Chikungaunya are disease-causing agents that are disseminated through arthropods and are known to cause serious public health issues [6]. The worldwide occurrence of characterized arbovirus incidence has spiked in the recent few decades, emphasizing the demand for efficient control. Biological control is a promising option, wherein the application of Wolbachia surfaces is a potent option. Owing to its appreciable control potential against insects and viral pathogens, Wolbachia proved itself as a potential management strategy against insect vectors [7,8,9].
Wolbachia, one of the best heritable groups of endosymbionts, is extensively distributed among members of arthropods and a few nematodes [10,11,12]. They are the most diverse and abundantly found group of symbiont bacteria present on the globe: around 40% to 60% of the insect species are infected with different strains of Wolbachia [13,14]. During the last couple of decades, the most extensively studied aspects of Wolbachia involved their potential to trigger manipulations in reproduction [15] as well as their employment in the control of vector-borne diseases [16,17]. Identical to some other symbiotic organisms, the present phylogeny of Wolbachia is the result of three important processes: switching between the host species, diversification along with the host clade, and symbiont destruction [18,19]. Wolbachia’s dynamic localization and presence during Drosophila oogenesis emphasize complex interactions between the endosymbiont and host germline cells (Figure 1), offering important insights into the mechanisms controlling Wolbachia persistence and distribution throughout the egg’s developmental stages [4,20] as well as in a few strains invading bedbugs (super group F) [21]. Numerous studies could not provide evidence of mutual diversification between arthropods and strains of Wolbachia fig wasp [22], ants [23], butterflies [24] and bees [25].
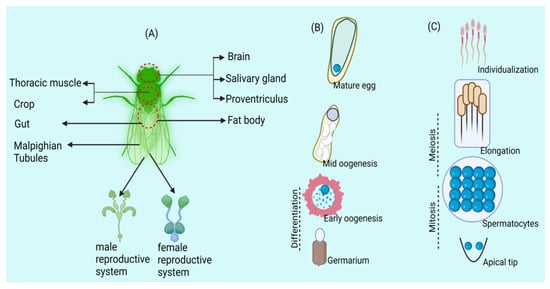
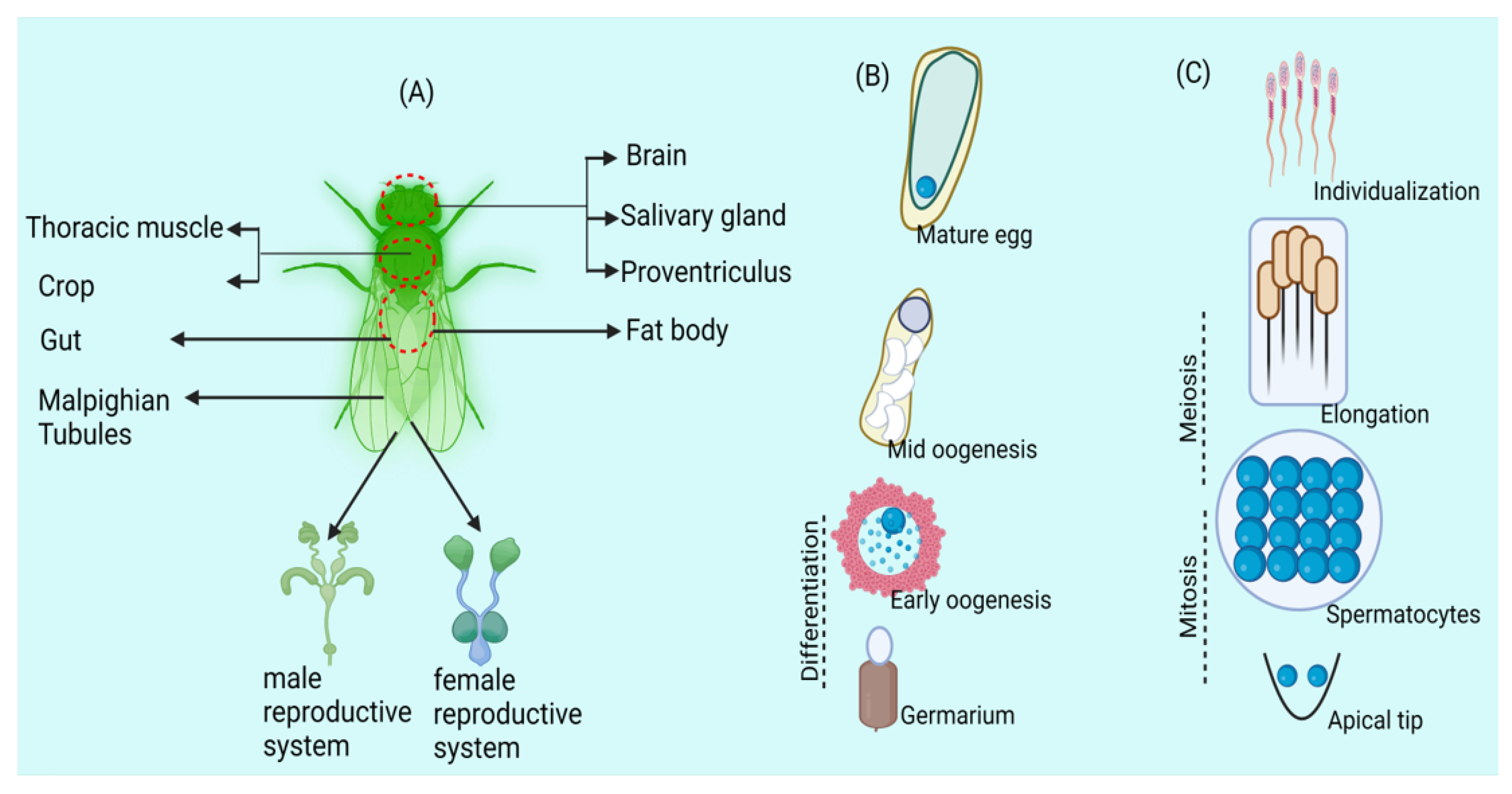
Figure 1.
Wolbachia exhibits tissue tropism in arthropods, influencing germline stem cells. (A) The arthropod host Drosophila has Wolbachia labelled in its somatic and reproductive tissues. (B) Wolbachia in Drosophila oogenesis: Germline stem cells host infection, influencing egg chamber formation. Microtubules aid oocyte entry; posterior localization persists in mature eggs. (C) Wolbachia in Drosophila spermatogenesis: Germline stem cells carry infection, mitotic divisions yield Wolbachia-uneven spermatocytes, with individualization discarding Wolbachia into a waste bag.
Shifting of host is another hypothesis to justify the prevailing distribution of Wolbachia in dearth of co-diversification [26]. It shifts hosts, preferably through horizontal transfer when a strain of Wolbachia infects a new species of arthropod [27] and, at times, possibly through the process of hybridization [28,29]. It has been confirmed through various transmission studies that the event of host switching occurs by artificial inoculation of an uninfected species by a strain of Wolbachia [30], and the presence of “super predator strains” that have the potential to infect hosts distantly related in phylogeny (e.g., ST41 strain type in Lepidoptera) [31]. The transmission of Wolbachia physically to an uninfected host from the infected is the initial step during host switching, accomplished through different means of transmission, and is mostly assisted through biological vectors or an appropriate environmental factor [32,33]. The various means of transmission reported until date include the interaction of host parasites [33,34,35], the interaction of predator prey [36], and usual food sharing [37].
The interaction of a number of factors governs the population density of Wolbachia within a host population. These factors involve the sex, development stages, species involved, host genotypes, environmental factors, and strains of Wolbachia [38,39,40]. It is therefore very important to characterize the population density of Wolbachia and related factors to have an insight into the interaction between the symbiont bacteria and their hosts; the data so collected might also facilitate the standardization of management. The species of leaf hopper Yamatotettix flavovittatus is known as the vector of a phytoplasma disease of sugarcane called white leaf disease [41]. It is one of the most serious concerns about the sugarcane crop, causing appreciable yield destruction in Southeast Asian sugarcane producing countries [42,43]. Infections of Wolbachia with a very high frequency (>80%) have been recorded in the leafhopper natural population [44]. Furthermore, perfect vertical transfer and incompatibility in reproduction resulted from Wolbachia infections in leaf hoppers [45].
Parasitism of the reproductive system is the most widely reported impact of Wolbachia in arthropods, involving the alteration of reproduction in hosts that favour the persistence and transmission of symbionts generally through the increase in the relative population of bacterium in females of infected and uninfected hosts. Among the sexes, females typically transfer Wolbachia and the related heritable bacteria, though exceptions may exist but are very rare [46,47,48]. All four types of alteration of reproductive systems are involved in the case of Wolbachia [15,49,50]. In orders Lepidoptera, Isopoda and Hemipteran, genetic males, through the process of Feminization, convert into functionally active females. Parthenogenesis is triggered by Wolbachia in haplodiploid arthropod hosts such as Hymenoptera, Thysanoptera and Acari, in which females are produced from unfertilized eggs that were supposed to develop into males. To favour surviving female siblings infected with bacteria, the infected males occur during Male killing and are reported in Diptera, Pseudoscorpionida, Lepidoptera and Coleoptera. In cytoplasmic incompatibility (CI) [51], infected males are prevented from producing viable progenies on mating with uninfected females (missing Wolbachia or its compatible strains), which is believed to be the most widely occurring reproductive manipulation triggered by Wolbachia and is reported in Coleoptera, Isopoda, Acari, Hemiptera, Hymenoptera, Diptera, Orthoptera and Lepidoptera. The purpose of this review is to improve our understanding of Wolbachia ecological dynamics and its use in insect population control by highlighting important research gaps and outlining future initiatives. In order to ensure a more thorough knowledge of Wolbachia involvement across a variety of insect hosts, we suggest possible topics for inquiry and emphasise the necessity for joint efforts to overcome problems and develop techniques.
2. Wolbachia Biology: Host Ecology, Diversity, and Genomics
Alphaproteobacterium Wolbachia is a vital member of symbiotic microbes throughout the globe [1,2]. Wolbachia, a class of symbiont bacteria, is subdivided into various super groups A, B, E, H, and F with a variety of associations with host vectors, like WPip in Culex pipiens, WalbA/WalbB in Aedes albopictus, and Wmel in Drosophila melanogaster [52,53,54]. Various species of Wolbachia such as WRi, WPip, WBm, and wMel, have had their morphologies clarified during the genetic investigations [55]. Appreciable control has been revealed against RNA viruses by some of these strains, whereas few others had impacted the mosquito’s lifespan. These distinctions are related to environmental features and adaptability to hosts [56,57]. The genome of WMelPop is worth mentioning on account of its CG-rich sequence bias, rendering it highly pathogenic. Reproductive parasitism of Wolbachia relies on its intimacy in cytoplasmic incompatibility, which involves unidirectional as well as bidirectional mechanisms [58]. Intrinsic genes of immunity and those related to structural, metabolic and stress-linked mechanisms are also regulated by the bacteria [59]. Cases have been observed of both horizontal and vertical transmission of host reproductive alterations that involve feminization, male death, parthenogenesis, and cytoplasmic incompatibility [60,61,62,63,64]. Convincing results were revealed by Wolbachia in managing arthropod vectors. However, its impact on the native confrontation among males should also be considered [65]. Wolbachia has a vital ecological relationship with the host due to its eclectic effects on complex genetic features, reproductive alterations, and hosts. Its effective application in the management of disease vectors and other areas is emphasized due to all these important features.
Wolbachia is a universal facultative symbiotic bacterium that plays a vital role in arthropods and insects within the intricate domain of bacteria transmitted maternally. These inherent symbionts, commonly invading arthropods, and insects, are vital in tailoring the ecology of the host. They are highly dependent for their transmission and survival on hosts when transmitted vertically (Table 1), with affiliations ranging from obligate (concerning nutrition) to facultative (involving mutualism in parasitic relationships) [14,66,67]. In this domain, Wolbachia enjoys the key position as the most widely occurring facultative symbiont for a period as long as 200 million years ago [68]. Genus Alphaproteobacteria, a diverse group of intracellular G-ve bacteria, belongs to the order Rickettsiales and has affiliations with filarial nematodes and arthropods. Wolbachia has small, unique spherical cells, occupies testes and ovaries predominantly, and is associated with the female germline [69,70]. It has a ubiquitous presence in the insect species infecting 40 to 66% of the population with a range of prevalence and infection over time and space within insect populations [13,71,72]. The multiplex interrelationship that is present between Wolbachia and insect hosts spotlights the intrinsic symbiotic relationships that have repercussions both for the symbionts as well as for arthropod hosts under various ecological environments.

Table 1.
A thorough explanation of the host impacts and ways that Wolbachia spreads throughout several insect species in a range of settings and interactions.
Genus Wolbachia, belonging to the family Rickettsaceae of the phylum Alphaproteobacteria, was identified for the first time in a mosquito (Culex pipiens) by Hertig and Wolbachia in 1924. Although initially there were many named taxa, Wolbachia primarily attributes to Wolbachia pipientis. Specific genome (DNA) sequences are targeted for recognition of the different isolates of Wolbachia, such as 16S rDNA, ftsZ, wsp and groEl [85]. Employing these genes, isolates were categorized into eight super groups (A, B, C, D, E, F, G, H), with each group having a unique host proclivity [86]. One must be very cautious while interpreting the phylogeny between Wolbachia isolates, taking into account the potential recombination and gene selection [87,88,89].
Initially, the Wolbachia’s genetic diversity was conducted based on the 16S rRNA gene [90] as well as wsp (the variable surface protein gene) [86]. As a result of extensive recombination in the wsp gene and the challenges faced in strains resolution and phylogenies due to reduced pace of 16S rRNA evolutionary rate, the MLST (multilocus sequence typing) system was introduced and established, involving housekeeping conserved genes [91]. All reported strains of Wolbachia were classified into at least 17 different (A–F, H–Q, and S) phylogenetic super groups, with most of the sequences from A and B super group strains [92]. With latest developments in the target enrichment protocols we are still in the phase of having a good insight on the genetic diversity [93,94]. The genetic landscape of Wolbachia is very complicated, as it is divided into various super groups based on the DNA sequences of few genes. To this group, the primary representative is Wolbachia pipientis. A two-tier method has been suggested to improve the clarity of phylogeny, exploring the evolution of Wolbachia, and to figure out the complicated relationships with reproduction in arthropod host. The present genetic research work being conducted on Wolbachia is helping to gain a clear understanding of the diversity of symbionts among the host arthropod species.
The intrinsic categorization of Wolbachia into fourteen super groups (A to O) is based on molecular characterization of important genes, such as multilocus sequence typing (MLST) loci, 16S rRNA gene, and wsp (surface protein gene) of Wolbachia [91]. Among the fourteen classified super groups (A to O) across different host taxa, most of the arthropod-related strains are in super groups A and B, nematode-associated strains are grouped in super groups C and D, whereas the rest of the super groups were observed infecting diverse hosts [95]. The latest developments in technologies employed for sequencing the DNA have facilitated the complete genome sequencing of Wolbachia strains with the complete sequence of wMel strain infecting Drosophila melanogaster to start with [96]. Strain wMel belongs to the super group A and has disclosed as little as 1.3 Mb (mega base) genome littered with free-moving genetic components.
Further insight on the host biology influencing genes and differences specific to strains was provided on subsequent sequencing of strains wAlbB of Aedes albopictus [97], wRi of Drosophila simulans [98], wPip of Culex pipiens [60] and wBm of Brugia malayi [99,100,101,102]. The data bank now has the genome sequence of more than one hundred genomes of Wolbachia infecting a variety of nematode and arthropod species [103]. Importantly, the developments in the field of DNA sequencing resulted in a change in the research field of Wolbachia that has unearthed complete genomes of many strains, including the wMel groundbreaking strain infecting D. Melanogaster. The research findings have laid the ground for comprehensive analysis that provides detailed insight into each specific strain, key genes that affect the symbiont–host interactions, and the complicated genome picture that is the basis of coevolution of Wolbachia, along with different nematode and arthropod hosts.
3. Wolbachia for Nature’s Collaborations to Control Insect Populations
In quest for innovative control strategies, Wolbachia proved to be an efficient as a biocontrol of mosquitoes, as it not only degrades the competency of vector but also exhibits appreciable amount of maternal transmission in various species like St. aegypti. The promising occurrence of the wAlbB strain in St. aegypti was observed in the case of embryo cytoplasm transfer from related mosquito specie (St. albopicta) [104]. Later, trans-infection involving wMel and wMelPop strains infecting D. melanogaster drastically reduced the St. aegypti’s competency for DENV during a laboratory assay [105,106,107]. The appreciable impact of Wolbachia stretches further to inhibit DENV in saliva of mosquitos, hence revealing its potential as a biocontrol agent. It is obligatory to invade the population of wild mosquitoes for efficient biocontrol. The infected females must transmit Wolbachia vertically (Table 1) at a very high rate to the offspring. Incredibly, all three strains of Wolbachia viz wMel, wAlbB, and wMelPop display a very high frequency of female transmission (close to 100%), and trigger increased CI (cytoplasmic incompatibility) (Table 2) in St. aegypti [104,105].

Table 2.
An Extensive Review of Wolbachia Action Mechanisms and Their Effects on Host Physiology.
The increasing risk of vector-transmitted diseases demands significant investments in devising the alternative means of control. Biological control is among the oldest approaches recorded, ever since the ancient China, having the potential to address concurrent challenges. Deciphering the coordination between biocontrol and population cognizance, collective approaches appears more efficient in managing vector-transmitted diseases [122,123]. Wolbachia, particularly, protrudes in the management of Aedes mosquito population, extending a viable solution to the proliferating threat of arboviruses. In this context, the use of a chemical, such as larvicide, provides a meaningful solution to control mosquitos during the initial phase when young, but has limitations while combating the adults at the stage during which the vectors actually transmit virus. Wolbachia surfaces as an environmentally friendly and efficient substitute to control mosquitoes. The invaded mosquitoes not only furnish the management of arbovirus but also play a key role in controlling other similarly transmitted pathologies, and hence are instrumental for public heath safety [111,124].
The emerging realm of Wolbachia research, specifically against dengue and other related arboviruses, has potential for extended applications to manage different types of pathologies. Microinjection and transinfection are the two primary techniques used that operate as the integral tools. Trans-infection is the simple mechanical transfer of the symbiont however; micro-injection needs a qualified skilled person and also is costly. It is worth mentioning that the strains employed during this process are either extracted from eggs or procured from cell culture [30]. The most widely employed strain is wMel that presents appreciable amount of viability for the field release. wMelPop a transient strain of Wolbachia, has the potential to lowers the fecundity and viability of females of Aedes and hence reduce the population density in a locality. In addition, it has been revealed that strains of symbionts also restrict the multiplication of virus within the host, thereby further enhancing their efficacy as efficient biocontrol [109,125]. The vector competence of DENV is reduced when Wolbachia, applied as biological control agent, efficiently attacks St. aegypti. The strains of Wolbachia (wMel, wAlbB and wMelPop) transinfected result in cytoplasmic incompatibility and exhibit appreciable amount of maternal transmission. No matter when the vector-transmitted diseases are getting a big menace, Wolabachia offers an efficient and environmentally friendly tool of vector control. This, along with conventional biocontrol strategies, enhances the efficiency of disease management. Their methods of application such as transinfection have exhibited inspiring potentials for extensive pathogen management.
4. Wolbachia Dynamics in Lepidopteran and Dipteran Insects: Geographic Structure and Male Killing Aspects
The order of Lepidoptera contains many pests of agricultural significance besides containing modal species across the disciplines of biology [126]. Moreover, larvae of lepidopteran also act as hosts for parasitic wasps and flies [127,128]. It is a diverse group of insects and many of these have affiliations with other organisms however, there is very little know how about the bacterial populations affiliated with the members of order Lepidoptera. There is evidence of having mutualistic affiliations yet it is established that the Wolbachia has parasitic association with its arthropod host’s reproductive system [15]. It has both mutualistic as well as parasitic relationships with Lepidoptera hosts. Cytoplasmic incompatibility, androcide and feminization are the well established impacts of Wolbachia on Lepidopteran’s hosts reproductive system [129,130,131,132,133,134]. It has been reported that a species of Wolbachia is known to increase susceptibility of a lepidopteran host against baculoviruses, depicting it as an efficient biocontrol agent against Spodoptera exempta. The present study revealed an infection of a high proportion of Lepidopteran pests with Wolbachia, exhibiting the potential of Wolbachia in butterfly and moth biology [15].
It has been reported in an extensive survey that among 300 reported species of Lepidopteran, 43% were found invaded with Wolbachia. The reported figures noticeably exceed the previous regional reports of 16.2% infection frequency reported from Panama (n = 43 species [135]), 14.3% in the US (n = 21 [136]), 35.2% in the UK (n = 34 [137]), 45% in Japan (n = 49 [138]), 17% in Uganda (n = 24 [139]), 52% in India (n = 56 [140], and 43% in Western Europe (n = 7 [141]). It has been reported during a study of prevalence and distribution across the species that huge population of Lepidoptera are invaded with Wolbachia (approx. 80%), which is much higher than the estimated frequency across the insects when taken together [14,67]. Globally, there is an appreciable correlation between the host geography and the frequency of Wolbachia’s infection. The infection rate is usually high at lesser latitudes, depicting a tendency for warm temperatures. These findings were in contrast to some previous studies, where varied infection rates were recorded with no clear-cut correlation to the climatic conditions [142,143,144,145]. There was evidence that with an increased temperature, there was a reduction in infections caused by Wolbachia [146,147,148,149]. A significant prevalence of Wolbachia has been revealed in Lepidoptera during global investigations, defying preliminary findings. Additionally, besides many impacts of Wolbachia on Lepidoptera, the prevalent frequency of symbiont and geography of the arthropod hosts emphasises the complexity of the correlation and demands a dire need for further research to have a comprehensive insight of its subtleness.
Higher number of female infected offspring is produced as a result of infection due to Wolbachia through the process of feminization, parthenogenesis, male killing, or cytoplasmic incompatibility. Theses reported alterations are believed to be adaptive that promote the population of Wolbachia [4,5]. The elements of Wolbachia that were involved in cytoplasmic incompatibility of Drosophila melanogaster have been characterized recently However, the part of the genome responsible for governing the other three alterations is still unknown. The latest comparative genomics strategy recognized a candidate gene involved in male killing, which was found conserved in various male-killing strains of Wolbachia. The putative gene wmk (WO-mediated killing) encodes a presumed transcription factor having two DNA helix-turn-helix binding domains and is present within the eukaryotic affiliation module of Wolbachia prophage WO in numerous strains of Wolbachia so are the CI genes cifA as well as cifB [150,151]. The expression of wmk (codon optimized) trans-genetically in D. melanogaster triggers a number of cytological changes that ultimately results in the death of an embryo and also induces an appreciable female bias (average sex ratio of male: female is 0.65:1).
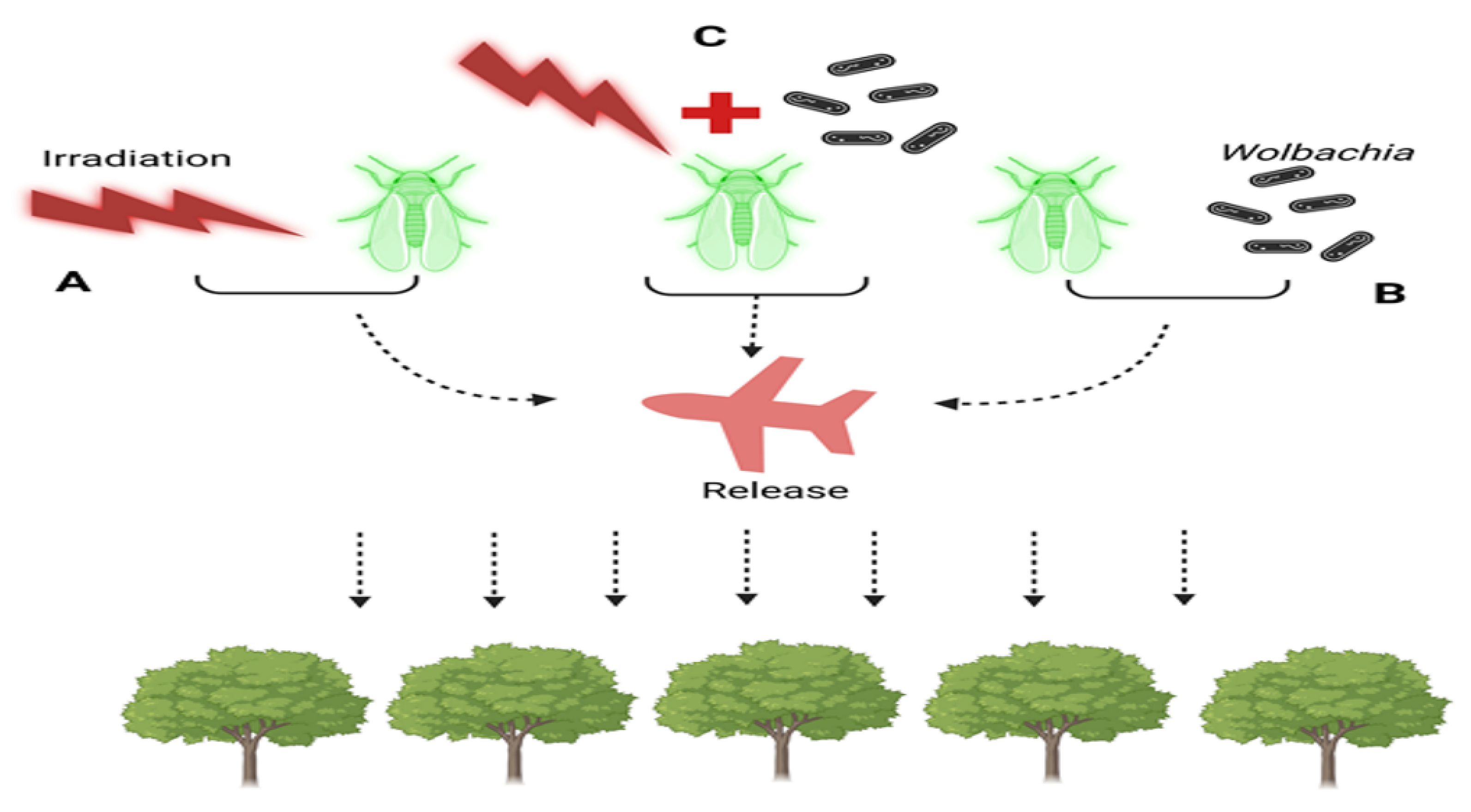
A species-specific and ecologically friendly method for controlling or eliminating pest populations is the Sterile Insect Technique (SIT), as seen in (Figure 2). Using this technique, sterile insects that are mass-produced are repeatedly released. Although successful deployments of both sexes have been reported, the majority of the insects released are males. Ionising radiation is the main method used to sterilise sperm, causing dominantly fatal mutations. To put it briefly, the SIT entails mass raising of the target species, releasing males (and maybe females) into the target region after separating and sterilising them. The treatment of D. suzukii is based on the SIT principles, which are illustrated in (Figure 2).
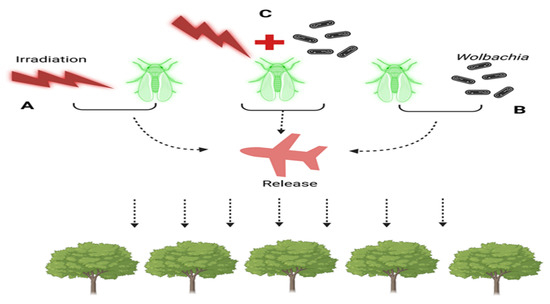
Figure 2.
Strategies for Population Control in Sterile Insect Techniques (SIT) and Incompatible Insect Techniques (IIT): (A) Sterile Insect Technique (SIT): Males undergo sterilization through irradiation. (B) Incompatible Insect Technique (IIT): Males are sterilized through Wolbachia trans-infection. (C) Combination of SIT and IIT: male sterility is achieved through both irradiation and Wolbachia infection. In all three cases (A–C), sterilized males are released into the field to target and sterilize wild females of the population.
We thoroughly examined the tenets of the Incompatible IIT and SIT, investigating their possible application in the control of Drosophila suzukii. To successfully decrease the D. suzukii populations, we suggest including SIT as a crucial component of an area-wide integrated pest-control plan, taking into account the limitations posed by SIT and IIT [152]. Male killing has also been observed quite often in Lepidopterans (moths, butterflies, fruit flies). A very novel type of Wolbachia-triggered male killing has been reported in Ostrinia scapulalis and its related specie O. furnacalis [153,154], which is quite different from the male killings observed in other types of moths and butterflies [155,156]. The killing of Wolbachia by application of antibiotics resulted in all male progenies [153,154], revealing that the Wolbachia possessed the factor responsible for inhibiting masculinization or feminization in Ostrinia. Furthermore, the feminization factor in Ostrinia itself might have been disintegrated or hampered as a result of extended periods of invasion by male killing strains of Wolbachia. A protein of Wolbachia, Oscar’s, was manifested to engage with Masc through its Ankyrin repeats. The embryonic expression of Oscar’s has been revealed to hamper the masculinization trigger of Masc in two Lepidoptera insects: Ostrinia furnacalis and silkworm (Bombyx mori) resulting in the death of males [157]. Male death caused by Wolbachia is accomplished through a number of alterations besides other reproductive abnormalities occurring in Lepidoptera and Drosophila hosts involving the Oscar protein and discovery of candidate gene wmk (WO-moderated killing).
5. The Function of Scale Insect Companion Species in Wolbachia Host Fluctuations
The present study, using the superfamily Coccoidea as a model system, reveals the dynamics of Wolbachia in scale insects by exploring the complicated world of host switching. The super family Coccoidea (the scale insects) is distributed across the globe with >8200 species and 24 families [158,159]. Scale insects, just like numerous other Sternorrhyncha suborder members (including whiteflies, psyllids and aphids), feed exclusively on plants and are believed to be serious pests of agricultural crops [160]. They have been found in association with a large number of other arthropod species. Particularly, a number of these have been found in proximity with ants owing to trophallaxis (ants are attracted to the honeydew of scale insects) [161,162]. Although there are numerous similarities with other hemipterans, it has been reported that many of the species are anticipated to have small-to-moderate prevalence of Wolbachia [26] contrary to a u-shaped (majority of the species have very high or very low prevalence) predicted distribution reported for other groups [67]. The positive interaction between ants and their associated Wolbachia strains infecting scale insects also indicates a credible transfer route [26].
These preclusive data reveal a wider view of the dynamics of Wolbachia infection in scale insects and hence encourages us to explore strain diversity of Wolbachia and henceforth host-switching in scale insects. Another probable justification for having a weak impact of host geographical distance on Wolbachia sharing is host-switching through ecological vectors. These vectors may cause either temporary or permanent infection and transfer it to a far geographical place to recipient species from the donor species. There are many indirect or direct pathways that may act as the routes of transfer of Wolbachia from prey-predator [36,163] to host-parasitoid [164,165] and trophallaxis interactions [166]. Familiarity of physical direct interactions of scale insects and ants may furnish a mean for exchange of microbes as observed in other hemipteran groups [167,168]. Furthermore, it has been observed that credible means of horizontal transfer of Cardinium do exist between parasitoid and scale insects [169]. These ecological routes may have been used by Wolbachia for its spread within the communities of scale insects. The positive association of Wolbachia infection within members of scale insects and the associates exhibited that the ants might have a role in host-switching [170]. This innovative research provided the initial data about the strains of Wolbachia in scale insects and the noteworthy frequency of confected samples. It is interesting that wSph 1 was recorded as the most frequently observed strain of Wolbachia in scale insects. In addition, it was observed that distance effect of host phylogeny is very important for promoting host switching in scale insects [171]. These findings help to unveil the complicated interactions observed among the scale insects.
6. The Use of Wolbachia in Leafhopper Vectors to Regulate Plant Hopper Populations
The plant hoppers are among the most threatening and destructive pest of rice. They suck the sap and oviposit the tissues of rice, and hence are a serious threat to rice production. Besides infestations of rice crop, these plant hoppers are also the vectors of various important plant viruses, including rice black-streaked dwarf virus, southern rice black-streaked dwarf virus rice stripe virus, grassy stunt virus, and rugged stunt virus [172]. The successful control of mosquitoes through Wolbachia prompts the application of a similar approach to manage plant hoppers. The sugarcane white leaf disease caused by a phytoplasma is also vectored by leafhopper Yamatotettix flavovittatus Matsumura (Hemiptera: Cicadellidae) [41]. It is believed to be among the most serious issues of sugarcane production resulting in significant yield losses in many sugarcane cultivating areas of Southeast Asia [42,43]. In natural populations of leafhoppers Wolbachia infection has been reported with high frequencies (>80%) [44]. Furthermore, the reproductive incompatibility along with the immaculate vertical transmission in leaf hoppers were caused after infection with Wolbachia [45]. The results stresses on the probability of future prospects of management of leafhoppers using Wolbachia. Research on the quantity of Wolbachia seeks to gain insight on the relationship of a symbiont with its hosts. To investigate the number of Wolbachia, the wsp gene was chosen to express the surface protein and measure Wolachia through qPCR. On the basis of the fact that a single copy of the wsp gene is present in each of the genomes, its absolute copy number was measured to predict the number of Wolbachia. The same method has been employed to determine the population of Wolbachia in many arthropod hosts [173,174,175]. D. citri, the psyllid, the population density of Wolbachia was raised during the succeeding nymphal instars [176]. The adults and nymphs of leafhopper Wolbachia concentrated and localized in bacteriomes [175] and hence, its multiplication was hampered by the available space in cells or the tissues being invaded. The latest research findings revealed that Wolbachia furnishes beneficent impacts to BPH (Brown Plant hopper). There is a high rate of egg production in BPH females infected with Wolbachia compared to uninfected females.
Nevertheless, the lifespan of BPH infected with Wolbachia is quite shorter than the uninfected BPHs, which might also explain the production of a very high number of eggs and lesser prevalence of the symbiont in the wild type BPH. Analogous to the BPH, Wolbachia also significantly enhanced the fecundity of SBPH (Small Brown Plant Hopper), which may also be affiliated with the increased number of Ovarioles containing mitotic germ cells and apoptotic nurse cells [177,178]. Additionally, Wolbachia impacts the expression of miRNA in SBPH to manipulate the gene expression associated with fecundity [179]. More genomic and experimental testimonies established that Wolbachia improves the fecundity of SBPH and BPH females through synthesis of key nutrients riboflavin and biotin [180]. This symbiont is an encouraging tool to devise efficient management practices against important agricultural pests, as is evident from the divergent impacts it has on arthropod vectors including leafhoppers (Yamatotettix flavovittatus) and plant hoppers of rice. The detrimental impacts range from ideal vertical transfer and incompatibility of the reproduction system to impacting fecundity via the synthesis of nutrients.
7. Future Prospective and Challenges
The control strategies of Wolbachia-based systems have been shown to be effective, and their application might lead to a drastically different and dynamic environment. Decoding the complex of changing aspects of Wolbachia in a variety of insect orders, such as dipterans, lepidopterans and hemipterans, is one major area of research. It examines control strategies, with a focus on the incompatible IIT and SIT. To overcome obstacles and efficiently manage D. suzukii populations, it is recommended that SIT be included in area-wide management and that the contingency plan of merging SIT/IIT be taken into consideration. A potential new approach that combines these two methods has been created and is presently being evaluated in open-field experiments against populations of Aedes mosquitoes.
This has indicated the necessity of funding efficient control measures. Biological control vector techniques are one of these acts, and Wolbachia has proven to have beneficial potential in the agriculture field. The efficacy of Wolbachia-based control relies on maternal transmission and induced cytoplasmic incompatibility, ensuring rapid spread through the pest population. Despite its success in mosquito control, progress has extended to protecting plants from rice pests. This synthesis explores Wolbachia phenotypic effects in mosquito control and its interactions with rice pest plant hopper, aiming to bridge findings from mosquito programs to potential applications in plant hopper control.
Wolbachia are ubiquitous endosymbiotic bacteria that are highly prevalent, especially in arthropods, because they can generate a variety of phenotypes in their hosts and move between host species with ease. Although the phenotypic impacts have been better understood, there is still much to learn about the processes underpinning Wolbachia host-shifting. A significant incidence of co-infected samples was shown by a recent study on the variety of Wolbachia strains in scale insects, with a particular emphasis on the common wSph1 strain. Produce that the host’s evolutionary distance was a significant factor affecting the host-shifting behaviour of scale insects, suggesting possible transmission pathways and possible host changes. This highlights the significance of ecological connectedness in the complex dynamics of Wolbachia host-shifting and lays the groundwork for further worldwide research investigating similar phenomena. The research’s suggested technique may be applied to larger datasets, providing information on the variables affecting Wolbachia host changes in various environments.
Maternally inherited endosymbionts, known as Wolbachia, are ubiquitous in the animal kingdom and exhibit amazing mutualistic and parasitic adaptations. A centennial investigation shows Wolbachia global abundance in nematodes and arthropods, establishing it as a key paradigm for studying symbiosis and fighting illnesses in humans and agriculture. The host range, phylogenetic diversity, genomics, and medical uses of Wolbachia are all included in this synthesis. The importance of the mobilome, in particular phage WO, for reproductive phenotypes is discussed. The system is a shining example of science education that is focused on discovery. Manuals on symbiosis may change as a result of recent developments in multiomics, gene functional assays, and human health applications. The molecular underpinnings of reproductive parasitism, genetic engineering for reductionist research, and the influence of Wolbachia on human illnesses and speciation are among the concerns of the future. The coming century promises revolutionary advancements, emphasizing the evolution of Wolbachia from a scientific curiosity to a model for microbial symbiosis and showing how fundamental science contributes to beneficial outcomes in education and translational research.
8. Conclusions
In conclusion, the influence of Wolbachia on evolution is highlighted by its ability to manipulate insect sex determination systems, as shown by the new protein Oscar. Wolbachia prevalence in Lepidoptera indicates predictability based on ecological parameters, with a latitudinal gradient and regional variance. The commercial fruit pest Drosophila suzukii may be managed with the use of the IIT and SIT. The ability of Wolbachia to suppress pests is dependent on both cytoplasmic incompatibility and maternal transmission. Using Wolbachia-based methods to protect plants from pests goes beyond only controlling mosquitoes. Molecular biology, ecology, and public policy must be combined by interdisciplinary teams in order to fully realise the potential of Wolbachia-based therapeutics.
Author Contributions
The first draft was written by M.H. and A.M.L., who also made the tables. The document was rewritten several times before it was finalized by J.G., A.B. and R.M. The data were prepared by Z.W. After reading the published version of the article, all writers gave their approval. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the GDAS Special Project of Science and Technology Development (2022GDASZH-2022010106, 2022GDASZH-2022030501-08), National Science Foundation of China (32202276), Guangdong Province Rural Science and Technology Commissioner Project (KTP20210352), and the Open Competition Program of Top Ten Critical Priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2023SDZG06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of Holobionts and Hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- McFall-Ngai, M. Are biologists in ‘‘future shock’’? Symbiosis integrates biology across domains. Nat. Rev. Microbiol. 2008, 6, 789–792. [Google Scholar] [CrossRef]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-like micro-organisms in insects. J. Med. Res. 1924, 44, 329–374.7. [Google Scholar]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Frost, C.L. Reproductive parasitism: Maternally inherited symbionts in a biparental world. Cold Spring Harb. Perspect. Biol. 2015, 7, a017699. [Google Scholar]
- Bliman, P.A.; Aronna, M.S.; Coelho, F.C.; Da Silva, M.A.H.B. Ensuring successful introduction of Wolbachia in natural populations of Aedes aegypti by means of feedback control. J. Math. Biol. 2018, 76, 1269–1300. [Google Scholar] [CrossRef]
- Kean, J.; Rainey, S.M.; Mcfarlane, M.; Donald, C.L.; Schnettler, E.; Kohl, A.; Pondeville, E. Fighting arbovirus transmission: Natural and engineered control of vector competence in Aedes mosquitoes. Insects 2015, 1, 236–278. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Walker, T.; O’Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef]
- Aliota, M.T.; Walker, E.C.; Yepes, A.U.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef] [PubMed]
- Hertig, M. The rickettsia, Wolbachia pipientis and associated inclusions of the mosquito, Culex Pipiens. Parasitology 1936, 28, 453–486. [Google Scholar] [CrossRef]
- Sironi, M.; Bandi, C.; Sacchi, L.; Di, B.S.; Damiani, G.; Genchi, C. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in a filarial worm. Mol. Biochem. Parasitol. 1995, 74, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H. Biology of wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150249. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Ross, P.A.; Rašić, G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015, 8, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary ecology of Wolbachia releases for disease control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef]
- Charleston, M.A.; Perkins, S.L. Traversing the tangle: Algorithms and applications for cophylogenetic studies. J. Bio. Infor. 2006, 39, 62–71. [Google Scholar] [CrossRef]
- Thompson, J.N. Symbiont-induced speciation. Biol. J. Linn. Soc. 1987, 32, 385–393. [Google Scholar] [CrossRef]
- Fenn, K.; Blaxter, M. Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol. Evol. 2004, 19, 163–166. [Google Scholar] [CrossRef]
- Balvín, O.; Roth, S.; Talbot, B.; Reinhardt, K. Co-speciation in bedbug Wolbachia parallel the pattern in nematode hosts. Sci. Rep. 2018, 8, 8797. [Google Scholar] [CrossRef]
- Shoemaker, D.D.; Machado, C.A.; Molbo, D.; Werren, J.H.; Windsor, D.M.; Herre, E.A. The distribution of Wolbachia in fig wasps: Correlations with host phylogeny, ecology and population structure. Proc. R. Soc. Lond. B Biol. 2002, 269, 2257–2267. [Google Scholar] [CrossRef]
- Frost, C.L.; Fernandez-Marin, H.; Smith, J.E.; Hughes, W.O.H. Multiple gains and losses of Wolbachia symbionts across a tribe of fungus-growing ants. Mol. Ecol. 2010, 19, 4077–4085. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Breinholt, J.W.; Kawahara, A.Y. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evol. Biol. 2016, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Gerth, M.; Röthe, J.; Bleidorn, C. Tracing horizontal Wolbachia movements among bees (Anthophila): A combined approach using multilocus sequence typing data and host phylogeny. Mol. Ecol. 2013, 22, 6149–6162. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, E.; Charlat, S.; Engelstädter, J. Wolbachia host shifts: Routes, mechanisms, constraints and evolutionary consequences. Biol. Rev. 2021, 96, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Heath, B.D.; Butcher, R.D.J.; Whitfield, W.G.F.; Hubbard, S.F. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr. Biol. 2021, 9, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Vanderpool, D.; Conner, W.R.; Matute, D.R.; Turelli, M. Wolbachia acquisition by drosophila yakuba-clade hosts and transfer of incompatibility loci between distantly related Wolbachia. Genetics 2019, 212, 1399–1419. [Google Scholar] [CrossRef] [PubMed]
- Turelli, M.; Cooper, B.S.; Richardson, K.M.; Ginsberg, P.S.; Peckenpaugh, B.; Antelope, C.X.; Kim, K.J.; May, M.R.; Abrieux, A.; Wilson, D.A.; et al. Rapid global spread of wRi-like Wolbachia across multiple drosophila. Curr. Biol. 2018, 28, 963–971.e8. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigate Wolbachia–host interactions and control arthropodborne disease. Insect Mol. Biol. 2015, 23, 141–151. [Google Scholar] [CrossRef]
- Ilinsky, Y.; Kosterin, O.E. Molecular diversity of Wolbachia in Lepidoptera: Prevalent allelic content and high recombination of MLST genes. Mol. Phylogenetics Evol. 2017, 109, 164–179. [Google Scholar] [CrossRef]
- Riegler, M.; Charlat, S.; Stauffer, C.; Merçot, H. Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: Investigating the outcomes of host-symbiont coevolution. Appl. Environ. Microbiol. 2004, 70, 273–279. [Google Scholar] [CrossRef]
- Vavre, F.; Fleury, F.; Lepetit, D.; Fouillet, P.; Boulétreau, M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 1999, 16, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Li, S.J.; Xue, X.; Yin, X.J.; Ren, S.X.; Jiggins, F.M.; Greeff, J.M.; Qiu, B.L. The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 2015, 11, e1004672. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.M.; Butcher, R.D.J. The transmission and effects of Wolbachia bacteria in parasitoids. Res. Popul. Ecol. 1999, 41, 15–28. [Google Scholar] [CrossRef]
- Le Clec’h, W.; Chevalier, F.D.; Genty, L.; Bertaux, J.; Bouchon, D.; Sicard, M. Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS ONE 2013, 8, e60232. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Ahmed, M.Z.; Lv, N.; Shi, P.Q.; Wang, X.M.; Huang, J.L.; Qiu, B.L. Plantmediated horizontal transmission of Wolbachia between whiteflies. ISME J. 2017, 11, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Mouton, L.H.; Henri, M.; Bouletreau Vavre, F. Strain-specifc regulation of intracellular Wolbachia density in multiply infected insects. Mol. Ecol. 2003, 12, 3459–3465. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.C.; Ballard, J.W. Wolbachia gonadal density in female and male Drosophila vary with laboratory adaptation and respond differently to physiological and environmental challenges. J. Invertebr. Pathol. 2012, 111, 197–204. [Google Scholar] [CrossRef] [PubMed]
- López-Madrigal, S.; Duarte, E.H. Titer regulation in arthropod Wolbachia symbioses. FEMS Microbiol. Lett. 2019, 366, 1–9. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Ritthison, W.; Choosai, C.; Sirithorn, P. Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector. J. Econ. Entomol. 2006, 99, 1531–1537. [Google Scholar] [CrossRef]
- Thein, M.M.; Jamjanya, T.; Kobori, Y.; Hanboonsong, Y. Dispersal of the leafhoppers Matsumuratettix hiroglyphicus and Yamatotettix flavovittatus (Homoptera: Cicadellidae), vectors of sugarcane white leaf disease. Appl. Entomol. Zool. 2012, 47, 255–262. [Google Scholar] [CrossRef]
- Youichi, K.; Hanboonsong, Y. Effect of temperature on the development and reproduction of the sugarcane white leaf insect vector, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae). J. Asia Pac. Entomol. 2017, 20, 281–284. [Google Scholar]
- Wangkeeree, J.; Tewaruxsa, P.; Roddee, J.; Hanboonsong, Y. Wolbachia (Rickettsiales: Alphaproteobacteria) infection in the leafhopper vector of sugarcane white leaf disease. J. Insect Sci. 2020, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Wangkeeree, J.; Suwanchaisri, K.; Roddee, J.; Hanboonsong, Y. Effect of Wolbachia infection states on the life history and reproductive traits of the leafhopper Yamatotettix flavovittatus Matsumura. J. Invertebr. Pathol. 2020, 177, 107490. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Turelli, M. Unidirectional incompatibility in Drosophila simulans: Inheritance, geographic variation and fitness effects. Genetics 1998, 119, 435444. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.; Dunbar, A.H.E. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl. Acad. Sci. USA 2006, 103, 12803–12806. [Google Scholar] [CrossRef] [PubMed]
- Chafee, M.; Funk, E.; Harrison, D.J.; Bordenstein, R.G. Lateral phage transfer in obligate intracellular bacteria (Wolbachia): Verification from natural populations. Mol. Biol. Evol. 2010, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Saridaki, A.; Bourtzis, K. Wolbachia: More than just a bug in insects genitals. Curr. Opin. Microbiol. 2010, 13, 67–72. [Google Scholar] [CrossRef]
- Schneider, D.; Miller, W.J.; Riegler, M. Arthropods shopping for Wolbachia. In Manipulative Tenants Bacteria Associated with Arthropods; Zchori-Fein, E., Bourtzis, K., Eds.; CRC Press: Boca Raton, Fl, USA, 2011; pp. 149–173. [Google Scholar]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef]
- LePage, D.; Bordenstein, S. Wolbachia: Can we save lives with a great pandemic? Trends Parasitol. 2013, 29, 385–393. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2013, 9, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Baton, L.A.; Pacidônio, E.C.; Gonçalves, D.d.S.; Moreira, L.A. WFLU: Characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS ONE 2013, 8, e59619. [Google Scholar] [CrossRef]
- Duplouy, A.; Iturbe-Ormaetxe, I.; A Beatson, S.; Szubert, J.M.; Brownlie, J.C.; McMeniman, C.J.; A McGraw, E.; Hurst, G.D.D.; Charlat, S.; O’neill, S.L.; et al. Draft Genome Sequence of the Male-Killing Wolbachia Strain wBol1 Reveals Recent Horizontal Gene Transfers from Diverse Sources. BMC Genom. 2013, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, F.D.; Robinson, J.; Young, P.R.; McGraw, E.A.; O’Neill, S.L. Wolbachia-Mediated Resistance to Dengue Virus Infection and Death at the Cellular Level. PLoS ONE 2010, 5, e13398. [Google Scholar] [CrossRef]
- Machkovech, H.M.; Bedford, T.; Suchard, M.A.; Bloom, J.D. Positive Selection in CD8+ T-Cell Epitopes of Influenza Virus Nucleoprotein Revealed by a Comparative Analysis of Human and Swine Viral Lineages. J. Virol. 2013, 87, 7066–7078. [Google Scholar] [CrossRef]
- Pan American Health Organization. Evaluation of Innovative Strategies for Aedes aegypti Control: Challenges for their Introduction and Impact Assessment; PAHO: Washington, DC, USA, 2019. [Google Scholar]
- Audsley, M.D.; Ye, Y.H.; McGraw, E.A. The Microbiome Composition of Aedes aegypti is not Critical for Wolbachia-Mediated Inhibition of Dengue Virus. PLoS Neglected Trop. Dis. 2017, 11, e0005426. [Google Scholar] [CrossRef]
- Klasson, L.; Walker, T.; Sebaihia, M.; Sanders, M.J.; Quail, M.A.; Lord, A.; Sanders, S.; Earl, J.; O’Neill, S.L.; Thomson, N.; et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol. Biol. Evol. 2009, 26, 1877–1887. [Google Scholar] [CrossRef]
- Kambris, Z.; Blagborough, A.M.; Pinto, S.B.; Blagrove, M.S.C.; Godfray, H.C.J.; Sinden, R.E.; Sinkins, S.P. Wolbachia Stimulates Immune Gene Expression and Inhibits Plasmodium Development in Anopheles gambiae. PLoS Pathog. 2010, 6, e1001143. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Hochstrasser, M. The Biochemistry of Cytoplasmic Incompatibility Caused by Endosymbiotic Bacteria. Genes 2020, 8, 852. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia Induces Reactive Oxygen Species-Dependent Activation of the Toll Pathway to Control Dengue Virus in the Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Werren, J.H. Bidirectional Incompatibility among divergent Wolbachia and incompatibility level differences among closely related Wolbachia in Nasonia. Heredity 2007, 99, 278–287. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Hurst, G.D. Arthropods and inherited bacteria: From counting the symbionts to understanding how symbionts count. BMC Biol. 2013, 11, 45. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?–A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Gerth, M.; Bleidorn, C. Comparative genomics provides a timeframe for Wolbachia evolution and exposes a recent biotin synthesis operon transfer. Nat. Microbiol. 2016, 2, 16241. [Google Scholar] [CrossRef]
- Harris, H.L.; Brennan, L.J.; Keddie, B.A.; Braig, H.R. Bacterial symbionts in insects: Balancing life and death. Symbiosis 2010, 51, 37–53. [Google Scholar] [CrossRef]
- Sacchi, L.; Genchi, M.; Clementi, E.; Negri, I.; Alma, A.; Ohler, S.; Sassera, D.; Bourtzis, K.; Bandi, C. Bacteriocyte-like cells harbour Wolbachia in the ovary of Drosophila melanogaster (Insecta, Diptera) and Zyginidia pullula (Insecta, Hemiptera). Tissue Cell 2010, 42, 328–333. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.D.; Goncalves, D.S.; Baton, L.A.; Shimabukuro, P.H.; Carvalho, F.D.; Moreira, L.A. Broader prevalence of Wolbachia in insects including potential human disease vectors. Bull. Entomol. Res. 2015, 105, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kriesner, P.; Conner, W.R.; Weeks, A.R.; Turelli, M.; Hoffmann, A.A. Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution 2016, 70, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Han, X.; Hong, X.Y. Various infection status and molecular evidence for horizontal transmission and recombination of Wolbachia and Cardinium among rice planthoppers and related species. Insect Sci. 2013, 20, 329–344. [Google Scholar] [CrossRef]
- Huigens, M.E.; de Almeida, R.P.; Boons, P.A.H.; Luck, R.F.; Stouthamer, R. Natural interspecifc and intraspecifc horizontal transfer of parthenogenesisinducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Gehrer, L.; Vorburger, C. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol. Lett. 2012, 8, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Deng, C.; Yun, Y.L.; Jian, C.; Peng, Y. Molecular detection and the phylogenetics of Wolbachia in Chinese spiders (Araneae). J. Arachnol. 2010, 38, 237–241. [Google Scholar] [CrossRef]
- Goya, L.F.; Lanteri, A.A.; Confalonieri, V.A.; Rodriguero, M.S. New host-parasitoid interactions in Naupactus cervinus (Coleoptera, Curculionidae) raise the question of Wolbachia horizontal transmission. Symbiosis 2022, 86, 325–336. [Google Scholar] [CrossRef]
- Schuler, H.; Bertheau, C.; Egan, S.P.; Feder, J.L.; Riegler, M.; Schlick-Steiner, B.C.; Steiner, F.M.; Johannesen, J.; Kern, P.; Tuba, K.; et al. Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol. Ecol. 2013, 22, 4101–4111. [Google Scholar] [CrossRef]
- Serbina, L.S.; Gajski, D.; Malenovsky, I.; Corretto, E.; Schuler, H.; Dittmer, J. Wolbachia infection dynamics in a natural population of the pear psyllid Cacopsylla pyri (Hemiptera: Psylloidea) across its seasonal generations. Sci. Rep. 2022, 12, 16502. [Google Scholar] [CrossRef] [PubMed]
- Shaikevich, E.; Bogacheva, A.; Rakova, V.; Ganushkina, L.; Ilinsky, Y. Wolbachia symbionts in mosquitoes: Intra- and intersupergroup recombinations, horizontal transmission and evolution. Mol. Phylogenet. Evol. 2019, 134, 24–34. [Google Scholar] [CrossRef]
- Su, Q.H.; Hu, G.W.; Yun, Y.L.; Peng, Y. Horizontal transmission of Wolbachia in Hylyphantes graminicola is more likely via intraspecies than interspecies transfer. Symbiosis 2019, 79, 123–128. [Google Scholar] [CrossRef]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef]
- Fast, E.M.; Toomey, M.E.; Panaram, K.; Desjardins, D.; Kolaczyk, E.D.; Frydman, H.M. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 2011, 334, 990–992. [Google Scholar] [CrossRef]
- Newton, I.L.; Savytskyy, O.; Sheehan, K.B. Wolbachia utilize host actin for efcient maternal transmission in Drosophila melanogaster. PLoS Pathog. 2015, 11, e1004798. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Rosengaus, R.B. Discovery of a novel Wolbachia Supergroup in Isoptera. Curr. Microbiol. 2005, 51, 393–398. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neill, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Jiggins, F.M.; Schulenburg, J.H.G.; Hurst, G.D.D.; Majerus, M.E.N. Recombination confounds interpretations of Wolbachia evolution. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 14231427. [Google Scholar] [CrossRef]
- Werren, J.H.; Bartos, J.D. Recombination in Wolbachia. Curr. Biol. 2001, 11, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bandi, C.; Bourtzis, K.; Braig, H.; Lo, N.; O’Neill, S.; Stouthamer, R.; Vandekerckove, T.T.M.; Werren, J.H. Wolbachia Nomenclatural System: A Discussion Paper. News and Forums. 2003. Available online: www.wolbachia.sols.uq.edu.au/news.cfm?action=thread&id=25 (accessed on 14 February 2024).
- O’Neill, S.L.; Hoffmann, A.A.; Werren, J.H. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction and Development; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Baldo, L.; Dunning Hotopp, J.C.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.J.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Bleidorn, C.; Gerth, M. A critical re-evaluation of multilocus sequence typing (MLST) efforts in Wolbachia. FEMS Microbiol. Ecol. 2017, 94, fix163. [Google Scholar] [CrossRef] [PubMed]
- Hotopp, J.C.D.; Slatko, B.E.; Foster, J.M. Targeted enrichment and sequencing of recent endosymbiont-host lateral gene transfers. Sci. Rep. 2017, 7, 857. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.N.; Bordenstein, S.R. Phage WO of Wolbachia: Lambda of the endosymbiont world. Trends Microbiol. 2010, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beck, C.R.; English, A.C.; Meng, Q.; Buhay, C.; Han, Y.; Doddapaneni, H.V.; Yu, F.; Boerwinkle, E.; Lupski, J.R.; et al. PacBio-LITS: A large-insert targeted sequencing method for characterization of human disease-associated chromosomal structural variations. BMC Genom. 2015, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004, 2, E69. [Google Scholar]
- Mavingui, P.; Valiente Moro, C.V.; Tran-Van, V.; Wisniewski-Dye, F.; Raquin, V.; Minard, G.; Tran, F.H.; Voronin, D.; Rouy, Z.; Bustos, P.; et al. Whole-genome sequence of Wolbachia strain wALbB, an endosymbiont of tiger mosquito vector Aedes albopictus. J. Bacteriol. 2012, 194, 18–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klasson, L.; Westberg, J.; Sapountzis, P.; Naslund, K.; Lutnaes, Y.; Darby, E.A.C.; Veneti, Z.; Chen, L.; Braig, H.R.; Garrett, R.; et al. The mosaicgenome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc. Natl. Acad. Sci. USA 2009, 106, 5725–5730. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef] [PubMed]
- Ishmael, N.; Hotopp, J.C.D.; Ioannidis, P.; Biber, S.; Sakamoto, J.; Siozios, S.; Nene, V.; Werren, J.; Bourtzis, K.; Bordenstein, S.R.; et al. Extensive genomic diversity of closely related Wolbachia strains. Microbiology 2009, 155, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, J.I.; Bordenstein, S.R.; Unckless, R.L.; LePage, D.P.; Metcalf, J.A.; Hill, T.; Martinez, J.; Jiggins, F.M.; Bordenstein, S.R. The phage gene wmk is a candidate for male killing by a bacterial endosymbiont. PLoS Pathog. 2019, 15, e1007936. [Google Scholar] [CrossRef]
- Rice, D.W.; Sheehan, K.B.; Newton, I.L.G. Large-scale identification of Wolbachia pipientis effectors. Genome Biol. Evol. 2017, 9, 1925–1937. [Google Scholar] [CrossRef]
- Scholz, M.; Albanese, D.; Tuohy, K.; Donati, C.; Segata, N.; Rota-Stabelli, O. Large scale genome reconstructions illuminate Wolbachia evolution. Nat. Commun. 2020, 11, 5235. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Marinotti, O.; de Brito, M.; Moreira, C.K. Apyrase and alpha-glucosidase in the salivary glands of Aedes albopictus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 113, 675–679. [Google Scholar] [CrossRef]
- Kremer, N.; Voronin, D.; Charif, D.; Mavingui, P.; Mollereau, B.; Vavre, F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009, 5, e1000630. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White, V.L.; EndersbyHarshman, N.M.; Hofmann, A.A. Wolbachia infections in Aedes aegypti difer markedly in their response to cyclical heat stress. PLoS Pathog. 2017, 13, e1006006. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.H.; Carrasco, A.M.; Dong, Y.; Sgró, C.M.; Mcgraw, E.A. The effect of temperature on Wolbachia-mediated dengue virus blocking in Aedes aegypti. Am. J. Trop. Med. Hyg. 2016, 94, 812. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.; Mcelroy, K.; Day, A.; Higgs, S.; O’neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLOS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- Hussain, M.; Frentiu, F.D.; Moreira, L.A.; O’Neill, S.L.; Asgari, S. Wolbachia uses host MicroRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 9250–9255. [Google Scholar] [CrossRef]
- Moreira, L.A.; Ye, H.Y.; Turner, K.; Eyles, D.W.; Mcgraw, E.A.; O’neill, S.L. The wMelPop strain of Wolbachia interferes with dopamine levels in Aedes aegypti. Parasit. Vectors 2011, 4, 28. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.C.; Moreira, L.A. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microbial Cell 2016, 3, 293–295. [Google Scholar] [CrossRef]
- Pietri, J.E.; Debruhl, H.; Sullivan, W. The rich somatic life of Wolbachia. Microbiol. Open. 2016, 5, 923. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Makepeace, B.L.; Benjamin, L.; Baylis, M.; Solomon, T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 2017, 30, 108–116. [Google Scholar] [CrossRef]
- Caragata, E.P.; Pais, F.S.; Baton, L.A.; Silva, J.B.L.; Sorgine, M.H.F.; Moreira, L.A. The transcriptome of the mosquito Aedes fuviatilis (Diptera: Culicidae), and transcriptional changes associated with its native Wolbachia infection. BMC Genom. 2017, 18, 6. [Google Scholar] [CrossRef]
- Rancés, E.; Yh, Y.E.; Woolft, M.; Mcgraw, E.A.; O’neill, S.L. The relative importance of innate immune priming in Wolbachiamediated dengue. Interf. PLoS Pathog. 2012, 8, e1002548. [Google Scholar]
- Koh, C.; Islam, M.N.; Ye, Y.H.; Chotiwan, N.; Graham, B.; Belisle, J.T.; Kouremenos, K.A.; Dayalan, S.; Tull, D.L.; Klatt, S.; et al. Dengue virus dominates lipid metabolismo modulations in Wolbachia coinfected Aedes aegypti. Commun. Biol. 2020, 3, 518. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Ramos, L.F.C.; Murillo, J.R.; Torres, A.; de Carvalho, S.S.; Domont, G.B.; de Oliveira, D.M.P.; Mesquita, R.D.; Nogueira, F.C.S.; Maciel-de-Freitas, R.; et al. Comprehensive quantitative proteome analysis of Aedes aegypti identifies proteins and pathways involved in wolbachia pipientis and zika virus interference phenomenon. Front. Physiol. 2021, 12, 642237. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, S.T.; Meehan, M.T.; Adekunle, A.I.; Rojas, D.P.; Adegboye, O.A.; McBryde, E.S. A review: Aedes-borne arboviral infections, controls and Wolbachia-based strategies. Vaccines 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.N.; Beier, J.C.; Devine, G.J.; Hugo, L.E. Heat Sensitivity of wMelWolbachia during Aedes aegypti development. PLOS Negl. Trop. Dis. 2016, 10, e0004873. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Ferguson, N.M.; Harris, E.; Holmes, E.C.; Mcgraw, E.A.; O’neill, S.L.; Ooi, O.O.; Ritchie, A.S.; Ryan, P.A.; Scott, T.W.; et al. Assessing the epidemiological impact of Wolbachia deployment for dengue control. Lancet Infect. Dis. 2015, 15, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Christophel, E.M.; Gopinath, D.; Abdur, R.M. Challenges and future perspective for dengue, vector control in the Western Pacifc Region. WPSAR 2011, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.; Turelli, M. Wolbachia versus dengue evolutionary forecasts. Evol. Med. Public Health 2013, 2013, 197–207. [Google Scholar] [CrossRef]
- Roe, A.D.; Weller, S.J.; Baixeras, J.; Brown, J.W.; Cummings, M.P.; Davis, D.; Kawahara, A.; Parr, C.; Regier, J.; Rubinoff, D. Evolutionary framework for Lepidoptera model systems. In Genetics and Molecular Biology of Lepidoptera, Contemporary Topics in Entomology; Goldsmith, M., Marec, F., Eds.; CRC Press: Gainesville, FL, USA, 2009. [Google Scholar]
- Feener, D.H., Jr.; Brown, B.V. Diptera as parasitoids. Annu. Rev. Entomol. 1997, 42, 73–97. [Google Scholar] [CrossRef]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef]
- Narita, S.; Shimajiri, Y.; Nomura, M. Strong cytoplasmic incompatibility and high vertical transmission rate can explain the high frequencies of Wolbachia infection in Japanese populations of Colias erate poliographus (Lepidoptera: Pieridae). Bull. Entomol. Res. 2009, 99, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Dyson, E.A.; Hurst, G.D.D. Persistence of an extreme sex-ratio bias in a natural population. Proc. Natl. Acad. Sci. USA 2004, 101, 6520–6523. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishikawa, H. Wolbachia infections and cytoplasmic incompatibility in the almond moth and the mediterranean flour moth. Zoo. Sci. 1999, 16, 739–744. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae). Heredity 2002, 88, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.; Huguet, E.; Casas, J.; Commin, C.; Giron, D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. R. Soc. B-Biol. Sci. 2010, 277, 2311–2319. [Google Scholar] [CrossRef]
- Graham, R.I.; Grzywacz, D.; Mushobozi, W.L.; Wilson, K. Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol. Lett. 2012, 15, 9931000. [Google Scholar] [CrossRef]
- Werren, J.H.; Windsor, D.; Guo, L.R. Distribution of Wolbachia among Neotropical Arthropods. Proc. R. Soc. B-Biol. Sci. 1995, 1364, 197–204. [Google Scholar]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. B Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef]
- West, S.A.; Cook, J.M.; Werren, J.H.; Godfray, H.C.J. Wolbachia in two insect hostparasitoid communities. Mol. Ecol. 1998, 7, 1457–1465. [Google Scholar] [CrossRef]
- Tagami, Y.; Miura, K. Distribution and prevalence of Wolbachia in Japanese populations of Lepidoptera. Insect Mol. Biol. 2004, 13, 359–364. [Google Scholar] [CrossRef]
- Jiggins, F.M.; Hurst, G.D.D.; Schulenburg, J.; Majerus, M.E.N. Two male-killing Wolbachia strains coexist within a population of the butterfly Acraea encedon. Heredity 2001, 86, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Salunke, B.K.; Salunkhe, R.C.; Dhotre, D.P.; Walujkar, S.A.; Khandagale, A.B.; Chaudhari, R.; Chandode, R.K.; Ghate, H.V.; Patole, M.S.; Werren, J.H.; et al. Determination of Wolbachia diversity in butterflies from western Ghats, India, by a multigene approach. Appl. Environ. Microbiol. 2012, 78, 4458–4467. [Google Scholar] [CrossRef]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstaedter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biology 2008, 6, 27. [Google Scholar] [CrossRef]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, H.; Hong, X.Y. Distribution of the endosymbiotic bacterium Cardinium in Chinese populations of the carmine spider mite Tetranychus cinnabarinus (Acari: Tetranychidae). J. Appl. Entomol. 2006, 130, 523–529. [Google Scholar] [CrossRef]
- Morag, N.; Klement, E.; Saroya, Y.; Lensky, I.; Gottlieb, Y. Prevalence of the symbiont Cardinium in Culicoides (Diptera: Ceratopogonidae) vector species is associated with land surface temperature. Am. Fasten. J. 2012, 26, 4025–4034. [Google Scholar] [CrossRef]
- Sumi, T.; Miura, K.; Miyatake, T. No seasonal trend in infection of the pale grass blue butterfly, Zizeeria maha (Lepidoptera: Lycaenidae), by Wolbachia. Appl. Entomol. Zool. 2013, 48, 35–38. [Google Scholar] [CrossRef]
- Van Opijnen, T.; Breeuwer, J.A.J. High temperatures eliminate Wolbachia, a cytoplasmic incompatibility inducing endosymbiont, from the two-spotted spider mite. Exp. Appl. Acarol. 1999, 23, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D.; Jiggins, F.M.; Robinson, S.J.W. What causes inefficient transmission of male-killing Wolbachia in Drosophila? Heredity 2001, 87, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.X.; Yang, M.S.; Yang, W.J.; Wang, J.J. Influence of Continuous High Temperature Conditions on Wolbachia Infection Frequency and the Fitness of Liposcelis tricolor (Psocoptera: Liposcelididae). Environ. Entomol. 2009, 38, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, N.M.; Mouton, L.; Puttaraju, H.P. Effect of Wolbachia infection and temperature variations on the fecundity of the Uzifly Exorista sorbillans (Diptera: Tachinidae). Symbiosis 2011, 54, 151–158. [Google Scholar] [CrossRef]
- LePage, D.P.; Metcalf, J.A.; Bordenstein, S.R.; On, J.; Perlmutter, J.I.; Shropshire, J.D.; Layton, E.M.; Funkhouser-Jones, L.J.; Beckmann, J.F.; Bordenstein, S.R. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 2017, 543, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.F.; Ronau, J.A.; Hochstrasser, M.A. Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2017, 2, 17007. [Google Scholar] [CrossRef] [PubMed]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R.; et al. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J. Pest. Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef]
- Fukui, T.; Kawamoto, M.; Shoji, K.; Kiuchi, T.; Sugano, S.; Shimada, T.; Suzuki, Y.; Katsuma, S. The endosymbiotic bacterium Wolbachia selectively kills male hosts by targeting the masculinizing gene. PLoS Pathog. 2015, 11, e1005048. [Google Scholar] [CrossRef]
- Sugimoto, T.N.; Ishikawa, Y.A. male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sexdetermining system of its host. Biol. Lett. 2012, 8, 412–415. [Google Scholar] [CrossRef]
- Charlat, S.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Disrupting the timing of Wolbachia-induced male-killing. Biol. Lett. 2007, 3, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Hirano, T.; Akizuki, N.; Abe, A.; Nakai, M.; Kunimi, Y.; Inoue, M.N. Multiple infection and reproductive manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae). Micro. Ecol. 2019, 77, 257–266. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirota, K.; Matsuda-Imai, N.; Fukui, T.; Muro, T.; Nishino, K.; Kosako, H.; Shoji, K.; Takanashi, H.; Fujii, T.; et al. A Wolbachia factor for male killing in lepidopteran Insects. Nat. Commun. 2022, 13, 6764. [Google Scholar] [CrossRef]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cook, L.G. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 2007, 1668, 413–425. [Google Scholar] [CrossRef]
- Kondo, T.; Gullan, P.J.; Williams, D.J. Coccidology. The study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Corpoica. Cienc. Y Tecnol. Agropecu. 2008, 9, 55–61. [Google Scholar] [CrossRef]
- Buckley, R.; Gullan, P. More aggressive ant species (Hymenoptera: Formicidae) provide better protection for soft scales and mealybugs (Homoptera: Coccidae, Pseudococcidae). Biotropica 1991, 23, 282–286. [Google Scholar] [CrossRef]
- Gullan, P.J.; Buckley, R.C.; Ward, P.S. Ant-tended scale insects (Hemiptera: Coccidae: Myzolecanium) within lowland rain Forest trees in Papua New Guinea. J. Trop. Ecol. 1993, 9, 81–91. [Google Scholar] [CrossRef]
- Johanowicz, D.L.; Hoy, M.A. Wolbachia in a predator–prey system: 16 S ribosomal Dna analysis of two Phytoseiids (Acari: Phytoseiidae) and their prey (Acari: Tetranychidae). Ann. Entomol. Soc. Am. 1996, 89, 435–441. [Google Scholar] [CrossRef]
- Kageyama, D.; Narita, S.; Imamura, T.; Miyanoshita, A. Detection and identification of Wolbachia endosymbionts from laboratory stocks of stored-product insect pests and their parasitoids. J. Stored Prod. Res. 2010, 46, 13–19. [Google Scholar] [CrossRef]
- Tzuri, N.; Caspi-Fluger, A.; Betelman, K.; Rohkin Shalom, S.; Chiel, E. Horizontal transmission of microbial symbionts within a Guild of fly Parasitoids. Microb. Ecol. 2020, 81, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.O.; Moreau, C.S. The evolution and biogeography of Wolbachia in ants (Hymenoptera: Formicidae). Diversity 2020, 12, 426. [Google Scholar] [CrossRef]
- Ivens, A.B.F.; Gadau, A.; Kiers, E.T.; Kronauer, D.J.C. Can social partnerships influence the microbiome? Insights from ant farmers and their trophobiont mutualists. Mol. Ecol. 2018, 27, 1898–1914. [Google Scholar] [CrossRef]
- Pringle, E.G.; Moreau, C.S. Community analysis of microbial sharing and specialization in a Costa Rican ant–plant–hemipteran symbiosis. Proc. R. Soc. B 2017, 284, 20162770. [Google Scholar] [CrossRef]
- Gruwell, M.E.; Wu, J.; Normark, B.B. Diversity and phylogeny of Cardinium (Bacteroidetes) in armored scale insects (Hemiptera: Diaspididae). Ann. Entomol. Soc. Am. 2009, 102, 1050–1061. [Google Scholar] [CrossRef]
- Sanaei, E.; Lin, Y.P.; Cook, L.G.; Engelstädter, J. Wolbachia in scale insects: A distinct pattern of infection frequencies and potential transfer routes via ant associates. Environ. Microbiol. 2021, 23, 457441. [Google Scholar] [CrossRef]
- Sanaei, E.; Albery, G.; Yeoh, Y.K.; Lin, Y.P.; Cook, L.; Engelstädter, J. Host phylogeny and ecological associations best explain Wolbachia host shifts in scale insects. Mol. Ecol. 2023, 32, 2351–2363. [Google Scholar] [CrossRef]
- Hibino, H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 1996, 34, 249–274. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.J.; Hong, X.Y. Population dynamics of noncytoplasmic incompatibility-inducing Wolbachia in Nilaparvata lugens and its effects on host adult life span and female ftness. Environ. Entomol. 2010, 39, 1801–1809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, X.F.; Li, Z.X. Establishment of the cytoplasmic incompatibility-inducing Wolbachia strain wMel in an important agricultural pest insect. Sci. Rep. 2016, 6, 39200. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Shams-Bakhsh, M.; Mann, M.; Fattah-Hosseini, S.; Bagheri, A.; Mehrabadi, M.; Heck, M. Distribution and variation of bacterial endosymbiont and ‘Candidatus Liberibacter asiaticus’ titer in the Huanglongbing insect vector, Diaphorina citri Kuwayama. Microb. Ecol. 2019, 78, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.L.; Li, Y.H.; Ou, D.; Guo, Y.J.; Qureshi, J.A.; Stansly, P.A.; Qiu, B.L. Localization and dynamics of Wolbachia infection in Asian citrus psyllid Diaphorina citri, the insect vector of the causal pathogens of Huanglongbing. Microbio. Open. 2018, 7, e00561. [Google Scholar] [CrossRef]
- Guo, Y.; Hoffmann, A.A.; Xu, X.Q.; Mo, P.W.; Huang, H.J.; Gong, J.T.; Ju, J.F.; Hong, X.Y. Vertical transmission of Wolbachia is associated with host vitellogenin in Laodelphax striatellus. Front. Microbiol. 2018, 9, 2016. [Google Scholar] [CrossRef]
- Guo, Y.; Khan, J.; Zheng, X.Y.; Wu, Y. Wolbachia increase germ cell mitosis to enhance the fecundity of Laodelphax striatellus. Insect Biochem. Mol. Biol. 2020, 127, 103471. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shiyuan, M.; Zhengyan, W.; Sibao, W. Prevalence of Wolbachia in 10 Tenebrionidae stored-product insects and spatiotemporal infection dynamics in Tribolium confusum (Jaquelin Du Val) (Coleoptera: Tenebrionidae). Grain Oil Sci. Tech. 2019, 2, 85–90. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.Y.; Hoffmann, A.A.; Zhang, K.-J.; Huang, H.-J.; Gong, J.-T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).