Long-Term Monitoring Reveals Changes in the Small Mammal Community Composition and Co-Occurrence Patterns in the Diannan Area of Yunnan, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
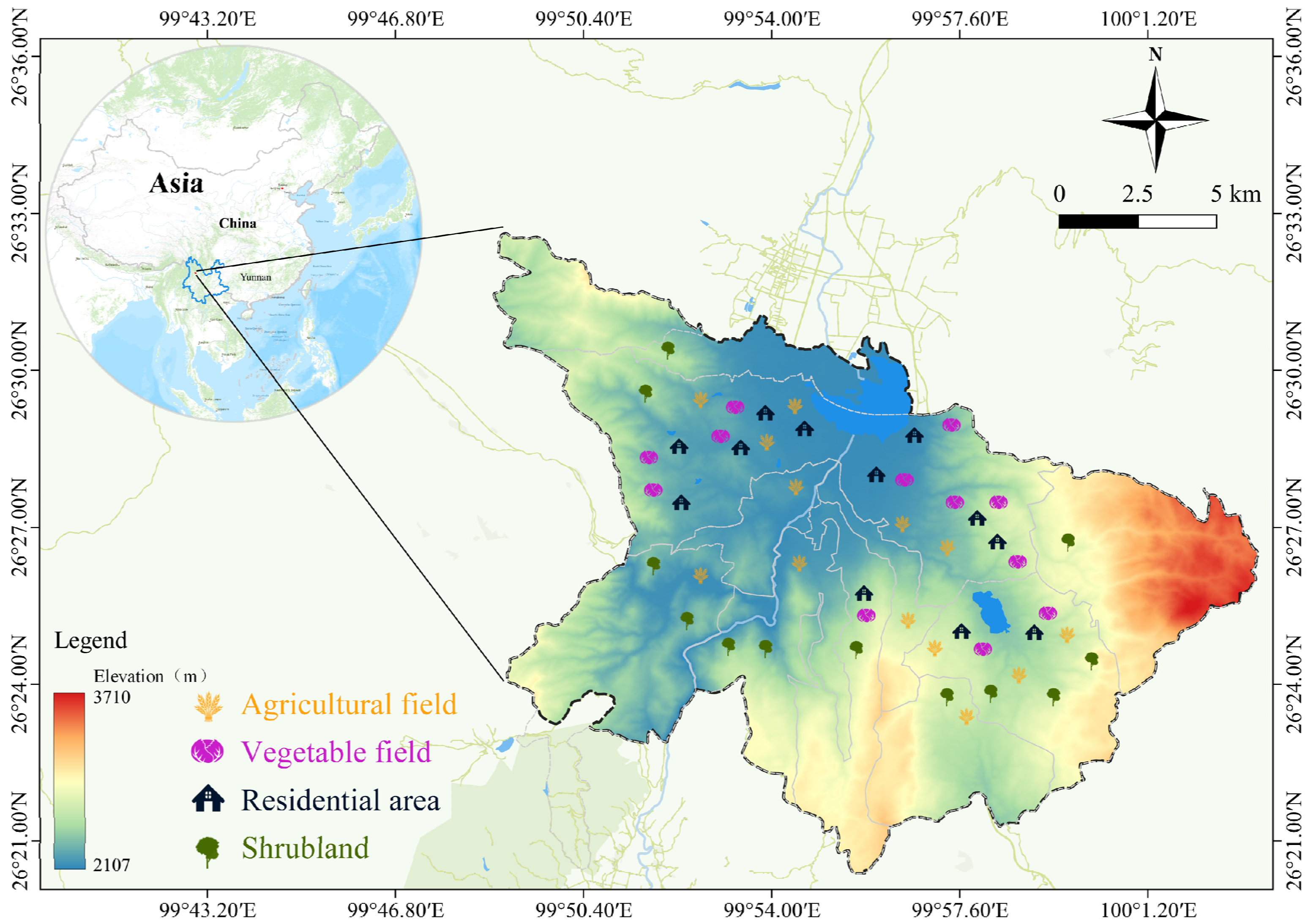
2.1. Study Area
2.2. Field Methods
2.3. Climatic Data
2.4. Data Processing and Analysis
3. Results
3.1. Species Collected During the Surveys
3.2. Variations in Species Composition
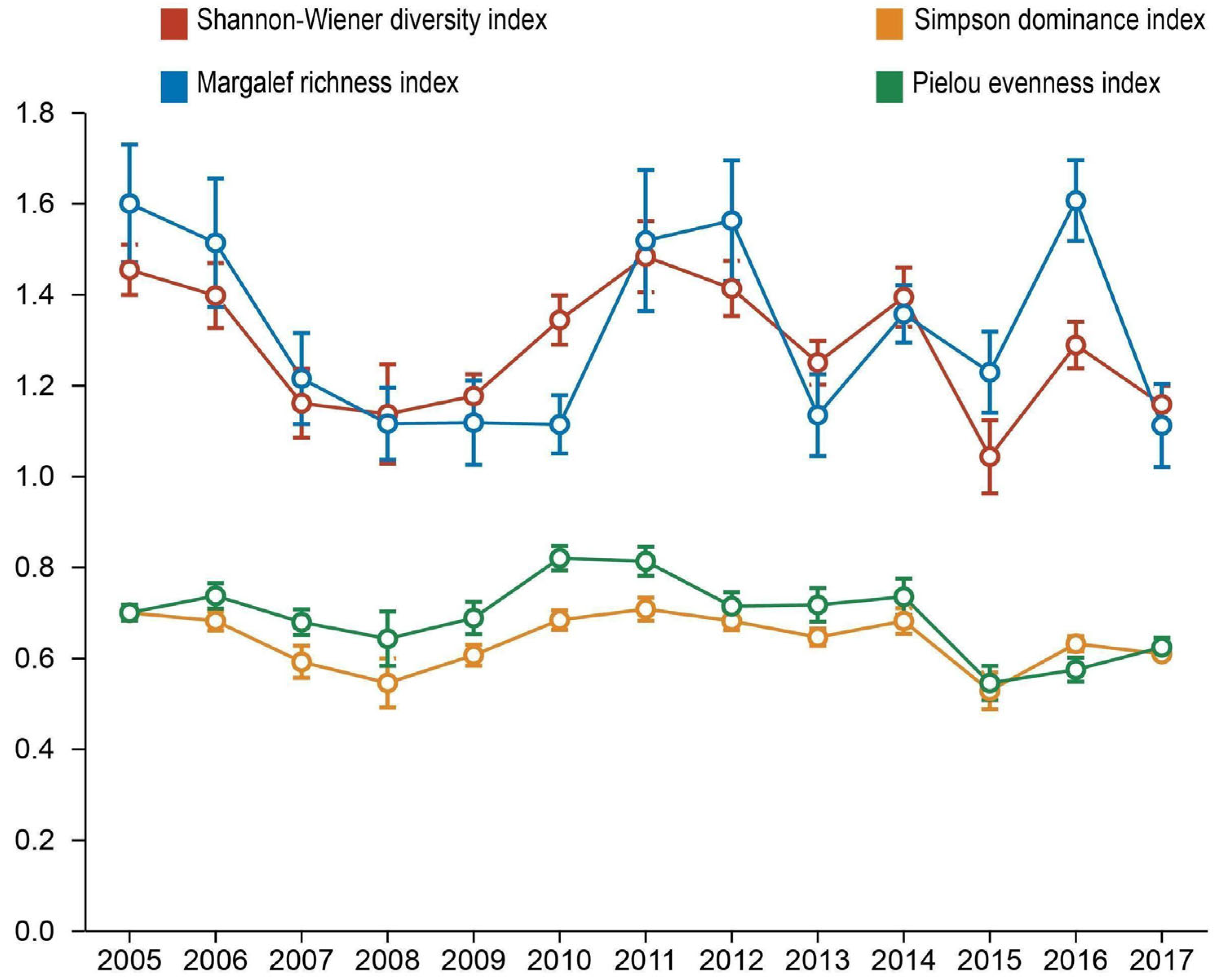
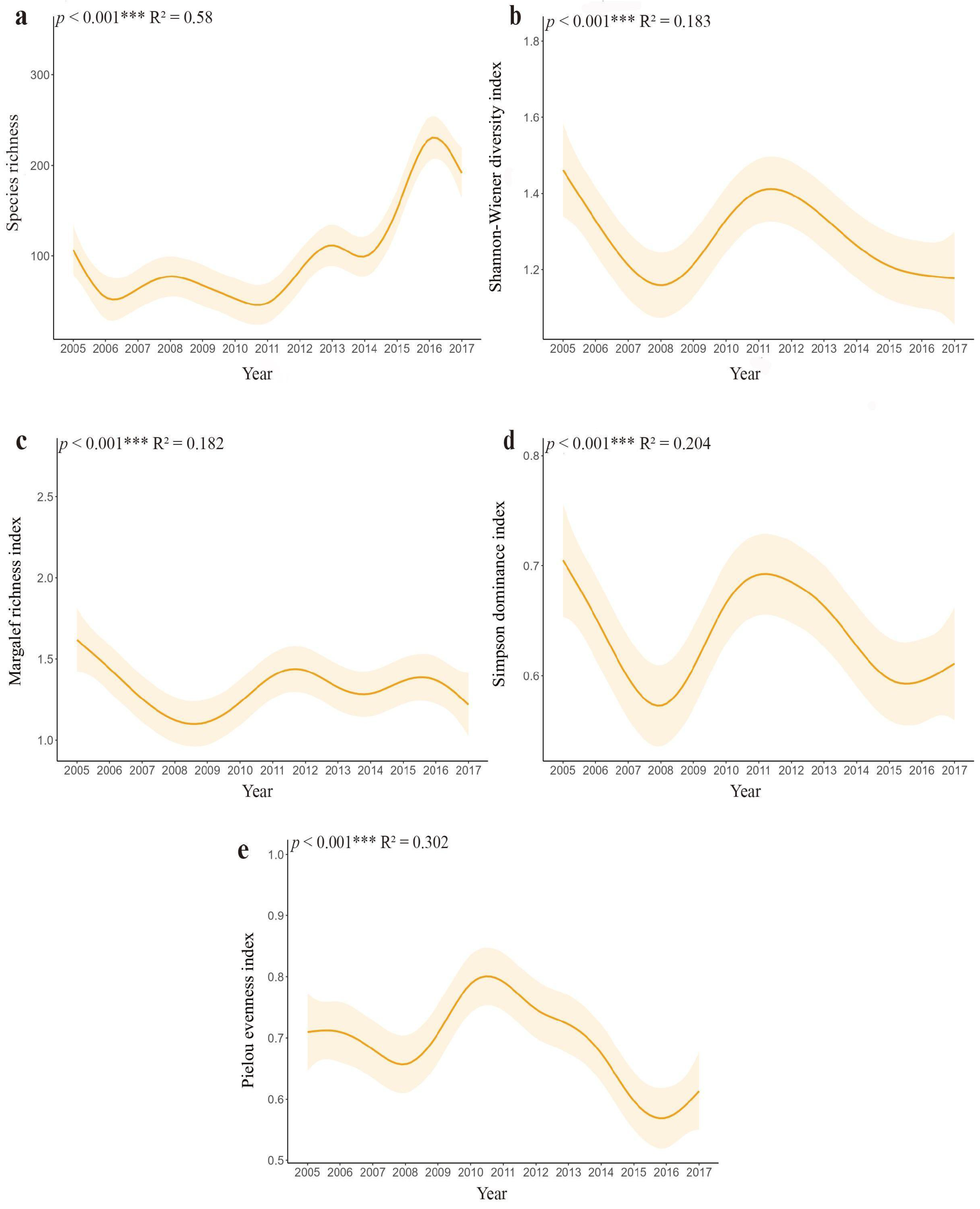
3.3. Variations in Species Diversity
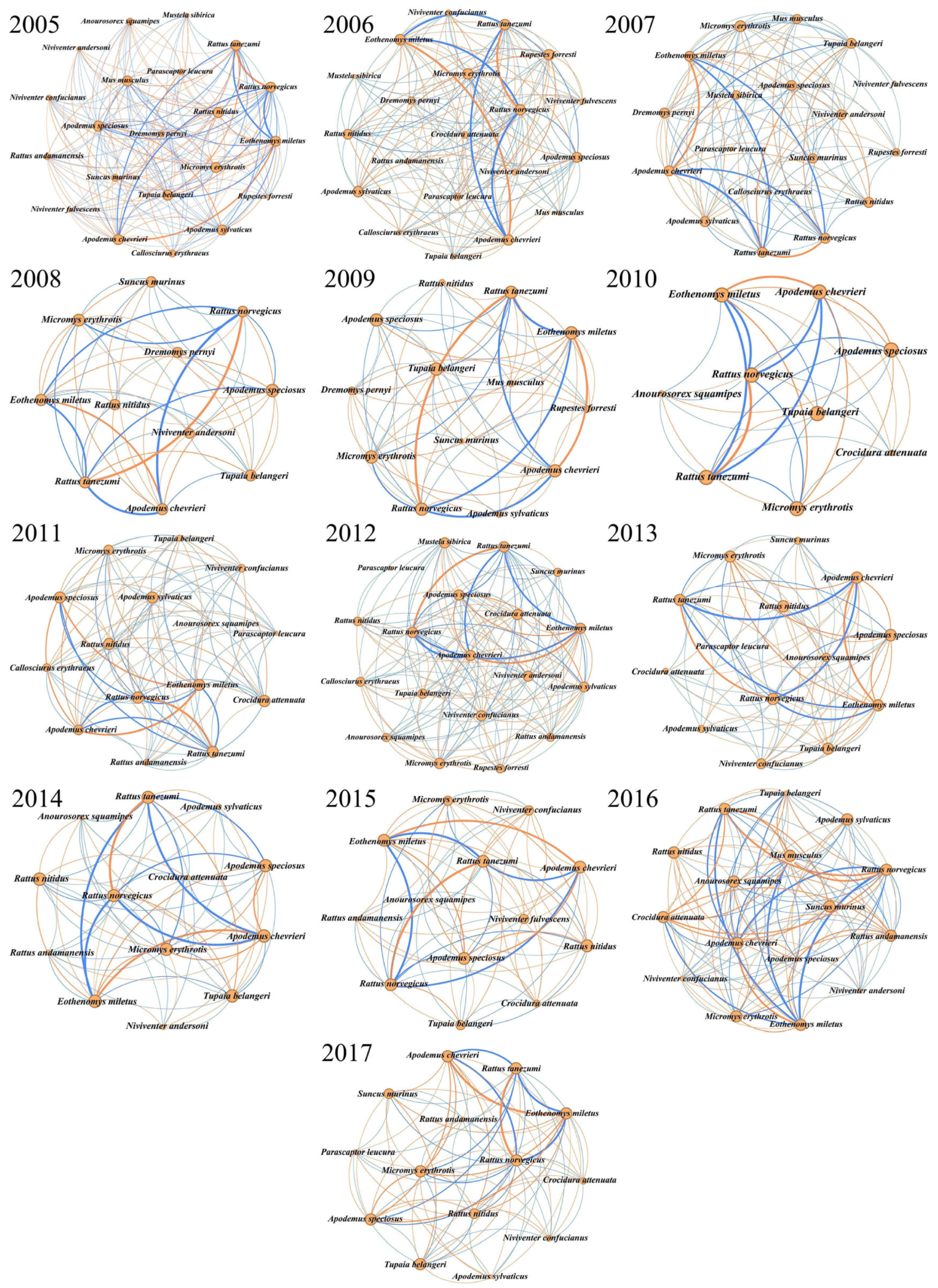
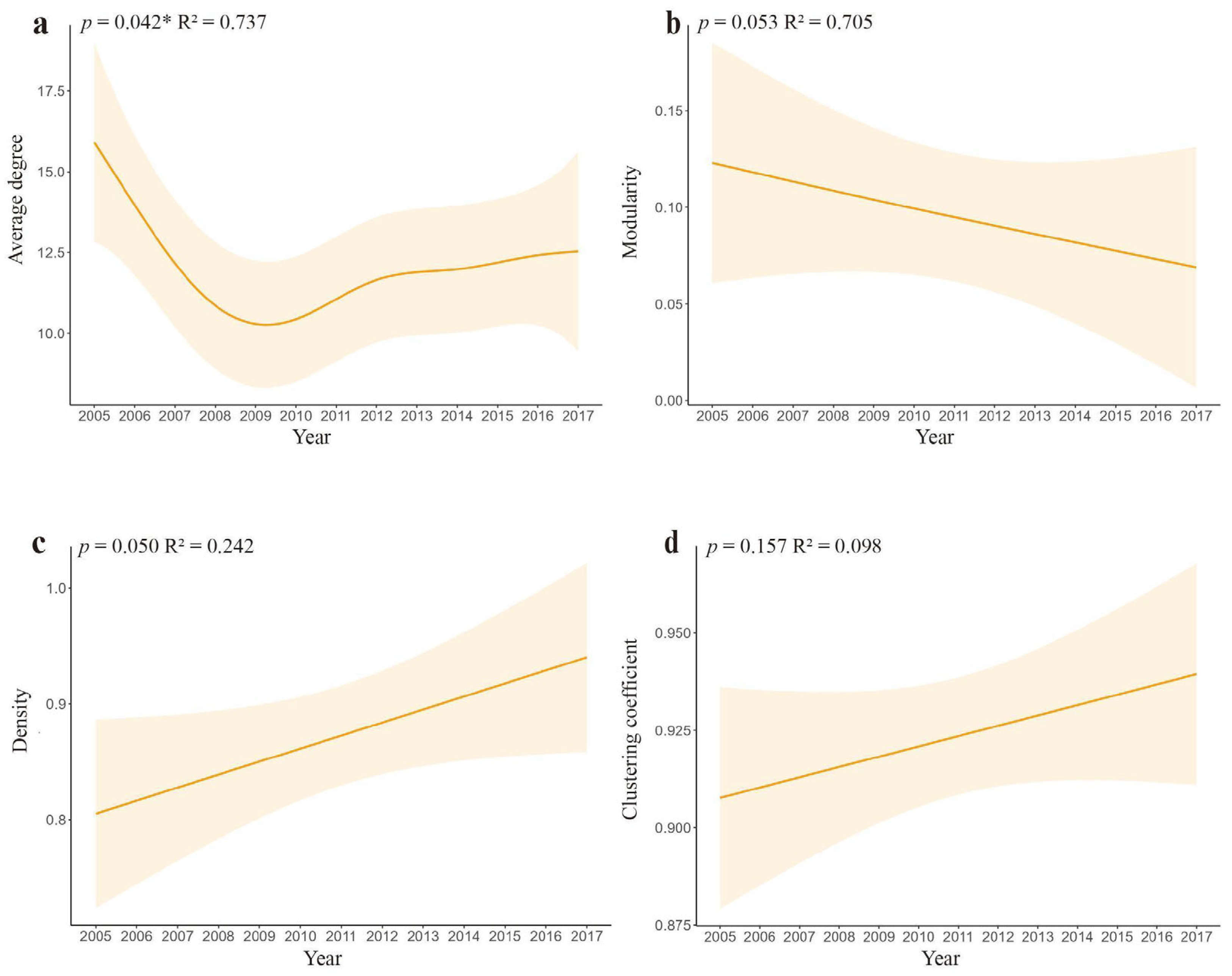
3.4. Changes in Species Co-Occurrence and Networks
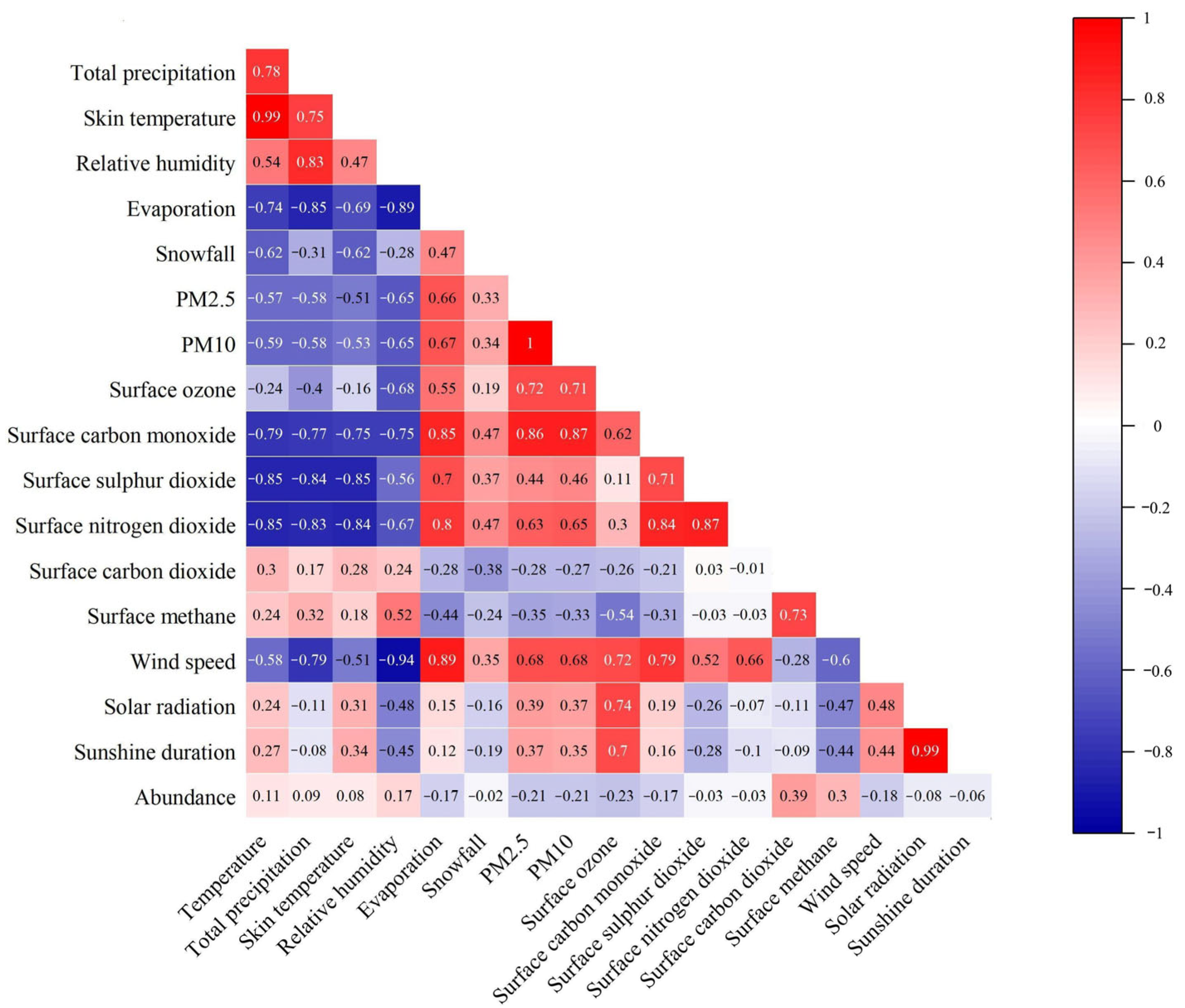
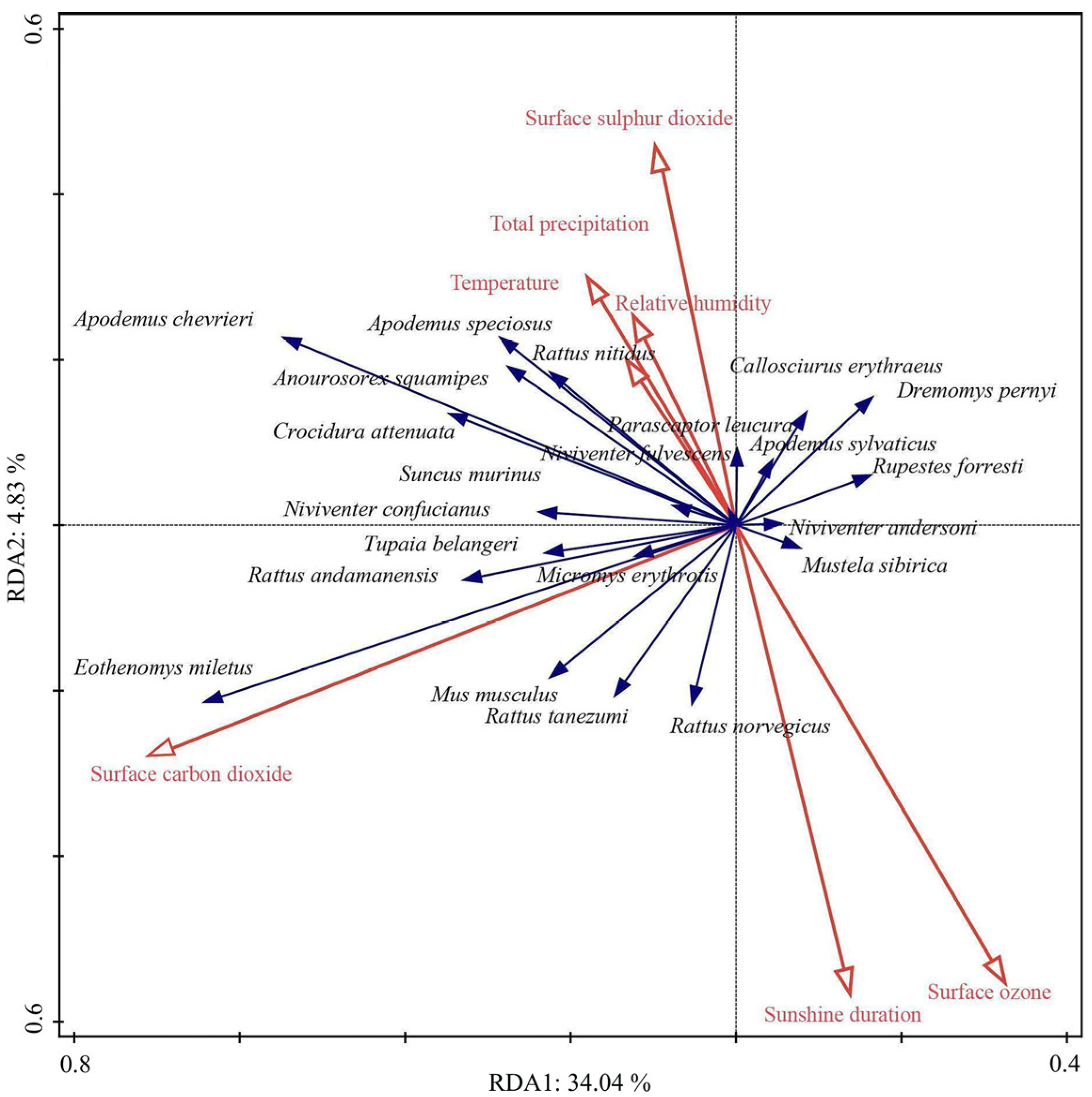
3.5. Association Patterns Between Climate Factors and Small Mammal Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Liu, H.Z.; Li, Q.Q.; Wang, X.Q.; Ma, W.; Liu, C.L.; Fang, X.; Tang, Y.Z.; Shi, T.Z.; Wang, Q.B.; et al. Human expansion into Asian highlands in the 21st Century and its effects. Nat. Commun. 2022, 13, 4955. [Google Scholar] [CrossRef]
- Yang, L.Y.; Xu, H.; Pan, S.P.; Chen, W.X.; Zeng, J. Identifying the impact of global human activities expansion on natural habitats. J. Clean. Prod. 2024, 434, 140247. [Google Scholar] [CrossRef]
- Wei, G.; He, B.J.; Liu, Y.B.; Li, R. How does rapid urban construction land expansion affect the spatial inequalities of ecosystem health in China? Evidence from the country, economic regions and urban agglomerations. Environ. Impact Assess. Rev. 2024, 106, 107533. [Google Scholar] [CrossRef]
- Xiong, S.W.; Yang, F. Multiscale exploration of spatiotemporal dynamics in China’s largest urban agglomeration: An interactive coupling perspective on human activity intensity and ecosystem health. J. Environ. Manag. 2025, 376, 124375. [Google Scholar] [CrossRef] [PubMed]
- Li, G.D.; Fang, C.L.; Li, Y.J.; Wang, Z.B.; Sun, S.; He, S.W.; Qi, W.; Bao, C.; Ma, H.T.; Fan, Y.P.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef]
- Stevens, C.J.; Gowing, D.J.G.; Wotherspoon, K.A.; Alard, D.; Aarrestad, P.A.; Bleeker, A.; Bobbink, R.; Diekmann, M.; Dise, N.B.; Dupre, C.; et al. Addressing the impact of atmospheric nitrogen deposition on western European grasslands. Environ. Manag. 2011, 48, 885–894. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Pacifici, M.; Visconti, P.; Butchart, S.; Butchart, S.H.M.; Watson, J.E.M.; Cassola, F.M.; Rondinini, C. Species’ traits influenced their response to recent climate change. Nat. Clim. Change 2017, 7, 205–208. [Google Scholar] [CrossRef]
- Christie, A.P.; Amano, T.; Martin, P.A.; Shackelford, G.E.; Simmons, B.I.; Sutherland, W.J. Simple study designs in ecology produce inaccurate estimates of biodiversity responses. J. Appl. Ecol. 2019, 56, 2742–2754. [Google Scholar] [CrossRef]
- Laméris, D.W.; Tagg, N.; Kuenbou, J.K.; Sterck, E.H.M.; Willie, J. Drivers affecting mammal community structure and functional diversity under varied conservation efforts in a tropical rainforest in Cameroon. Anim. Conserv. 2020, 23, 182–191. [Google Scholar] [CrossRef]
- Cook, R.N.; Ramirez-Parada, T.; Browne, L. Environmental correlates of richness, community composition, and functional traits of terrestrial birds and mammals in a fragmented tropical landscape. Landsc. Ecol. 2020, 35, 2825–2841. [Google Scholar] [CrossRef]
- Cárdenas, P.A.; Christensen, E.; Ernest, S.K.M.; Lightfoot, D.C.; Schooley, R.L.; Stapp, P.; Rudgers, J.A. Declines in rodent abundance and diversity track regional climate variability in North American drylands. Glob. Change Biol. 2021, 27, 4005–4023. [Google Scholar] [CrossRef]
- Vitalijus, S.; Linas, B.; Laima, B. Spatiotemporal variation of small mammal communities in commercial orchards across the small country. Agriculture 2022, 12, 632. [Google Scholar] [CrossRef]
- Mason-Romo, E.D.; Farías, A.A.; Ceballos, G. Two decades of climate driving the dynamics of functional and taxonomic diversity of a tropical small mammal community in western Mexico. PLoS ONE 2017, 12, e0189104. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granados, C.; Schuchmann, K.-L. The sound of the illegal: Applying bioacoustics for long-term monitoring of illegal cattle in protected areas. Ecol. Inform. 2023, 74, 101981. [Google Scholar] [CrossRef]
- Aschwanden, J.; Holzgang, O.; Jenni, L. Importance of ecological compensation areas for small mammals in intensively farmed areas. Wildl. Biol. 2007, 13, 150–158. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Benedek, M.A. Advances in diversity and conservation of terrestrial small mammals. Diversity 2023, 15, 884. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow-forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Marques, F.C.; dos Anjos, L. Differences in mammal communities between forest fragments and restoration areas in the Atlantic Forest. Austral Ecol. 2023, 48, 1779–1796. [Google Scholar] [CrossRef]
- Afonso, B.C.; Rosalino, L.M.; Henriques, J.; Tinoco Torres, R.; Wauters, J.; Carvalho, J. The effects of wild ungulates on small mammals: A systematic review and meta-analysis. Mammal. Rev. 2024, 54, 121–132. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Jasiulionis, M.; Balčiauskienė, L.; Remeikis, V. Immediate increase in isotopic enrichment in small mammals following the expansion of a great cormorant colony. Biogeosciences 2018, 15, 3883–3891. [Google Scholar] [CrossRef]
- Karasov-Olson, A.; Kelt, D.A. Small mammal assemblage composition and habitat associations across an elevational gradient in southern California. J. Mammal. 2020, 101, 92–106. [Google Scholar] [CrossRef]
- Loggins, A.A.; Shrader, A.M.; Monadjem, A.; McCleery, R.A. Shrub cover homogenizes small mammals’ activity and perceived predation risk. Sci. Rep. 2019, 9, 16857. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.A.D.S.; Alvarez, M.R.D.V.; Mariano-Neto, E.; Cassano, C.R. Is shadier better? The effect of agroforestry management on small mammal diversity. Biotropica 2020, 52, 470–479. [Google Scholar] [CrossRef]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat suitability for small mammals in Mediterranean landscapes: How and why shrubs matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- McCain, C.M.; King, S.R.B.; Szewczyk, T.; Beck, J. Small mammal species richness is directly linked to regional productivity, but decoupled from food resources, abundance, or habitat complexity. J. Biogeogr. 2018, 45, 2533–2545. [Google Scholar] [CrossRef]
- Chaudhary, V.; Goswami, V.R.; Jones, G.; Ben, K.; Tapi, T.; Ri, C.; Kery, M.; Oli, M.K. Using population monitoring programs to detect changes in mammalian communities. Biol. Conserv. 2022, 276, 109778. [Google Scholar] [CrossRef]
- Ghimirey, Y.P.; Tietje, W.D.; Polyakov, A.Y.; Hines, J.E.; Oli, M.K. Decline in small mammal species richness in coastal-central California, 1997–2013. Ecol. Evol. 2023, 13, e10611. [Google Scholar] [CrossRef]
- Hurlbert, S.H. The nonconcept of species diversity: A critique and alternative parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef]
- Monadjem, A.; Perrin, M. Population fluctuations and community structure of small mammals in a Swaziland grassland over a three-year period. Afr. Zool. 2003, 38, 127–137. [Google Scholar] [CrossRef]
- Kay, G.M.; Tulloch, A.; Barton, P.S.; Cunningham, S.A.; Driscoll, D.A.; Lindenmayer, D.B. Species co-occurrence networks show reptile community reorganization under agricultural transformation. Ecography 2018, 41, 113–125. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rozenfeld, A.; Rahbek, C.; Marquet, P.A. Using species co-occurrence networks to assess the impacts of climate change. Ecography 2011, 34, 897–908. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Poisot, T.; Stouffer, D.B.; Gravel, D. Beyond species: Why ecological interaction networks vary through space and time. Oikos 2015, 124, 243–251. [Google Scholar] [CrossRef]
- Li, D.; Poisot, T.; Waller, D.M.; Baiser, B. Homogenization of species composition and species association networks are decoupled. Global Ecol. Biogeogr. 2018, 27, 1481–1491. [Google Scholar] [CrossRef]
- Wang, J.Q.; Gao, C.X.; Tian, S.Q.; Han, D.Y.; Ma, J.; Dai, L.B.; Ye, S. Shifts in composition and co-occurrence patterns of the fish community in the south inshore of Zhejiang, China. Global Ecol. Conserv. 2023, 44, e02502. [Google Scholar] [CrossRef]
- Cordero, R.D.; Jackson, D.A. Abiotic factors influence species co-occurrence patterns of lake fishes. J. Anim. Ecol. 2021, 90, 2859–2874. [Google Scholar] [CrossRef]
- Echevarria, G.; Rodriguez, J.P. Co-occurrence patterns of fish species in two aquatic habitats of the Arauca River floodplain, Venezuela. Community Ecol. 2017, 18, 137–148. [Google Scholar] [CrossRef]
- García-Navas, V.; Sattler, T.; Schmid, H.; Ozgul, A. Bird species co-occurrence patterns in an alpine environment supports the stress-gradient hypothesis. Oikos 2021, 130, 1905–1918. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Chadès, I.; Lindenmayer, D. Species co-occurrence analysis predicts management outcomes for multiple threats. Nat. Ecol. Evol. 2018, 2, 465–474. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Li, X.Y.; Song, W.Y.; Li, Q.; Onditi, K.; Khana, L.; Jiang, X.L. Small mammal species richness and turnover along elevational gradient in Yulong Mountain, Yunnan, Southwest China. Ecol. Evol. 2020, 10, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.D.; Wu, H.Y.; Duan, X.D.; Feng, X.G.; Zhang, Y.Z.; Liu, Q. The species diversity and distribution trends of small mammals in Hengduan Mountains, Yunnan. Biodivers. Sci. 2001, 9, 73–79. [Google Scholar] [CrossRef]
- Wu, Y.J.; Yang, Q.S.; Wen, Z.X.; Xia, L.; Zhang, Q.A.; Zhou, H.M. What drives the species richness patterns of non-volant small mammals along a subtropical elevational gradient? Ecography 2013, 36, 185–196. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhu, W.L. Impact of habitat variation and human activities on small mammal community structure and diversity in Diannan Town, Jianchuan County, Yunnan. Biodivers. Sci. 2023, 31, 113–122. [Google Scholar] [CrossRef]
- Zhu, W.L.; Zhang, H.; Meng, L.H.; Cai, J.H.; Wang, Z.K. Composition and diversity of small mammals community in Shilong, Jianchuan area of Yunnan Province. J. Biol. 2016, 33, 1–4. [Google Scholar]
- Bekele, A. Population dynamics of the Ethiopian endemic rodent Praomys albipes in Menagesha state forest. J. Zool. Soc. Lond. 1996, 238, 1–12. [Google Scholar] [CrossRef]
- Wei, F.W.; Yang, Q.S.; Wu, Y.; Jiang, X.L.; Liu, S.Y.; Li, B.G.; Yang, G.; Li, M.; Zhou, J.; Li, S.; et al. Catalogue of mammals in China (2021). Acta Theriol. Sin. 2021, 41, 487–501. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of planktonic foraminifera in deep-sea sediments. Sci. Total Environ. 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 2004, 99, 673–686. [Google Scholar] [CrossRef]
- Wood, S.N.; Goude, Y.; Shaw, S. Generalized additive models for large data sets. J. R. Stat. Soc. Ser. C-Appl. Stat. 2015, 64, 139–155. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Gong, Z.D.; Feng, X.G.; Duan, X.D.; Wu, H.Y.; Weng, X.; Lü, Y. The community structure and vertical distribution of small mammals in Baicaoling Mt., Yunnan Province, China. Chin. J. Zool. 2002, 37, 63–66. [Google Scholar]
- Song, W.Y.; Li, X.Y.; Wang, H.J.; Chen, Z.Z.; He, S.W.; Jiang, X.L. Multi-dimensional evaluation of small mammal diversity in Tree Line Habitats across the Three Parallel Rivers of Yunnan Protected Areas: Implications for conservation. Biodivers. Sci. 2021, 29, 1215–1228. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Gong, Z.D.; Wu, H.Y.; Cai, Q.S.; Luo, H.K.; Li, C.C.; Li, Z.P. Community structure and vertical distribution of small mammals in Wuliang Mountain Nature Reserve, Yunnan Province, China. Endem. Dis. Bull. 2005, 20, 13-15, 17. [Google Scholar]
- Zárybnická, M.; Riegert, J.; Bejček, V.; Sedláček, F.; Šťastný, K.; Šindelář, J.; Heroldová, M.; Vilímová, J.; Zima, J. Long-term changes of small mammal communities in heterogeneous landscapes of Central Europe. Eur. J. Wildl. Res. 2017, 63, 89. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring biological diversity. Curr. Biol. 2021, 69, R1174–R1177. [Google Scholar] [CrossRef]
- Huang, W.; Zheng, B.; Wen, D.; Wang, F.; Fan, L.; Yu, Z.; Liu, W.; Zhao, S. Community characteristics and potential risk of Nekton in waters adjacent to Ningde Nuclear Power Plant in Fujian, China. Biology 2025, 14, 481. [Google Scholar] [CrossRef]
- García-Baquero, G.; Crujeiras, R.M. Can environmental constraints determine random patterns of plant species co-occurrence? Ecol. Evol. 2015, 5, 1088–1099. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. Chapter two—The effects of invasive species on the decline in species richness: A global meta-analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar]
- Tylianakis, J.M.; Laliberté, E.; Nielsen, A.; Bascompte, J. Conservation of species interaction networks. Biol. Conserv. 2010, 143, 2270–2279. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 2605–2611. [Google Scholar] [CrossRef]
- Rivero-Castro, G.A.; Beninato, V.A.; Giannoni, S.M.; Borghi, C.E. Diversity and co-occurrence of small and medium sized mammals in rock outcrops of the Desert Puna. Biodiversity 2023, 24, 242–251. [Google Scholar] [CrossRef]
- Liang, R.J.; Lin, Z.S.; Cheng, L.L. The competition model of species at different types of habitat and simulation studies and applications. Acta Ecol. Sin. 2006, 26, 3308–3316. [Google Scholar]
- Lopes, K.G.; Bicca-Marques, J.C. Ambient temperature and humidity modulate the behavioural thermoregulation of a small arboreal mammal (Callicebus bernhardi). J. Therm. Biol. 2017, 69, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Gaines, S.D.; García Molinos, J.; Zhang, M.; Xu, J. Climate change and fishing are pulling the functional diversity of the world’s largest marine fisheries to opposite extremes. Glob. Ecol. Biogeogr. 2022, 31, 1616–1629. [Google Scholar] [CrossRef]
- Bagne, K.E.; Finch, D.M. Response of small mammal populations to fuel treatment and precipitation in a Ponderosa Pine Forest, New Mexico. Restor. Ecol. 2010, 18, 409–417. [Google Scholar] [CrossRef]
- Zhu, W.L.; Cai, J.H.; Xiao, L.; Wang, Z.K. Effects of photoperiod on energy intake, thermogenesis and body mass in Eothenomys miletus in Hengduan Mountain region. J. Therm. Biol. 2011, 36, 380–385. [Google Scholar] [CrossRef]
- Setterstrom, C. Effects of sulfur dioxide on plants and animals. Ind. Eng. Chem. 2022, 32, 473–479. [Google Scholar] [CrossRef]
- Hughes, B.B.; Beas-Luna, R.; Barner, A.K.; Brewitt, K.; Brumbaugh, D.R.; Cerny, C.E.B.; Close, S.L.; Coblentz, K.E.; Nesnera, K.L.; Drobnitch, S.T.; et al. Long-term studies contribute disproportionately to ecology and policy. Bioscience 2016, 67, 81–271. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Gilbert, B.S.; Kenney, A.J.; Boutin, S. Impact of climate change on the small mammal community of the Yukon boreal forest. Integr. Zool. 2019, 14, 528–541. [Google Scholar] [CrossRef]
- Lovegrove, B.G.; Canale, C.; Levesque, D.; Fluch, G.; Reháková-Petrů, M.; Ruf, T. Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 2014, 87, 30–45. [Google Scholar] [CrossRef]
- Mason-Romo, E.D.; Ceballos, G.; Lima, M.; Martínez-Yrízar, A.; Jaramillo, V.J.; Maass, M. Long-term population dynamics of small mammals in tropical dry forests, effects of unusual climate events, and implications for management and conservation. Forest Ecol. Manag. 2018, 426, 123–133. [Google Scholar] [CrossRef]
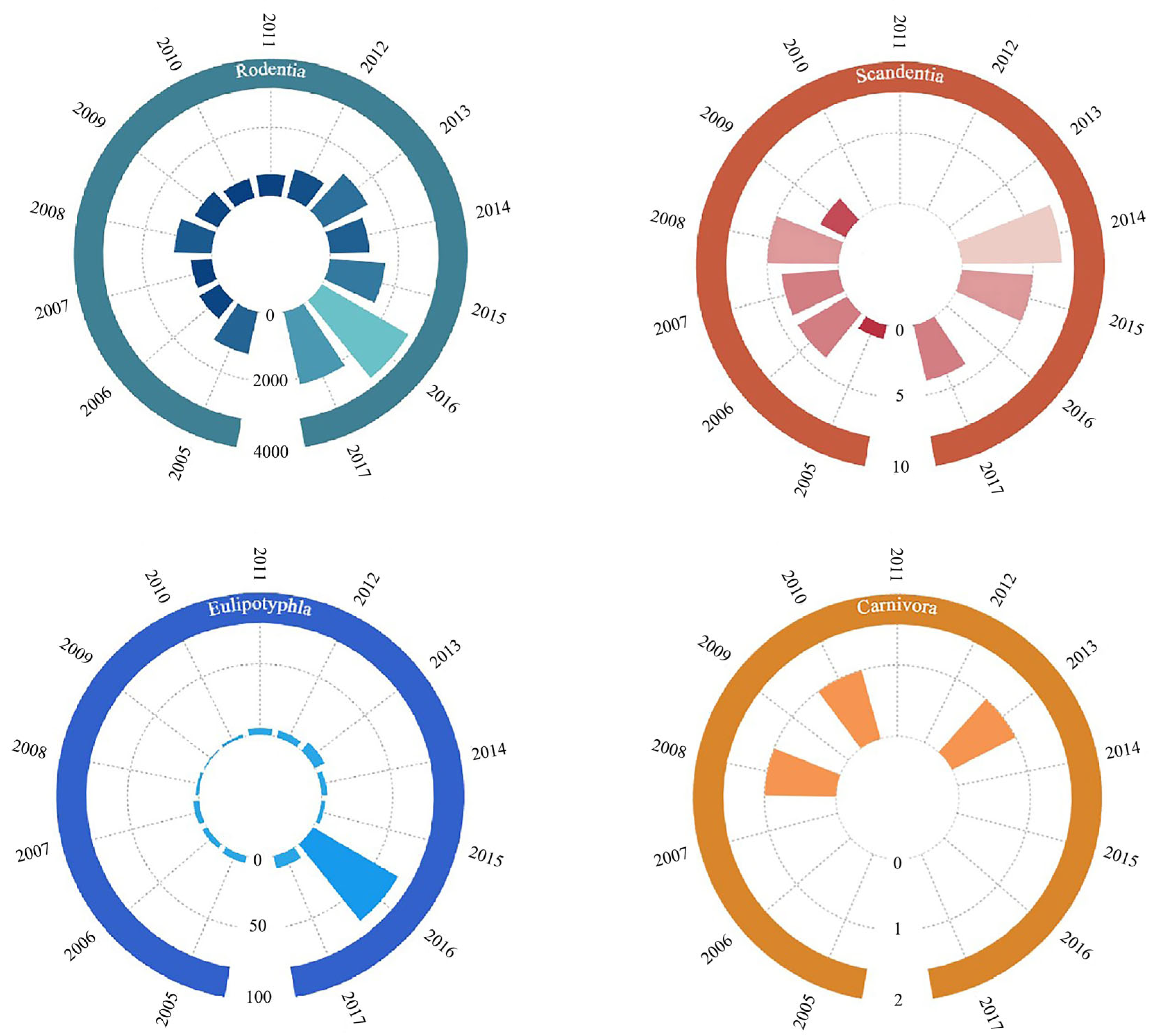
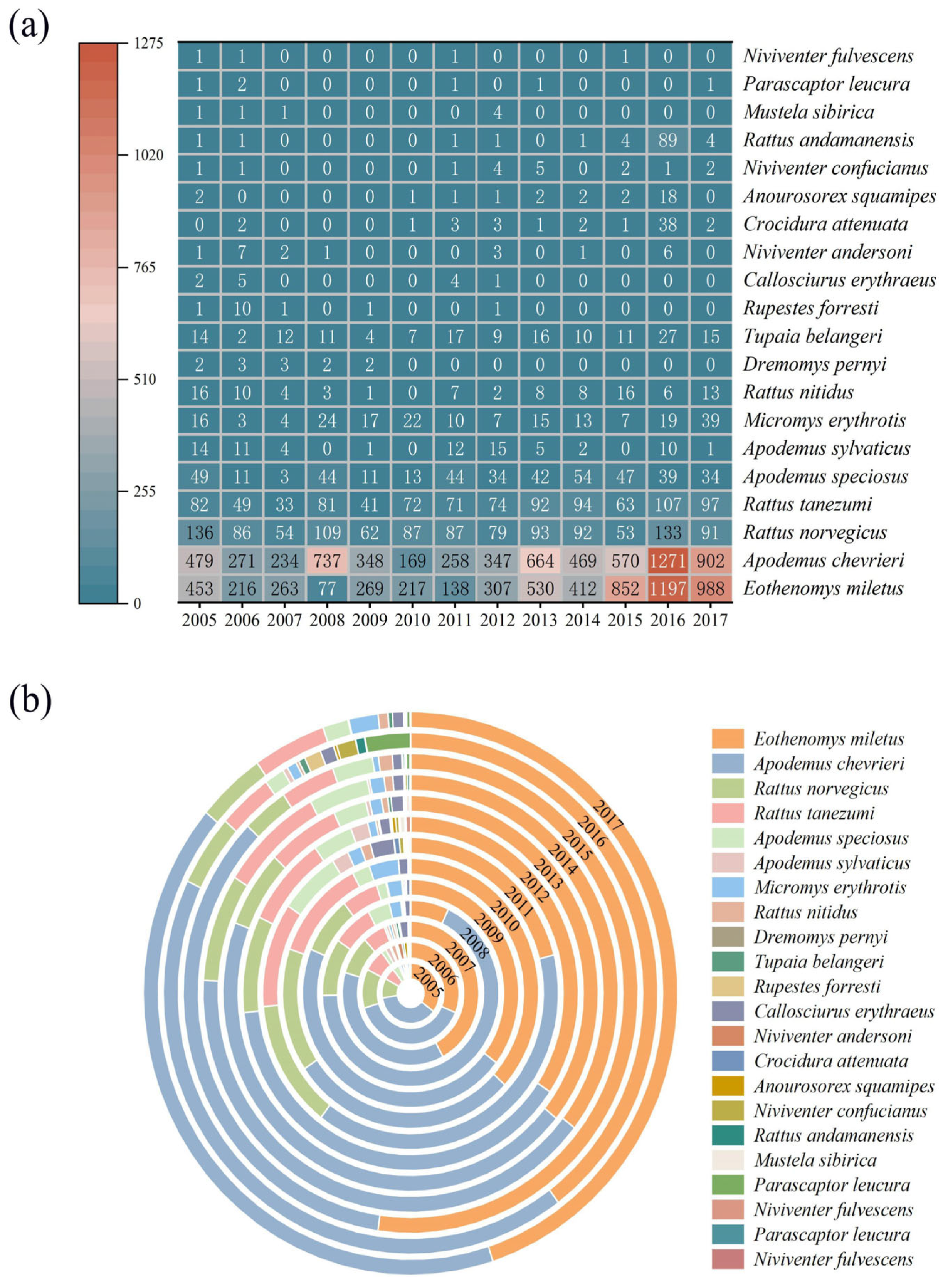
| Order | Family | Genus | Species | Common Name |
|---|---|---|---|---|
| Rodentia | Cricetidae | Eothenomys | Eothenomys miletus | Large Chinese vole |
| Muridae | Apodemus | Apodemus chevrieri | Chevrier’s field mouse | |
| Apodemus speciosus | Korean field mouse | |||
| Apodemus sylvaticus | Wood mouse | |||
| Rattus | Rattus norvegicus | Brown rat | ||
| Rattus tanezumi | Oriental house rat | |||
| Rattus nitidus | White-footed Indochinese rat | |||
| Rattus andamanensis | Indochinese forest rat | |||
| Mus | Mus musculus | House mouse | ||
| Micromys | Micromys erythrotis | Red-eared harvest mouse | ||
| Niviventer | Niviventer confucianus | Confucian niviventer | ||
| Niviventer andersoni | Anderson’s niviventer | |||
| Niviventer fulvescens | Chestnut white-bellied rat | |||
| Sciuridae | Dremomys | Dremomys pernyi | Perny’s long-nosed squirrel | |
| Callosciurus | Callosciurus erythraeus | Pallas’s squirrel | ||
| Suncus | Suncus murinus | Asian house shrew | ||
| Scandentia | Tupalldae | Tupaia | Tupaia belangeri | Northern tree shrew |
| Eulipotyphla | Talpidae | Parascaptor | Parascaptor leucura | White-tailed mole |
| Soricidae | Crocidura | Crocidura attenuata | Asian gray white-toothed shrew | |
| Suncus | Suncus murinus | Asian house shrew | ||
| Anourosorex | Anourosorex squamipes | Chinese mole shrew | ||
| Carnivora | Mustelidae | Mustela | Mustela sibirica | Siberian weasel |
| Years | Average Degree | Modularity | Density | Clustering Coefficient |
|---|---|---|---|---|
| 2005 | 16.190 | 0.129 | 0.810 | 0.892 |
| 2006 | 15.900 | 0.226 | 0.837 | 0.891 |
| 2007 | 11.000 | 0.120 | 0.647 | 0.918 |
| 2008 | 9.455 | 0.015 | 0.945 | 0.945 |
| 2009 | 10.154 | 0.056 | 0.846 | 0.900 |
| 2010 | 7.778 | 0.019 | 0.972 | 0.972 |
| 2011 | 12.667 | 0.131 | 0.905 | 0.919 |
| 2012 | 14.632 | 0.151 | 0.813 | 0.914 |
| 2013 | 11.714 | 0.099 | 0.901 | 0.928 |
| 2014 | 10.462 | 0.020 | 0.872 | 0.907 |
| 2015 | 11.077 | 0.106 | 0.923 | 0.930 |
| 2016 | 14.750 | 0.098 | 0.983 | 0.983 |
| 2017 | 11.571 | 0.070 | 0.890 | 0.907 |
| Stable | Lost | Gained | ||||
|---|---|---|---|---|---|---|
| n | %Links | n | %Links | n | %Links | |
| 2005→2006 | 122 | 71.76 | 48 | 28.24 | 37 | 23.27 |
| 2006→2007 | 81 | 50.94 | 78 | 49.06 | 18 | 18.19 |
| 2007→2008 | 52 | 52.52 | 47 | 47.48 | 0 | 0 |
| 2008→2009 | 41 | 78.85 | 11 | 21.15 | 25 | 37.88 |
| 2009→2010 | 21 | 31.82 | 45 | 68.18 | 14 | 40.00 |
| 2010→2011 | 33 | 94.29 | 2 | 5.71 | 62 | 65.26 |
| 2011→2012 | 83 | 87.37 | 12 | 12.63 | 56 | 40.29 |
| 2012→2013 | 71 | 51.08 | 68 | 48.92 | 11 | 13.41 |
| 2013→2014 | 52 | 63.41 | 30 | 36.59 | 16 | 23.53 |
| 2014→2015 | 51 | 75.00 | 17 | 25.00 | 21 | 29.17 |
| 2015→2016 | 60 | 83.33 | 12 | 16.67 | 58 | 49.15 |
| 2016→2017 | 71 | 60.17 | 47 | 39.83 | 10 | 12.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Jia, T.; Zhu, W.; Yang, X. Long-Term Monitoring Reveals Changes in the Small Mammal Community Composition and Co-Occurrence Patterns in the Diannan Area of Yunnan, China. Biology 2025, 14, 897. https://doi.org/10.3390/biology14070897
Yang J, Jia T, Zhu W, Yang X. Long-Term Monitoring Reveals Changes in the Small Mammal Community Composition and Co-Occurrence Patterns in the Diannan Area of Yunnan, China. Biology. 2025; 14(7):897. https://doi.org/10.3390/biology14070897
Chicago/Turabian StyleYang, Jinyu, Ting Jia, Wanlong Zhu, and Xiaomi Yang. 2025. "Long-Term Monitoring Reveals Changes in the Small Mammal Community Composition and Co-Occurrence Patterns in the Diannan Area of Yunnan, China" Biology 14, no. 7: 897. https://doi.org/10.3390/biology14070897
APA StyleYang, J., Jia, T., Zhu, W., & Yang, X. (2025). Long-Term Monitoring Reveals Changes in the Small Mammal Community Composition and Co-Occurrence Patterns in the Diannan Area of Yunnan, China. Biology, 14(7), 897. https://doi.org/10.3390/biology14070897





