Effects of Salinity on the Reproductive and Lifespan Traits of Artemia Parthenogenetic Lineages with Different Ploidy Levels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Within-Lineage
3.2. Between-Lineages
3.3. Mixed Groups
4. Discussion
4.1. Reproductive Traits
4.2. Lifespan
4.3. Reproductive Mode
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asem, A.; Yang, C.; Eimanifar, A.; Hontoria, F.; Varó, I.; Mahmoudi, F.; Fu, C.; Shen, C.; Rastegar-Pouyani, N.; Wang, P.; et al. Phylogenetic analysis of problematic Asian species of Artemia Leach, 1819 (Crustacea, Anostraca), with the descriptions of two new species. J. Crustac. Biol. 2023, 43, ruad002. [Google Scholar] [CrossRef]
- Asem, A.; Gajardo, G.; Hontoria, F.; Yang, C.; Shen, C.; Rastegar-Pouyani, N.; Padhye, S.M.; Sorgeloos, P. The species problem in Artemia Leach, 1819 (Crustacea: Anostraca), a genus with sexual species and obligate parthenogenetic lineages. Zool. J. Linn. Soc. 2024, 202, zlad192. [Google Scholar] [CrossRef]
- Maniatsi, S.; Baxevanis, A.D.; Kappas, I.; Deligiannidis, P.; Triantafyllidis, A.; Papakostas, S.; Bougiouklis, D.; Abatzopoulos, T.J. Is polyploidy a persevering accident or an adaptive evolutionary pattern? The case of the brine shrimp Artemia. Mol. Phyl. Evol. 2011, 58, 353–364. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Sun, S.C. Genetic variation and evolutionary origins of parthenogenetic Artemia (Crustacea: Anostraca) with different ploidies. Zool. Scr. 2016, 45, 421–436. [Google Scholar] [CrossRef]
- Asem, A.; Yang, C.; De Vos, S.; Mahmoudi, F.; Xia, L.; Shen, C.; Hontoria, F.; Rogers, C.; Gajardo, G. Mitogenomic phylogeny and divergence time estimation of Artemia Leach, 1819 (Branchiopoda: Anostraca) with emphasis on parthenogenetic lineages. BMC Genom. 2025, 26, 228. [Google Scholar] [CrossRef]
- Ghomari, S.; Ghalem, S.; Hontoria, F.; Moncef, M.; Amat, F. Note on the biogeography of the genus Artemia biodiversity in the north western region of Africa (Algeria, Morocco and Tunisia). Ecol. Mediterr. 2012, 38, 29–38. [Google Scholar]
- Timms, B.V. An Identification guide to the brine shrimps (Crustacea: Anostraca: Artemiina) of Australia. Mus. Vic. Sci. Rep. 2012, 16, 1–36. [Google Scholar] [CrossRef][Green Version]
- Zheng, B.; Sun, S.C. Review of the biogeography of Artemia Leach, 1819 (Crustacea: Anostraca) in China. Int. J. Artemia Biol. 2013, 3, 20–50. [Google Scholar][Green Version]
- Rogers, D.C.; Timms, B.V. Anostracan (Crustacea: Branchiopoda) zoogeography III Australian bioregions. Zootaxa 2014, 3881, 453–487. [Google Scholar] [CrossRef]
- De los Ríos-Escalante, P.; Amarouayache, M. Crustacean zooplankton assemblages in Algerian saline lakes: A comparison with their Chilean Altiplano counterparts. Crustaceana 2016, 89, 1485–1500. [Google Scholar] [CrossRef]
- Hontoria, F.; Redón, S.; Maccari, M. A revision of Artemia biodiversity in Macaronesia. Aquat. Biosyst. 2012, 8, 25. [Google Scholar] [CrossRef]
- Asem, A.; Eimanifar, A.; Wink, M. Species diversity and distribution of Artemia (Crustacea, Anostraca) in Iran: Historical contexts and updated review. Zoodiversity 2024, 58, 269–298. [Google Scholar] [CrossRef]
- Sorgeloos, P. The use of the brine shrimp Artemia in aquaculture. In The Brine Shrimp Artemia. Ecology, Culturing, Use in Aquaculture, 1st ed.; Persoone, G., Sorgeloos, P., Roels, O., Jaspers, E., Eds.; Universa Press: Wetteren, Belgium, 1980; Volume 3, pp. 25–46. [Google Scholar]
- Litvinenko, L.; Litvinenko, A.; Boyko, E.; Kuzanov, K. Artemia in the Salt Lakes of Russia: The Productivity of Populations, the Reserves of the Cysts and the Fisheries. Acta Geol. Sin. 2014, 88, 87–88. [Google Scholar] [CrossRef]
- Litvinenko, L.; Litvinenko, A.; Boiko, E.; Kutsanov, K. Artemia cyst production in Russia. J. Oceanol. Limnol. 2015, 33, 1436–1450. [Google Scholar] [CrossRef]
- Amat, F.; Hontoria, F.; Navarro, J.C.; Vieira, N.; Mura, G. Biodiversity loss in the genus Artemia in the western Mediterranean region. Limnetica 2007, 26, 387–404. [Google Scholar] [CrossRef]
- Sánchez, M.I.; Paredes, I.; Lebouvier, M.; Green, A.J. Functional role of native and invasive filter feeders, and the effect of parasites: Learning from hypersaline ecosystems. PLoS ONE 2016, 11, e0161478. [Google Scholar] [CrossRef]
- El-Bermawi, N. Responses of Artemia franciscana and Egyptian parthenogenetica Artemia from Wadi Mariout Lake to West Alexandria conditions. Egypt. J. Nut. Feeds 2014, 17, 501–510. [Google Scholar]
- Browne, R.A.; Sallee, S.E.; Grosch, D.S.; Segreti, W.O.; Purser, S.M. Partitioning genetic and environmental components of reproduction and lifespan in Artemia. Ecology 1984, 65, 949–960. [Google Scholar] [CrossRef]
- Gajardo, G.; Beardmore, J.A. Ability to switch reproductive mode in Artemia is related to maternal heterozygosity. Mar. Ecol. Prog. Ser. 1989, 55, 191–195. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Poulopoulou, K.; Abatzopoulos, T.J.; Perez, C.A.; Sorgeloos, P. International study on Artemia XLIX, Salinity effect on survival, maturity, growth, biometrics, reproductive and lifespan characteristics of a bisexual and a parthenogenetic population of Artemia. Hydrobiologia 1995, 302, 215–227. [Google Scholar] [CrossRef]
- Camara, M.R.; Reis, L.G.; Costa, M.F. Reproductive performance of three Artemia franciscana Kellogg (Crustacea, Anostraca) populations in north-eastern Brazil pond culture conditions. J. Biol. Res. Thessalon. 2005, 4, 173–179. [Google Scholar]
- EI-Magsodi, M.; EI-Ghebli, H.M.; Hamza, E.M.; Drebika, U.A.; Sorgeloos, P. Reproductive and lifespan characteristics of Artemia from Libyan Abu Kammash Sabkha. Int. J. Mar. Sci. 2005, 10, 1–8. [Google Scholar]
- Browne, R.A.; Wanigasekera, G. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J. Exp. Mar. Biol. Ecol. 2000, 244, 29–44. [Google Scholar] [CrossRef]
- Abatzopoulos, T.J.; El-Bermawi, N.; Vasdekis, C.; Baxevanis, A.D.; Sorgeloos, P. Effects of salinity and temperature on reproductive and lifespan characteristics of clonal Artemia (International Study on Artemia, LXVI). Hydrobiologia 2003, 492, 191–199. [Google Scholar] [CrossRef]
- Baxevanis, A.D.; El-Bermawi, N.; Abatzopoulos, T.J.; Sorgeloos, P. Salinity effects on maturation, reproductive and lifespan characteristics of four Egyptian Artemia populations (International Study on Artemia. LXVIII). Hydrobiologia 2004, 513, 87–100. [Google Scholar] [CrossRef]
- Agh, N.; Van Stappen, G.; Bossier, P.; Sepehri, H.; Lotfi, V.; Rouhani, S.M.; Sorgeloos, P. Effects of salinity on survival, growth, reproductive and lifespan characteristics of Artemia populations from Urmia Lake and neighboring lagoons. Pak. J. Biol. Sci. 2007, 11, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.R.; Goenaga, J.; Hontoria, F.; Cohen, G.; Amat, F. Effects of temperature and salinity on pre-reproductive lifespan and reproductive traits of two species of Artemia (Branchiopoda, Anostraca) from Argentina: Artemia franciscana and A. persimilis. Hydrobiologia 2007, 579, 41–53. [Google Scholar] [CrossRef]
- Ben Naceur, H.; Jenhani, A.B.R.; Romdhane, M.S. Reproduction characteristics, survival rate and sex-ratio of four brine shrimp Artemia salina (Linnaeus, 1758) populations from Tunisia cultured under laboratory conditions. Invertebr. Reprod. Dev. 2012, 57, 156–164. [Google Scholar] [CrossRef]
- Mohebbi, F.; Hafezieh, M.; Seidgar, M.; Hosseinzadeh Sahhafi, H.; Mohsenpour Azari, A.; Ahmadi, R. The growth, survival rate and reproductive characteristics of Artemia urmiana fed by Dunaliella tertiolecta, Tetraselmis suecica, Nannochloropsis oculata, Chaetoceros sp., Chlorella sp. and Spirolina sp. as feeding microalgae. Iran. J. Fish. Sci. 2016, 15, 727–737. [Google Scholar]
- Manaffar, R.; Abdolahzadeh, N.; Moosavi Toomatari, G.; Zare, S.; Sorgeloos, P.; Bossier, P.; Van Stappen, G. Reproduction and lifespan characterization of Artemia urmiana in Lake Urmia, Iran (Branchiopoda: Anostraca). Iran. J. Fish. Sci. 2020, 19, 1344–1358. [Google Scholar]
- Yang, J.; Sun, S. Combined effects of temperature, photoperiod, and salinity on reproduction of the brine shrimp Artemia sinica (Crustacea: Anostraca). PeerJ 2023, 11, e15945. [Google Scholar] [CrossRef]
- Browne, R. Reproductive Pattern and Mode in the Brine Shrimp. Ecology 1980, 61, 466–470. [Google Scholar] [CrossRef]
- Barigozzi, C. Artemia: A survey of its significance in genetic problems. In Evolutionary Biology; Dobzhansky, T., Hecht, M.K., Steere, W.C., Eds.; Springer: Boston, MA, USA, 1974; pp. 221–252. [Google Scholar]
- Ubaskin, A.; Akhmetov, K.; Abylkhassanov, T.; Lunkov, A.; Akimbekova, N. Revisiting the methods of Artemia reproductive performance test (Anostraca: Crustacea). Nat. Croat. 2022, 31, 325–333. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, G.; Shu, H.; Wang, W.; Huang, X. Effects of Temperature and Salinity on the Growth, Reproduction, and Carotenoid Accumulation in Artemia sinica and Transcriptome Analysis. Fishes 2024, 9, 437. [Google Scholar] [CrossRef]
- Asem, A.; Sun, S.C. Biometric characterization of Chinese parthenogenetic Artemia (Crustacea: Anostraca) cysts, focusing on its relationship with ploidy and habitat altitude. North-West. J. Zool. 2014, 10, 149–157. [Google Scholar]
- Zhang, C.; Lv, A.; Zhu, W.; Yao, G.; Qi, S. Using Multisource Satellite Data to Investigate Lake Area, Water Level, and Water Storage Changes of Terminal Lakes in Ungauged Regions. Remote Sens. 2021, 13, 3221. [Google Scholar] [CrossRef]
- Maccari, M.; Amat, F.; Gomez, A. Origin and genetic diversity of diploid parthenogenetic Artemia in Eurasia. PLoS ONE 2013, 8, e83348. [Google Scholar] [CrossRef] [PubMed]
- Maccari, M.; Gomez, A.; Hontoria, F.; Amat, F. Functional rare males in diploid parthenogenetic Artemia. J. Evol. Biol. 2013, 26, 1934–1948. [Google Scholar] [CrossRef]
- Google Earth. Google. 2024. Available online: https://earth.google.com (accessed on 17 June 2025).
- Asem, A.; Sun, S.C. Morphological differentiation of seven parthenogenetic Artemia (Crustacea: Branchiopoda) populations from China, with special emphasis on ploidy degrees. Microsc. Res. Tech. 2016, 79, 258–266. [Google Scholar] [CrossRef]
- Triantaphyllidis, G.V.; Abatzopoulos, T.J.; Miasa, E.; Sorgeloos, P. International study on Artemia. LVI. Characterization of two Artemia populations from Namibia and Madagascar: Cytogenetics, biometry, hatching characteristics and fatty acid profiles. Hydrobiologia 1996, 335, 97–106. [Google Scholar] [CrossRef]
- Xu, J.; Bian, B.; Li, M. Preliminary studies on the biology of Artemia salina from different localities of P. R. China. J. Ocean Uni. Qingdao 1993, 23, 109–118. [Google Scholar]
- Yarmohammadi, M.; Pourkazemi, M. Cytogenetic study of Artemia from Urmiah, Maharloo and Incheborun Lakes. Hydrobiologia 2004, 529, 99–104. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Lavens, P.; Leger, P.; Tackaert, W.; Versichele, D. Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture; Ghent University Press: Ghent, Belgium, 1986; p. 319. [Google Scholar]
- Coutteau, P.; Brendonck, L.; Lavens, P.; Sorgeloos, P. The use of manipulated baker’s yeast as an algal substitute for the laboratory culture of Anostraca. Hydrobiologia 1992, 234, 25–32. [Google Scholar] [CrossRef]
- Barata, C.; Hontoria, F.; Amat, F. Life history, resting egg formation, and hatching may explain the temporal-geographical distribution of Artemia strains in the Mediterranean basin. Hydrobiologia 1995, 298, 295–305. [Google Scholar] [CrossRef]
- Dana, G.L.; Lenz, P.H. Effects of increasing salinity on an Artemia population from Mono Lake, California. Oecologia 1986, 68, 428–436. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, R.S. Effects of temperature and salinity on the biological character of Artemia strains. Acta Ecol. Sin. 1995, 15, 214–220. [Google Scholar]
- Jia, Q.X.; Wang, Y.Y.; Lu, J.R.; Zhou, W.S.; Yang, M.K. Effects of salinity on Artemia sinica population in Yanchi waters of Shanxi. J. Fish. China 1995, 19, 172–176. [Google Scholar]
- Sui, L.Y.; Wang, J.; He, H.; Deng, Y.G. Effect of salinity on survival and reproductive performance of different Artemia strains. Mar. Sci. 2013, 37, 41–47. [Google Scholar]
- Bian, B.Z. Practical Artemia Culture and Application Technology; Agriculture Press: Beijing, China, 1990; p. 152. [Google Scholar]
- Chang, M.S.; Asem, A.; Sun, S.C. The incidence of rare males in seven parthenogenetic Artemia (Crustacea: Anostraca) populations. Turk. J. Zool. 2017, 41, 138–143. [Google Scholar] [CrossRef]
- Vahdat, S. Long-term effects of different concentrations of vinasse on growth and survival, reproductive and lifespan characteristics of Artemia franciscana (Kellogg, 1906) and Artemia parthenogenetica. J. Appl. Aquac. 2023, 36, 498–513. [Google Scholar] [CrossRef]
- Berthélémy-Okazaki, N.J.; Hedgecock, D. Effect of environmental factors on cyst formation in the brine shrimp Artemia. In Artemia Research and Its Applications; Ecology, culturing, use in aquaculture; Sorgeloos, P., Bengtson, D.A., Decleir, W., Jaspers, E., Eds.; Universa Press: Wetteren, Belgium, 1987; Volume 3, pp. 167–182. [Google Scholar]
- Wang, Z.C.; Asem, A.; Sun, S.C. Coupled effects of photoperiod, temperature and salinity on diapause induction of the parthenogenetic Artemia (Crustacea: Anostraca) from Barkol Lake, China. North-West. J. Zool. 2017, 13, 12–17. [Google Scholar]
- Drinkwater, L.E.; Clegg, J.S. Experimental biology of cyst diapause. In Artemia Biology; Brwone, R.A., Sorgeloos, P., Trotman, C.N.A., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 93–117. [Google Scholar]
- Clegg, J.S.; Trotman, C.N.A. Physiological and biochemical aspects of Artemia ecology. In Artemia Basic and Applied Biology; Abatzopoulos, T.J., Beardmore, J.A., Clegg, J.S., Sorgeloos, P., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 129–170. [Google Scholar]
- Nambu, Z.; Tanaka, S.; Nambu, F. Influence of photoperiod and temperature on reproductive mode in the Brine shrimp, Artemia franciscana. J. Exp. Zool. 2004, 301, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhao, Y.; Dai, Z.M.; Chen, H.M.; Yang, W.J. Formation of diapause cyst shell in brine shrimp, Artemia parthenogenetica, and its resistance role in environmental stresses. J. Biol. Chem. 2009, 284, 16931–16938. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yang, Y.T.; Chen, J.H.; Wang, F. Effects of feeding rate on reproductive mode of brine shrimp Artemia sinica in Xiechi Lake. Fish. Sci. 2019, 38, 86–91. [Google Scholar]
- Zhang, Z.W.; Li, L.; Luo, Y.M.; Zhan, J.; Zhag, X.F.; Pan, P.P. Several key techniques in family construction of Artemia sinica. Hebei Fish. 2020, 12, 9–13. [Google Scholar]
- Zhang, L.; King, C.E. Life history, divergence of sympatric diploid and polyploid populations of brine shrimp Artemia parthenogenetica. Oecologia 1993, 93, 177–183. [Google Scholar] [CrossRef]
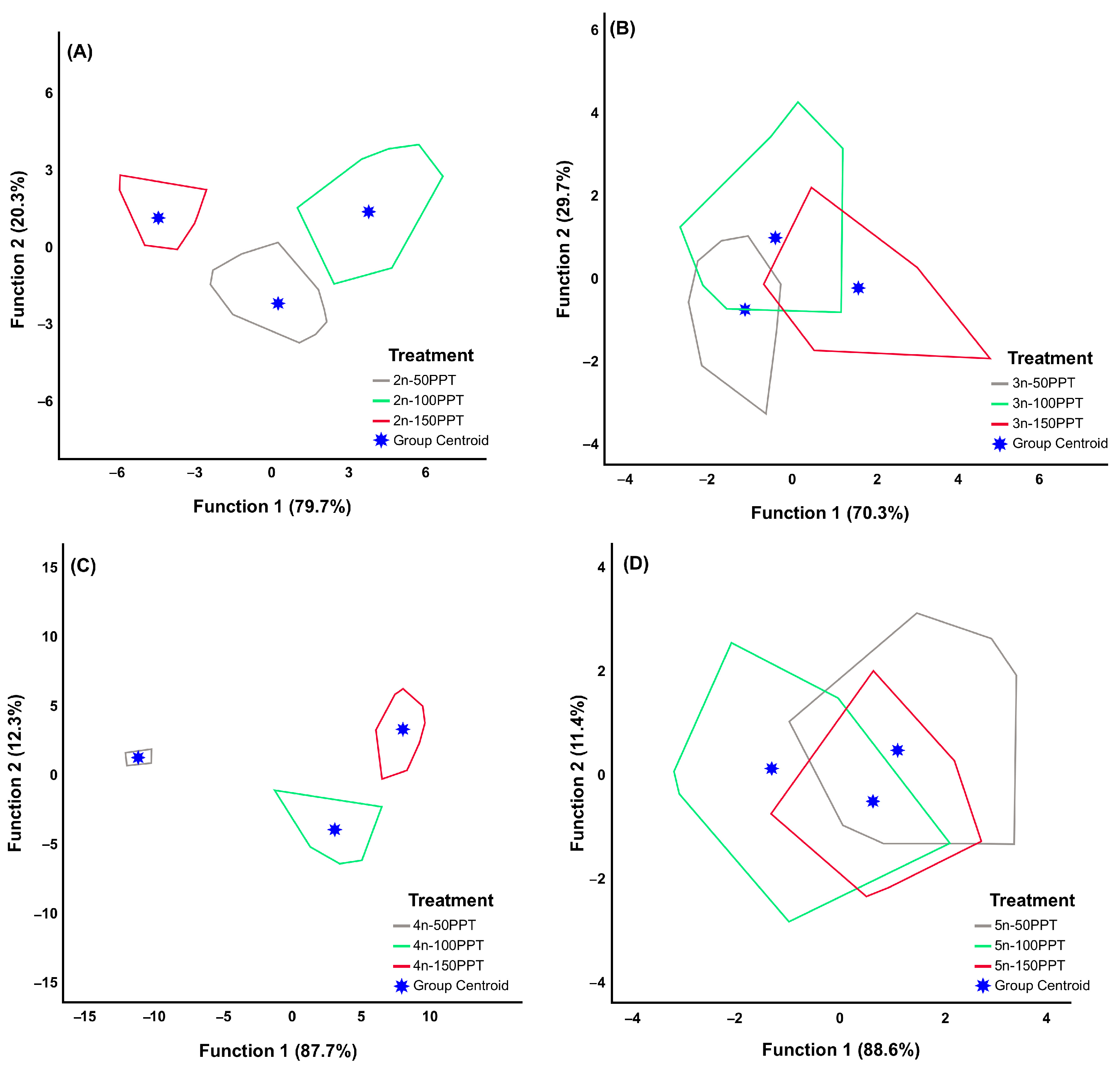
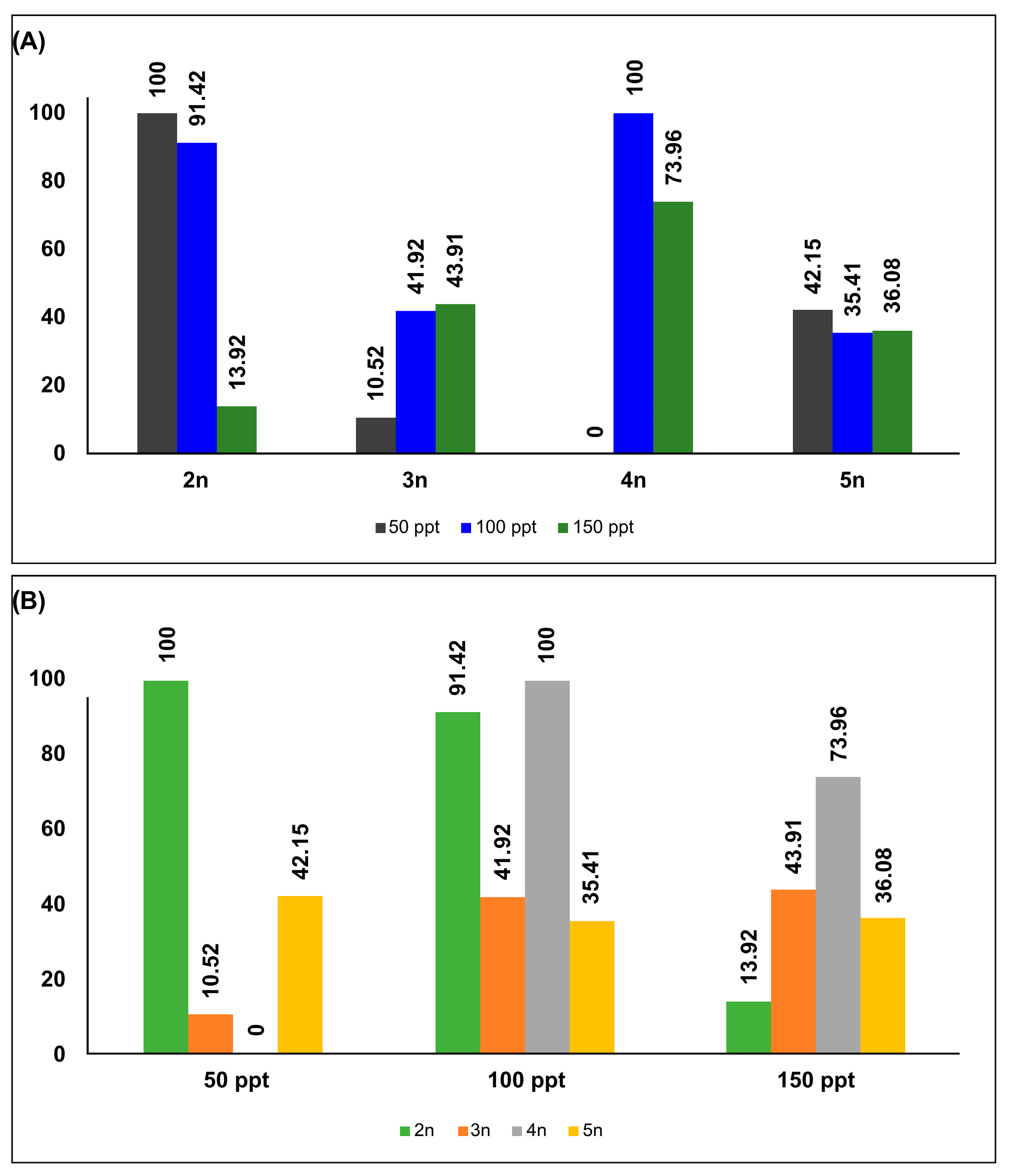
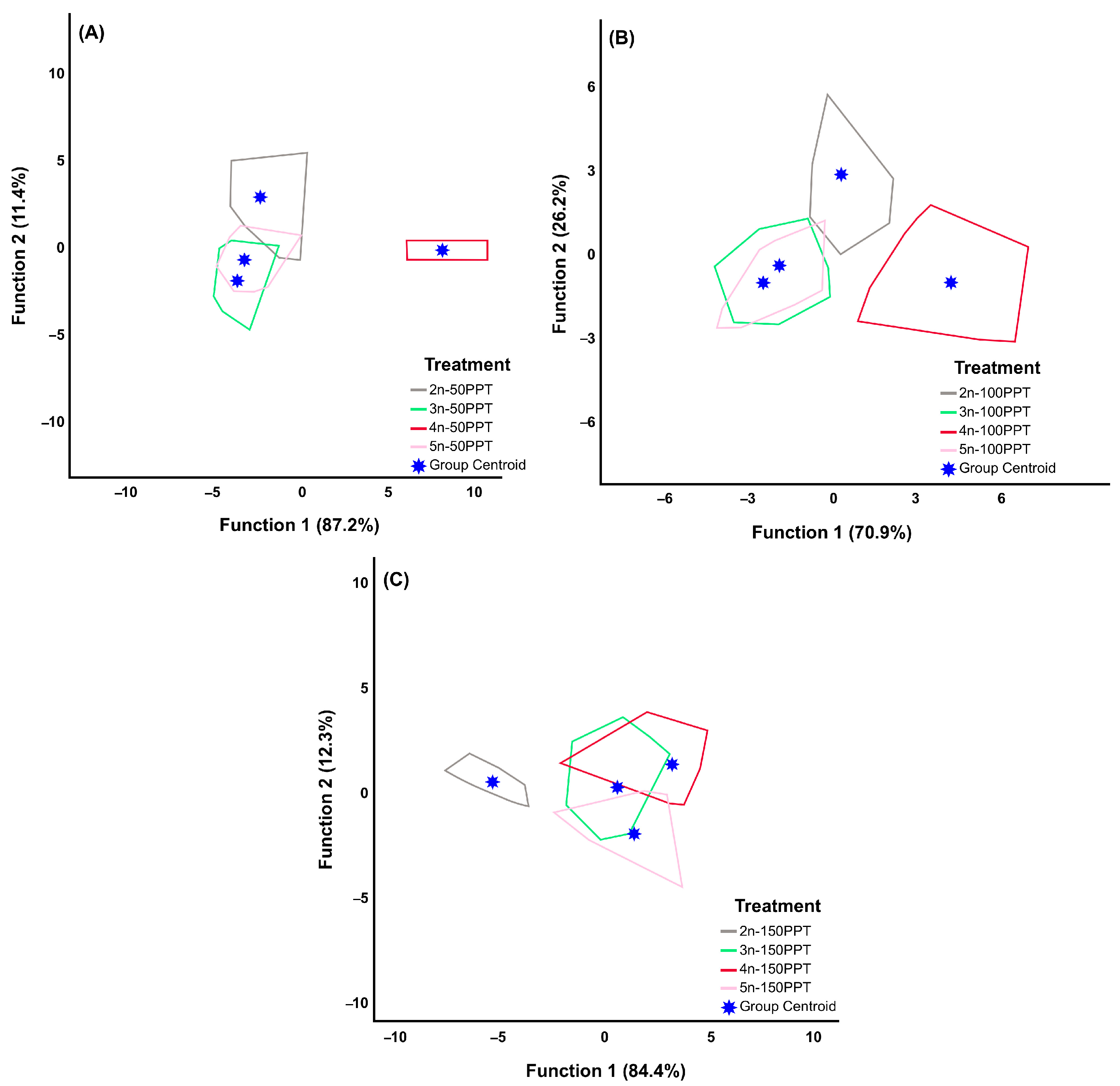
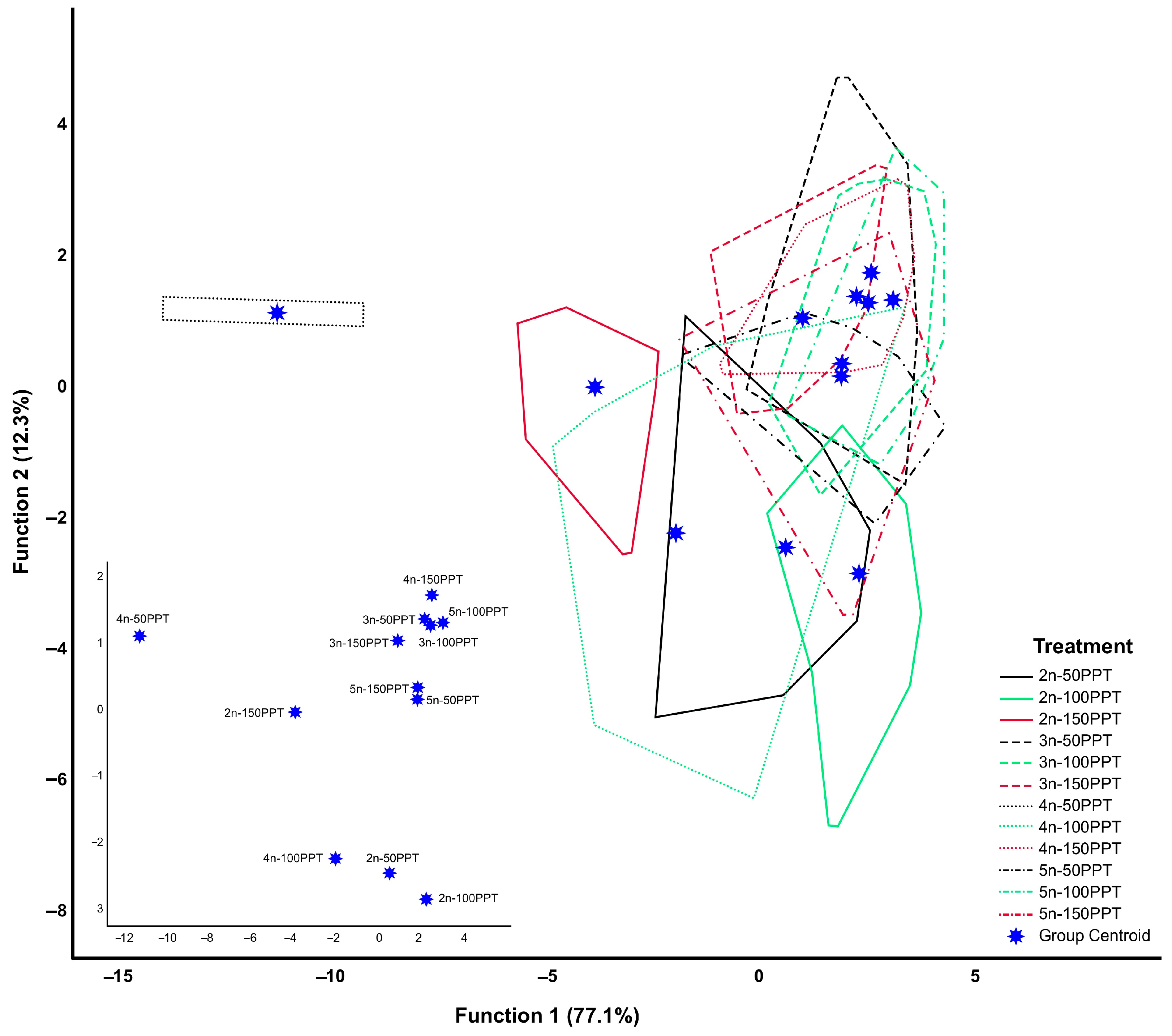
| Ploidy Level | Locality | Geographic Coordinates | Abb. | Egg Bank Accession |
|---|---|---|---|---|
| Diploid lineage | Ga Hai Lake, China | 37°08′ N, 97°33′ E | 2n | OUC 1, China |
| Triploid lineage | Ankiembe Saltern, Madagascar | 23°23′ S, 43°42′ E | 3n | ARC 2, Belgium |
| Tetraploid lineage | Hoh Lake, China | 36°56′ N, 98°12′ E | 4n | OUC, China |
| Pentaploid lineage | Yinggehai Saltern, China | 18°31′ N, 108°44′ E | 5n | OUC, China |
| 2n | 3n | 4n | 5n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | 50 ppt n = 35 | 100 ppt n = 35 | 150 ppt n = 31 | 50 ppt n = 30 | 100 ppt n = 35 | 150 ppt n = 32 | 50 ppt n = 35 | 100 ppt n = 35 | 150 ppt n = 35 | 50 ppt n = 28 | 100 ppt n = 35 | 150 ppt n = 28 |
| A | 26.29 a (6.11) | 21.14 b (3.73) | 49.16 c (13.73) | 21.77 a (4.47) | 22.31 a (3.11) | 30.94 b (4.45) | 88.80 a (9.41) | 45.57 b (8.12) | 23.54 c (5.63) | 24.11 b (3.83) | 19.29 a (2.31) | 22.79 b (3.32) |
| B | 18.31 a (10.62) | 46.51 b (9.06) | 7.39 c (8.79) | 37.60 a (27.54) | 54.51 b (28.00) | 47.81 ab (19.55) | 0.00 a (0.00) | 15.57 b (10.94) | 65.23 c (13.51) | 12.54 a (13.84) | 39.77 b (17.91) | 21.82 a (17.35) |
| C | 13.66 b (8.91) | 15.37 b (8.11) | 6.29 a (5.16) | 2.03 a (4.00) | 4.91 a (5.55) | 4.13 a (4.80) | 0.00 a (0.00) | 21.51 b (14.97) | 9.51 c (5.42) | 5.96 a (6.30) | 7.91 a (9.46) | 5.00 a (9.49) |
| D | 2.94 a (1.14) | 5.37 b (0.77) | 2.19 c (1.42) | 7.83 a (5.43) | 10.69 b (4.98) | 9.31 ab (3.45) | 0.00 a (0.00) | 2.94 b (0.97) | 13.17 c (2.35) | 3.18 a (2.64) | 9.31 b (4.00) | 5.11 a (3.42) |
| E | 8.07 a (4.07) | 10.06 b (2.29) | 2.26 c (2.71) | 3.91 a (2.15) | 4.44 a (0.96) | 4.61 a (0.84) | 0.00 a (0.00) | 6.03 b (3.79) | 4.27 c (0.62) | 2.83 a (2.30) | 3.50 a (1.08) | 3.47 a (1.63) |
| F | 0.00 a (0.00) | 9.89 ab (33.34) | 14.35 b (13.28) | 160.90 a (126.62) | 114.83 a (79.34) | 105.41 a (70.41) | 0.00 a (0.00) | 0.00 a (0.00) | 82.80 b (39.51) | 39.96 a (44.38) | 132.51 b (109.71) | 55.86 a (59.44) |
| G | 30.60 a (13.69) | 108.54 b (42.68) | 2.13 c (6.26) | 22.80 a (45.28) | 111.40 b (108.69) | 93.34 b (87.05) | 0.00 a (0.00) | 46.14 b (21.91) | 232.97 c (58.30) | 30.00 a (64.26) | 72.60 a (114.27) | 36.79 a (51.80) |
| H | 30.60 a (13.69) | 118.43 b (26.90) | 16.48 c (13.48) | 183.70 a (138.04) | 226.23 a (111.20) | 198.75 a (87.50) | 0.00 a (0.00) | 46.14 b (21.91) | 315.77 c (59.72) | 69.96 a (66.83) | 205.11 b (98.59) | 92.64 a (68.83) |
| I | 10.48 a (2.97) | 22.10 b (4.26) | 6.97 c (4.02) | 21.84 a (5.88) | 21.09 a (4.69) | 20.64 a (4.12) | 0.00 a (0.00) | 15.37 b (3.16) | 24.14 c (3.41) | 20.63 ab (8.29) | 21.47 b (5.33) | 17.07 a (5.56) |
| J | 58.26 a (11.40) | 83.03 b (9.02) | 67.00 c (10.29) | 64.63 a (33.47) | 82.66 b (30.64) | 84.09 b (22.88) | 89.80 a (9.41) | 82.66 a (16.05) | 98.29 b (17.17) | 43.21 a (18.35) | 67.69 b (21.52) | 50.46 a (21.95) |
| Ploidy Level | Category | Treatment | 50 ppt | 100 ppt | 150 ppt | Total |
|---|---|---|---|---|---|---|
| 2n a | Count | 50 ppt | 35 | 0 | 0 | 35 |
| 100 ppt | 1 | 34 | 0 | 35 | ||
| 150 ppt | 0 | 0 | 31 | 31 | ||
| % | 50 ppt | 100 | 0 | 0 | 100 | |
| 100 ppt | 2.9 | 97.1 | 0 | 100 | ||
| 150 ppt | 0 | 0 | 100 | 100 | ||
| 3n b | Count | 50 ppt | 25 | 3 | 2 | 30 |
| 100 ppt | 7 | 25 | 3 | 35 | ||
| 150 ppt | 1 | 2 | 29 | 32 | ||
| % | 50 ppt | 83.3 | 10 | 6.7 | 100 | |
| 100 ppt | 20 | 71.4 | 8.6 | 100 | ||
| 150 ppt | 3.1 | 6.3 | 90.6 | 100 | ||
| 4n c | Count | 50 ppt | 35 | 0 | 0 | 35 |
| 100 ppt | 0 | 35 | 0 | 35 | ||
| 150 ppt | 0 | 0 | 35 | 35 | ||
| % | 50 ppt | 100 | 0 | 0 | 100 | |
| 100 ppt | 0 | 100 | 0 | 100 | ||
| 150 ppt | 0 | 0 | 100 | 100 | ||
| 5n d | Count | 50 ppt | 16 | 1 | 11 | 28 |
| 100 ppt | 2 | 28 | 5 | 35 | ||
| 150 ppt | 6 | 4 | 18 | 28 | ||
| % | 50 ppt | 57.1 | 3.6 | 39.3 | 100 | |
| 100 ppt | 5.7 | 80 | 14.3 | 100 | ||
| 150 ppt | 21.4 | 14.3 | 64.3 | 100 |
| 50 ppt | 100 ppt | 150 ppt | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | 2n n = 35 | 3n n = 30 | 4n n = 35 | 5n n = 28 | 2n n = 35 | 3n n = 35 | 4n n = 35 | 5n n = 35 | 2n n = 31 | 3n n = 32 | 4n n = 35 | 5n n = 28 |
| A | b | a | c | ab | a | a | b | a | c | b | a | a |
| B | b | c | a | b | bc | c | a | b | a | b | c | d |
| C | c | a | a | b | b | a | b | a | ab | a | b | a |
| D | b | c | a | b | b | c | a | c | a | b | c | d |
| E | c | b | a | b | c | a | b | a | a | c | bc | b |
| F | a | b | a | a | a | b | a | b | a | c | bc | b |
| G | b | ab | a | b | b | b | a | ab | a | b | c | a |
| H | ab | c | a | b | b | c | a | c | a | b | c | d |
| I | b | c | a | c | b | b | a | b | a | b | c | d |
| J | b | b | c | a | b | b | b | a | a | b | c | d |
| Treatment | Category | Ploidy Level | 2n | 3n | 4n | 5n | Total |
|---|---|---|---|---|---|---|---|
| 50 ppt a | Count | 2n | 33 | 0 | 0 | 2 | 35 |
| 3n | 0 | 18 | 0 | 12 | 30 | ||
| 4n | 0 | 0 | 35 | 0 | 35 | ||
| 5n | 1 | 1 | 0 | 26 | 28 | ||
| % | 2n | 94.3 | 0 | 0 | 5.7 | 100 | |
| 3n | 0 | 60 | 0 | 40 | 100 | ||
| 4n | 0 | 0 | 100 | 0 | 100 | ||
| 5n | 3.6 | 3.6 | 0 | 92.9 | 100 | ||
| 100 ppt b | Count | 2n | 35 | 0 | 0 | 0 | 35 |
| 3n | 0 | 23 | 0 | 12 | 35 | ||
| 4n | 0 | 0 | 35 | 0 | 35 | ||
| 5n | 1 | 6 | 0 | 28 | 35 | ||
| % | 2n | 100 | 0 | 0 | 0 | 100 | |
| 3n | 0 | 65.7 | 0 | 34.3 | 100 | ||
| 4n | 0 | 0 | 100 | 0 | 100 | ||
| 5n | 2.9 | 17.1 | 0 | 80 | 100 | ||
| 150 ppt c | Count | 2n | 31 | 0 | 0 | 0 | 31 |
| 3n | 0 | 24 | 3 | 5 | 32 | ||
| 4n | 0 | 1 | 34 | 0 | 35 | ||
| 5n | 0 | 1 | 1 | 26 | 28 | ||
| % | 2n | 100 | 0 | 0 | 0 | 100 | |
| 3n | 0 | 75 | 9.4 | 15.6 | 100 | ||
| 4n | 0 | 2.9 | 97.1 | 0 | 100 | ||
| 5n | 0 | 3.6 | 3.6 | 92.9 | 100 |
| 2n | 3n | 4n | 5n | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Ploidy Level | Treatment | 50 ppt | 100 ppt | 150 ppt | 50 ppt | 100 ppt | 150 ppt | 50 ppt | 100 ppt | 150 ppt | 50 ppt | 100 ppt | 150 ppt | Total |
| Count | 2n | 50 ppt | 32 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 35 |
| 100 ppt | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 35 | ||
| 150 ppt | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 31 | ||
| 3n | 50 ppt | 0 | 0 | 0 | 14 | 0 | 3 | 0 | 0 | 1 | 7 | 1 | 4 | 30 | |
| 100 ppt | 1 | 0 | 0 | 3 | 12 | 0 | 0 | 0 | 10 | 5 | 2 | 2 | 35 | ||
| 150 ppt | 0 | 0 | 0 | 1 | 2 | 20 | 0 | 0 | 3 | 1 | 0 | 5 | 32 | ||
| 4n | 50 ppt | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 0 | 0 | 0 | 0 | 0 | 35 | |
| 100 ppt | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 27 | 0 | 1 | 0 | 1 | 35 | ||
| 150 ppt | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 29 | 0 | 1 | 0 | 35 | ||
| 5n | 50 ppt | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 17 | 0 | 7 | 28 | |
| 100 ppt | 0 | 0 | 0 | 10 | 3 | 0 | 0 | 0 | 7 | 3 | 8 | 4 | 35 | ||
| 150 ppt | 1 | 0 | 1 | 2 | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 14 | 28 | ||
| % | 2n | 50 ppt | 91.4 | 0 | 2.9 | 0 | 0 | 0 | 0 | 2.9 | 0 | 2.9 | 0 | 0 | 100 |
| 100 ppt | 2.9 | 94.3 | 0 | 0 | 0 | 0 | 0 | .0 | 0 | 2.9 | 0 | 0 | 100 | ||
| 150 ppt | 0 | 0 | 93.5 | 0 | 0 | 0 | 0 | 6.5 | 0 | .0 | 0 | 0 | 100 | ||
| 3n | 50 ppt | 0 | 0 | 0 | 46.7 | 0 | 10 | 0 | 0 | 3.3 | 23.3 | 3.3 | 13.3 | 100 | |
| 100 ppt | 2.9 | 0 | 0 | 8.6 | 34.3 | 0 | 0 | 0 | 28.6 | 14.3 | 5.7 | 5.7 | 100 | ||
| 150 ppt | 0 | 0 | 0 | 3.1 | 6.3 | 62.5 | 0 | 0 | 9.4 | 3.1 | 0 | 15.6 | 100 | ||
| 4n | 50 ppt | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 100 ppt | 8.6 | 2.9 | 5.7 | 0 | 0 | 0 | 0 | 77.1 | 0 | 2.9 | 0 | 2.9 | 100 | ||
| 150 ppt | 0 | 0 | 0 | 0 | 11.4 | 2.9 | 0 | 0 | 82.9 | 0 | 2.9 | 0 | 100 | ||
| 5n | 50 ppt | 0 | 3.6 | 3.6 | 0 | 3.6 | 0 | 0 | 0 | 3.6 | 60.7 | 0 | 25 | 100 | |
| 100 ppt | 0 | 0 | 0 | 28.6 | 8.6 | 0 | 0 | 0 | 20 | 8.6 | 22.9 | 11.4 | 100 | ||
| 150 ppt | 3.6 | 0 | 3.6 | 7.1 | 10.7 | 0 | 0 | 0 | 3.6 | 10.7 | 10.7 | 50 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asem, A.; Li, Y.; Yan, X.; Zhang, Y.; Zhu, Y.; Kangarloei, B.A.; Yang, C. Effects of Salinity on the Reproductive and Lifespan Traits of Artemia Parthenogenetic Lineages with Different Ploidy Levels. Biology 2025, 14, 1055. https://doi.org/10.3390/biology14081055
Asem A, Li Y, Yan X, Zhang Y, Zhu Y, Kangarloei BA, Yang C. Effects of Salinity on the Reproductive and Lifespan Traits of Artemia Parthenogenetic Lineages with Different Ploidy Levels. Biology. 2025; 14(8):1055. https://doi.org/10.3390/biology14081055
Chicago/Turabian StyleAsem, Alireza, Yuxin Li, Xintong Yan, Yaojia Zhang, Yunlong Zhu, Behrooz Atashbar Kangarloei, and Chaojie Yang. 2025. "Effects of Salinity on the Reproductive and Lifespan Traits of Artemia Parthenogenetic Lineages with Different Ploidy Levels" Biology 14, no. 8: 1055. https://doi.org/10.3390/biology14081055
APA StyleAsem, A., Li, Y., Yan, X., Zhang, Y., Zhu, Y., Kangarloei, B. A., & Yang, C. (2025). Effects of Salinity on the Reproductive and Lifespan Traits of Artemia Parthenogenetic Lineages with Different Ploidy Levels. Biology, 14(8), 1055. https://doi.org/10.3390/biology14081055





