Immune Tuning in Extreme Environments: Protein Citrullinome and Extracellular Vesicle Signatures Comparing Hibernating Versus Active States in the Heterothermic and Heterometabolic Tenrec (Tenrec ecaudatus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
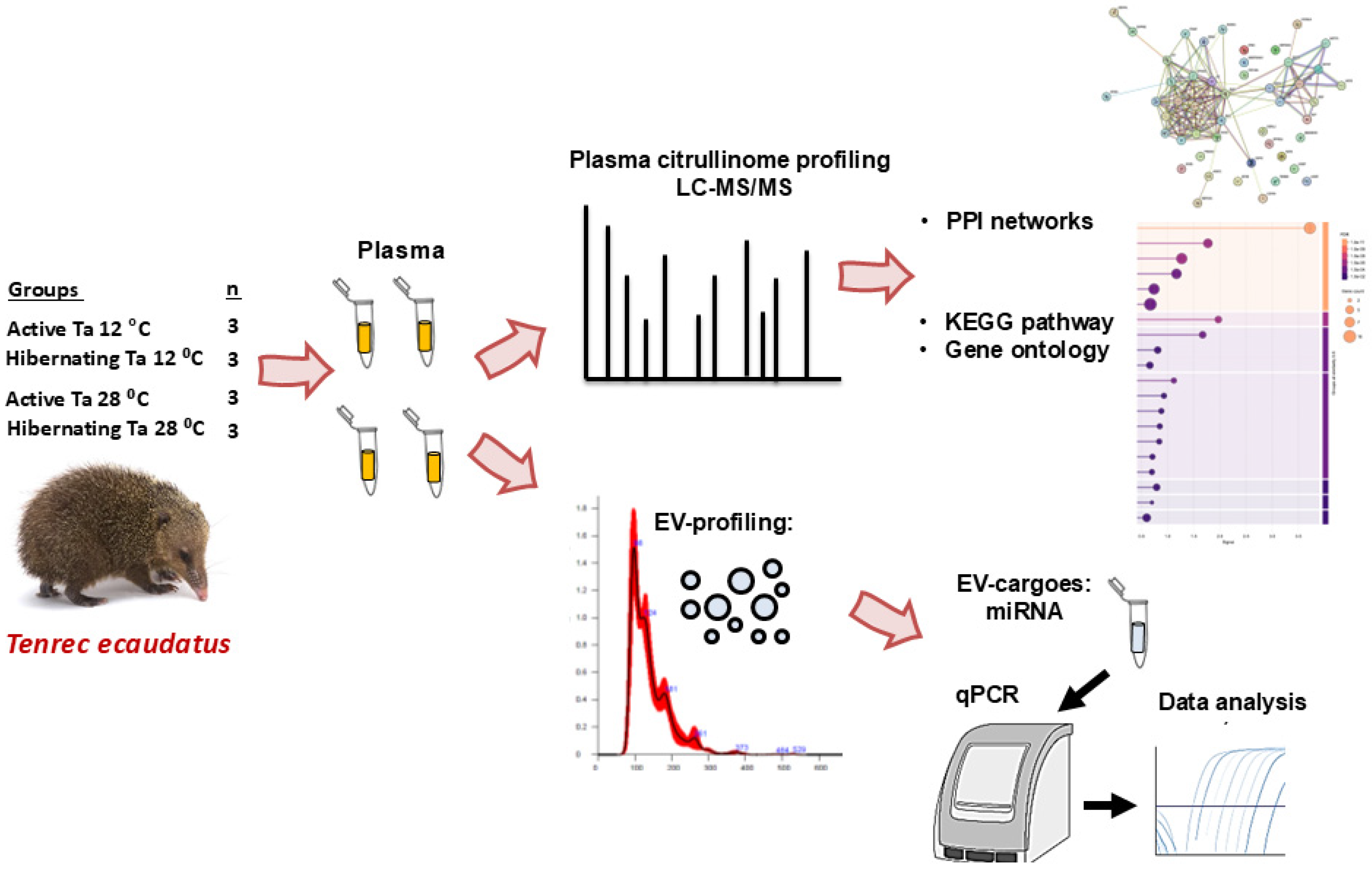
2.1. Animals and Plasma Samples
2.2. Analysis of the Tenrec Plasma Citrullinomes
2.3. LC-MS/MS and STRING Pathway Analysis of the Tenrec Plasma Citrullinomes
2.4. Western Blotting for PAD and Histone H3 Citrullination in Tenrec Plasma and EV Surface Markers
2.5. Isolation and Characterisation of Extracellular Vesicles from Tenrec Plasma
2.6. Analysis of miRNAs miR-21, miR-155, miR-210 and miR-206 in Tenrec Plasma-EVs
2.7. Statistical Analysis
3. Results
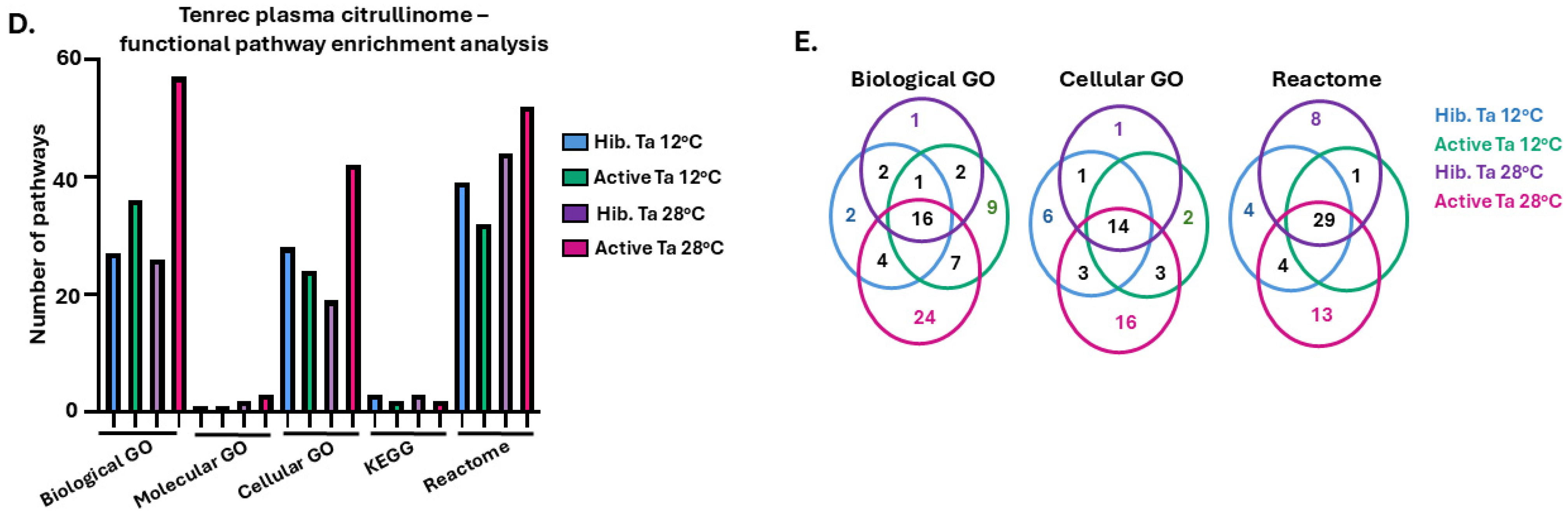
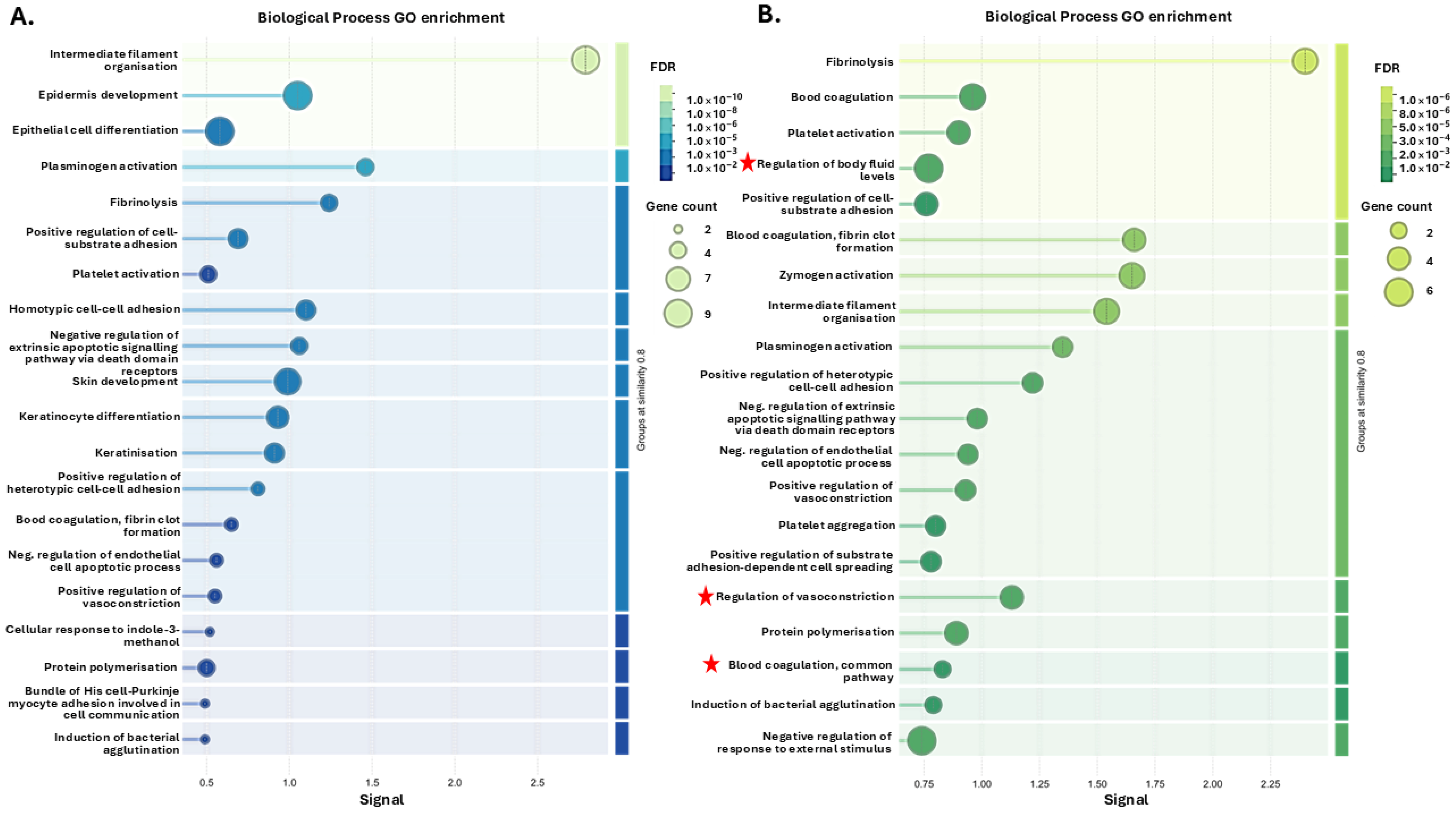
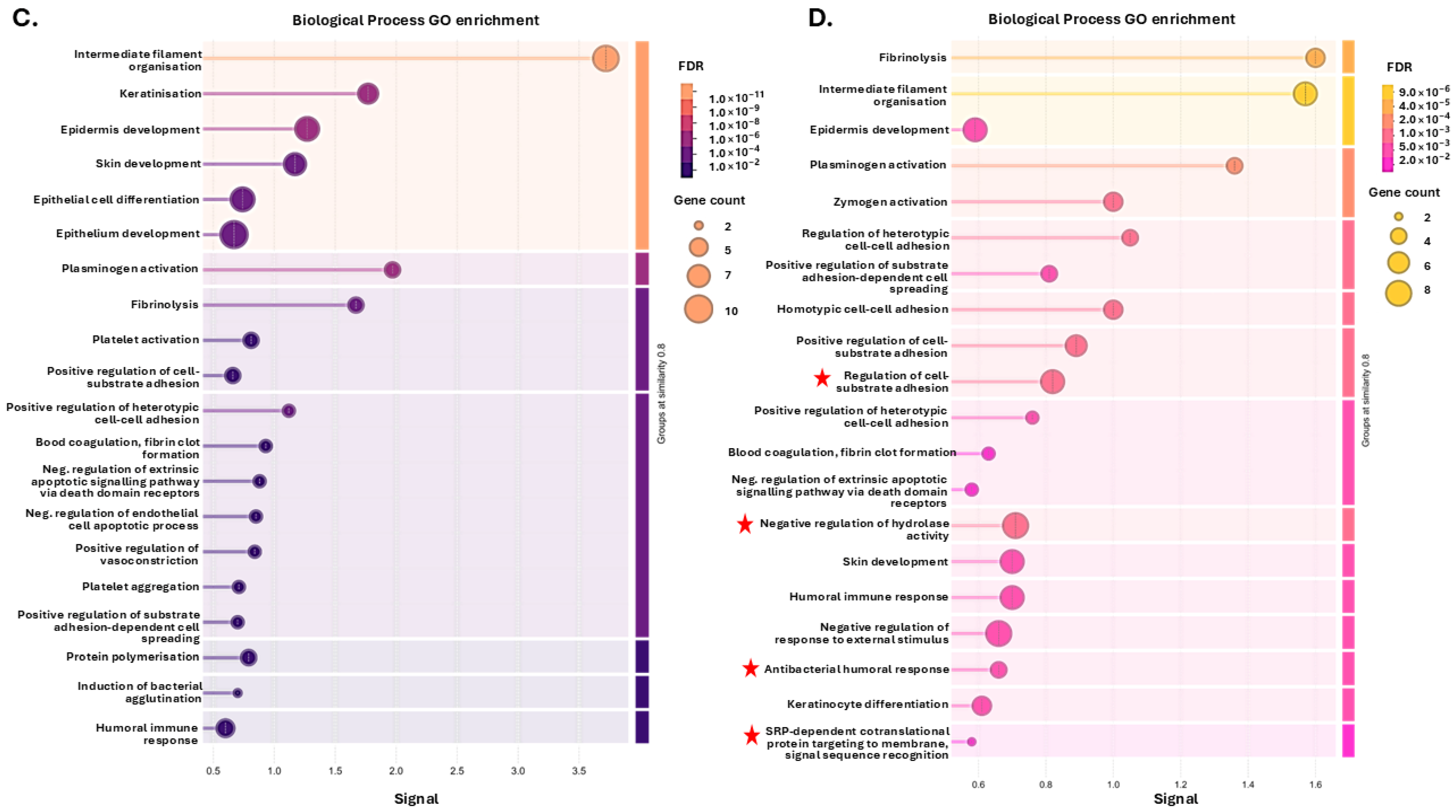
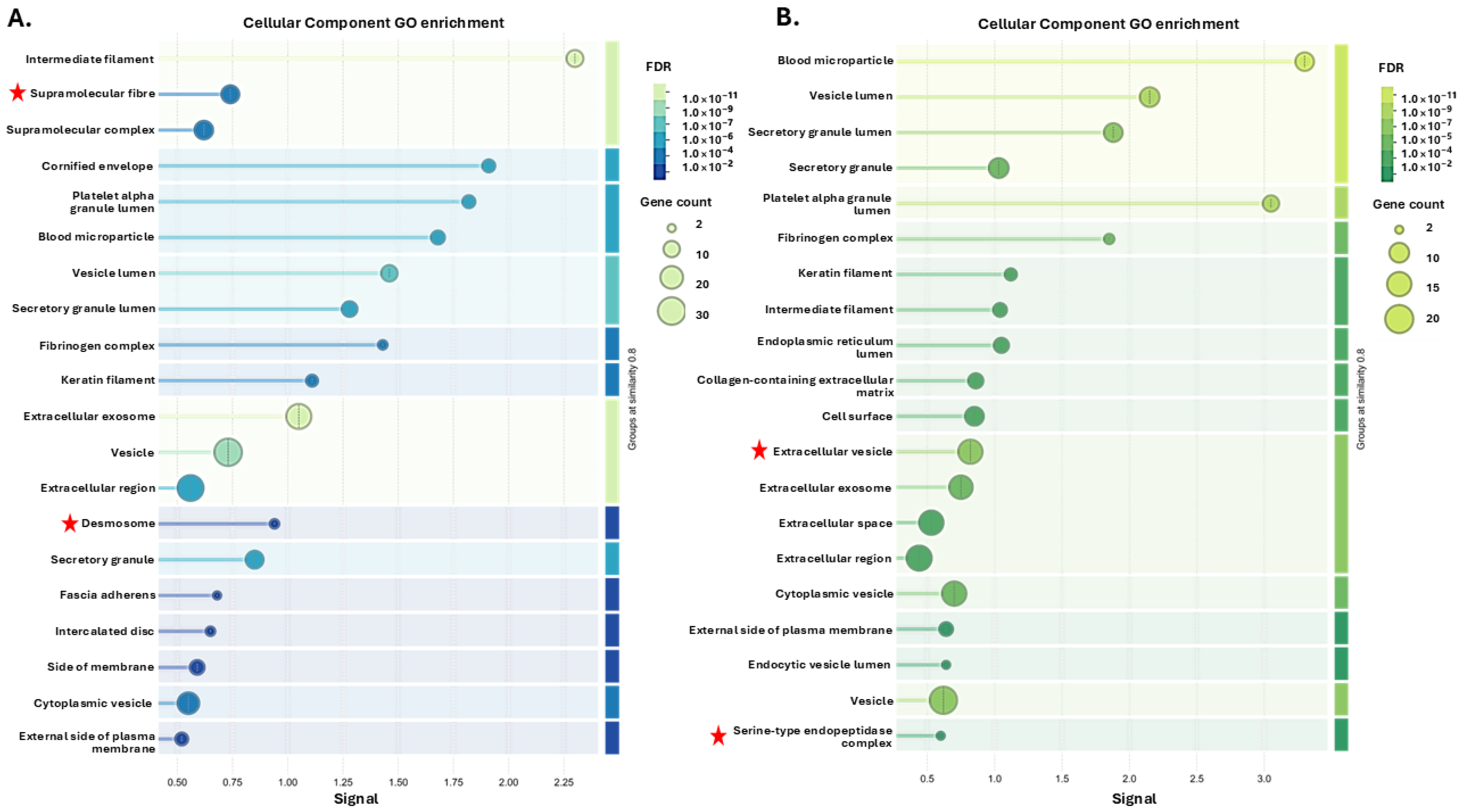
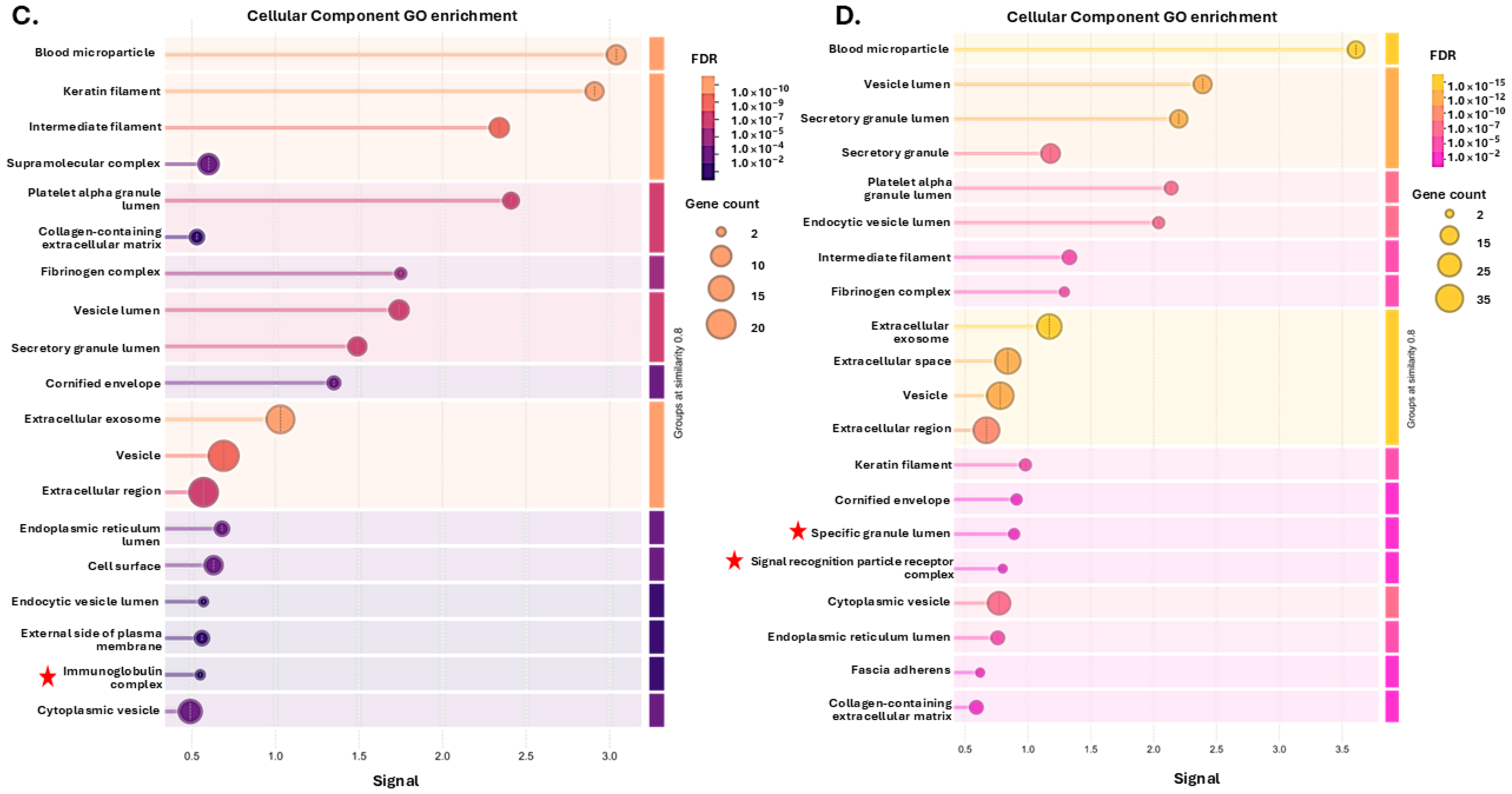
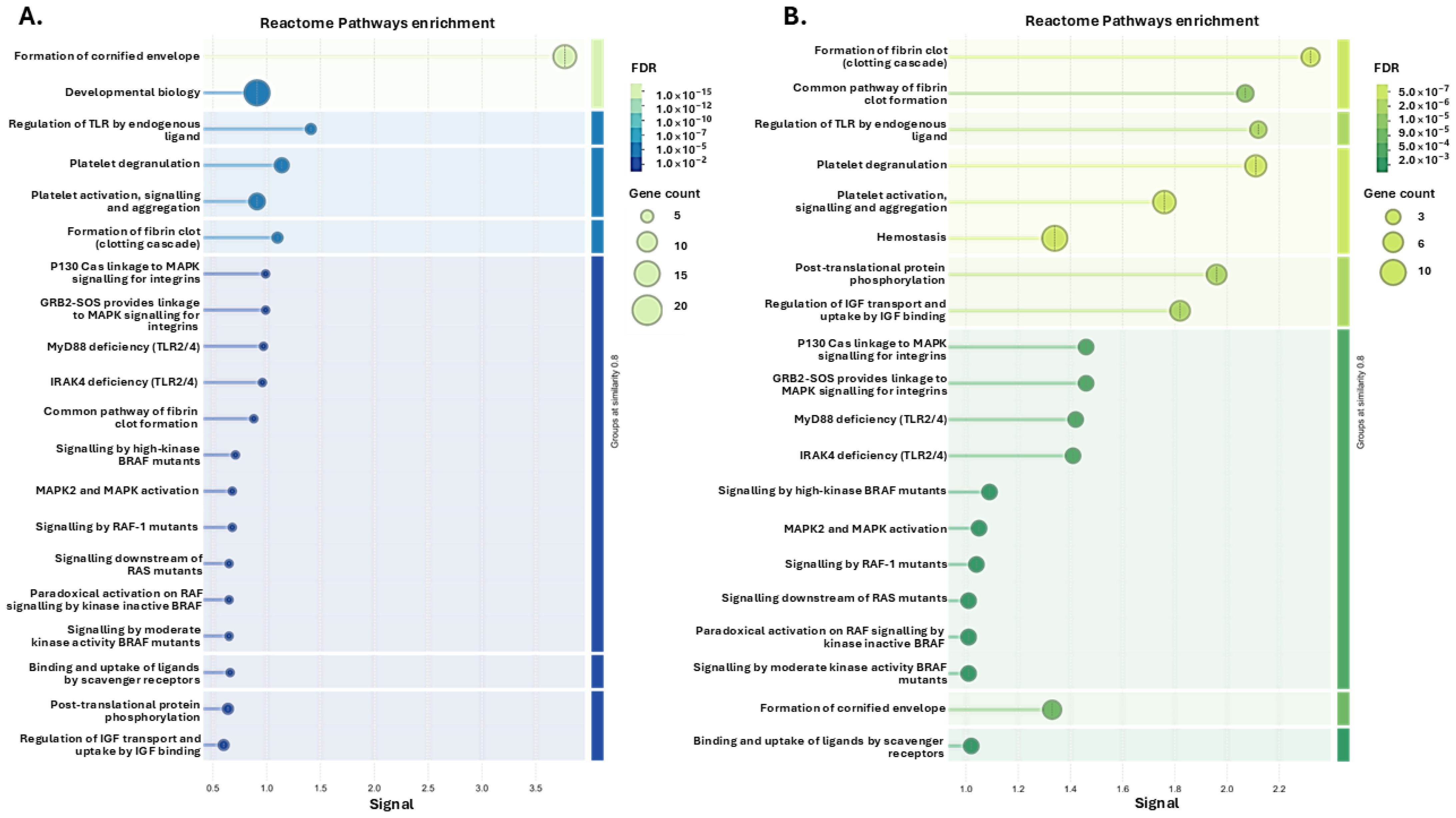
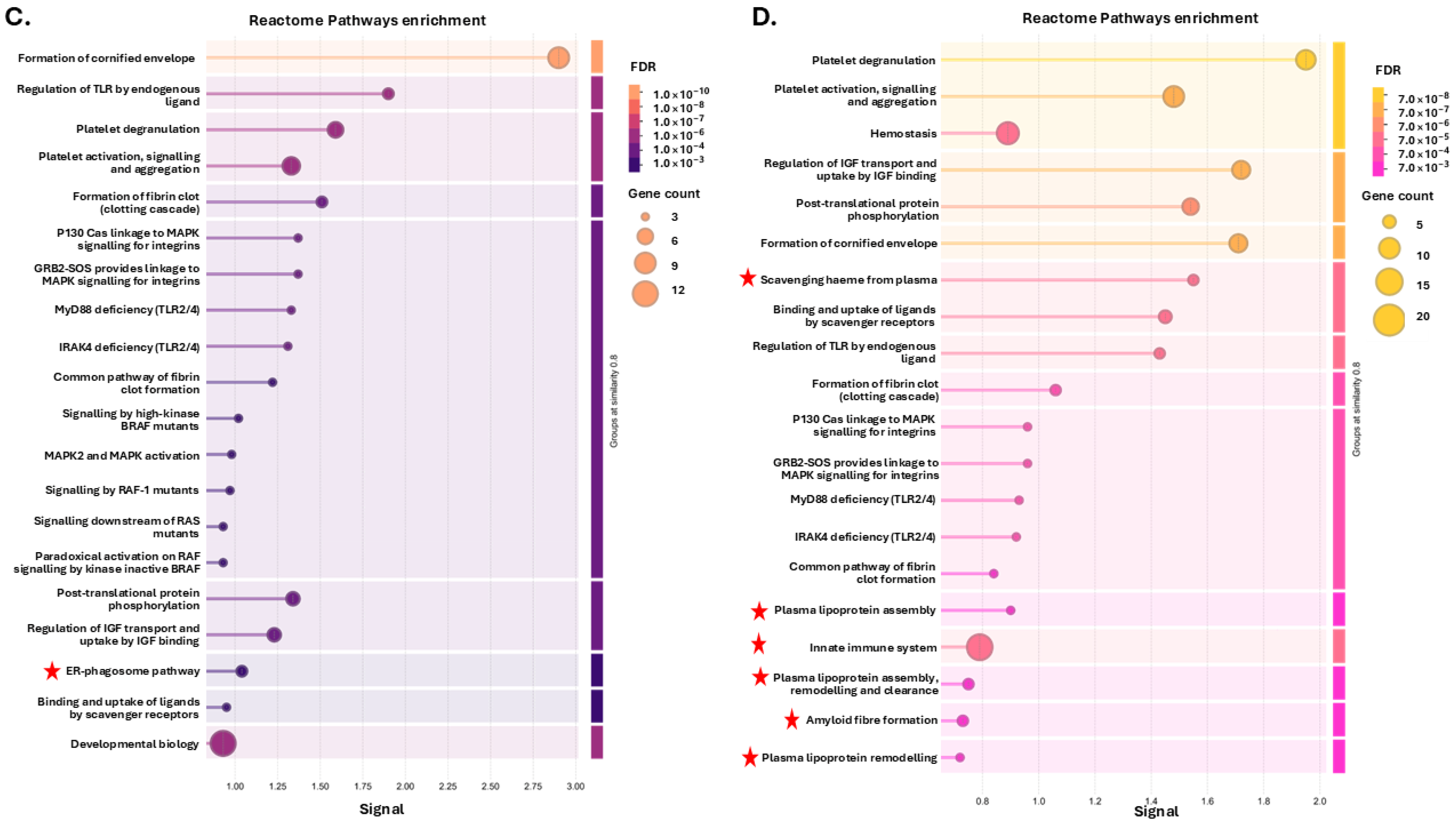
3.1. Tenrec Plasma Citrullinomes and Pathway Enrichment Analysis in Hibernating Versus Active States at Ta 12 °C and Ta 28 °C
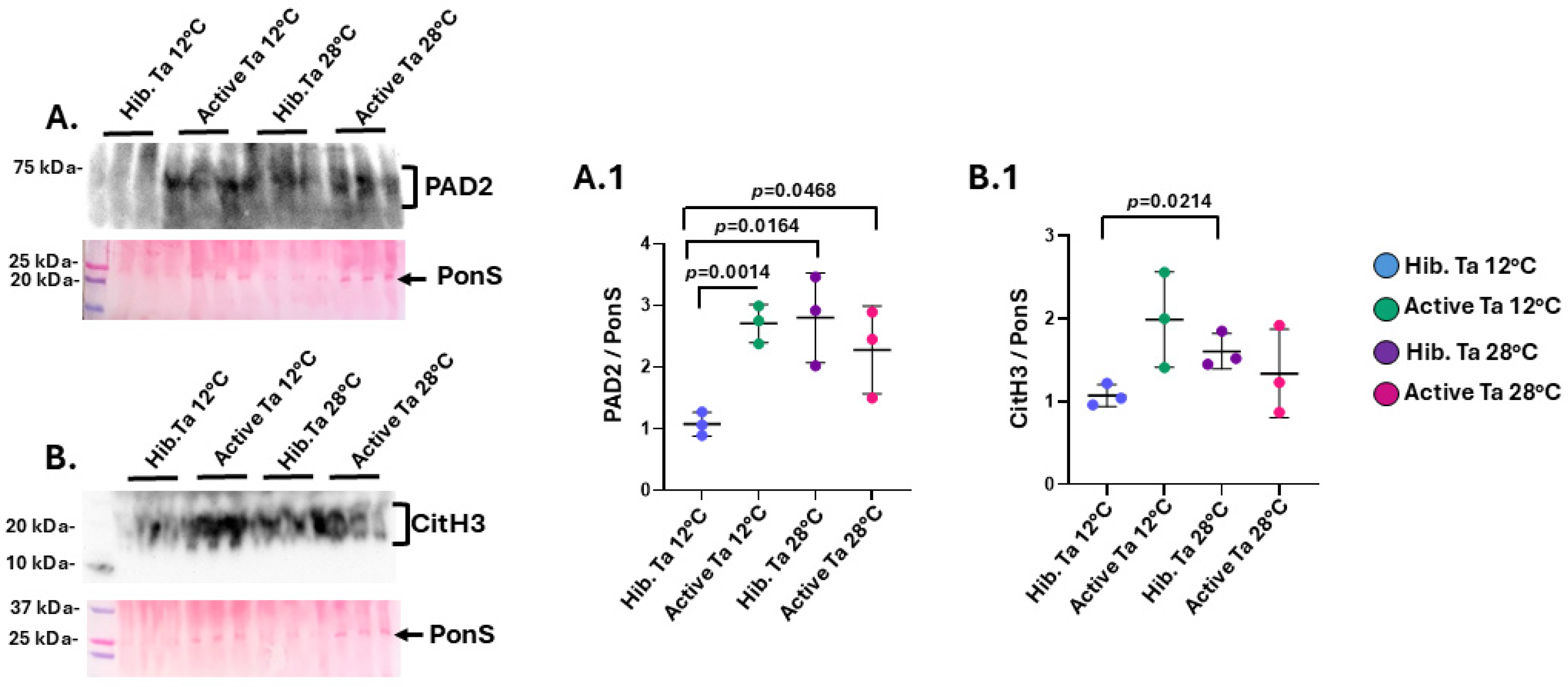
3.2. PAD and CitH3 Detection in Tenrec Plasma in Hibernating Versus Active States at Ta 12 °C and Ta 28 °C
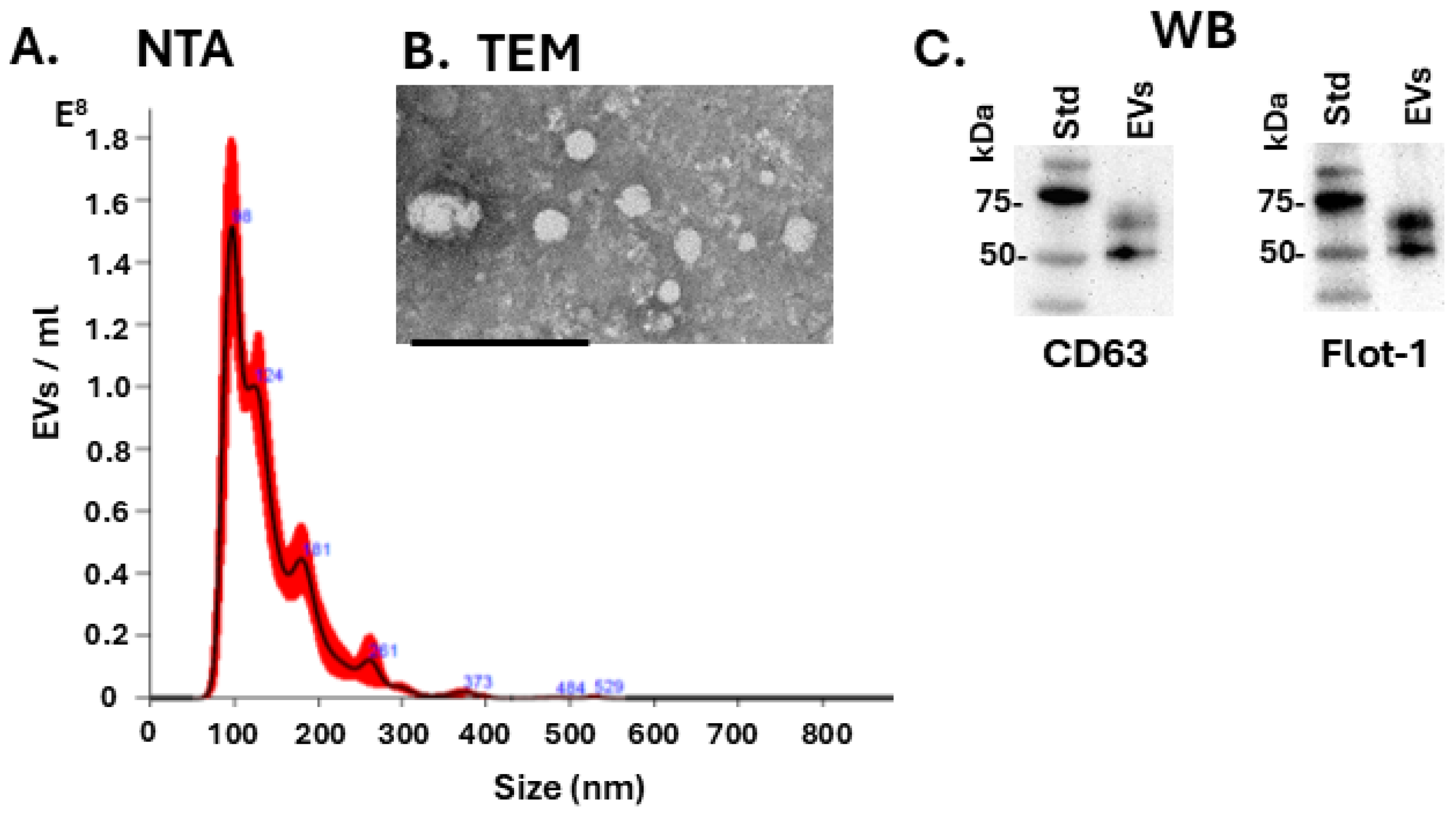
3.3. EV Characterisation and EV Profiles in Tenrec Plasma in Hibernating Versus Active States at 12 °C and 28 °C
3.4. Oncogenic, Inflammatory-, Hypoxia- and Metabolic-Related microRNA EV Cargoes in Tenrec Plasma of Hibernating Versus Active States at Ta 12 °C and Ta 28 °C
4. Discussion
4.1. The Tenrec Plasma Citrullinome Reflects Temperature and State Specific Immune Modulation
4.2. EV-Mediated miRNA Signalling Indicates a Flexible Immune State
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva Rubio, C.; Kim, A.B.; Milsom, W.K.; Pamenter, M.E.; Smith, G.R.; van Breukelen, F. Common tenrecs (Tenrec ecaudatus) reduce oxygen consumption in hypoxia and in hypercapnia without concordant changes to body temperature or heart rate. J. Comp. Physiol. B 2024, 194, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Treat, M.D.; Scholer, L.; Barrett, B.; Khachatryan, A.; McKenna, A.J.; Reyes, T.; Rezazadeh, A.; Ronkon, C.F.; Samora, D.; Santamaria, J.F.; et al. Extreme physiological plasticity in a hibernating basoendothermic mammal, Tenrec ecaudatus. J. Exp. Biol. 2018, 221 Pt 20, jeb185900. [Google Scholar] [CrossRef]
- Romiguier, J.; Ranwez, V.; Delsuc, F.; Galtier, N.; Douzery, E.J. Less is more in mammalian phylogenomics: AT-rich genes minimize tree conflicts and unravel the root of placental mammals. Mol. Biol. Evol. 2013, 30, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, J.I.; Treat, M.D.; Shanafelt, M.C.; Deyarmin, J.S.; Neely, B.A.; van Breukelen, F. Liver proteome response to torpor in a basoendothermic mammal, Tenrec ecaudatus, provides insights into the evolution of homeothermy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R614–R624. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Ten Donkelaar, H.J.; Nicholson, C. The Central Nervous System of Vertebrates; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Bloch, J.I.; Flynn, J.J.; Gaudin, T.J.; Giallombardo, A.; Giannini, N.P.; Goldberg, S.L.; Kraatz, B.P.; Luo, Z.X.; Meng, J.; et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 2013, 339, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Gassen, J.; Nowak, T.J.; Henderson, A.D.; Muehlenbein, M.P. Dynamics of temperature change during experimental respiratory virus challenge: Relationships with symptoms, stress hormones, and inflammation. Brain Behav. Immun. 2022, 99, 157–165. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Buttari, F.; Gilio, L.; Simonelli, I.; Carbone, F.; Micillo, T.; De Rosa, V.; Sica, F.; Furlan, R.; et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult. Scler. 2020, 26, 1237–1246. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Neto, A.; Fernandes, A.; Baraterio, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 60, 1352–1364. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.I.N.; Ali, M.M.; McGuire, G.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–984. [Google Scholar] [PubMed]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of server influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Quach, T.T. COVID-19 cytokine storm syndrome: A threshold concept. Lancet Microbe 2021, 2, e49–e50. [Google Scholar] [CrossRef] [PubMed]
- Witalison, E.E.; Thompson, P.R.; Hofseth, L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets 2015, 16, 700–710. [Google Scholar] [CrossRef]
- Biron, B.M.; Chung, C.S.; O’Brien, X.M.; Chen, Y.; Reichner, J.S.; Ayala, A. Cl-Amidine Prevents Histone 3 Citrullination and Neutrophil Extracellular Trap Formation, and Improves Survival in a Murine Sepsis Model. J. Innate Immun. 2017, 9, 22–32. [Google Scholar] [CrossRef]
- Tian, Y.; Qu, S.; Alam, H.B.; Williams, A.M.; Wu, Z.; Deng, Q.; Pan, B.; Zhou, J.; Liu, B.; Duan, X.; et al. Peptidylarginine deiminase 2 has potential as both a biomarker and therapeutic target of sepsis. JCI Insight. 2020, 5, e138873. [Google Scholar] [CrossRef]
- Sancandi, M.; Uysal-Onganer, P.; Kraev, I.; Mercer, A.; Lange, S. Protein deimination signatures in plasma and plasma-EVs and protein deimination in the brain vasculature in a rat model of pre-motor Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 2743. [Google Scholar] [CrossRef]
- Arisan, E.D.; Uysal-Onganer, P.; Lange, S. Putative Roles for Peptidylarginine Deiminases in COVID-19. Int. J. Mol. Sci. 2020, 21, 4662. [Google Scholar] [CrossRef]
- Wang, J.; Miao, J.; Zhu, P. Insights into the complexities of Citrullination: From immune regulation to autoimmune disease. Autoimmun. Rev. 2025, 24, 103734. [Google Scholar] [CrossRef]
- Lange, S.; Gögel, S.; Leung, K.Y.; Vernay, B.; Nicholas, A.P.; Causey, C.P.; Thompson, P.R.; Greene, N.D.; Ferretti, P. Protein deiminases: New players in the developmentally regulated loss of neural regenerative ability. Dev. Biol. 2011, 355, 205–214. [Google Scholar] [CrossRef]
- Lange, S.; Rocha-Ferreira, E.; Thei, L.; Mawjee, P.; Bennett, K.; Thompson, P.R.; Subramanian, V.; Nicholas, A.P.; Peebles, D.; Hristova, M.; et al. Peptidylarginine deiminases: Novel drug targets for prevention of neuronal damage following hypoxic ischemic insult (HI) in neonates. J. Neurochem. 2014, 130, 555–562. [Google Scholar] [CrossRef]
- Sase, T.; Arito, M.; Onodera, H.; Omoteyama, K.; Kurokawa, M.S.; Kagami, Y.; Ishigami, A.; Tanaka, Y.; Kato, T. Hypoxia-induced production of peptidylarginine deiminases and citrullinated proteins in malignant glioma cells. Biochem. Biophys. Res. Commun. 2017, 482, 50–56. [Google Scholar] [CrossRef]
- D’Alessio, S.; Cheng, H.; Eaton, L.; Kraev, I.; Pamenter, M.E.; Lange, S. Acute Hypoxia Alters Extracellular Vesicle Signatures and the Brain Citrullinome of Naked Mole-Rats (Heterocephalus glaber). Int. J. Mol. Sci. 2022, 23, 4683. [Google Scholar] [CrossRef] [PubMed]
- Kholia, S.; Jorfi, S.; Thompson, P.R.; Causey, C.P.; Nicholas, A.P.; Inal, J.M.; Lange, S. A novel role for peptidylarginine deiminases in microvesicle release reveals therapeutic potential of PAD inhibition in sensitizing prostate cancer cells to chemotherapy. J. Extracell. Vesicles. 2015, 4, 26192. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Uysal-Onganer, P.; MacLatchy, A.; Kraev, I.; Chatterton, N.P.; Nicholas, A.P.; Inal, J.M.; Lange, S. Peptidylarginine deiminases post-translationally deiminate prohibitin and modulate extracellular vesicle release and microRNAs in glioblastoma multiforme. Int. J. Mol. Sci. 2018, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Uysal-Onganer, P.; MacLatchy, A.; Mahmoud, R.; Kraev, I.; Thompson, P.R.; Inal, J.M.; Lange, S. Peptidylarginine deiminase isozyme-specific PAD2, PAD3 and PAD4 inhibitors differentially modulate extracellular vesicle signatures and cell invasion in two glioblastoma multiforme cell lines. Int. J. Mol. Sci. 2020, 21, 1495. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. BioEssays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Magnadóttir, B.; Hayes, P.; Hristova, M.; Bragason, B.T.; Nicholas, A.P.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Post-translational protein deimination in cod (Gadus morhua L.) ontogeny novel roles in tissue remodelling and mucosal immune defences? Dev. Comp. Immunol. 2018, 87, 157–170. [Google Scholar] [CrossRef]
- Magnadóttir, B.; Bragason, B.T.; Bricknell, I.R.; Bowden, T.; Nicholas, A.P.; Hristova, M.; Guðmundsdóttir, S.; Dodds, A.W.; Lange, S. Peptidylarginine deiminase and deiminated proteins are detected throughout early halibut ontogeny—Complement components C3 and C4 are post-translationally deiminated in halibut (Hippoglossus hippoglossus L.). Dev. Comp. Immunol. 2019, 92, 1–19. [Google Scholar] [CrossRef]
- Criscitiello, M.F.; Kraev, I.; Lange, S. Deiminated proteins in extracellular vesicles and plasma of nurse shark (Ginglymostoma cirratum)—Novel insights into shark immunity. Fish Shellfish Immunol. 2019, 92, 249–255. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Silva-Álvarez, V.; Kraev, I.; Uysal-Onganer, P.; Lange, S. Extracellular vesicles and citrullination signatures are novel biomarkers in sturgeon (Acipenser gueldenstaedtii) during chronic stress due to seasonal temperature challenge. Fish Shellfish Immunol. 2024, 154, 109974. [Google Scholar] [CrossRef]
- Novák, L.; Zubáčová, Z.; Karnkowska, A.; Kolisko, M.; Hroudová, M.; Stairs, C.W.; Simpson, A.G.; Keeling, P.J.; Roger, A.J.; Čepička, I.; et al. Arginine deiminase pathway enzymes: Evolutionary history in metamonads and other eukaryotes. BMC Evol. Biol. 2016, 16, 197. [Google Scholar] [CrossRef]
- Gavinho, B.; Sabatke, B.; Feijoli, V.; Rossi, I.V.; da Silva, J.M.; Evans-Osses, I.; Palmisano, G.; Lange, S.; Ramirez, M.I. Peptidylarginine Deiminase Inhibition Abolishes the Production of Large Extracellular Vesicles From Giardia intestinalis, Affecting Host-Pathogen Interactions by Hindering Adhesion to Host Cells. Front. Cell. Infect. Microbiol. 2020, 10, 417. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shindia, A.A.; AbouZaid, A.A.; Yassin, A.M.; Ali, G.S.; Sitohy, M.Z. Biochemical characterization of peptidylarginine deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzyme Microb. Technol. 2019, 124, 41–53. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Matewele, P.; Mastroianni, G.; Kraev, I.; Brotherton, D.; Awamaria, B.; Nicholas, A.P.; Lange, S.; Inal, J.M. Peptidylarginine Deiminase Inhibitors Reduce Bacterial Membrane Vesicle Release and Sensitize Bacteria to Antibiotic Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 227. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Uysal-Onganer, P.; Huynh, K.W.; Kraev, I.; Lange, S. Post-Translational Deimination of Immunological and Metabolic Protein Markers in Plasma and Extracellular Vesicles of Naked Mole-Rat (Heterocephalus glaber). Int. J. Mol. Sci. 2019, 20, 5378. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Erratum in J. Extracell. Vesicles 2024 13 e12451. https://doi.org/10.1002/jev2.12451. [Google Scholar] [CrossRef]
- Wessler, S.; Meisner-Kober, N. On the road: Extracellular vesicles in intercellular communication. Cell Commun. Signal. 2025, 23, 95. [Google Scholar] [CrossRef]
- Di Naro, M.; Petronio Petronio, G.; Mukhtar, F.; Cutuli, M.A.; Magnifico, I.; Falcone, M.; Brancazio, N.; Guarnieri, A.; Di Marco, R.; Nicolosi, D. Extracellular Vesicles in Bacteria, Archaea, and Eukaryotes: Mechanisms of Inter-Kingdom Communication and Clinical Implications. Microorganisms 2025, 13, 636. [Google Scholar] [CrossRef]
- Lange, S. Extracellular Vesicles in Phylogeny. Int. J. Mol. Sci. 2023, 24, 10466. [Google Scholar] [CrossRef]
- Magnadóttir, B.; Uysal-Onganer, P.; Kraev, I.; Svansson, V.; Hayes, P.; Lange, S. Deiminated proteins and extracellular vesicles—Novel serum biomarkers in whales and orca. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100676. [Google Scholar] [CrossRef]
- Criscitiello, M.F.; Kraev, I.; Petersen, L.H.; Lange, S. Deimination Protein Profiles in Alligator mississippiensis Reveal Plasma and Extracellular Vesicle-Specific Signatures Relating to Immunity, Metabolic Function, and Gene Regulation. Front. Immunol. 2020, 11, 651. [Google Scholar] [CrossRef]
- Criscitiello, M.F.; Kraev, I.; Lange, S. Deiminated proteins in extracellular vesicles and serum of llama (Lama glama)-Novel insights into camelid immunity. Mol. Immunol. 2020, 117, 37–53. [Google Scholar] [CrossRef]
- Vistro, W.A.; Huang, Y.; Bai, X.; Yang, P.; Haseeb, A.; Chen, H.; Liu, Y.; Yue, Z.; Tarique, I.; Chen, Q. In Vivo Multivesicular Body and Exosome Secretion in the Intestinal Epithelial Cells of Turtles During Hibernation. Microsc. Microanal. 2019, 25, 1341–1351. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Yadav, P.; Tamilselvan, R.; Mani, H.; Singh, K.K. MicroRNA-mediated regulation of nonsense-mediated mRNA decay factors: Insights into microRNA prediction tools and profiling techniques. Biochim. Biophys. Acta Gene Regul. Mech. 2024, 1867, 195022. [Google Scholar] [CrossRef]
- Capraro, A.; O’Meally, D.; Waters, S.A.; Patel, H.R.; Georges, A.; Waters, P.D. MicroRNA dynamics during hibernation of the Australian central bearded dragon (Pogona vitticeps). Sci. Rep. 2020, 10, 17854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, R.; Liang, J.; Izaz, A.; Shu, Y.; Pan, T.; Wu, X. Molecular mechanism of Chinese alligator (Alligator sinensis) adapting to hibernation. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Biggar, Y.; Ingelson-Filpula, W.A.; Storey, K.B. Pro- and anti-apoptotic microRNAs are differentially regulated during estivation in Xenopus laevis. Gene 2022, 819, 146236. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Storey, K.B. Identification and expression of microRNA in the brain of hibernating bats, Myotis lucifugus. Gene 2014, 544, 67–74. [Google Scholar] [CrossRef]
- Ingelson-Filpula, W.A.; Storey, K.B. Hibernation-Induced microRNA Expression Promotes Signaling Pathways and Cell Cycle Dysregulation in Ictidomys tridecemlineatus Cardiac Tissue. Metabolites 2023, 13, 1096. [Google Scholar] [CrossRef]
- Fazzalari, A.; Basadonna, G.; Kucukural, A.; Tanriverdi, K.; Koupenova, M.; Pozzi, N.; Kakuturu, J.; Friedrich, A.-K.U.; Korstanje, R.; Fowler, N.; et al. A Translational Model for Venous Thromboembolism: MicroRNA Expression in Hibernating Black Bears. J. Surg. Res. 2021, 257, 203–212. [Google Scholar] [CrossRef]
- Angelopoulos, A.; Oikonomou, E.; Antonopoulos, A.; Theofilis, P.; Zisimos, K.; Katsarou, O.; Gazouli, M.; Lazaros, G.; Papanikolaou, P.; Siasos, G.; et al. Expression of Circulating miR-21 and -29 and their Association with Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Curr. Med. Chem. 2024, 31, 3987–3996. [Google Scholar] [CrossRef]
- Giordo, R.; Ahmadi, F.A.M.; Husaini, N.A.; Al-Nuaimi, N.R.A.M.; Ahmad, S.M.S.; Pintus, G.; Zayed, H. microRNA 21 and long non-coding RNAs interplays underlie cancer pathophysiology: A narrative review. Noncoding RNA Res. 2024, 9, 831–852. [Google Scholar] [CrossRef]
- Sinha, S.; Rajendran, B.; Vasagam, S.; Balakrishnan, J. Correlation between microRNA-21 expression and overweight/obesity. Folia. Med. 2024, 66, 825–833. [Google Scholar] [CrossRef]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Khalilian, S.; Bijanvand, A.; Abedinlou, H.; Ghafouri-Fard, S. A review on the role of miR-210 in human disorders. Pathol. Res. Pract. 2023, 241, 154244. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, D.; Tao, K.; Lin, F.; Wang, H.; Sun, H.; Liu, R.; Li, Z. The Role of MicroRNA-206 in the Regulation of Diabetic Wound Healing via Hypoxia-Inducible Factor 1-Alpha. Biochem. Genet. 2025, 63, 393–410. [Google Scholar] [CrossRef]
- Millozzi, F.; Milán-Rois, P.; Sett, A.; Carpini, G.D.; De Bardi, M.; Gisbert-Garzarán, M.; Sandonà, M.; Rodríguez-Díaz, C.; Martínez-Mingo, M.; Pardo, I.; et al. Aptamer-conjugated gold nanoparticles enable oligonucleotide delivery into muscle stem cells to promote regeneration of dystrophic muscles. Nat. Commun. 2025, 16, 577. [Google Scholar] [CrossRef]
- Qi, W.; Guan, W. A Comprehensive Review on the Importance of MiRNA-206 in the Animal Model and Human Diseases. Curr. Neuropharmacol. 2024, 22, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Tarver, J.E.; Dos Reis, M.; Mirarab, S.; Moran, R.J.; Parker, S.; O’Reilly, J.E.; King, B.L.; O’Connell, M.J.; Asher, R.J.; Warnow, T.; et al. The Interrelationships of Placental Mammals and the Limits of Phylogenetic Inference. Genome Biol. Evol. 2016, 8, 330–344. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, S.; Thorgeirsdóttir, S.; Kraev, I.; Skírnisson, K.; Lange, S. Post-Translational Protein Deimination Signatures in Plasma and Plasma EVs of Reindeer (Rangifer tarandus). Biology 2021, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Retter, A.; Singer, M.; Annane, D. “The NET effect”: Neutrophil extracellular traps-a potential key component of the dysregulated host immune response in sepsis. Crit. Care 2025, 29, 59. [Google Scholar] [CrossRef]
- Thienel, M.; Müller-Reif, J.B.; Zhang, Z.; Ehreiser, V.; Huth, J.; Shchurovska, K.; Kilani, B.; Schweizer, L.; Geyer, P.E.; Zwiebel, M.; et al. Immobility-associated thromboprotection is conserved across mammalian species from bear to human. Science 2023, 380, 178–187. [Google Scholar] [CrossRef]
- Smith, G.R.; Silva Rubio, C.; van Breukelen, F. The use of micro-computed tomography to investigate splenic control of resting oxygen consumption in Tenrec caudatus. Physiology 2024, 39, 981. [Google Scholar] [CrossRef]
- Zimmerman, L.M.; Vogel, L.A.; Bowden, R.M. Understanding the vertebrate immune system: Insights from the reptilian perspective. J. Exp. Bio. 2010, 213, 661–671. [Google Scholar] [CrossRef]
- Drake, K.; Aiello, C.M.; Bowen, L.; Lewison, R.L.; Esque, T.C.; Nussear, K.E.; Waters, S.C.; Hudson, P.J. Complex immune responses and molecular reactions to pathogens and disease in a desert reptile (Gopherus agassizii). Ecol. Evol. 2019, 9, 2516–2534. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, Q.; Pan, B.; Alam, H.B.; Tian, Y.; Bhatti, U.F.; Liu, B.; Mondal, S.; Thompson, P.R.; Li, Y. Inhibition of PAD2 Improves Survival in a Mouse Model of Lethal LPS-Induced Endotoxic Shock. Inflammation 2020, 43, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Qiu, R.; Kittisopikul, M.; Vahabikashi, A.; Goldman, A.E.; Goldman, R.D.; Wagner, D.D.; Waterman, C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. USA 2020, 117, 7326–7337. [Google Scholar] [CrossRef]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Briot, J.; Simon, M.; Méchin, M.C. Deimination, Intermediate Filaments and Associated Proteins. Int. J. Mol. Sci. 2020, 21, 8746. [Google Scholar] [CrossRef]
- Coudane, F.; Mechin, M.C.; Huchenq, A.; Henry, J.; Nachat, R.; Ishigami, A.; Adoue, V.; Sebbag, M.; Serre, G.; Simon, M. Deimination and expression of peptidylarginine deiminases during cutaneous wound healing in mice. Eur. J. Dermatol. 2011, 21, 376–384. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chen, Y.M.; Hsieh, T.Y.; Hsieh, C.W.; Lin, C.C.; Lan, J.L. Significant effects of biologic therapy on lipid profiles and insulin resistance in patients with rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Damiana, T.; Damgaard, D.; Sidelmann, J.J.; Nielsen, C.H.; de Maat, M.P.M.; Münster, A.B.; Palarasah, Y. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin. Chim. Acta 2020, 501, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zollet, V.; Arenas Hoyos, I.; Hirsiger, S.; Brahim, B.B.; Petrucci, M.F.; Casoni, D.; Wang, J.; Spirig, R.; Nettelbeck, K.; Garcia, L.; et al. Neutrophil extracellular traps and citrullinated fibrinogen contribute to injury in a porcine model of limb ischemia and reperfusion. Front. Immunol. 2024, 15, 1436926. [Google Scholar] [CrossRef]
- Yuzhalin, A.E. Citrullination in Cancer. Cancer Res. 2019, 79, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Thompson, P.R. Current insights into the role of citrullination in thrombosis. Curr. Opin. Chem. Biol. 2023, 75, 102313. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, G.; Rao, L.; Tian, C. Citrullination in health and disease: From physiological function to gene regulation. Genes Dis. 2024, 12, 101355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tetz, Z.A.; Zeng, X.; Go, S.J.; Ouyang, W.; Lee, K.E.; Dong, T.; Li, Y.; Ma, J. CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction. Pharmaceutics 2025, 17, 809. [Google Scholar] [CrossRef]
- Bruggeman, Y.; Sodré, F.M.C.; Buitinga, M.; Mathieu, C.; Overbergh, L.; Kracht, M.J.L. Targeting citrullination in autoimmunity: Insights learned from preclinical mouse models. Expert Opin. Ther. Targets 2021, 25, 269–281. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Chong, D.L.W.; Sriskandan, S. Pro-Inflammatory Mechanisms in Sepsis. In Sepsis—Pro-Inflammatory and Anti-Inflammatory Responses: Good, Bad or Ugly? Karger Medical and Scientific Publishers: Basel, Switzerland, 2011; Volume 17, pp. 86–107. [Google Scholar]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Valsamaki, A.; Vazgiourakis, V.; Mantzarlis, K.; Stamatiou, R.; Makris, D. MicroRNAs in Sepsis. Biomedicines 2024, 12, 2049. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Yamamoto, Y.; Ito, K.; Otsuka, K.; Soekmadji, C.; Egawa, S.; Kimura, T.; Ochiya, T. Metastatic prostate cancer-derived extracellular vesicles facilitate osteoclastogenesis by transferring the CDCP1 protein. J. Extracell. Vesicles 2023, 12, 12312. [Google Scholar] [CrossRef]
- Kornfeld, S.F.; Biggar, K.K.; Storey, K.B. Differential expression of mature microRNAs involved in muscle maintenance of hibernating little brown bats, Myotis lucifugus: A model of muscle atrophy resistance. Genom. Proteom. Bioinform. 2012, 10, 295–301. [Google Scholar] [CrossRef]
- Taiwo, O.O.; Rehman, S.; Storey, K.B. Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers. Biomolecules 2025, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Pan, W.; Qian, J.F.; Liu, F.; Dong, H.Q.; Liu, Q.J. MicroRNA-21 contributes to the puerarin-induced cardioprotection via suppression of apoptosis and oxidative stress in a cell model of ischemia/reperfusion injury. Mol. Med. Rep. 2019, 20, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wang, J.; Fu, K.; Dai, S.; Wu, R.; Peng, C.; Li, Y. The role of miR-155 on liver diseases by modulating immunity, in-flammation and tumorigenesis. Int. Immunopharmacol. 2023, 116, 109775. [Google Scholar] [CrossRef]
- Ke, F.; Wang, H.; Geng, J.; Jing, X.; Fang, F.; Fang, C.; Zhang, B.H. MiR-155 promotes inflammation and apoptosis via targeting SIRT1 in hypoxic-ischemic brain damage. Exp. Neurol. 2023, 362, 114317. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Xia, H.; Yao, S. MicroRNA-155: Regulation of Immune Cells in Sepsis. Mediat. Inflamm. 2021, 2021, 8874854. [Google Scholar] [CrossRef]
- Devereaux, M.E.M.; Silva Rubio, C.; van Breukelen, F.; Pamenter, M.E. Physiological responses to hypoxia are constrained by environmental temperature in heterothermic tenrecs. J. Exp. Biol. 2023, 226, jeb245324. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Zhang, H.; Huang, P.; Luthra, R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 2010, 29, 4362–4368. [Google Scholar] [CrossRef]
- Voloboueva, L.A.; Sun, X.; Xu, L.; Ouyang, Y.-B.; Giffard, R.G. Distinct effects of miR-210 reduction on neurogenesis: Increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J. Neurosci. 2017, 37, 3072–3084. [Google Scholar] [CrossRef]
- Favaro, E.; Ramachandran, A.; McCormick, R.; Gee, H.; Blancher, C.; Crosby, M.; Devlin, C.; Blick, C.; Buffa, F.; Li, J.-L.; et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS ONE 2010, 5, e10345. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Le, Q.-T.; Giaccia, A.J. MiR-210—Micromanager of the hypoxia pathway. Trends Mol. Med. 2010, 16, 230–237. [Google Scholar] [CrossRef]
- Magnadóttir, B.; Uysal-Onganer, P.; Kraev, I.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.). Aquacult. Rep. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef]
- Ackermann, M.; Anders, H.J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef] [PubMed]
- Aymonnier, K.; Ng, J.; Fredenburgh, L.E.; Zambrano-Vera, K.; Münzer, P.; Gutch, S.; Fukui, S.; Desjardins, M.; Subramaniam, M.; Baron, R.M.; et al. Inflammasome activation in neutrophils of patients with severe COVID-19. Blood Adv. 2022, 6, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Heger, L.A. Thromboinflammation: From Atherosclerosis to COVID-19. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1103–1112. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, G.R.; Uysal-Onganer, P.; Kraev, I.; van Breukelen, F.; Lange, S. Immune Tuning in Extreme Environments: Protein Citrullinome and Extracellular Vesicle Signatures Comparing Hibernating Versus Active States in the Heterothermic and Heterometabolic Tenrec (Tenrec ecaudatus). Biology 2025, 14, 1056. https://doi.org/10.3390/biology14081056
Smith GR, Uysal-Onganer P, Kraev I, van Breukelen F, Lange S. Immune Tuning in Extreme Environments: Protein Citrullinome and Extracellular Vesicle Signatures Comparing Hibernating Versus Active States in the Heterothermic and Heterometabolic Tenrec (Tenrec ecaudatus). Biology. 2025; 14(8):1056. https://doi.org/10.3390/biology14081056
Chicago/Turabian StyleSmith, Gilbecca Rae, Pinar Uysal-Onganer, Igor Kraev, Frank van Breukelen, and Sigrun Lange. 2025. "Immune Tuning in Extreme Environments: Protein Citrullinome and Extracellular Vesicle Signatures Comparing Hibernating Versus Active States in the Heterothermic and Heterometabolic Tenrec (Tenrec ecaudatus)" Biology 14, no. 8: 1056. https://doi.org/10.3390/biology14081056
APA StyleSmith, G. R., Uysal-Onganer, P., Kraev, I., van Breukelen, F., & Lange, S. (2025). Immune Tuning in Extreme Environments: Protein Citrullinome and Extracellular Vesicle Signatures Comparing Hibernating Versus Active States in the Heterothermic and Heterometabolic Tenrec (Tenrec ecaudatus). Biology, 14(8), 1056. https://doi.org/10.3390/biology14081056








