Menstrual Cycle Phase Influences Cognitive Performance in Women and Modulates Sex Differences: A Combined Longitudinal and Cross-Sectional Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
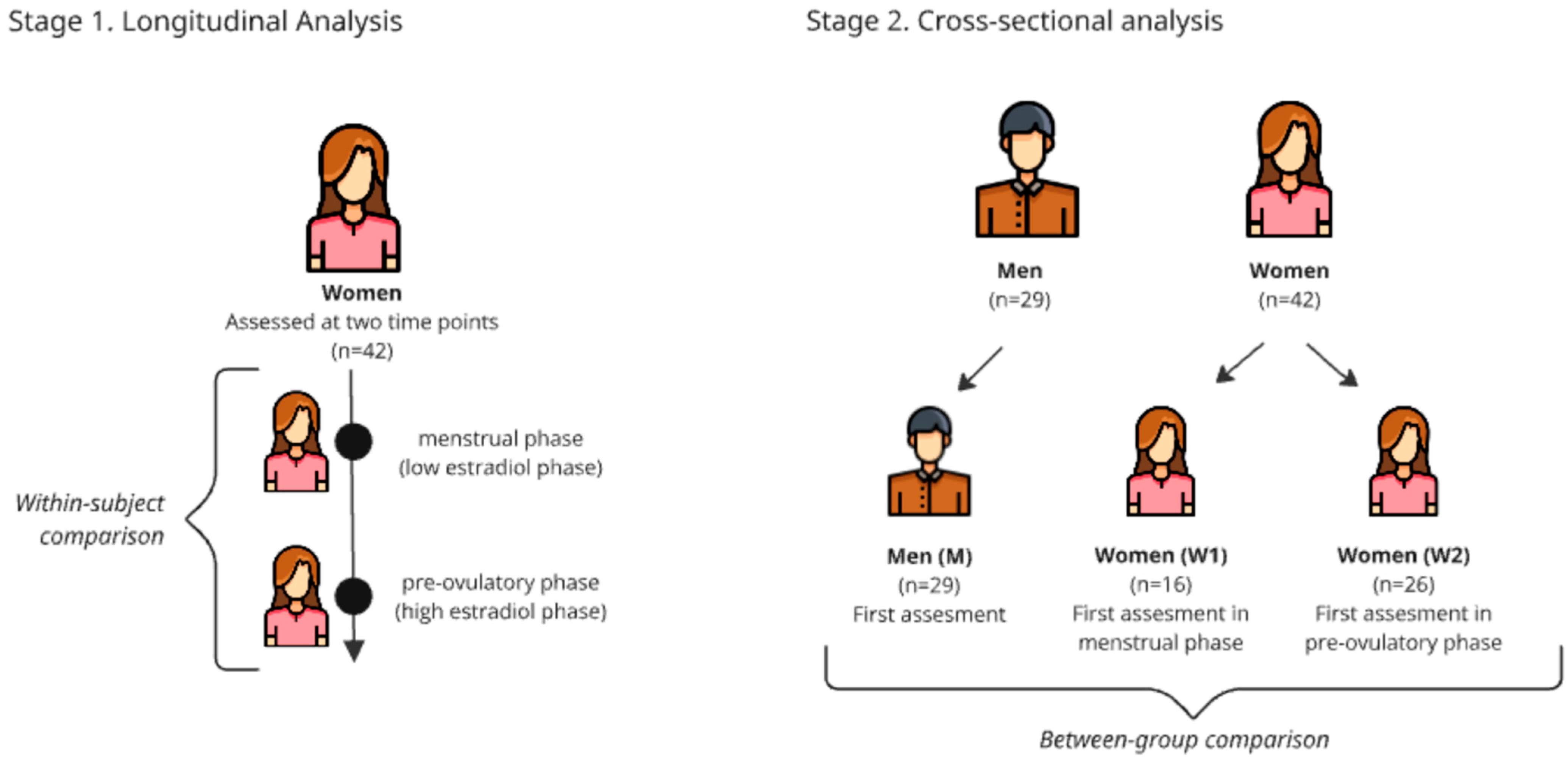
2.3. Study Design
2.4. Assessment Timing
2.5. Hormone Measurements
2.6. Cognitive Tests
2.7. Statistical Analysis
3. Results
3.1. Hormonal Data
3.2. Changes in Cognitive Performance Between Women in the Menstrual and Pre-Ovulatory Phases
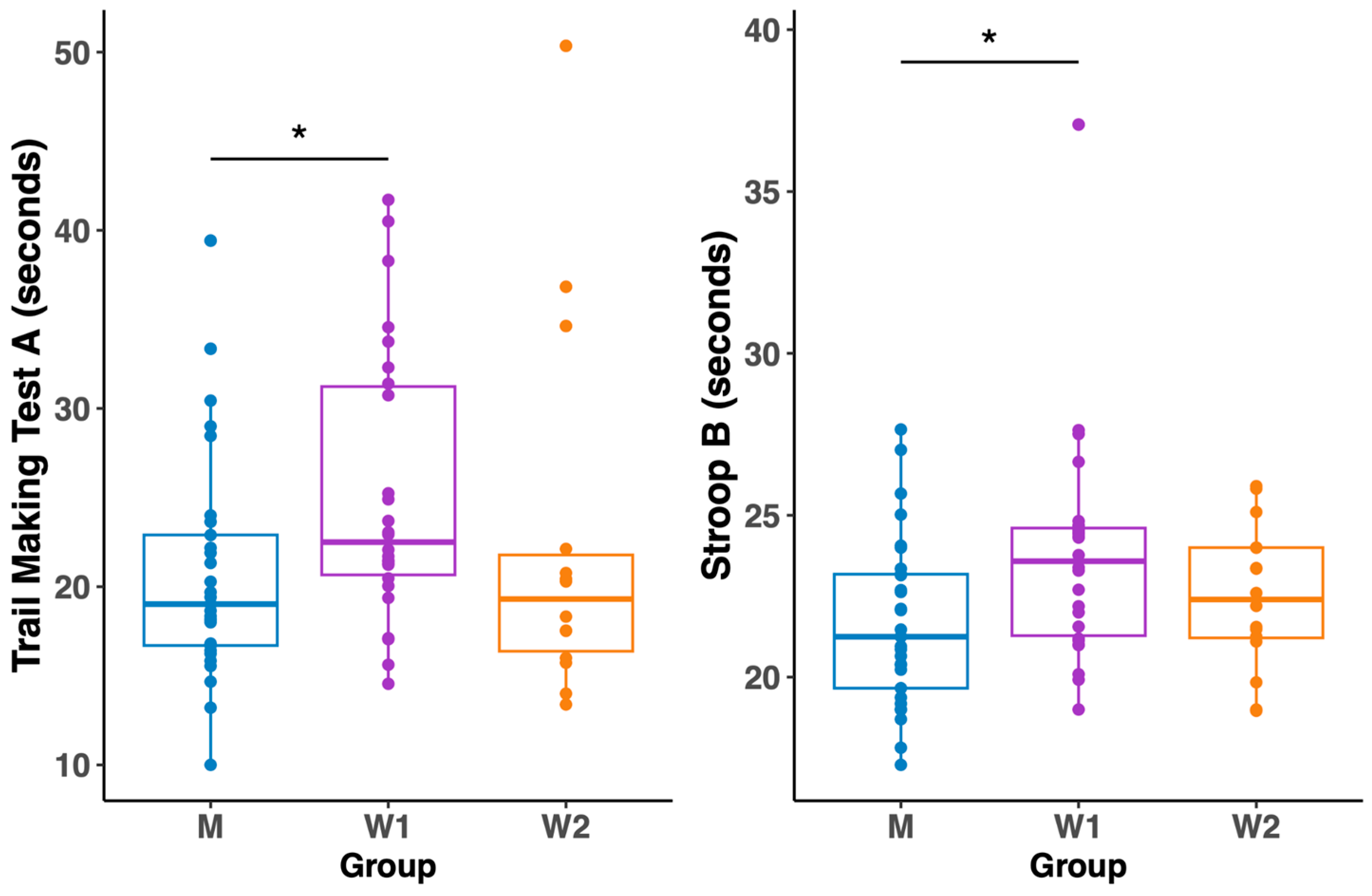
3.3. Differences in Cognitive Performance Between Men and Women in Two Phases of the Menstrual Cycle
3.4. Correlation Between Hormone Concentration and Cognitive Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, G.; Phillips, T.J. Estrogen and Skin: The Effects of Estrogen, Menopause, and Hormone Replacement Therapy on the Skin. J. Am. Acad. Dermatol. 2005, 53, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.; Eberhart, R.; Chevailler, M.-C.; Quinn, F.A.; Bischof, P.; Stricker, R. Establishment of Detailed Reference Values for Luteinizing Hormone, Follicle Stimulating Hormone, Estradiol, and Progesterone during Different Phases of the Menstrual Cycle on the Abbott ARCHITECT® Analyzer. Clin. Chem. Lab. Med. (CCLM) 2006, 44, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone Receptors: Form and Function in Brain. Front. Neuroendocr. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Gleason, C.E.; Sandra, O.; Carlsson, C.M.; Asthana, S. Neurobiological Underpinnings of the Estrogen—Mood Relationship. Curr. Psychiatry Rev. 2012, 8, 247–256. [Google Scholar] [CrossRef]
- Farage, M.A.; Osborn, T.W.; MacLean, A.B. Cognitive, Sensory, and Emotional Changes Associated with the Menstrual Cycle: A Review. Arch. Gynecol. Obs. Obstet. 2008, 278, 299–307. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex Hormones Affect Neurotransmitters and Shape the Adult Female Brain during Hormonal Transition Periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Sundström Poromaa, I.; Gingnell, M. Menstrual Cycle Influence on Cognitive Function and Emotion Processing—from a Reproductive Perspective. Front. Neurosci. 2014, 8, 380. [Google Scholar] [CrossRef]
- Gegenhuber, B.; Wu, M.V.; Bronstein, R.; Tollkuhn, J. Gene Regulation by Gonadal Hormone Receptors Underlies Brain Sex Differences. Nature 2022, 606, 153–159. [Google Scholar] [CrossRef]
- Protopopescu, X.; Butler, T.; Pan, H.; Root, J.; Altemus, M.; Polanecsky, M.; McEwen, B.; Silbersweig, D.; Stern, E. Hippocampal Structural Changes across the Menstrual Cycle. Hippocampus 2008, 18, 985–988. [Google Scholar] [CrossRef]
- Luine, V.N. Sex Steroids and Cognitive Function. J. Neuroendocrinol. 2008, 20, 866–872. [Google Scholar] [CrossRef]
- Hao, J.; Rapp, P.R.; Janssen, W.G.M.; Lou, W.; Lasley, B.L.; Hof, P.R.; Morrison, J.H. Interactive Effects of Age and Estrogen on Cognition and Pyramidal Neurons in Monkey Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 11465–11470. [Google Scholar] [CrossRef]
- Keenan, P.A.; Ezzat, W.H.; Ginsburg, K.; Moore, G.J. Prefrontal Cortex as the Site of Estrogen’s Effect on Cognition. Psychoneuroendocrinology 2001, 26, 577–590. [Google Scholar] [CrossRef]
- Hatta, T.; Nagaya, K. Menstrual Cycle Phase Effects on Memory and Stroop Task Performance. Arch. Sex. Behav. 2009, 38, 821–827. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, M.; Huang, S.; Guo, W. Association between Serum Estradiol Levels and Cognitive Function in Older Women: A Cross-Sectional Analysis. Front. Aging Neurosci. 2024, 16, 1356791. [Google Scholar] [CrossRef]
- Hampson, E.; Morley, E.E. Estradiol Concentrations and Working Memory Performance in Women of Reproductive Age. Psychoneuroendocrinology 2013, 38, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Estrogen and Memory in Women: How Can We Reconcile the Findings? Horm. Behav. 2005, 47, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Šimić, N.; Santini, M. Verbal and Spatial Functions during Different Phases of the Menstrual Cycle. Psychiatr. Danub. 2012, 24, 73–79. [Google Scholar]
- Solís-Ortiz, S.; Corsi-Cabrera, M. Sustained Attention Is Favored by Progesterone during Early Luteal Phase and Visuo-Spatial Memory by Estrogens during Ovulatory Phase in Young Women. Psychoneuroendocrinology 2008, 33, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Avila-Varela, D.S.; Hidalgo-Lopez, E.; Dagnino, P.C.; Acero-Pousa, I.; del Agua, E.; Deco, G.; Pletzer, B.; Escrichs, A. Whole-Brain Dynamics across the Menstrual Cycle: The Role of Hormonal Fluctuations and Age in Healthy Women. npj Women’s Health 2024, 2, 8. [Google Scholar] [CrossRef]
- Berent-Spillson, A.; Briceno, E.; Pinsky, A.; Simmen, A.; Persad, C.C.; Zubieta, J.-K.; Smith, Y.R. Distinct Cognitive Effects of Estrogen and Progesterone in Menopausal Women. Psychoneuroendocrinology 2015, 59, 25–36. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone and the Brain. Aging Male 2006, 9, 195–199. [Google Scholar] [CrossRef]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in Women—The Clinical Significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Dratva, M.A.; Banks, S.J.; Panizzon, M.S.; Galasko, D.; Sundermann, E.E. Low Testosterone Levels Relate to Poorer Cognitive Function in Women in an APOE-Ε4-Dependant Manner. Biol. Sex Differ. 2024, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Ostatníková, D.; Hodosy, J. On the Effects of Testosterone on Brain Behavioral Functions. Front. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Thilers, P.P.; MacDonald, S.W.S.; Herlitz, A. The Association between Endogenous Free Testosterone and Cognitive Performance: A Population-Based Study in 35 to 90 Year-Oldmen and Women. Psychoneuroendocrinology 2006, 31, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Leeners, B.; Krüger, T.; Geraedts, K.; Tronci, E.; Mancini, T.; Ille, F.; Egli, M.; Röblitz, S.; Wunder, D.; Saleh, L.; et al. Cognitive Function in Association with High Estradiol Levels Resulting from Fertility Treatment. Horm. Behav. 2021, 130, 104951. [Google Scholar] [CrossRef]
- Leeners, B.; Kruger, T.H.C.; Geraedts, K.; Tronci, E.; Mancini, T.; Ille, F.; Egli, M.; Röblitz, S.; Saleh, L.; Spanaus, K.; et al. Lack of Associations between Female Hormone Levels and Visuospatial Working Memory, Divided Attention and Cognitive Bias across Two Consecutive Menstrual Cycles. Front. Behav. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Sundström-Poromaa, I. The Menstrual Cycle Influences Emotion but Has Limited Effect on Cognitive Function. Vitam. Horm. 2018, 107, 349–376. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S. The Two FIGO Systems for Normal and Abnormal Uterine Bleeding Symptoms and Classification of Causes of Abnormal Uterine Bleeding in the Reproductive Years: 2018 Revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef]
- Landgren, B.M.; Unden, A.L.; Diczfalusy, E. Hormonal Profile of the Cycle in 68 Normally Menstruating Women. Acta Endocrinol. 1980, 94, 89–98. [Google Scholar] [CrossRef]
- Erdodi, L.A.; Sagar, S.; Seke, K.; Zuccato, B.G.; Schwartz, E.S.; Roth, R.M. The Stroop Test as a Measure of Performance Validity in Adults Clinically Referred for Neuropsychological Assessment. Psychol. Assess. 2018, 30, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Sawyer, R.J.; Roper, B.L.; Baughman, B.C. Expansion and Re-Examination of Digit Span Effort Indices on the WAIS-IV. Clin. Neuropsychol. 2012, 26, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct Validity of the Trail Making Test: Role of Task-Switching, Working Memory, Inhibition/Interference Control, and Visuomotor Abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Arce, T.; McMullen, K. The Corsi Block-Tapping Test: Evaluating Methodological Practices with an Eye towards Modern Digital Frameworks. Comput. Hum. Human. Behav. Rep. 2021, 4, 100099. [Google Scholar] [CrossRef]
- Guariglia, C.C. Spatial Working Memory in Alzheimer’s Disease: A Study Using the Corsi Block-Tapping Test. Dement. Neuropsychol. 2007, 1, 392–395. [Google Scholar] [CrossRef]
- Berch, D.B.; Krikorian, R.; Huha, E.M. The Corsi Block-Tapping Task: Methodological and Theoretical Considerations. Brain Cogn. 1998, 38, 317–338. [Google Scholar] [CrossRef]
- McInerney, V. Review of Visual Patterns Test. In The Seventeenth Mental Measurements Yearbook; Buros Institute of Mental Measurements: Lincoln, NE, USA, 2007; pp. 842–845. [Google Scholar]
- Della Sala, S.; Gray, C.; Baddeley, A.; Allamano, N.; Wilson, L. Pattern Span: A Tool for Unwelding Visuo–Spatial Memory. Neuropsychologia 1999, 37, 1189–1199. [Google Scholar] [CrossRef]
- Colosimo, S.; Brown, T. Examining the Convergent Validity of the Test of Visual Perceptual Skills—Fourth Edition (TVPS-4) in the Australian Context. J. Occup. Ther. Sch. Early Interv. 2022, 15, 90–110. [Google Scholar] [CrossRef]
- Hampson, E. Variations in Sex-Related Cognitive Abilities across the Menstrual Cycle. Brain Cogn. 1990, 14, 26–43. [Google Scholar] [CrossRef]
- Phillips, S.M.; Sherwin, B.B. Variations in Memory Function and Sex Steroid Hormones across the Menstrual Cycle. Psychoneuroendocrinology 1992, 17, 497–506. [Google Scholar] [CrossRef]
- Hausmann, M.; Slabbekoorn, D.; Van Goozen, S.H.M.; Cohen-Kettenis, P.T.; Güntürkün, O. Sex Hormones Affect Spatial Abilities during the Menstrual Cycle. Behav. Neurosci. 2000, 114, 1245–1250. [Google Scholar] [CrossRef]
- Schöning, S.; Engelien, A.; Kugel, H.; Schäfer, S.; Schiffbauer, H.; Zwitserlood, P.; Pletziger, E.; Beizai, P.; Kersting, A.; Ohrmann, P.; et al. Functional Anatomy of Visuo-Spatial Working Memory during Mental Rotation Is Influenced by Sex, Menstrual Cycle, and Sex Steroid Hormones. Neuropsychologia 2007, 45, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, B.; Harris, T.A.; Ortner, T. Sex and Menstrual Cycle Influences on Three Aspects of Attention. Physiol. Behav. 2017, 179, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Park, S. Verbal and Spatial Functions across the Menstrual Cycle in Healthy Young Women. Psychoneuroendocrinology 2002, 27, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, B.; Harris, T.A.; Scheuringer, A.; Hidalgo-Lopez, E. The Cycling Brain: Menstrual Cycle Related Fluctuations in Hippocampal and Fronto-Striatal Activation and Connectivity during Cognitive Tasks. Neuropsychopharmacology 2019, 44, 1867–1875. [Google Scholar] [CrossRef]
- Khan, M.M.; Dhandapani, K.M.; Zhang, Q.; Brann, D.W. Estrogen Regulation of Spine Density and Excitatory Synapses in Rat Prefrontal and Somatosensory Cerebral Cortex. Steroids 2013, 78, 614–623. [Google Scholar] [CrossRef]
- Yankova, M.; Hart, S.A.; Woolley, C.S. Estrogen Increases Synaptic Connectivity between Single Presynaptic Inputs and Multiple Postsynaptic CA1 Pyramidal Cells: A Serial Electron-Microscopic Study. Proc. Natl. Acad. Sci. USA 2001, 98, 3525–3530. [Google Scholar] [CrossRef]
- Jacobs, E.; D’Esposito, M. Estrogen Shapes Dopamine-Dependent Cognitive Processes: Implications for Women’s Health. J. Neurosci. 2011, 31, 5286–5293. [Google Scholar] [CrossRef]
- Feng, Q.; Zheng, Y.; Zhang, X.; Song, Y.; Luo, Y.; Li, Y.; Talhelm, T. Gender Differences in Visual Reflexive Attention Shifting: Evidence from an ERP Study. Brain Res. 2011, 1401, 59–65. [Google Scholar] [CrossRef]
- Evans, K.L.; Hampson, E. Sex-Dependent Effects on Tasks Assessing Reinforcement Learning and Interference Inhibition. Front. Psychol. 2015, 6, 1044. [Google Scholar] [CrossRef]
- Murray, S.O.; Schallmo, M.P.; Kolodny, T.; Millin, R.; Kale, A.; Thomas, P.; Rammsayer, T.H.; Troche, S.J.; Bernier, R.A.; Tadin, D. Sex Differences in Visual Motion Processing. Curr. Biol. 2018, 28, 2794–2799.e3. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Jin, X.; Niu, D.; Zhang, L.; Jiang, S.Y.; Ruan, H.D.; Ho, G.W. Sex Differences in Hemispheric Lateralization of Attentional Networks. Psychol. Res. 2021, 85, 2697–2709. [Google Scholar] [CrossRef]
- Voyer, D. Time Limits and Gender Differences on Paper-and-Pencil Tests of Mental Rotation: A Meta-Analysis. Psychon. Bull. Rev. 2011, 18, 267–277. [Google Scholar] [CrossRef]
- Voyer, D.; Voyer, S.D.; Saint-Aubin, J. Sex Differences in Visual-Spatial Working Memory: A Meta-Analysis. Psychon. Bull. Rev. 2017, 24, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Bandelow, S.; Hogervorst, E. Testosterone Levels and Cognition in Elderly Men: A Review. Maturitas 2011, 69, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jiang, H.; Li, S.; Zhang, D. Low Serum Testosterone Concentrations Are Associated With Poor Cognitive Performance in Older Men but Not Women. Front. Aging Neurosci. 2021, 13, 712237. [Google Scholar] [CrossRef] [PubMed]
- Giannos, P.; Prokopidis, K.; Church, D.D.; Kirk, B.; Morgan, P.T.; Lochlainn, M.N.; Macpherson, H.; Woods, D.R.; Ispoglou, T. Associations of Bioavailable Serum Testosterone With Cognitive Function in Older Men: Results From the National Health and Nutrition Examination Survey. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2023, 78, 151–157. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Vermeulen, A. The Decline of Androgen Levels in Elderly Men and Its Clinical and Therapeutic Implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Halari, R.; Hines, M.; Kumari, V.; Mehrotra, R.; Wheeler, M.; Ng, V.; Sharma, T. Sex Differences and Individual Differences in Cognitive Performance and Their Relationship to Endogenous Gonadal Hormones and Gonadotropins. Behav. Neurosci. 2005, 119, 104–117. [Google Scholar] [CrossRef]
- Puts, D.A.; Cárdenas, R.A.; Bailey, D.H.; Burriss, R.P.; Jordan, C.L.; Breedlove, S.M. Salivary Testosterone Does Not Predict Mental Rotation Performance in Men or Women. Horm. Behav. 2010, 58, 282–289. [Google Scholar] [CrossRef]
- Scheuringer, A.; Pletzer, B. Sex Differences and Menstrual Cycle Dependent Changes in Cognitive Strategies during Spatial Navigation and Verbal Fluency. Front. Psychol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Gouchie, C.; Kimura, D. The Relationship between Testosterone Levels and Cognitive Ability Patterns. Psychoneuroendocrinology 1991, 16, 323–334. [Google Scholar] [CrossRef]
- Moffat, S.; Hampson, E. A Curvilinear Relationship between Testosterone and Spatial Cognition in Humans: Possible Influence of Hand Preference. Psychoneuroendocrinology 1996, 21, 323–337. [Google Scholar] [CrossRef]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, the Menstrual Cycle, and Premenstrual Disorders: A Review. Brain Sci 2020, 10, 198. [Google Scholar] [CrossRef]
- Yen, J.Y.; Lin, P.C.; Hsu, C.J.; Lin, C.; Chen, I.J.; Ko, C.H. Attention, Response Inhibition, Impulsivity, and Decision-Making within Luteal Phase in Women with Premenstrual Dysphoric Disorder. Arch. Womens Ment. Health 2023, 26, 321–330. [Google Scholar] [CrossRef] [PubMed]


| Women (n = 42) | |||
|---|---|---|---|
| Variable | M (SD) | Min Value | Max Value |
| Length of the menstrual cycle (days) | 30.05 (2.17) | 25 | 35 |
| Duration of the menstrual phase (days) | 5.52 (0.86) | 4 | 7 |
| Group/Phase | Testosterone (nmol/mL) M (SD) | Progesterone (ng/mL) M (SD) | Oestradiol (pg/mL) M (SD) |
|---|---|---|---|
| Women (n = 42) | |||
| Menstrual phase | 1.32 (0.45) | 0.37 (0.2) | 30.86 (16.74) |
| Pre-ovulatory phase | 1.77 (0.58) | 2.06 (3.92) | 163.98 (115.85) |
| Men (n = 29) | |||
| 18.95 (6.07) | 0.35 (0.19) | 24.79 (8.09) |
| Variables | Group | N | Mean | SD | Median | Mean Rank | Sum of Ranks | T | p | r |
|---|---|---|---|---|---|---|---|---|---|---|
| Digit span forward | W_M | 42 | 7.33 | 2.54 | 7 | Negative | 133.5 | 2.06 | 0.04 * | 0.22 |
| W_PO | 42 | 8.0 | 2.06 | 8 | Positive | 331.5 | ||||
| Digit span forward max | W_M | 42 | 6.24 | 1.38 | 6 | Negative | 93.0 | 2.61 | 0.01 * | 0.28 |
| W_PO | 42 | 6.74 | 1.27 | 7 | Positive | 313.0 | ||||
| Digit span backward | W_M | 42 | 7.0 | 2.35 | 7 | Negative | 167.5 | 1.84 | 0.07 | 0.20 |
| W_PO | 42 | 7.48 | 2.08 | 7 | Positive | 360.5 | ||||
| Digit span backward max | W_M | 42 | 4.98 | 1.3 | 5 | Negative | 96.0 | 2.32 | 0.02 * | 0.25 |
| W_PO | 42 | 5.43 | 1.21 | 5 | Positive | 282.0 | ||||
| TMT A time (s) | W_M | 42 | 22.83 | 7.41 | 21.45 | Negative | 525.5 | 1.55 | 0.12 | 0.17 |
| W_PO | 40 | 21.68 | 7.29 | 20.13 | Positive | 294.50 | ||||
| TMT B time (s) | W_M | 42 | 47.47 | 14.38 | 47.23 | Negative | 604.00 | 2.61 | 0.01 * | 0.28 |
| W_PO | 40 | 41.57 | 9.74 | 41.63 | Positive | 216.00 | ||||
| TMT B/A time (s) | W_M | 42 | 2.15 | 0.56 | 2.11 | Negative | 489.00 | 1.06 | 0.29 | 0.12 |
| W_PO | 40 | 2.02 | 0.57 | 1.89 | Positive | 331.00 | ||||
| Corsi block span forward | W_M | 42 | 6.33 | 1.07 | 6 | Negative | 228.5 | 0.98 | 0.33 | 0.11 |
| W_PO | 42 | 6.17 | 1.17 | 6 | Positive | 149.5 | ||||
| Corsi TSF | W_M | 42 | 61.9 | 21.01 | 60 | Negative | 409.0 | 0.87 | 0.39 | 0.09 |
| W_PO | 42 | 58.88 | 21.76 | 54 | Positive | 294.0 | ||||
| Corsi block span backward | W_M | 42 | 6.60 | 1.29 | 6.00 | Negative | 153.00 | 0.27 | 0.79 | 0.03 |
| W_PO | 42 | 6.57 | 0.80 | 6.00 | Positive | 172.00 | ||||
| Corsi TSB | W_M | 42 | 62.10 | 17.23 | 60.00 | Negative | 204.50 | 1.81 | 0.07 | 0.20 |
| W_PO | 42 | 67.05 | 17.71 | 60.00 | Positive | 425.50 | ||||
| VPT max | W_M | 41 | 10.32 | 1.86 | 10 | Negative | 172.0 | 1.52 | 0.13 | 0.17 |
| W_PO | 42 | 10.76 | 1.86 | 11 | Positive | 324.0 | ||||
| VPT mean | W_M | 41 | 9.76 | 1.68 | 10.30 | Negative | 202.50 | 1.85 | 0.07 | 0.20 |
| W_PO | 42 | 10.18 | 1.76 | 10.00 | Positive | 427.50 | ||||
| VMT Vis Mem | W_M | 41 | 16.22 | 1.26 | 16.00 | Negative | 221.50 | 0.53 | 0.60 | 0.06 |
| W_PO | 40 | 16.38 | 1.41 | 17.00 | Positive | 274.50 | ||||
| VMT Seq Mem | W_M | 41 | 14.61 | 1.52 | 15.00 | Negative | 163.50 | 1.91 | 0.06 | 0.21 |
| W_PO | 40 | 15.28 | 1.63 | 15.00 | Positive | 364.50 | ||||
| Stroop A time (s) | W_M | 42 | 28.28 | 4.67 | 27.92 | Negative | 517.5 | 0.83 | 0.41 | 0.09 |
| W_PO | 42 | 27.85 | 4.1 | 26.45 | Positive | 385.5 | ||||
| Stroop B time (s) | W_M | 42 | 22.89 | 3.50 | 22.16 | Negative | 477.50 | 0.33 | 0.75 | 0.04 |
| W_PO | 42 | 22.48 | 2.42 | 22.13 | Positive | 425.50 | ||||
| Stroop C time (s) | W_M | 42 | 44.45 | 9.89 | 44.75 | Negative | 606.00 | 1.93 | 0.05 | 0.21 |
| W_PO | 42 | 42.21 | 8.03 | 41.53 | Positive | 297.00 | ||||
| Stroop D time (s) | W_M | 42 | 49.10 | 9.61 | 48.65 | Negative | 540.00 | 1.11 | 0.27 | 0.12 |
| W_PO | 42 | 47.69 | 9.61 | 47.74 | Positive | 363.00 | ||||
| Stroop interference | W_M | 42 | 21.56 | 7.8 | 21.44 | Negative | 598.0 | 1.83 | 0.07 | 0.20 |
| W_PO | 42 | 19.72 | 7.69 | 19.79 | Positive | 305.00 | ||||
| Stroop interference a | W_M | 42 | 16.17 | 7.50 | 13.62 | Negative | 596.00 | 1.81 | 0.07 | 0.20 |
| W_PO | 42 | 14.35 | 6.08 | 13.70 | Positive | 307.00 | ||||
| Stroop interference b | W_M | 42 | −2.06 | 8.37 | −2.65 | Negative | 552.50 | 1.26 | 0.21 | 0.14 |
| W_PO | 42 | −2.65 | 7.84 | −3.04 | Positive | 350.50 | ||||
| Stroop interference c | W_M | 42 | 4.65 | 7.94 | 4.65 | Negative | 429.00 | 0.28 | 0.78 | 0.03 |
| W_PO | 42 | 5.48 | 8.44 | 5.21 | Positive | 474.00 |
| Cognitive Test | Group | Statistics | |||||
| N | Mean Rank | Median | IQR | KW Statistic | p | ||
| Digit span forward | |||||||
| W1 | 26 | 30.58 | 7 | 2 | 2.96 | 0.23 | |
| W2 | 16 | 40.22 | 8 | 4 | |||
| M | 29 | 38.53 | 8 | 4 | |||
| Digit span forward max | |||||||
| W1 | 26 | 30.88 | 6 | 2 | 2.67 | 0.26 | |
| W2 | 16 | 39.47 | 6.5 | 2 | |||
| M | 29 | 38.67 | 7 | 2 | |||
| Digit span backward | |||||||
| W1 | 26 | 31.27 | 7 | 3 | 2.21 | 0.33 | |
| W2 | 16 | 38.25 | 8 | 3 | |||
| M | 29 | 39.00 | 8 | 4 | |||
| Digit span backward max | |||||||
| W1 | 26 | 30.96 | 5 | 2 | 2.99 | 0.22 | |
| W2 | 16 | 36.38 | 5.5 | 2 | |||
| M | 29 | 40.31 | 6 | 3 | |||
| TMT A time (s) | |||||||
| W1 | 26 | 43.08 | 22.5 | 11.26 | 6.77 | 0.03 * | |
| W2 | 14 | 30.50 | 19.31 | 9.32 | |||
| M | 29 | 29.93 | 19.02 | 6.71 | |||
| TMT B time (s) | |||||||
| W1 | 26 | 37.27 | 47.75 | 16.76 | 0.54 | 0.76 | |
| W2 | 14 | 34.07 | 47.91 | 15.19 | |||
| M | 29 | 33.41 | 44.55 | 17.78 | |||
| TMT B/A time (s) | |||||||
| W1 | 26 | 30.88 | 1.89 | 0.56 | 1.97 | 0.37 | |
| W2 | 14 | 35.43 | 2.16 | 1.12 | |||
| M | 29 | 38.48 | 2.06 | 1.21 | |||
| Corsi block span forward | |||||||
| W1 | 26 | 37.12 | 6 | 2 | 0.15 | 0.93 | |
| W2 | 16 | 35.97 | 6 | 3 | |||
| M | 29 | 35.02 | 6 | 2 | |||
| Corsi TSF | |||||||
| W1 | 26 | 36.58 | 54 | 32 | 0.22 | 0.90 | |
| W2 | 16 | 37.44 | 57 | 50 | |||
| M | 29 | 34.69 | 54 | 30 | |||
| Corsi block span backward | |||||||
| W1 | 26 | 32.79 | 6 | 1 | 2.27 | 0.32 | |
| W2 | 16 | 42.00 | 7 | 2 | |||
| M | 29 | 35.57 | 6 | 1 | |||
| Corsi TSB | |||||||
| W1 | 26 | 29.87 | 57 | 12 | 4.37 | 0.11 | |
| W2 | 16 | 42.88 | 66.5 | 31 | |||
| M | 29 | 37.71 | 60 | 23 | |||
| VPT max | |||||||
| W1 | 26 | 34.62 | 11 | 3 | 1.03 | 0.60 | |
| W2 | 16 | 40.53 | 11 | 2 | |||
| M | 29 | 34.74 | 10 | 3 | |||
| VPT mean | |||||||
| W1 | 26 | 34.60 | 10.3 | 3.07 | 0.56 | 0.76 | |
| W2 | 16 | 39.31 | 10.15 | 2.6 | |||
| M | 29 | 35.43 | 9.67 | 2.16 | |||
| VMT Vis Mem | |||||||
| W1 | 26 | 33.87 | 16 | 2 | 0.80 | 0.67 | |
| W2 | 14 | 32.25 | 16 | 2 | |||
| M | 29 | 37.34 | 16 | 1 | |||
| VMT Seq Mem | |||||||
| W1 | 26 | 33.81 | 14.5 | 3 | 0.40 | 0.82 | |
| W2 | 14 | 33.57 | 14.5 | 3 | |||
| M | 29 | 36.76 | 15.00 | 2 | |||
| Stroop A time (s) | |||||||
| W1 | 26 | 39.69 | 28.88 | 6.8 | 1.35 | 0.51 | |
| W2 | 16 | 33.06 | 28.18 | 7.51 | |||
| M | 29 | 34.31 | 28.44 | 4.86 | |||
| Stroop B time (s) | |||||||
| W1 | 26 | 43.37 | 23.58 | 3.47 | 6.60 | 0.04 * | |
| W2 | 14 | 36.59 | 22.4 | 2.86 | |||
| M | 29 | 29.07 | 21.25 | 3.75 | |||
| Stroop C time (s) | |||||||
| W1 | 26 | 37.96 | 45.35 | 13.23 | 0.38 | 0.83 | |
| W2 | 16 | 34.38 | 46.27 | 15.17 | |||
| M | 29 | 35.14 | 44.00 | 9.85 | |||
| Stroop D time (s) | |||||||
| W1 | 26 | 36.38 | 52.70 | 13.76 | 0.35 | 0.84 | |
| W2 | 16 | 33.38 | 49.28 | 13.6 | |||
| M | 29 | 37.10 | 52.09 | 9.84 | |||
| Stroop interference | |||||||
| W1 | 26 | 35.92 | 22.37 | 13.95 | 0.45 | 0.80 | |
| W2 | 16 | 33.28 | 23.09 | 14.45 | |||
| M | 29 | 37.57 | 23.68 | 9.99 | |||
| Stroop interference a | |||||||
| W1 | 26 | 36.50 | 14.11 | 12.8 | 0.17 | 0.92 | |
| W2 | 16 | 34.13 | 15.54 | 10.59 | |||
| M | 29 | 36.59 | 15.61 | 8.71 | |||
| Stroop interference b | |||||||
| W1 | 26 | 32.38 | −2.82 | 15.18 | 2.44 | 0.30 | |
| W2 | 16 | 33.59 | 0.13 | 12.51 | |||
| M | 29 | 40.57 | 1.34 | 9.19 | |||
| Stroop interference c | |||||||
| W1 | 26 | 32.08 | 4.30 | 12.68 | 1.49 | 0.47 | |
| W2 | 16 | 37.81 | 6.96 | 9.55 | |||
| M | 29 | 38.52 | 7.28 | 9.59 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, A.K.; Michalak, K.M.; Naparło, B.; Bermudo-Gallaguet, A.; Mataró, M.; Winklewski, P.J.; Marcinkowska, A.B. Menstrual Cycle Phase Influences Cognitive Performance in Women and Modulates Sex Differences: A Combined Longitudinal and Cross-Sectional Study. Biology 2025, 14, 1060. https://doi.org/10.3390/biology14081060
Sawicka AK, Michalak KM, Naparło B, Bermudo-Gallaguet A, Mataró M, Winklewski PJ, Marcinkowska AB. Menstrual Cycle Phase Influences Cognitive Performance in Women and Modulates Sex Differences: A Combined Longitudinal and Cross-Sectional Study. Biology. 2025; 14(8):1060. https://doi.org/10.3390/biology14081060
Chicago/Turabian StyleSawicka, Angelika K., Katarzyna M. Michalak, Barbara Naparło, Adrià Bermudo-Gallaguet, Maria Mataró, Pawel J. Winklewski, and Anna B. Marcinkowska. 2025. "Menstrual Cycle Phase Influences Cognitive Performance in Women and Modulates Sex Differences: A Combined Longitudinal and Cross-Sectional Study" Biology 14, no. 8: 1060. https://doi.org/10.3390/biology14081060
APA StyleSawicka, A. K., Michalak, K. M., Naparło, B., Bermudo-Gallaguet, A., Mataró, M., Winklewski, P. J., & Marcinkowska, A. B. (2025). Menstrual Cycle Phase Influences Cognitive Performance in Women and Modulates Sex Differences: A Combined Longitudinal and Cross-Sectional Study. Biology, 14(8), 1060. https://doi.org/10.3390/biology14081060







