Author Contributions
Conceptualization, M.S. and H.C.O.; methodology, M.S. and H.C.O.; formal analysis, M.S.; investigation, M.S. and H.C.O.; resources, M.S., H.C.O., B.R.G., J.M.S., N.G., A.M. and S.A.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing H.C.O., B.R.G., J.M.S., N.G., A.M. and S.A.; supervision, H.C.O.; project administration, H.C.O.; funding acquisition, H.C.O., B.R.G. and N.G. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Comparison between the total number of target mosquitoes manually identified and those classified by the VECTRACK system in the Algarve region (coordinates: 37.10134887, −8.12356948), by genus and sex. The study was conducted between 24 June and 17 September 2021.
Figure 1.
Comparison between the total number of target mosquitoes manually identified and those classified by the VECTRACK system in the Algarve region (coordinates: 37.10134887, −8.12356948), by genus and sex. The study was conducted between 24 June and 17 September 2021.
Figure 2.
Comparison of the number of target mosquitoes manually identified and those classified by the VECTRACK system, by date of capture in the Algarve region (coordinates: 37.10134887, −8.12356948), by genus and date of capture. The study was conducted between 24 June and 17 September 2021.
Figure 2.
Comparison of the number of target mosquitoes manually identified and those classified by the VECTRACK system, by date of capture in the Algarve region (coordinates: 37.10134887, −8.12356948), by genus and date of capture. The study was conducted between 24 June and 17 September 2021.
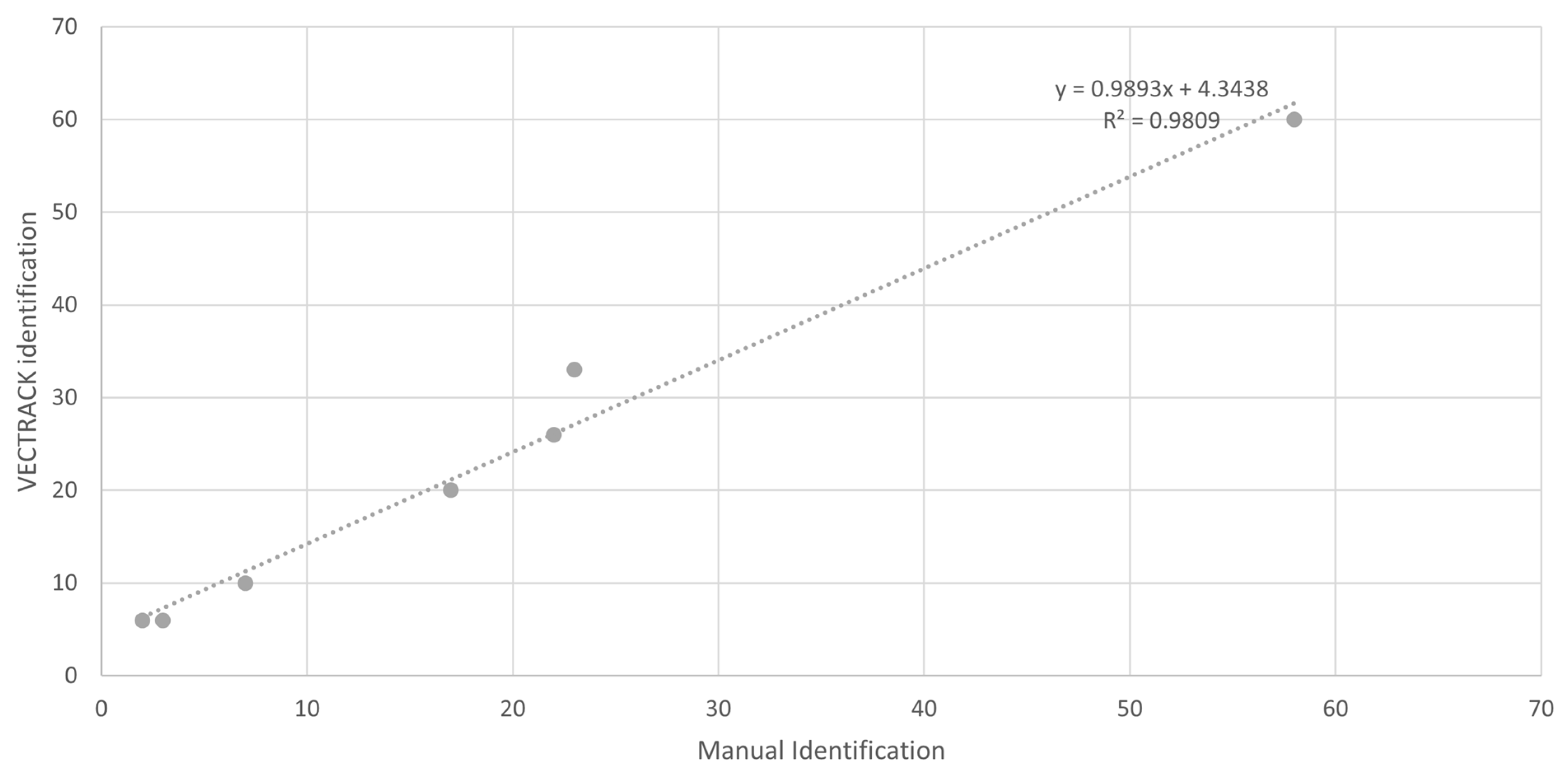
Figure 3.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Algarve (coordinates: 37.10134887, −8.12356948), based on total mosquito captures recorded between 8 June and 23 June 2021.
Figure 3.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Algarve (coordinates: 37.10134887, −8.12356948), based on total mosquito captures recorded between 8 June and 23 June 2021.
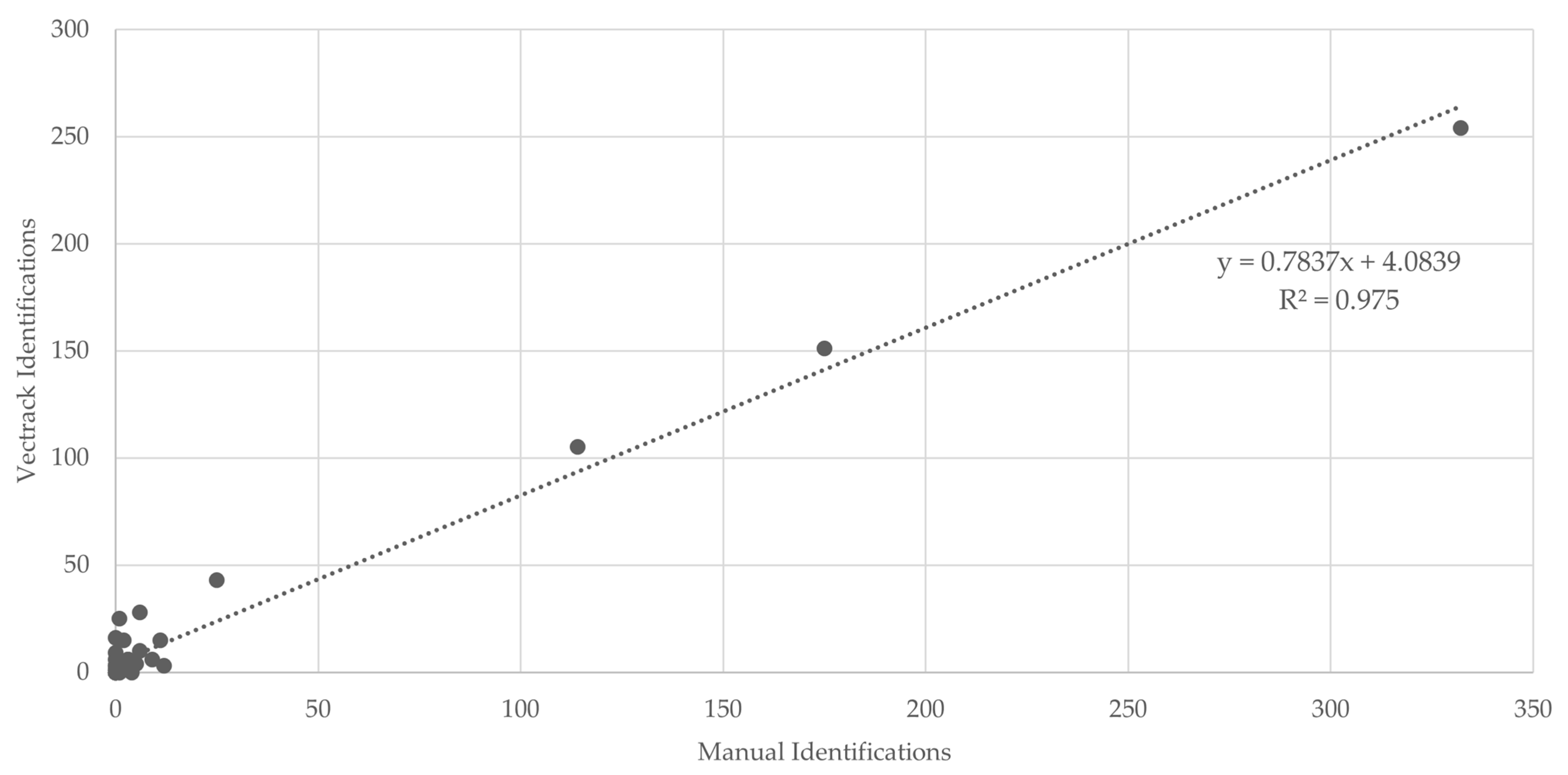
Figure 4.
Scatter plot and linear regression analysis comparing VECTRACK sensor identifications with manual identifications in Algarve (coordinates: 37.10134887, −8.12356948), considering both mosquito genus and sex, based on total mosquito captures. Recorded between 24 June and 17 September 2021.
Figure 4.
Scatter plot and linear regression analysis comparing VECTRACK sensor identifications with manual identifications in Algarve (coordinates: 37.10134887, −8.12356948), considering both mosquito genus and sex, based on total mosquito captures. Recorded between 24 June and 17 September 2021.
Figure 5.
Comparison of the total number of target mosquitoes captured and manually identified with those identified by the VECTRACK system in Palmela (coordinates: 38.583248, −8.689730), categorized by genus and sex. Both the captures and VECTRACK identifications were conducted between 8 June and 23 June 2021.
Figure 5.
Comparison of the total number of target mosquitoes captured and manually identified with those identified by the VECTRACK system in Palmela (coordinates: 38.583248, −8.689730), categorized by genus and sex. Both the captures and VECTRACK identifications were conducted between 8 June and 23 June 2021.
Figure 6.
Comparison of the number of target mosquitoes captured and manually identified with those identified by the VECTRACK system in Palmela (coordinates: 38.583248, −8.689730), by genus and date of capture. Both captures of VECTRACK identification were conducted between 8 June and 23 June 2021.
Figure 6.
Comparison of the number of target mosquitoes captured and manually identified with those identified by the VECTRACK system in Palmela (coordinates: 38.583248, −8.689730), by genus and date of capture. Both captures of VECTRACK identification were conducted between 8 June and 23 June 2021.
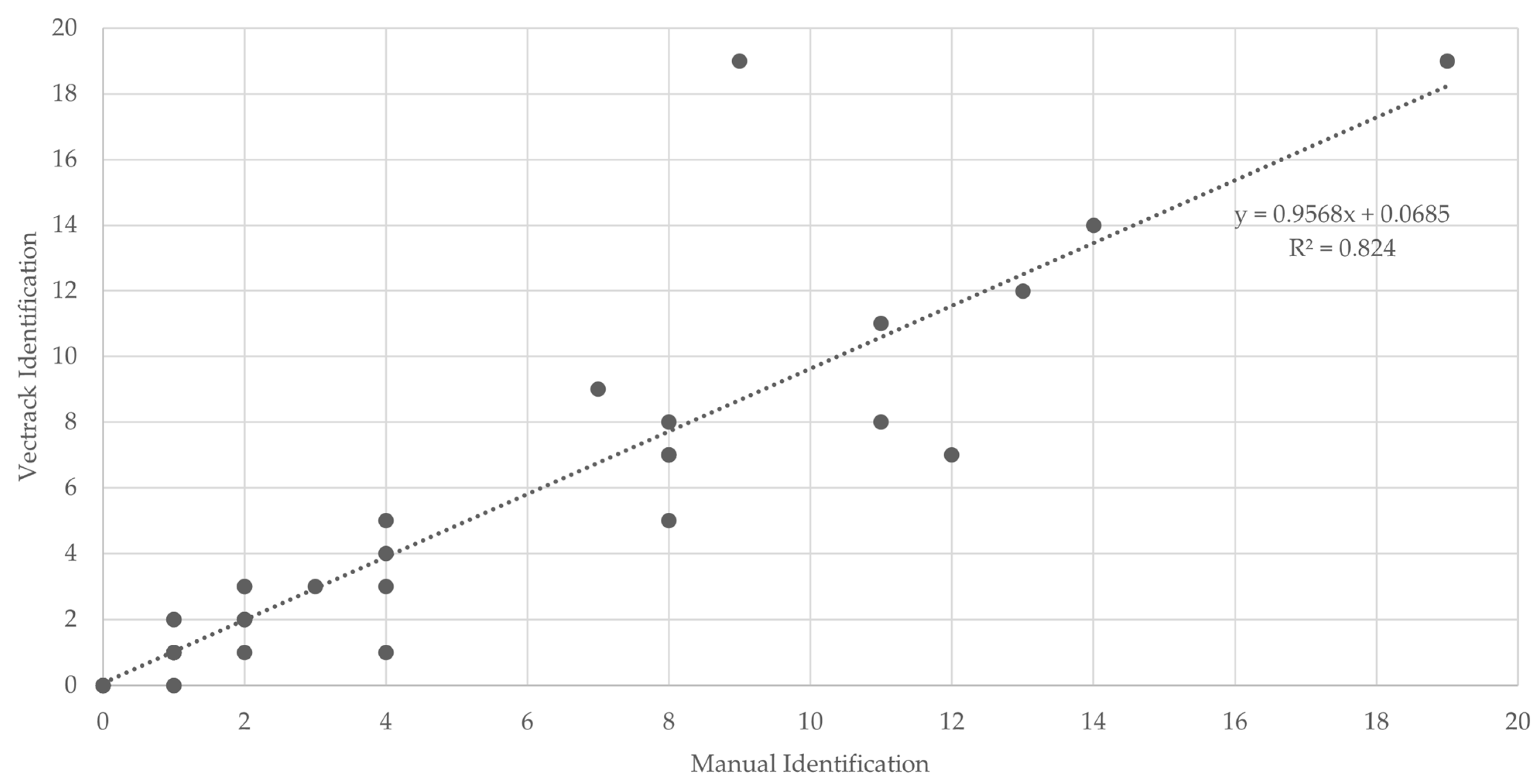
Figure 7.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Palmela (coordinates: 38.583248, −8.689730), based on total mosquito captures. Recorded between 8 June and 23 June 2021.
Figure 7.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Palmela (coordinates: 38.583248, −8.689730), based on total mosquito captures. Recorded between 8 June and 23 June 2021.
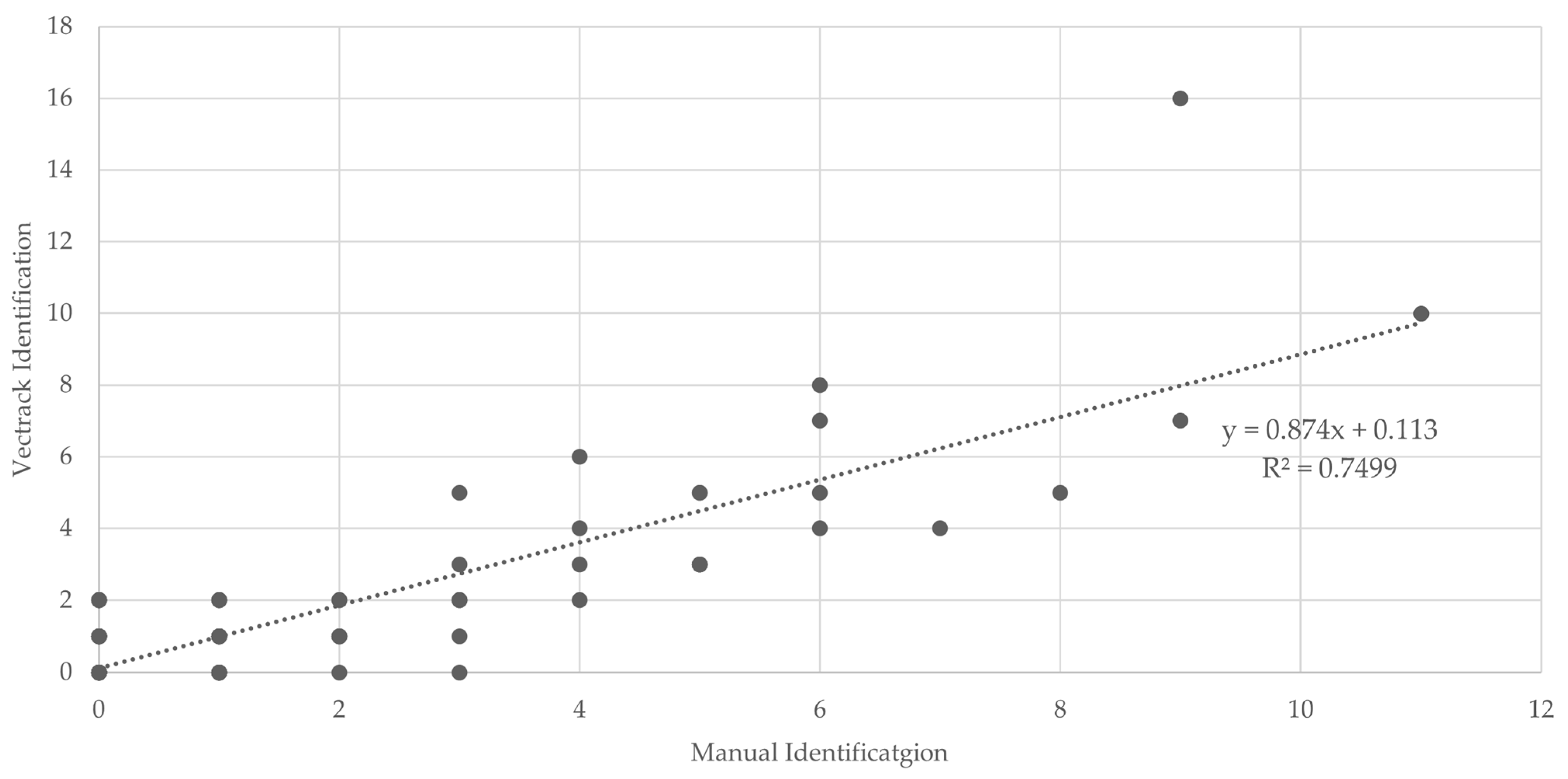
Figure 8.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Palmela (coordinates: 38.583248, −8.689730), considering both mosquito genus and sex, based on total mosquito captures. Recorded between 8 June and 23 June 2021.
Figure 8.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito counts in Palmela (coordinates: 38.583248, −8.689730), considering both mosquito genus and sex, based on total mosquito captures. Recorded between 8 June and 23 June 2021.
Figure 9.
Comparison between the total number of target mosquitoes manually identified and those classified by the VECTRACK system in Funchal (coordinates: 32.651318, −16.908259) by genus and sex. The study was conducted between 8 August and 28 June 2023.
Figure 9.
Comparison between the total number of target mosquitoes manually identified and those classified by the VECTRACK system in Funchal (coordinates: 32.651318, −16.908259) by genus and sex. The study was conducted between 8 August and 28 June 2023.
Figure 10.
Comparison of the number of target mosquitoes manually identified and those classified by the VECTRACK system, by date of capture in Funchal (coordinates: 32.651318, −16.908259), and by genus and date of capture. The study was conducted between 8 August and 28 June 2023.
Figure 10.
Comparison of the number of target mosquitoes manually identified and those classified by the VECTRACK system, by date of capture in Funchal (coordinates: 32.651318, −16.908259), and by genus and date of capture. The study was conducted between 8 August and 28 June 2023.
Figure 11.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito count, in Funchal (coordinates: 32.651318, −16.908259), based on total mosquito captures recorded between 8 June and 23 June 2021.
Figure 11.
Scatter plot and linear regression analyses of VECTRACK sensor count versus manual mosquito count, in Funchal (coordinates: 32.651318, −16.908259), based on total mosquito captures recorded between 8 June and 23 June 2021.
Figure 12.
Scatter plot and linear regression analyses comparing VECTRACK sensor identifications with manual identifications, in Funchal (coordinates: 32.651318, −16.908259), considering both genus and sex based on total mosquito captures. Recorded between 8 August 2022 and 28 June 2023.
Figure 12.
Scatter plot and linear regression analyses comparing VECTRACK sensor identifications with manual identifications, in Funchal (coordinates: 32.651318, −16.908259), considering both genus and sex based on total mosquito captures. Recorded between 8 August 2022 and 28 June 2023.
Table 1.
Total number of mosquitoes captured and identified by manual inspections in Algarve (coordinates: 37.10134887, −8.12356948), by species and sex. The captures occurred between 24 June and 17 September 2021.
Table 1.
Total number of mosquitoes captured and identified by manual inspections in Algarve (coordinates: 37.10134887, −8.12356948), by species and sex. The captures occurred between 24 June and 17 September 2021.
| Sample Composition | Number of Females | Number of Males | Total |
|---|
| Target species | Aedes albopictus | 15 (10.5%) | 0 | 15 (10.5%) |
| Culex pipiens | 113 (79.0%) | 4 (2.8%) | 117 (81.8%) |
| Non-target species | Culiseta longiareolata | 8 (5.6%) | 2 (1.4%) | 10 (7.0%) |
| Anopheles maculipennis | 1 (0.7%) | 0 | 1 (0.7%) |
| Total Mosquito species | 137 (95.8%) | 6 (4.2%) | 143 |
Table 2.
Summary of classification metrics on confusion matrices comparing manual identifications with those generated by the VECTRACK system identifications in the Algarve region (coordinates: 37.10134887, −8.12356948). The study took place between 24 June and 17 September 2021.
Table 2.
Summary of classification metrics on confusion matrices comparing manual identifications with those generated by the VECTRACK system identifications in the Algarve region (coordinates: 37.10134887, −8.12356948). The study took place between 24 June and 17 September 2021.
| Classification Task | Number of Samples | Accuracy | Sensitivity | Specificity | Precision |
|---|
| Target and Non-target insects | 983 | 0.971516 | 0.964 | 0.967 | 0.825 |
| Mosquito genus classification | 132 | 0.856 | 0.964 | 0.838 | 0.642 |
| Aedes sex classification | 15 | 0.000 | 0.500 | 0.000 | 0.250 |
| Culex sex classification | 117 | 0.816 | 0.885 | 0.925 | 0.595 |
Table 3.
Total number of mosquitoes captured and manually identified in Palmela (coordinates: 38.583248, −8.689730), categorized by species and sex. Sampling was conducted between 8 June 2021 and 23 June 2021.
Table 3.
Total number of mosquitoes captured and manually identified in Palmela (coordinates: 38.583248, −8.689730), categorized by species and sex. Sampling was conducted between 8 June 2021 and 23 June 2021.
| Sample Composition | Number of Females | Number of Males | Total |
|---|
| Target species | Aedes caspius | 29 (3.9%) | 1 (0.1%) | 30 (40.9%) |
| Culex pipiens | 28 (3.8%) | 7 (0.9%) | 35 (47.8%) |
| Culex theileri | 617 (84.3%) | 31(4.2%) | 648 (88.5%) |
| Non-target species | Culiseta longiareolata | 5 (0.7%) | 7 (0.9%) | 12 (16.4%) |
| Anopheles maculipennis | 4 (0.5%) | 3 (0.4%) | 7 (1.0%) |
| Total Mosquito Species | 683 (93.3%) | 49 (6.7%) | 732 |
Table 4.
Summary of classification parameters on confusion matrices made for the comparison between manual identifications and VECTRACK system identifications in Palmela (coordinates: 38.583248, −8.689730). Both the captures and the identifications performed by the VECTRACK sensor occurred between 8 June 2021 and 23 June 2021.
Table 4.
Summary of classification parameters on confusion matrices made for the comparison between manual identifications and VECTRACK system identifications in Palmela (coordinates: 38.583248, −8.689730). Both the captures and the identifications performed by the VECTRACK sensor occurred between 8 June 2021 and 23 June 2021.
| Classification Task | Number of Samples | Accuracy | Sensitivity | Specificity | Precision |
|---|
| Target and Non-target insects | 1598 | 0.996 | 1.000 | 0.992 | 0.990 |
| Mosquito genus classification | 713 | 0.967 | 0.989 | 0.977 | 0.742 |
| Aedes sex classification | 30 | 0.483 | 0.983 | 0.500 | 0.529 |
| Culex sex classification | 693 | 0.885 | 0.940 | 0.952 | 0.690 |
Table 5.
Total number of mosquitoes captured and identified by manual inspection in Funchal (coordinates: 32.651318, −16.908259) by species and sex. The captures occurred between 8 August 2022 and 28 June 2023.
Table 5.
Total number of mosquitoes captured and identified by manual inspection in Funchal (coordinates: 32.651318, −16.908259) by species and sex. The captures occurred between 8 August 2022 and 28 June 2023.
| Sample Composition | Number of Female | Number of Male | Unknown Sex | Total |
|---|
| Target species | Aedes aegypti | 65 (36.9%) | 83 (47.1%) | 12 (6.8%) | 160 (90.9%) |
| Culex pipiens | 13 (7.4%) | 1 (0.6%) | 2 (1.1%) | 16 (9.1%) |
| Total | 78 (44.3%) | 84 (47.7%) | 14 (8.0%) | 176 |
Table 6.
Summary of classification metrics on confusion matrices comparing manual identifications with those generated by VECTRACK system identifications in Funchal (coordinates: 32.651318, −16.908259). The study took place between 8 June and 23 June 2021.
Table 6.
Summary of classification metrics on confusion matrices comparing manual identifications with those generated by VECTRACK system identifications in Funchal (coordinates: 32.651318, −16.908259). The study took place between 8 June and 23 June 2021.
| Classification Task | Number of Samples | Accuracy | Sensitivity | Specificity | Precision |
|---|
| Target and Non-target insects | 367 | 0.986 | 0.97 | 1 | 0.974 |
| Mosquito genus classification | 176 | 0.928 | 0.953 | 0.968 | 0.758 |
| Aedes sex classification | 160 | 0.843 | 0.851 | 0.968 | 0.917 |
| Culex sex classification | 16 | 0.687 | 1 | 0.266 | 0.5 |