A Ubiquitous Volatile in Noctuid Larval Frass Attracts a Parasitoid Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
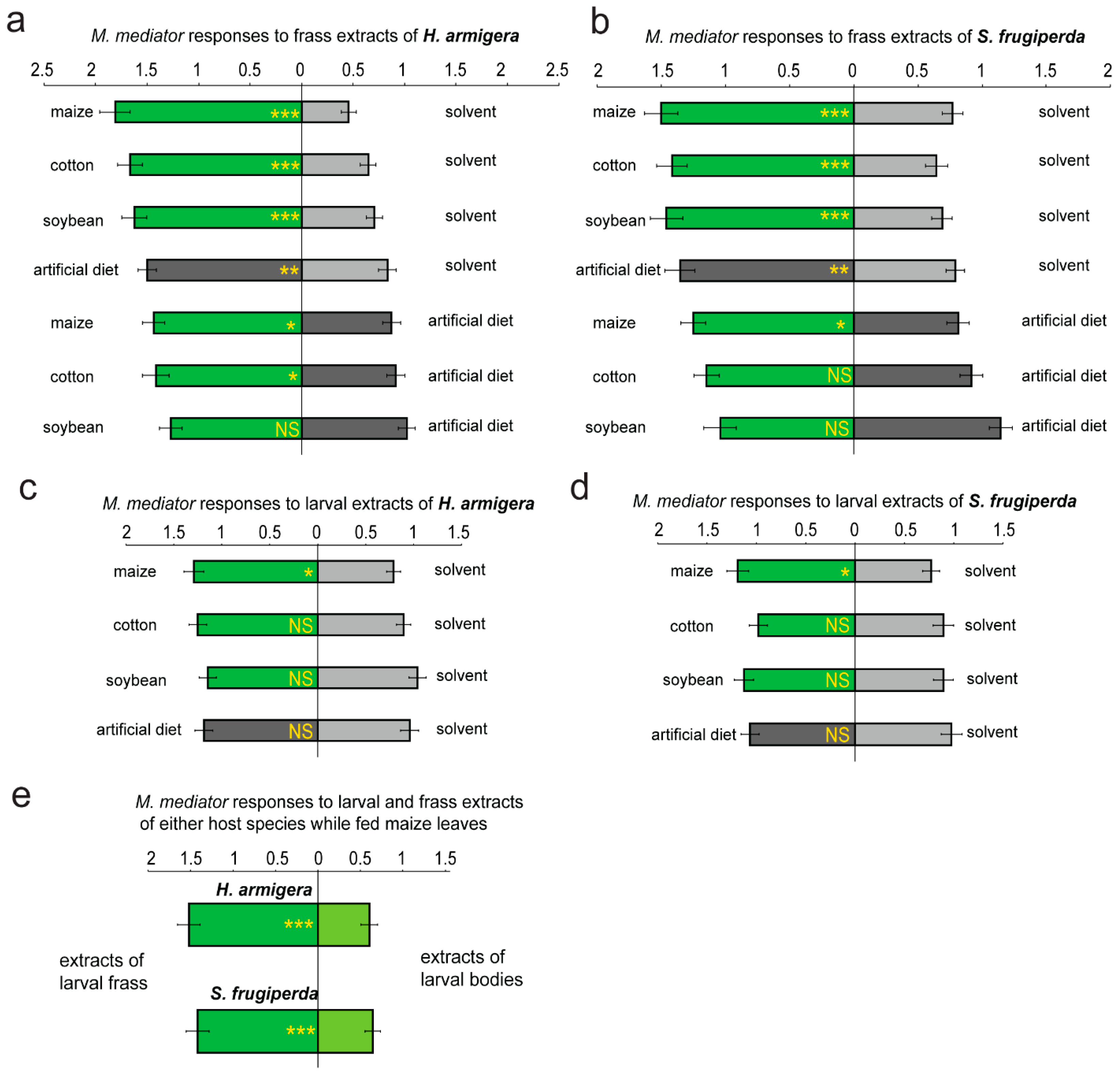
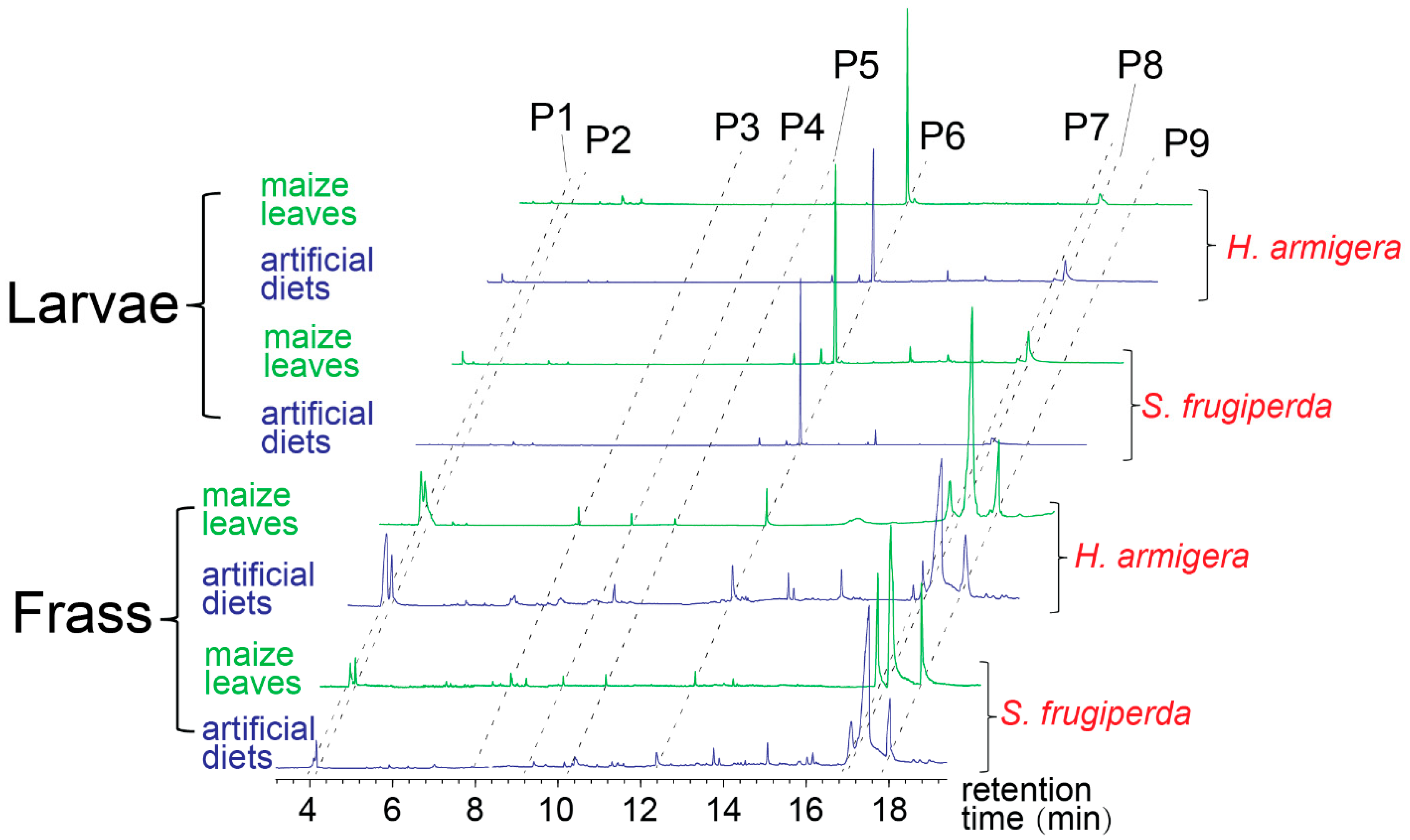
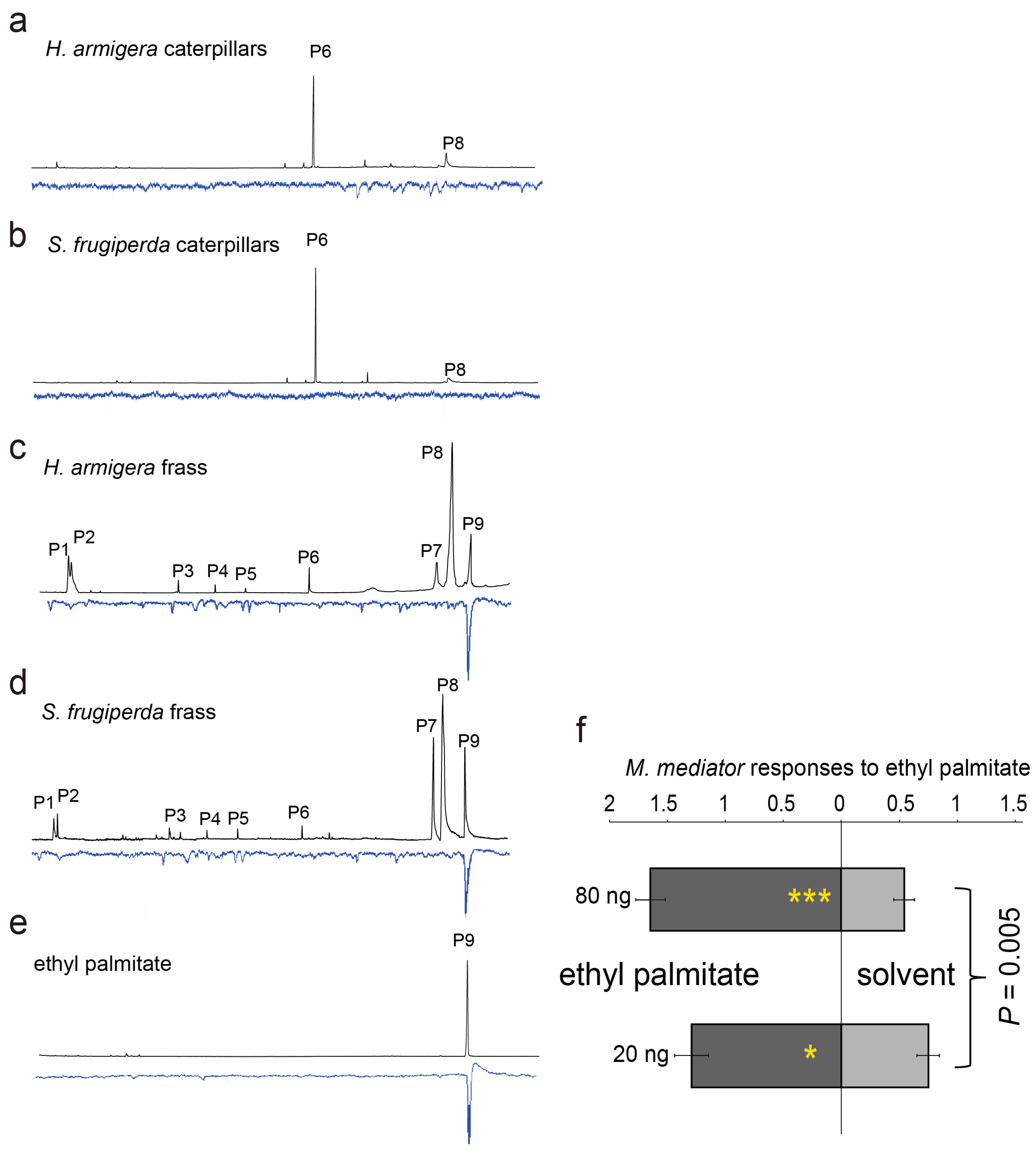
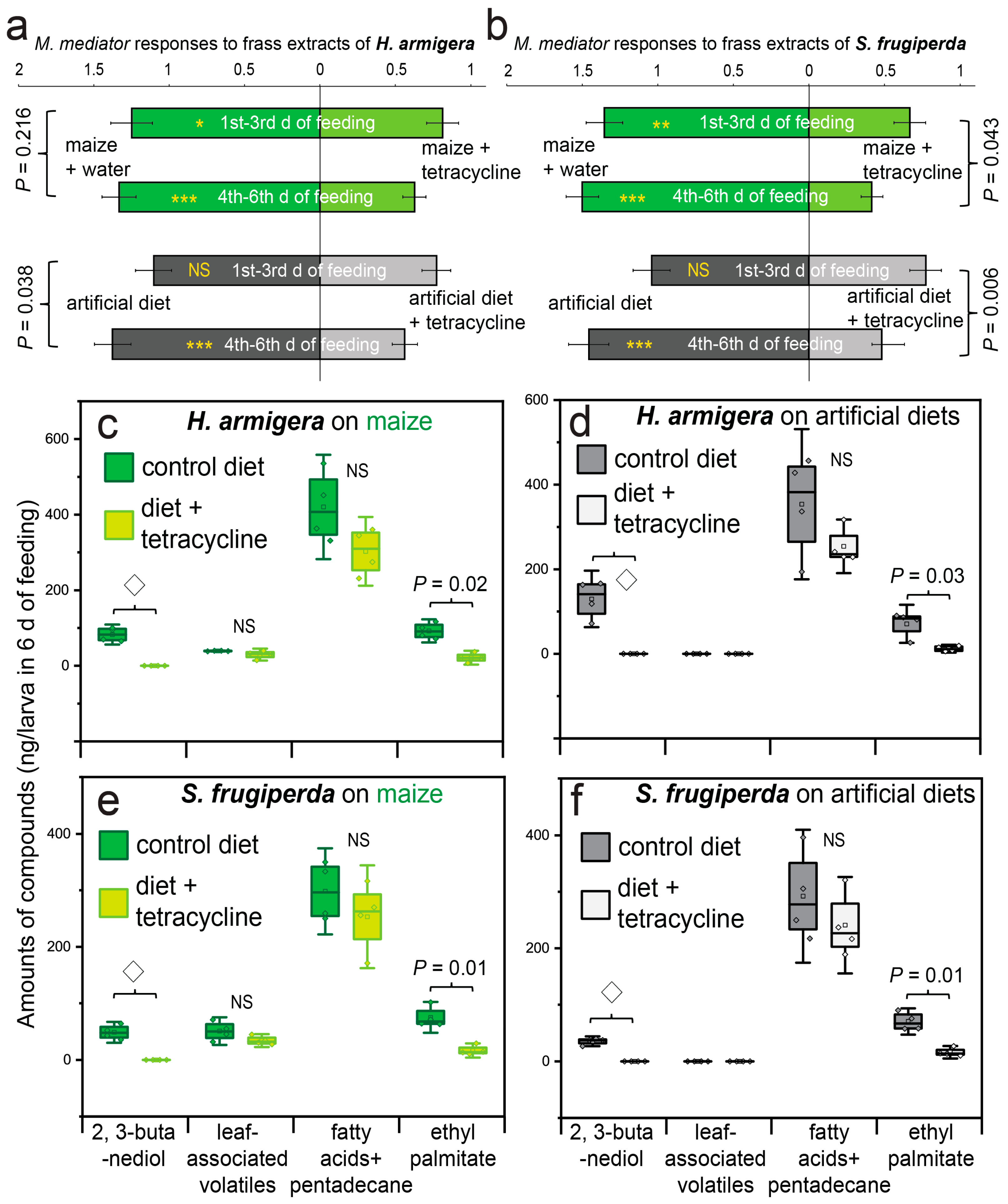
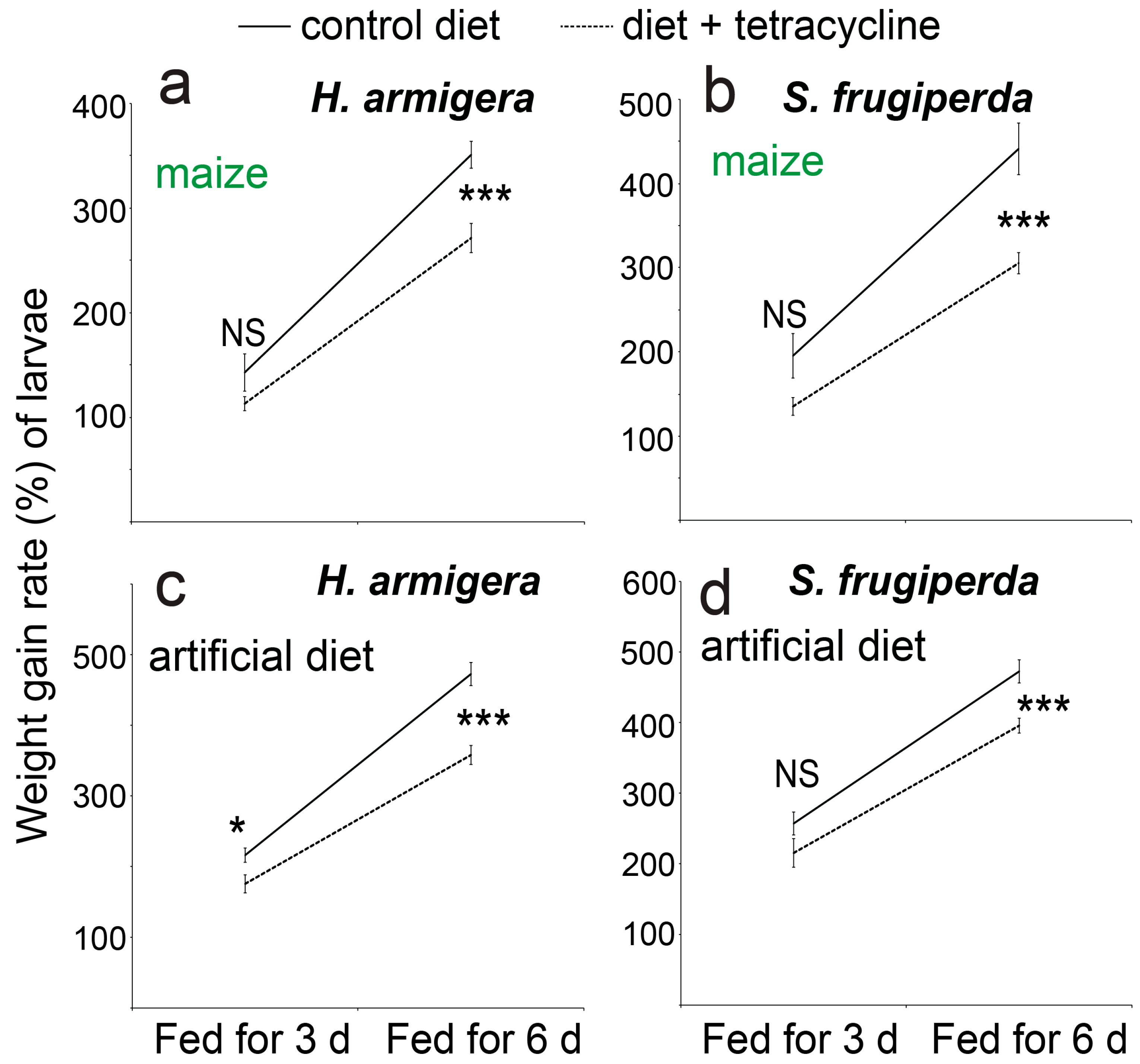
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; McCall, P.J.; Alborn, H.T.; Tumlinson, J.H. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J. Chem. Ecol. 1993, 19, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Ruther, J.; Tolasch, T.; Francke, W.; Hilker, M. Alcoholism in cockchafers: Orientation of male Melolontha melolontha towards green leaf alcohols. Naturwissenschaften 2002, 89, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R. Chemical communication in the true bugs and parasitoid exploitation. In Chemical Ecology of Insects 2; Ring, T.C., William, J.B., Eds.; Springer: Boston, MA, USA, 1995; pp. 318–363. [Google Scholar]
- Vet, L.E.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Tanaka, C.; Kainoh, Y.; Honda, H. Host frass as arrestant chemicals in locating host Mythimna separata by the tachinid fly Exorista japonica. Entomol. Exp. Appl. 2001, 100, 173–178. [Google Scholar] [CrossRef]
- Ha, D.-M.; Choi, S.-H.; Shim, J.-K.; Jung, D.-O.; Song, K.-S.; Nho, S.-K.; Lee, K.-Y. Behavioral attraction of two parasitoids, Venturia canescens and Bracon hebetor, to silk extracts of a host Plodia interpunctella. J. Asia-Pac. Entomol. 2006, 9, 287–292. [Google Scholar] [CrossRef]
- Filella, I.; Bosch, J.; Llusià, J.; Seco, R.; Peñuelas, J. The role of frass and cocoon volatiles in host location by Monodontomerus aeneus, a parasitoid of Megachilid solitary bees. Environ. Entomol. 2011, 40, 126–131. [Google Scholar] [CrossRef]
- Agelopoulos, N.G.; Dicke, M.; Posthumus, M.A. Role of volatile inforchemicals emitted by feces of larvae in host-searching behavior of parasitoid Cotesia rubecula (Hymenoptera: Braconidae): A behavioral and chemical study. J. Chem. Ecol. 1995, 21, 1789–1811. [Google Scholar] [CrossRef]
- Ngi-Song, A.J.; Overholt, W.A. Host location and acceptance by Cotesia flavipes Cameron and C. sesamiae (Cameron) (Hymenoptera: Braconidae), parasitoids of African gramineous stemborers: Role of frass and other host cues. Biol. Control 1997, 9, 136–142. [Google Scholar] [CrossRef]
- Afsheen, S.; Wang, X.; Li, R.; Zhu, C.-S.; Lou, Y.-G. Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci. 2008, 15, 381–397. [Google Scholar] [CrossRef]
- Dalen, M.; Knudsen, G.K.; Norli, H.R.; Thöming, G. Sources of volatiles mediating host location behaviour of Glypta haesitator, a larval parasitoid of Cydia nigricana. Biol. Control 2015, 90, 128–140. [Google Scholar] [CrossRef]
- Sime, K.R. Experimental studies of the host-finding behavior of Trogus pennator, a parasitoid of swallowtail butterflies. J. Chem. Ecol. 2002, 28, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.R. Good housekeeping: Why do shelter-dwelling caterpillars fling their frass? Ecol. Lett. 2003, 6, 361–370. [Google Scholar] [CrossRef]
- Weiss, M.R. Defecation behavior and ecology of insects. Annu. Rev. Entomol. 2006, 51, 635–661. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Huigens, M.E.; van Loon, J.J.A.; Dicke, M.; Hilker, M. Butterfly anti-aphrodisiac lures parasitic wasps. Nature 2005, 433, 704. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Khrimian, A.; Zhang, A.; Shearer, P.W. Bug pheromones (Hemiptera, Heteroptera) and tachinid fly host-finding. Denisia 2006, 19, 1015–1031. [Google Scholar]
- Reddy, G.; Holopainen, J.; Guerrero, A. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J. Chem. Ecol. 2002, 28, 131–143. [Google Scholar] [CrossRef]
- Obeysekara, P.T.; Legrand, A. The influence of host species and location in the host detection ability of Tiphiid (Hymenoptera: Tiphiidae) parasitoids. Environ. Entomol. 2014, 43, 1594–1602. [Google Scholar] [CrossRef]
- Seenivasagan, T.; Chander, S.; Paul, A.N. Orientation and behavioural responses of Cotesia plutellae Kurdjumov (Hymenoptera: Braconidae) to cruciferous host plants and host larval body extracts. J. Biol. Control 2009, 23, 365–373. [Google Scholar]
- Chiu-Alvarado, P.; Rojas, J.C. Host location behaviour by two Cephalonomia spp.(Hymenoptera: Bethylidae) wasps associated with the coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae). Int. J. Trop. Insect Sci. 2008, 28, 179–184. [Google Scholar]
- Jumean, Z.; Unruh, T.; Gries, R.; Gries, G. Mastrus ridibundus parasitoids eavesdrop on cocoon-spinning codling moth, Cydia pomonella, larvae. Naturwissenschaften 2005, 92, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Avila, G.A.; Withers, T.M.; Holwell, G.I. Olfactory cues used in host-habitat location and host location by the parasitoid Cotesia urabae. Entomol. Exp. Appl. 2016, 158, 202–209. [Google Scholar] [CrossRef]
- Glasser, S.; Farzan, S. Host-associated volatiles attract parasitoids of a native solitary bee, Osmia lignaria Say (Hymenoptera, megachilidae). J. Hymenopt. Res. 2016, 51, 249. [Google Scholar] [CrossRef]
- Rogers, M.E.; Potter, D.A. Kairomones from scarabaeid grubs and their frass as cues in below-ground host location by the parasitoids Tiphia vernalis and Tiphia pygidialis. Entomol. Exp. Appl. 2002, 102, 307–314. [Google Scholar] [CrossRef]
- Steidle, J.L.; Steppuhn, A.; Ruther, J. Specific foraging kairomones used by a generalist parasitoid. J. Chem. Ecol. 2003, 29, 131–143. [Google Scholar] [CrossRef]
- Steidle, J.L.; Fischer, A. Quantity does matter: How feces are used for host stage selection by granary weevil parasitoid Lariophagus distinguendus. J. Chem. Ecol. 2000, 26, 2657–2664. [Google Scholar] [CrossRef]
- Meiners, T.; Westerhaus, C.; Hilker, M. Specificity of chemical cues used by a specialist egg parasitoid during host location. Entomol. Exp. Appl. 2000, 95, 151–159. [Google Scholar] [CrossRef]
- Chuche, J.; Xuéreb, A.; Thiéry, D. Attraction of Dibrachys cavus (Hymenoptera: Pteromalidae) to its host frass volatiles. J. Chem. Ecol. 2006, 32, 2721–2731. [Google Scholar] [CrossRef]
- Ramachandran, R.; Norris, D.M.; Phillips, J.K.; Phillips, T.W. Volatiles mediating plant-herbivore-natural enemy interactions: Soybean looper frass volatiles, 3-octanone and guaiacol, as kairomones for the parasitoid Microplitis demolitor. J. Agric. Food Chem. 1991, 39, 2310–2317. [Google Scholar] [CrossRef]
- Röse, U.S.; Alborn, H.T.; Makranczy, G.; Lewis, W.J.; Tumlinson, J.H. Host recognition by the specialist endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae): Role of host-and plant-related volatiles. J. Insect Behav. 1997, 10, 313–330. [Google Scholar] [CrossRef]
- Mattiacci, L.; Hütter, E.; Dorn, S. Host location of Hyssopus pallidus, a larval parasitoid of the codling moth, Cydia pomonella. Biol. Control 1999, 15, 241–251. [Google Scholar] [CrossRef]
- Weinhold, A.; Baldwin, I.T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA 2011, 108, 7855–7859. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.-N.; Pan, Y.; Liao, L.-L.; Wu, Y.-K.; Zhang, X.-Q.; Jin, L.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Mandelonitrile produced by commensal bacteria protects the Colorado potato beetle against predation. Nat. Commun. 2024, 15, 10081. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef]
- Desneux, N.; Ramírez-Romero, R.; Bokonon-Ganta, A.H.; Bernal, J.S. Attraction of the parasitoid Cotesia marginiventris to host (Spodoptera frugiperda) frass is affected by transgenic maize. Ecotoxicology 2010, 19, 1183–1192. [Google Scholar] [CrossRef]
- Bourne, M.E.; Gloder, G.; Weldegergis, B.T.; Slingerland, M.; Ceribelli, A.; Crauwels, S.; Lievens, B.; Jacquemyn, H.; Dicke, M.; Poelman, E.H. Parasitism causes changes in caterpillar odours and associated bacterial communities with consequences for host-location by a hyperparasitoid. PLoS Pathog. 2023, 19, e1011262. [Google Scholar] [CrossRef]
- Leroy, P.D.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.J.; Francis, F.; Brostaux, Y.; Felton, G.W.; Haubruge, E. Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2011, 2, 348. [Google Scholar] [CrossRef]
- Frago, E.; Zytynska, S. Impact of herbivore symbionts on parasitoid foraging behaviour. Curr. Opin. Insect Sci. 2023, 57, 101027. [Google Scholar] [CrossRef]
- Cunningham, J.P.; Zalucki, M.P. Understanding Heliothine (Lepidoptera: Heliothinae) pests: What is a host plant? J. Econ. Entomol. 2014, 107, 881–896. [Google Scholar] [CrossRef]
- Mlambo, S.; Mubayiwa, M.; Tarusikirwa, V.L.; Machekano, H.; Mvumi, B.M.; Nyamukondiwa, C. The fall armyworm and larger grain borer pest invasions in Africa: Drivers, impacts and implications for food systems. Biology 2024, 13, 160. [Google Scholar] [CrossRef]
- Li, X.-J.; Wu, M.-F.; Ma, J.; Gao, B.-Y.; Wu, Q.-L.; Chen, A.-D.; Liu, J.; Jiang, Y.-Y.; Zhai, B.-P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2019, 76, 454–463. [Google Scholar] [CrossRef]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Xu, S.; Hu, X.; Liu, Y.; Wang, X.; Wang, Y.; Li, G.; Turlings, T.C.; Li, Y. The threat of the fall armyworm to Asian rice production is amplified by the brown planthopper. Plant Cell Environ. 2025, 48, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, F.; Coudron, T.A.; Pan, W.; Zhang, X.; Liu, X.; Zhang, Q. Field release of the parasitoid Microplitis mediator (Hymenoptera: Braconidae) for control of Helicoverpa armigera (Lepidoptera: Noctuidae) in cotton fields in Northwestern China’s Xinjiang province. Environ. Entomol. 2006, 35, 694–699. [Google Scholar] [CrossRef]
- Lu, Z.-y.; Yang, X.-f.; Ma, A.-h.; Ran, H.-f.; Liu, W.-x.; Li, J.-c. Parasitic potential of two Microplitis species on Spodoptera frugiperda larvae. J. Hebei Agric. Sci. 2020, 24, 37–39. [Google Scholar]
- Arthur, A.P.; Mason, P.G. Life history and immature stages of the parasitoid Microplitis mediator (Hymenoptera: Braconidae), reared on the bertha armyworm Mamestra configurata (Lepidoptera: Noctuidae). Can. Entomol. 1986, 118, 487–491. [Google Scholar] [CrossRef]
- Xu, H.; Desurmont, G.; Degen, T.; Zhou, G.; Laplanche, D.; Henryk, L.; Turlings, T. Combined use of herbivore-induced plant volatiles and sex pheromones for mate location in braconid parasitoids. Plant Cell Environ. 2017, 40, 330–339. [Google Scholar] [CrossRef]
- Xu, H.; Veyrat, N.; Degen, T.; Turlings, T.C. Exceptional use of sex pheromones by parasitoids of the genus Cotesia: Males are strongly attracted to virgin females, but are no longer attracted to or even repelled by mated females. Insects 2014, 5, 499–512. [Google Scholar] [CrossRef]
- Merkx-Jacques, M.; Bede, J.C. Influence of diet on the larval beet armyworm, Spodoptera exigua, glucose oxidase activity. J. Insect Sci. 2005, 5, 48. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Li, F.; Yan, Q.; Meng, L.; Li, B. Chemical polymorphism regulates the attractiveness to nymphs in the bean bug Riptortus pedestris. J. Pest Sci. 2021, 94, 463–472. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Xu, H.; Turlings, T.C. Powdery mildew suppresses herbivore-induced plant volatiles and interferes with parasitoid attraction in Brassica rapa. Plant Cell Environ. 2016, 39, 1920–1927. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, G.; Dötterl, S.; Schäffler, I.; Degen, T.; Chen, L.; Turlings, T.C.J. Distinct roles of cuticular aldehydes as pheromonal cues in two Cotesia parasitoids. J. Chem. Ecol. 2020, 46, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Mumm, R.; Ruther, J. Courtship pheromones in parasitic wasps: Comparison of bioactive and inactive hydrocarbon profiles by multivariate statistical methods. J. Chem. Ecol. 2007, 33, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, C.-W.; Zhang, R.; Liang, Y.; Li, M.-Q.; Ci, T.-T.; Wang, Y.-Y.; Wang, W.-J.; Zhang, Y.-N.; Pan, Z.-J.; et al. Harnessing Leguminosae typical scents for a more effective and eco-friendly pheromone trapping of pest. J. Agric. Food Chem. 2025, 73, 17519–17528. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1–23. Available online: https://cran.r-project.org/web/packages/lme4 (accessed on 1 May 2025).
- Xu, H.; Zhou, G.; Dötterl, S.; Schäffler, I.; von Arx, M.; Röder, G.; Degen, T.; Chen, L.; Turlings, T.C.J. The combined use of an attractive and a repellent sex pheromonal component by a gregarious parasitoid. J. Chem. Ecol. 2019, 45, 559–569. [Google Scholar] [CrossRef]
- D’alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Lewis, W.; Tumlinson, J.H. Host detection by chemically mediated associative learning in a parasitic wasp. Nature 1988, 331, 257. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; Auger, J.; Thibout, E.; Rousset, S.; Caicedo, A.M.; Calatayud, S.; Buschmann, H.; Guillaud, J.; Mandon, N.; Bellotti, A.C. Identification and synthesis of a kairomone mediating host location by two parasitoid species of the cassava mealybug Phenacoccus herreni. J. Chem. Ecol. 2001, 27, 2203–2217. [Google Scholar] [CrossRef]
- Chen, Y.; Ulyshen, M.D.; Poland, T.M. Abundance of volatile organic compounds in white ash phloem and emerald ash borer larval frass does not attract Tetrastichus planipennisi in a Y-tube olfactometer. Insect Sci. 2016, 23, 712–719. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Kuhelj, A.; Polajnar, J.; Šturm, R. Predator-prey interactions and eavesdropping in vibrational communication networks. Front. Ecol. Evol. 2019, 7, 203. [Google Scholar] [CrossRef]
- Cortesero, A.; De Moraes, C.M.; Stapel, J.; Tumlinson, J.; Lewis, W. Comparisons and contrasts in host-foraging strategies of two larval parasitoids with different degrees of host specificity. J. Chem. Ecol. 1997, 23, 1589–1606. [Google Scholar] [CrossRef]
- Sidorov, R.; Zhukov, A.; Pchelkin, V.; Tsydendambaev, V. Palmitic acid in higher plant lipids. In Palmitic Acid: Occurrence, Biochemistry and Health Effects; NOVA Publishers: New York, NY, USA, 2014; pp. 125–144. [Google Scholar]
- Tonelli, M.; Gomes, G.; Silva, W.D.; Magri, N.T.; Vieira, D.M.; Aguiar, C.L.; Bento, J.M.S. Spittlebugs produce foam as a thermoregulatory adaptation. Sci. Rep. 2018, 8, 4729. [Google Scholar] [CrossRef] [PubMed]
- Noushini, S.; Perez, J.; Park, S.J.; Holgate, D.; Jamie, I.; Jamie, J.; Taylor, P. Rectal gland chemistry, volatile emissions, and antennal responses of male and female banana fruit fly, Bactrocera musae. Insects 2020, 11, 32. [Google Scholar] [CrossRef]
- Evans, P.H.; Becerra, J.X.; Venable, D.L.; Bowers, W.S. Chemical analysis of squirt-gun defense in Bursera and counter defense by Chrysomelid beetles. J. Chem. Ecol. 2000, 26, 745–754. [Google Scholar] [CrossRef]
- Jing, T.-Z.; Qi, F.-H.; Wang, Z.-Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Basit, A.; Haq, I.U.; Hyder, M.; Humza, M.; Younas, M.; Akhtar, M.R.; Ghafar, M.A.; Liu, T.-X.; Hou, Y. Microbial symbiosis in Lepidoptera: Analyzing the gut microbiota for sustainable pest management. Biology 2025, 14, 937. [Google Scholar] [CrossRef]
| Compounds | Ha Frass | Sf Frass | Ha Larvae | Sf Larvae | ||||
|---|---|---|---|---|---|---|---|---|
| Maize | AD | Maize | AD | Maize | AD | Maize | AD | |
| 2,3-butanediol I ▲ | 63 ± 16 a | 95 ± 13 a | 23 ± 7 a’ | 11 ± 4 a’ | n.d. | n.d. | n.d. | n.d. |
| 2,3-butanediol II ▲ | 42 ± 12 a | 54 ± 17 a | 27 ± 9 a’ | 21 ± 8 a’ | n.d. | n.d. | n.d. | n.d. |
| Benzaldehyde ◇ | 13 ± 2 | n.d. | 11 ± 3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-ethylhexanol ◇ | 10 ± 2 | n.d. | 14 ± 5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| (E)-coniferol ◇ | 7 ± 1 | n.d. | 16 ± 5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pentadecane B | 17 ± 6 a | 19 ± 7 a | 16 ± 4 a’ | 13 ± 3 a’ | 183 ± 21 a’’ | 156 ± 28 a’’ | 245 ± 62 a’’’ | 215 ± 31 a’’’ |
| palmitoleic acid | 47 ± 10 a | 41 ± 13 a | 24 ± 10 a’ | 26 ± 6 a’ | n.d. | n.d. | n.d. | n.d. |
| palmitic acid F | 271 ± 51 a | 254 ± 34 a | 286 ± 45 a’ | 213 ± 31 a’ | 21 ± 6 a’’ | 33 ± 9 a’’ | 18 ± 6 a’’’ | 9 ± 3 a’’’ |
| ethyl palmitate | 81 ± 12 a | 73 ± 11 a | 93 ±17 a’ | 74 ± 22 a’ | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, X.; Kelehoun, S.T.O.; Dong, K.; Wang, Y.; Yin, M.; Li, J.; Gao, Y.; Xu, H. A Ubiquitous Volatile in Noctuid Larval Frass Attracts a Parasitoid Species. Biology 2025, 14, 1007. https://doi.org/10.3390/biology14081007
Wang C, Liu X, Kelehoun STO, Dong K, Wang Y, Yin M, Li J, Gao Y, Xu H. A Ubiquitous Volatile in Noctuid Larval Frass Attracts a Parasitoid Species. Biology. 2025; 14(8):1007. https://doi.org/10.3390/biology14081007
Chicago/Turabian StyleWang, Chaowei, Xingzhou Liu, Sylvestre T. O. Kelehoun, Kai Dong, Yueying Wang, Maozhu Yin, Jinbu Li, Yu Gao, and Hao Xu. 2025. "A Ubiquitous Volatile in Noctuid Larval Frass Attracts a Parasitoid Species" Biology 14, no. 8: 1007. https://doi.org/10.3390/biology14081007
APA StyleWang, C., Liu, X., Kelehoun, S. T. O., Dong, K., Wang, Y., Yin, M., Li, J., Gao, Y., & Xu, H. (2025). A Ubiquitous Volatile in Noctuid Larval Frass Attracts a Parasitoid Species. Biology, 14(8), 1007. https://doi.org/10.3390/biology14081007







