Injury-Driven Structural and Molecular Modifications in Nociceptors
Simple Summary
Abstract
1. Introduction
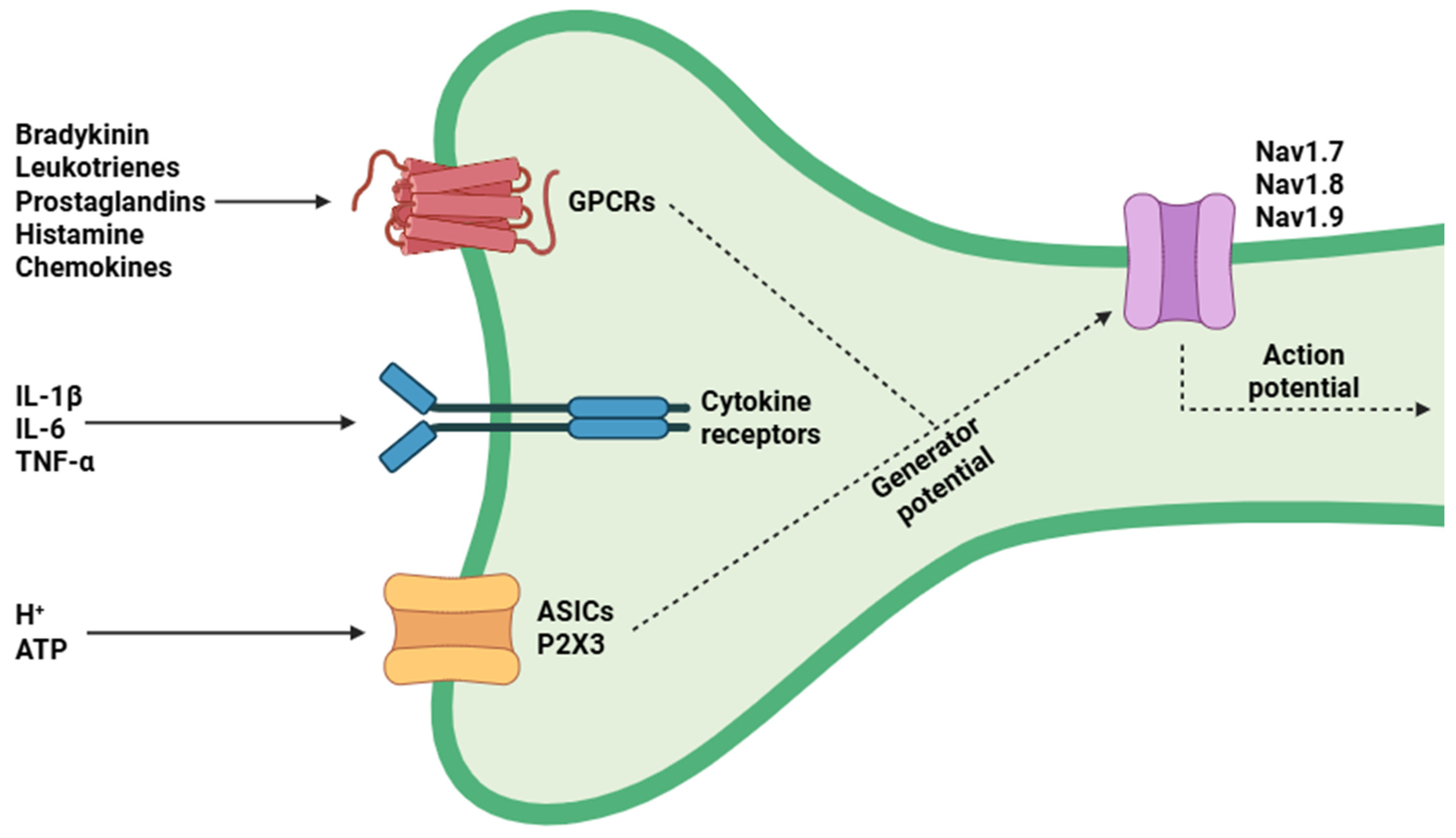
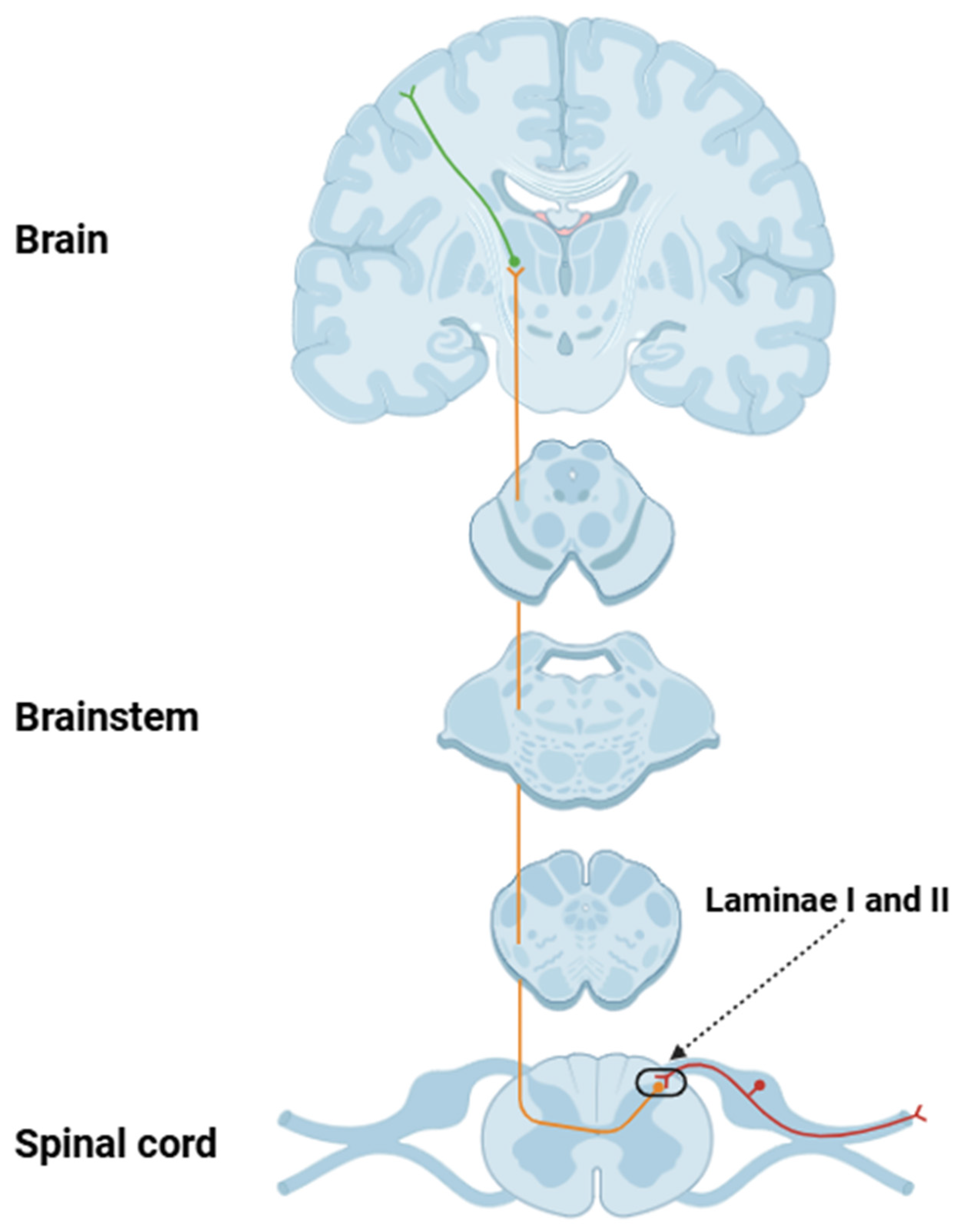
2. Structural and Functional Aspects of Nociceptors
3. Structural and Molecular Changes of Nociceptors Following an Injury
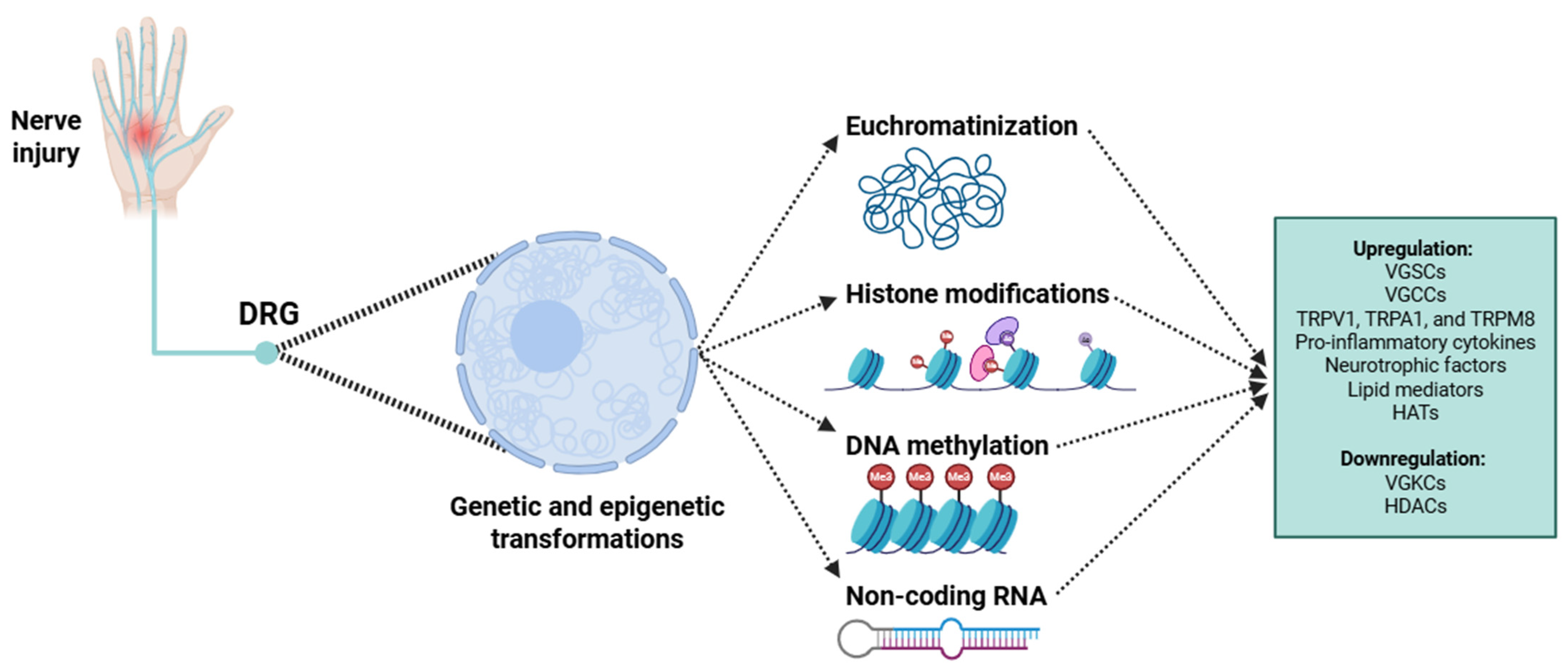
3.1. Molecular-Level Transformations
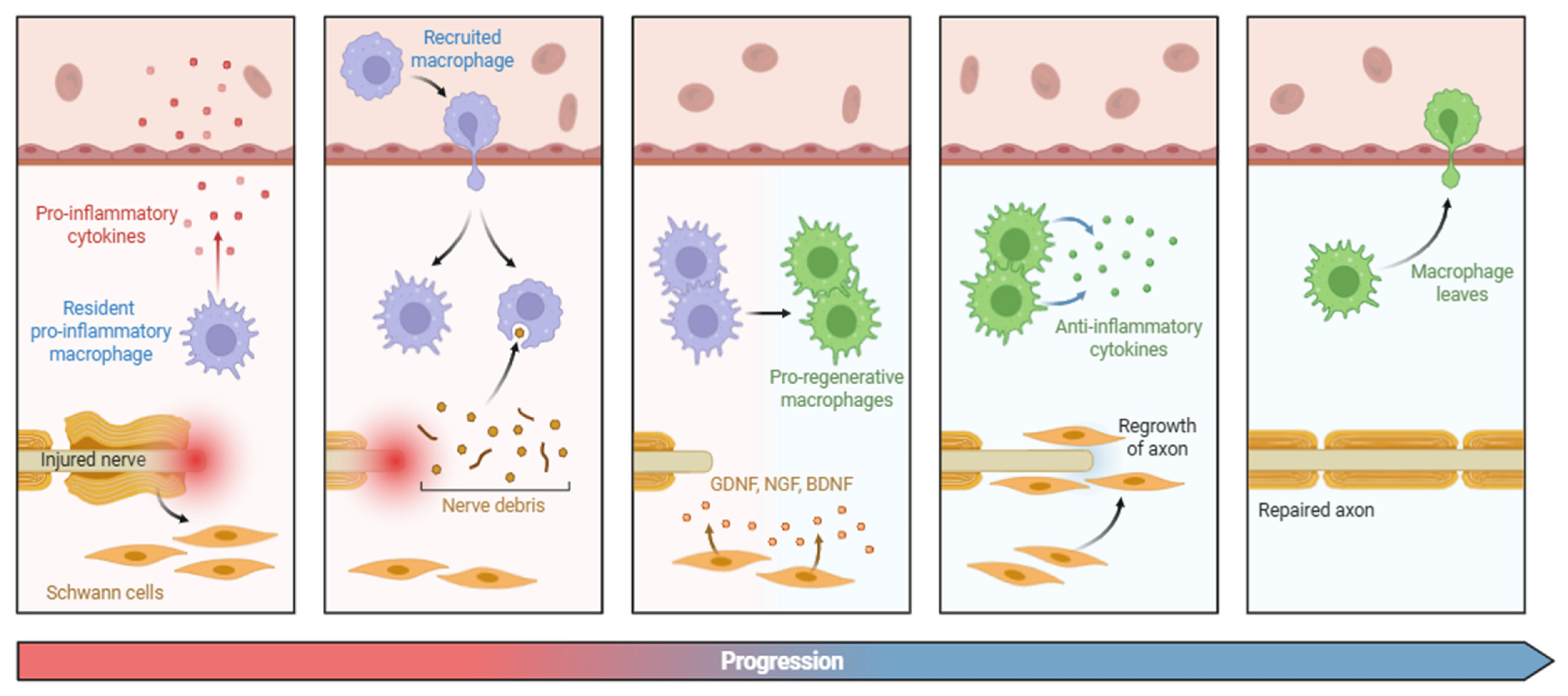
3.2. Structural-Level Transformations
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASIC | Acid-sensing ion channel |
| ATF3 | Activating transcription factor 3 |
| ATP | Adenosine triphosphate |
| BDNF | Brain-derived neurotrophic factor |
| CAM | Cell adhesion molecule |
| CaMK | Calcium/calmodulin-dependent protein kinase |
| cAMP | Cyclic adenosine monophosphate |
| Cav2.2 | Voltage-gated calcium channel, subtype 2.2 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCR2 | C-C Motif chemokine receptor 2 |
| Cdc42 | Cell division cycle 42 |
| CGRP | Calcitonin gene-related peptide |
| ChR2 | Channelrhodopsin-2 |
| circRNA | Circular RNA |
| c-Jun | Cellular Jun (a component of the AP-1 transcription factor complex) |
| CNO | Clozapine-N-oxide |
| CNS | Central nervous system |
| CREB | cAMP response element-binding protein |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CXCR2 | C-X-C Motif chemokine receptor 2 |
| Cx43 | Connexin 43 |
| DCZ | Deschloroclozapine |
| DNA | Deoxyribonucleic acid |
| DREADD | Designer receptor exclusively activated by designer drugs |
| DRG | Dorsal root ganglion |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FAK | Focal adhesion kinase |
| GAP-43 | Growth-associated protein 43 |
| GCN5 | General control non-derepressible 5 |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial Fibrillary Acidic Protein |
| H3K27me3 | Trimethylation of lysine 27 on histone H3 |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HDAC1 | Histone deacetylase 1 |
| HDAC2 | Histone deacetylase 2 |
| HDAC4 | Histone deacetylase 4 |
| HDAC5 | Histone deacetylase 5 |
| IASP | International Association for the Study of Pain |
| IKKβ | IκB kinase beta |
| IL-1β | Interleukin 1 beta |
| IL-1βR | Interleukin 1 beta receptor |
| IL-6 | Interleukin 6 |
| ILK | Integrin-linked kinase |
| JNK | c-Jun N-terminal kinase |
| Kcna2 | Potassium voltage-gated channel subfamily A member 2 |
| Kcnd2 | Potassium voltage-gated channel subfamily D member 2 |
| Kcnq2 | Potassium voltage-gated channel subfamily Q member 2 |
| L1CAM | L1 cell adhesion molecule |
| lncRNA | Long non-coding RNA |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| MAP | Microtubule-associated protein |
| MAP1B | Microtubule-associated protein 1B |
| MAPK | Mitogen-activated protein kinase |
| miRNA | MicroRNA |
| mRNA | Messenger ribonucleic acid |
| Nav1.7 | Voltage-gated sodium channel, subtype 1.7 |
| Nav1.8 | Voltage-gated sodium channel, subtype 1.8 |
| Nav1.9 | Voltage-gated sodium channel, subtype 1.9 |
| NCAM | Neural cell adhesion molecule |
| ncRNA | Non-coding RNA |
| Neat1 | Nuclear paraspeckle assembly transcript 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve growth factor |
| P2X | Purinergic receptor P2X |
| P2X3 | Purinergic Receptor P2X, ligand-gated ion channel 3 |
| p300/CBP | E1A binding protein p300/CREB-binding protein |
| PGE2 | Prostaglandin E2 |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| Piezo | Piezo-type mechanosensitive ion channel |
| Piezo1 | Piezo-type mechanosensitive ion channel 1 |
| Piezo2 | Piezo-type mechanosensitive ion channel 2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| Pten | Phosphatase and tensin homolog |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RAG | Regeneration-associated gene |
| Rho GTPase | Ras homolog family of GTPases |
| RhoA | Ras homolog gene family member A |
| ROS | Reactive oxygen species |
| rRNA | Ribosomal ribonucleic acid |
| scRNA-seq | Single-cell RNA-sequencing |
| SGC | Satellite glial cell |
| Sprr1a | Small proline-rich protein 1A |
| Spry2 | Sprouty RTK signaling antagonist 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TET | Ten-eleven translocation (dioxygenase family) |
| TET1 | Ten-eleven translocation methylcytosine dioxygenase 1 |
| TET3 | Ten-eleven translocation methylcytosine dioxygenase 3 |
| TG | Trigeminal ganglion |
| TNF-α | Tumor necrosis factor alpha |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TRAF6 | TNF receptor-associated factor 6 |
| TrkA | Tropomyosin receptor kinase A |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPM8 | Transient receptor potential melastatin 8 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| UPR | Unfolded protein response |
| VGCC | Voltage-gated calcium channel |
| VGKC | Voltage-gated potassium channel |
| VGSC | Voltage-gated sodium channel |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef]
- Malfliet, A.; Coppieters, I.; Van Wilgen, P.; Kregel, J.; De Pauw, R.; Dolphens, M.; Ickmans, K. Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur. J. Pain 2017, 21, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Hu, Y.; Meng, J. Pain Modulates Responses to Emotional Stimuli. Front. Psychol. 2020, 11, 595987. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Shelomentseva, E.M.; Tsvetkova, M.M.; Abdeeva, E.I.; Giller, D.B.; Babayeva, J.V.; Achkasov, E.E.; Gavryushova, L.V.; Sinelnikov, M.Y. Nociceptors: Their Role in Body’s Defenses, Tissue Specific Variations and Anatomical Update. J. Pain Res. 2022, 15, 867–877. [Google Scholar] [CrossRef]
- Middleton, S.J.; Barry, A.M.; Comini, M.; Li, Y.; Ray, P.R.; Shiers, S.; Themistocleous, A.C.; Uhelski, M.L.; Yang, X.; Dougherty, P.M.; et al. Studying human nociceptors: From fundamentals to clinic. Brain 2021, 144, 1312–1335. [Google Scholar] [CrossRef] [PubMed]
- McEntire, D.M.; Kirkpatrick, D.R.; Dueck, N.P.; Kerfeld, M.J.; Smith, T.A.; Nelson, T.J.; Reisbig, M.D.; Agrawal, D.K. Pain transduction: A pharmacologic perspective. Expert Rev. Clin. Pharmacol. 2016, 9, 1069–1080. [Google Scholar] [CrossRef]
- Gascon, E.; Moqrich, A. Heterogeneity in primary nociceptive neurons: From molecules to pathology. Arch. Pharm. Res. 2010, 33, 1489–1507. [Google Scholar] [CrossRef]
- Hanč, P.; von Andrian, U.H. No pain, no gain—How nociceptors orchestrate tissue repair. Cell Res. 2024, 34, 673–674. [Google Scholar] [CrossRef]
- Price, T.J.; Dussor, G. Evolution: The advantage of ‘maladaptive’ pain plasticity. Curr. Biol. 2014, 24, R384–R386. [Google Scholar] [CrossRef]
- Alvarez, P.; Bogen, O.; Green, P.G.; Levine, J.D. Nociceptor Overexpression of NaV1.7 Contributes to Chronic Muscle Pain Induced by Early-Life Stress. J. Pain 2021, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Barbosa, C.; Pei, Z.; Xie, W.; Strong, J.A.; Zhang, J.M.; Cummins, T.R. Increased Resurgent Sodium Currents in Nav1.8 Contribute to Nociceptive Sensory Neuron Hyperexcitability Associated with Peripheral Neuropathies. J. Neurosci. 2019, 39, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Kistner, K.; Carr, R.W.; Nassar, M.A.; Reeh, P.W.; Weidner, C. Reduced excitability and impaired nociception in peripheral unmyelinated fibers from Nav1.9-null mice. Pain 2017, 158, 58–67. [Google Scholar] [CrossRef]
- Duan, J.H.; Hodgdon, K.E.; Hingtgen, C.M.; Nicol, G.D. N-type calcium current, Cav2.2, is enhanced in small-diameter sensory neurons isolated from Nf1+/- mice. Neuroscience 2014, 270, 192–202. [Google Scholar] [CrossRef]
- Voolstra, O.; Huber, A. Post-Translational Modifications of TRP Channels. Cells 2014, 3, 258–287. [Google Scholar] [CrossRef] [PubMed]
- Viana, F. Nociceptors: Thermal allodynia and thermal pain. Handb. Clin. Neurol. 2018, 156, 103–119. [Google Scholar]
- Li, R.; Ye, J.J.; Gan, L.; Zhang, M.; Sun, D.; Li, Y.; Wang, T.; Chang, P. Traumatic inflammatory response: Pathophysiological role and clinical value of cytokines. Eur. J. Trauma Emerg. Surg. 2024, 50, 1313–1330. [Google Scholar] [CrossRef]
- Uçeyler, N.; Schäfers, M.; Sommer, C. Mode of action of cytokines on nociceptive neurons. Exp. Brain Res. 2009, 196, 67–78. [Google Scholar] [CrossRef]
- Illias, A.M.; Gist, A.C.; Zhang, H.; Kosturakis, A.K.; Dougherty, P.M. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin-induced mechanical hypersensitivity. Pain 2018, 159, 1308–1316. [Google Scholar] [CrossRef]
- Wang, F.; Stefano, G.B.; Kream, R.M. Epigenetic modification of DRG neuronal gene expression subsequent to nerve injury: Etiological contribution to complex regional pain syndromes (Part I). Med. Sci. Monit. 2014, 20, 1067–1077. [Google Scholar] [PubMed]
- Wang, F.; Stefano, G.B.; Kream, R.M. Epigenetic modification of DRG neuronal gene expression subsequent to nerve injury: Etiological contribution to complex regional pain syndromes (Part II). Med. Sci. Monit. 2014, 20, 1188–1200. [Google Scholar]
- Testa, L.; Dotta, S.; Vercelli, A.; Marvaldi, L. Communicating pain: Emerging axonal signaling in peripheral neuropathic pain. Front. Neuroanat. 2024, 18, 1398400. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Campbell, Z.T. RNA-binding proteins in pain. Wiley Interdiscip. Rev. RNA 2024, 15, e1843. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Tassorelli, C.; Sandrini, G.; Di Bella, P.; Buscone, S.; Nappi, G. Role of calcitonin gene-related peptide and substance P in different models of pain. Cephalalgia 2008, 28, 114–126. [Google Scholar]
- Techameena, P.; Feng, X.; Zhang, K.; Hadjab, S. The single-cell transcriptomic atlas iPain identifies senescence of nociceptors as a therapeutical target for chronic pain treatment. Nat. Commun. 2024, 15, 8585. [Google Scholar] [CrossRef]
- Miller, R.E.; Kim, Y.S.; Tran, P.B.; Ishihara, S.; Dong, X.; Miller, R.J.; Malfait, A.M. Visualization of Peripheral Neuron Sensitization in a Surgical Mouse Model of Osteoarthritis by In Vivo Calcium Imaging. Arthritis Rheumatol. 2018, 70, 88–97. [Google Scholar] [CrossRef]
- Kumar, P.A.; Stallman, J.; Kharbat, Y.; Hoppe, J.; Leonards, A.; Kerim, E.; Nguyen, B.; Adkins, R.L.; Baltazar, A.; Milligan, S.; et al. Chemogenetic Attenuation of Acute Nociceptive Signaling Enhances Functional Outcomes Following Spinal Cord Injury. J. Neurotrauma 2024, 41, 1060–1076. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Ding, H.; Cai, Y.; Yuan, X.; Lv, J.; Huang, J.; Huang, J.; Zhang, C.; Hong, Z.; et al. Optogenetic activation of mechanical nociceptions to enhance implant osseointegration. Nat. Commun. 2025, 16, 3093. [Google Scholar] [CrossRef]
- Berta, T.; Perrin, F.E.; Pertin, M.; Tonello, R.; Liu, Y.C.; Chamessian, A.; Kato, A.C.; Ji, R.R.; Decosterd, I. Gene Expression Profiling of Cutaneous Injured and Non-Injured Nociceptors in SNI Animal Model of Neuropathic Pain. Sci. Rep. 2017, 7, 9367. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Ma, Q. Nociceptors--noxious stimulus detectors. Neuron 2007, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lacinova, L. Electrophysiology of nociception: Understanding of signaling pathways forms a basis for potential treatment. Pflugers Arch. 2022, 474, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Motzkin, J.C.; Basbaum, A.I.; Crowther, A.J. Neuroanatomy of the nociceptive system: From nociceptors to brain networks. Int. Rev. Neurobiol. 2024, 179, 1–39. [Google Scholar]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef]
- Raz, N.; Granovsky, Y.; Defrin, R. Investigating the neural processing of spatial summation of pain: The role of A-delta nociceptors. Exp. Brain Res. 2015, 233, 405–413. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef]
- Emery, E.C.; Wood, J.N. Somatosensation a la mode: Plasticity and polymodality in sensory neurons. Curr. Opin. Physiol. 2019, 11, 29–34. [Google Scholar] [CrossRef]
- Kupari, J.; Ernfors, P. Molecular taxonomy of nociceptors and pruriceptors. Pain 2023, 164, 1245–1257. [Google Scholar] [CrossRef]
- Tracey, W.D., Jr. Nociception. Curr. Biol. 2017, 27, R129–R133. [Google Scholar] [CrossRef]
- Giniatullin, R. Ion Channels of Nociception. Int. J. Mol. Sci. 2020, 21, 3553. [Google Scholar] [CrossRef]
- Huang, J.; Korsunsky, A.; Yazdani, M.; Chen, J. Targeting TRP channels: Recent advances in structure, ligand binding, and molecular mechanisms. Front. Mol. Neurosci. 2024, 16, 1334370. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.; Parpaite, T.; Coste, B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron 2022, 110, 2713–2727. [Google Scholar] [CrossRef] [PubMed]
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The Form and Function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534. [Google Scholar] [CrossRef]
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 2022, 13, 816149. [Google Scholar] [CrossRef]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Burnstock, G. Purinergic Mechanisms and Pain. Adv. Pharmacol. 2016, 75, 91–137. [Google Scholar]
- Docherty, R.J.; Farmer, C.E. The pharmacology of voltage-gated sodium channels in sensory neurones. Handb. Exp. Pharmacol. 2009, 194, 519–561. [Google Scholar]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The Na(V)1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef]
- Heinle, J.W.; Dalessio, S.; Janicki, P.; Ouyang, A.; Vrana, K.E.; Ruiz-Velasco, V.; Coates, M.D. Insights into the voltage-gated sodium channel, NaV1.8, and its role in visceral pain perception. Front. Pharmacol. 2024, 15, 1398409. [Google Scholar] [CrossRef]
- Zhang, X.; Hartung, J.E.; Gold, M.S. Persistent (Na v 1.9) sodium currents in human dorsal root ganglion neurons. Pain 2025, 166, 448–459. [Google Scholar] [CrossRef]
- Correa, B.H.M.; Moreira, C.R.; Hildebrand, M.E.; Vieira, L.B. The Role of Voltage-Gated Calcium Channels in Basal Ganglia Neurodegenerative Disorders. Curr. Neuropharmacol. 2023, 21, 183–201. [Google Scholar] [CrossRef]
- Tan, S.; Faull, R.L.M.; Curtis, M.A. The tracts, cytoarchitecture, and neurochemistry of the spinal cord. Anat. Rec. (Hoboken) 2023, 306, 777–819. [Google Scholar] [CrossRef]
- Willis, W.D. Nociceptive pathways: Anatomy and physiology of nociceptive ascending pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 308, 253–270. [Google Scholar]
- Yarmolinsky, D.A.; Zeng, X.; MacKinnon-Booth, N.; Greene, C.A.; Kim, C.; Cheng, Y.T.; Lenfers Turnes, B.; Woolf, C.J. Differential modification of ascending spinal outputs in acute and chronic pain states. Neuron 2025, 113, 1223–1239.e5. [Google Scholar] [CrossRef]
- Pace, M.C.; Passavanti, M.B.; De Nardis, L.; Bosco, F.; Sansone, P.; Pota, V.; Barbarisi, M.; Palagiano, A.; Iannotti, F.A.; Panza, E.; et al. Nociceptor plasticity: A closer look. J. Cell Physiol. 2018, 233, 2824–2838. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Hanč, P.; Messou, M.A.; Ajit, J.; von Andrian, U.H. Setting the tone: Nociceptors as conductors of immune responses. Trends Immunol. 2024, 45, 783–798. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- García-Domínguez, M. The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life 2025, 15, 785. [Google Scholar] [CrossRef]
- Cheng, J.K.; Ji, R.R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem. Res. 2008, 33, 1970–1978. [Google Scholar] [CrossRef]
- Mizumura, K.; Murase, S. Role of nerve growth factor in pain. Handb. Exp. Pharmacol. 2015, 227, 57–77. [Google Scholar]
- von Hehn, C.A.; Baron, R.; Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012, 73, 638–652. [Google Scholar] [CrossRef]
- Jain, A.; Gyori, B.M.; Hakim, S.; Jain, A.; Sun, L.; Petrova, V.; Bhuiyan, S.A.; Zhen, S.; Wang, Q.; Kawaguchi, R.; et al. Nociceptor-immune interactomes reveal insult-specific immune signatures of pain. Nat. Immunol. 2024, 25, 1296–1305. [Google Scholar] [CrossRef]
- Hore, Z.; Denk, F. Neuroimmune interactions in chronic pain—An interdisciplinary perspective. Brain Behav. Immun. 2019, 79, 56–62. [Google Scholar] [CrossRef]
- St John Smith, E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, V.; Ringat, T.; Jurth, C.; Lichtner, G.; von Dincklage, F. Performance of the Nociception Level Index and the PainSensor to predict and detect responsiveness to nociceptive procedures in critical care patients. J. Crit. Care 2025, 88, 155090. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Walters, E.T. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: An adaptive-maladaptive hyperfunctional state hypothesis. Front. Physiol. 2012, 3, 309. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.C.; Willis, D.E. What makes a RAG regeneration associated? Front. Mol. Neurosci. 2015, 8, 43. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Snavely, A.; Barrett, L.B.; Zhang, X.; Herman, C.; Frost, D.J.; Riva, P.; Tochitsky, I.; Kawaguchi, R.; Singh, B.; et al. Topoisomerase I inhibition and peripheral nerve injury induce DNA breaks and ATF3-associated axon regeneration in sensory neurons. Cell. Rep. 2021, 36, 109666. [Google Scholar] [CrossRef]
- Starkey, M.L.; Davies, M.; Yip, P.K.; Carter, L.M.; Wong, D.J.; McMahon, S.B.; Bradbury, E.J. Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury. J. Comp. Neurol. 2009, 513, 51–68. [Google Scholar] [CrossRef]
- Ondarza, A.B.; Ye, Z.; Hulsebosch, C.E. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: Colocalization of GAP-43 and CGRP. Exp. Neurol. 2003, 184, 373–380. [Google Scholar] [CrossRef]
- Son, S.J.; Lee, K.M.; Jeon, S.M.; Park, E.S.; Park, K.M.; Cho, H.J. Activation of transcription factor c-jun in dorsal root ganglia induces VIP and NPY upregulation and contributes to the pathogenesis of neuropathic pain. Exp. Neurol. 2007, 204, 467–472. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in Axon Regeneration: Implications for the Treatment of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 567537. [Google Scholar] [CrossRef]
- Whitfield, J.; Neame, S.J.; Paquet, L.; Bernard, O.; Ham, J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 2001, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Herschkovitz, A.; Ben-Dror, I.; Holdengreber, V.; Ben-Shaul, Y.; Seger, R.; Vardimon, L. The cytoskeletal network controls c-Jun expression and glucocorticoid receptor transcriptional activity in an antagonistic and cell-type-specific manner. Mol. Cell. Biol. 1999, 19, 1742–1750. [Google Scholar] [CrossRef]
- Ruff, C.A.; Staak, N.; Patodia, S.; Kaswich, M.; Rocha-Ferreira, E.; Da Costa, C.; Brecht, S.; Makwana, M.; Fontana, X.; Hristova, M.; et al. Neuronal c-Jun is required for successful axonal regeneration, but the effects of phosphorylation of its N-terminus are moderate. J. Neurochem. 2012, 121, 607–618. [Google Scholar] [CrossRef]
- Bonilla, I.E.; Tanabe, K.; Strittmatter, S.M. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 2002, 22, 1303–1315. [Google Scholar] [CrossRef]
- Schäfer, M.K.; Frotscher, M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012, 349, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Quartu, M.; Serra, M.P.; Boi, M.; Ibba, V.; Melis, T.; Del Fiacco, M. Polysialylated-neural cell adhesion molecule (PSA-NCAM) in the human trigeminal ganglion and brainstem at prenatal and adult ages. BMC Neurosci. 2008, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, I.; Sah, S.; Keable, R.; Leshchyns’ka, I.; Janitz, M.; Sytnyk, V. Cell Adhesion Molecules and Protein Synthesis Regulation in Neurons. Front. Mol. Neurosci. 2020, 13, 592126. [Google Scholar] [CrossRef]
- Spiegel, I.; Adamsky, K.; Eisenbach, M.; Eshed, Y.; Spiegel, A.; Mirsky, R.; Scherer, S.S.; Peles, E. Identification of novel cell-adhesion molecules in peripheral nerves using a signal-sequence trap. Neuron Glia Biol. 2006, 2, 27–38. [Google Scholar] [CrossRef]
- Weledji, E.P.; Assob, J.C. The ubiquitous neural cell adhesion molecule (N-CAM). Ann. Med. Surg. (Lond.) 2014, 3, 77–81. [Google Scholar] [CrossRef]
- Pusey, M.A.; Pace, K.; Fascelli, M.; Linser, P.J.; Steindler, D.A.; Galileo, D.S. Ectopic expression of L1CAM ectodomain alters differentiation and motility, but not proliferation, of human neural progenitor cells. Int. J. Dev. Neurosci. 2019, 78, 49–64. [Google Scholar] [CrossRef]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, e2101329. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, G.; Hou, B.; Song, E.; Wen, J.; Ba, Y.; Zhu, D.; Wang, G.; Qin, F. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury. Front. Bioeng. Biotechnol. 2023, 11, 1133718. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Nodari, A.; Wrabetz, L.; Feltri, M.L. Role of integrins in peripheral nerves and hereditary neuropathies. Neuromol. Med. 2006, 8, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Santiago-Medina, M.; Gomez, T.M. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev. Neurobiol. 2011, 71, 901–923. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Leone, C.M.; Lenoir, C.; van den Broeke, E.N. Assessing signs of central sensitization: A critical review of physiological measures in experimentally induced secondary hyperalgesia. Eur. J. Pain 2025, 29, e4733. [Google Scholar] [CrossRef]
- Hu, G.; Huang, K.; Hu, Y.; Du, G.; Xue, Z.; Zhu, X.; Fan, G. Single-cell RNA-seq reveals distinct injury responses in different types of DRG sensory neurons. Sci. Rep. 2016, 6, 31851. [Google Scholar] [CrossRef]
- Cooper, A.H.; Barry, A.M.; Chrysostomidou, P.; Lolignier, R.; Wang, J.; Redondo Canales, M.; Titterton, H.F.; Bennett, D.L.; Weir, G.A. Peripheral nerve injury results in a biased loss of sensory neuron subpopulations. Pain 2024, 165, 2863–2876. [Google Scholar] [CrossRef]
- Bolívar, S.; Sanz, E.; Ovelleiro, D.; Zochodne, D.W.; Udina, E. Neuron-specific RNA-sequencing reveals different responses in peripheral neurons after nerve injury. eLife 2024, 12, RP91316. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Zamponi, G.W. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nat. Neurosci. 2014, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Kocsis, J.D.; Black, J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 1994, 72, 466–470. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, Q.; Shao, J.; Zhang, J.; Li, L.; Ren, X.; Su, S.; Bai, Q.; Li, M.; Chen, X.; et al. MicroRNA-182 Alleviates Neuropathic Pain by Regulating Nav1.7 Following Spared Nerve Injury in Rats. Sci. Rep. 2018, 8, 16750. [Google Scholar] [CrossRef] [PubMed]
- Thakor, D.K.; Lin, A.; Matsuka, Y.; Meyer, E.M.; Ruangsri, S.; Nishimura, I.; Spigelman, I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol. Pain 2009, 5, 14. [Google Scholar] [CrossRef]
- Sidaway, P. Pain: Gain-of-function Nav1.9 mutations are associated with painful peripheral neuropathy. Nat. Rev. Neurol. 2014, 10, 306. [Google Scholar] [CrossRef]
- Fan, L.; Guan, X.; Wang, W.; Zhao, J.Y.; Zhang, H.; Tiwari, V.; Hoffman, P.N.; Li, M.; Tao, Y.X. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol. Pain 2014, 10, 8. [Google Scholar] [CrossRef]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621–640. [Google Scholar] [CrossRef]
- Yang, J.; Xie, M.X.; Hu, L.; Wang, X.F.; Mai, J.Z.; Li, Y.Y.; Wu, N.; Zhang, C.; Li, J.; Pang, R.P.; et al. Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav. Immun. 2018, 71, 52–65. [Google Scholar] [CrossRef]
- Antunes, F.T.T.; Campos, M.M.; Carvalho, V.P.R.; da Silva Junior, C.A.; Magno, L.A.V.; de Souza, A.H.; Gomez, M.V. Current Drug Development Overview: Targeting Voltage-Gated Calcium Channels for the Treatment of Pain. Int. J. Mol. Sci. 2023, 24, 9223. [Google Scholar] [CrossRef]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Lüscher, C.; Mahn, M.; Pan, Z.H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2022, 2, 55. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Liang, W.; Li, Y.; Zou, Z.; Xie, Y.; Liao, Y.; Yu, L.; Lin, Q.; Huang, M.; et al. The Roles of Optogenetics and Technology in Neurobiology: A Review. Front. Aging Neurosci. 2022, 14, 867863. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Gereau, R.W. 4th. A bright future? Optogenetics in the periphery for pain research and therapy. Pain 2018, 159 (Suppl. 1), S65–S73. [Google Scholar] [CrossRef]
- Daou, I.; Beaudry, H.; Ase, A.R.; Wieskopf, J.S.; Ribeiro-da-Silva, A.; Mogil, J.S.; Séguéla, P. Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro 2016, 3, ENEURO.0140-15.2016. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Kouroki, S.; Kurogi, M.; Hidaka, K.; Koshida, T.; Miura, A.; Nakagawa, H.; Yanagita, T.; Takeya, R.; Tsuneyoshi, I. Comparison of Nocifensive Behavior in NaV1.7-, NaV1.8-, and NaV1.9-Channelrhodopsin-2 Mice by Selective Optogenetic Activation of Targeted Sodium Channel Subtype-Expressing Afferents. J. Neurosci. Res. 2024, 102, e25386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Campbell, E.J.; Marchant, N.J. The use of chemogenetics in behavioural neuroscience: Receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018, 175, 994–1003. [Google Scholar] [CrossRef]
- Poth, K.M.; Texakalidis, P.; Boulis, N.M. Chemogenetics: Beyond Lesions and Electrodes. Neurosurgery 2021, 89, 185–195. [Google Scholar] [CrossRef]
- Hsiao, I.H.; Yen, C.M.; Hsu, H.C.; Liao, H.Y.; Lin, Y.W. Chemogenetics Modulation of Electroacupuncture Analgesia in Mice Spared Nerve Injury-Induced Neuropathic Pain through TRPV1 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 1771. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef]
- García-Domínguez, M. NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2025, 47, 93. [Google Scholar] [CrossRef]
- Merighi, A. Brain-Derived Neurotrophic Factor, Nociception, and Pain. Biomolecules 2024, 14, 539. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflamm. 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Sugiura, T.; Katanosaka, K.; Banik, R.K.; Kozaki, Y. Excitation and sensitization of nociceptors by bradykinin: What do we know? Exp. Brain Res. 2009, 196, 53–65. [Google Scholar] [CrossRef]
- Kanngiesser, M.; Häussler, A.; Myrczek, T.; Küsener, N.; Lim, H.Y.; Geisslinger, G.; Niederberge, E.; Tegeder, I. Inhibitor kappa B kinase beta dependent cytokine upregulation in nociceptive neurons contributes to nociceptive hypersensitivity after sciatic nerve injury. J. Pain 2012, 13, 485–497. [Google Scholar] [CrossRef]
- Melemedjian, O.K.; Tillu, D.V.; Moy, J.K.; Asiedu, M.N.; Mandell, E.K.; Ghosh, S.; Dussor, G.; Price, T.J. Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol. Pain 2014, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Patodia, S.; Raivich, G. Role of transcription factors in peripheral nerve regeneration. Front. Mol. Neurosci. 2012, 5, 8. [Google Scholar] [CrossRef]
- Espinoza, N.; Papadopoulos, V. Role of Mitochondrial Dysfunction in Neuropathy. Int. J. Mol. Sci. 2025, 26, 3195. [Google Scholar] [CrossRef]
- Lim, T.K.; Rone, M.B.; Lee, S.; Antel, J.P.; Zhang, J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol. Pain 2015, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Silva Santos Ribeiro, P.; Willemen, H.L.D.M.; Eijkelkamp, N. Mitochondria and sensory processing in inflammatory and neuropathic pain. Front. Pain Res. (Lausanne) 2022, 3, 1013577. [Google Scholar] [CrossRef] [PubMed]
- Inceoglu, B.; Bettaieb, A.; Trindade da Silva, C.A.; Lee, K.S.; Haj, F.G.; Hammock, B.D. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc. Natl. Acad. Sci. USA 2015, 112, 9082–9087. [Google Scholar] [CrossRef]
- Oñate, M.; Court, F.A.; Hetz, C. Bursting the unfolded protein response accelerates axonal regeneration. Neural Regen. Res. 2016, 11, 892–893. [Google Scholar]
- Ohtake, Y.; Matsuhisa, K.; Kaneko, M.; Kanemoto, S.; Asada, R.; Imaizumi, K.; Saito, A. Axonal Activation of the Unfolded Protein Response Promotes Axonal Regeneration Following Peripheral Nerve Injury. Neuroscience 2018, 375, 34–48. [Google Scholar] [CrossRef]
- Perner, C.; Krüger, E. Endoplasmic Reticulum Stress and Its Role in Homeostasis and Immunity of Central and Peripheral Neurons. Front. Immunol. 2022, 13, 859703. [Google Scholar] [CrossRef] [PubMed]
- Meza-Menchaca, T.; Albores-Medina, A.; Heredia-Mendez, A.J.; Ruíz-May, E.; Ricaño-Rodríguez, J.; Gallegos-García, V.; Esquivel, A.; Vettoretti-Maldonado, G.; Campos-Parra, A.D. Revisiting Epigenetics Fundamentals and Its Biomedical Implications. Int. J. Mol. Sci. 2024, 25, 7927. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Cho, Y. Epigenetic Regulation of Axon Regeneration after Neural Injury. Mol. Cells 2017, 40, 10–16. [Google Scholar] [CrossRef]
- Zhang, W.; Jiao, B.; Yu, S.; Zhang, C.; Zhang, K.; Liu, B.; Zhang, X. Histone deacetylase as emerging pharmacological therapeutic target for neuropathic pain: From epigenetic to selective drugs. CNS Neurosci. Ther. 2024, 30, e14745. [Google Scholar] [CrossRef]
- Gölzenleuchter, M.; Kanwar, R.; Zaibak, M.; Al Saiegh, F.; Hartung, T.; Klukas, J.; Smalley, R.L.; Cunningham, J.M.; Figueroa, M.E.; Schroth, G.P.; et al. Plasticity of DNA methylation in a nerve injury model of pain. Epigenetics 2015, 10, 200–212. [Google Scholar] [CrossRef]
- Liang, L.; Gu, X.; Zhao, J.Y.; Wu, S.; Miao, X.; Xiao, J.; Mo, K.; Zhang, J.; Lutz, B.M.; Bekker, A.; et al. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci. Rep. 2016, 6, 37704. [Google Scholar] [CrossRef]
- Pollema-Mays, S.L.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Expression of DNA methyltransferases in adult dorsal root ganglia is cell-type specific and up regulated in a rodent model of neuropathic pain. Front. Cell Neurosci. 2014, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Chamessian, A.G.; Qadri, Y.J.; Cummins, M.; Hendrickson, M.; Berta, T.; Buchheit, T.; Van de Ven, T. 5-Hydroxymethylcytosine (5hmC) and Ten-eleven translocation 1-3 (TET1-3) proteins in the dorsal root ganglia of mouse: Expression and dynamic regulation in neuropathic pain. Somatosens. Mot. Res. 2017, 34, 72–79. [Google Scholar] [CrossRef]
- Nagata, K.; Hama, I.; Kiryu-Seo, S.; Kiyama, H. microRNA-124 is down regulated in nerve-injured motor neurons and it potentially targets mRNAs for KLF6 and STAT3. Neuroscience 2014, 256, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Suzuki, H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem. Biophys. Res. Commun. 2013, 435, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, Z.; Zhang, H.; Atianjoh, F.E.; Zhao, J.Y.; Liang, L.; Wang, W.; Guan, X.; Kao, S.C.; Tiwari, V.; et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 2013, 16, 1024–1031. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Liu, W.; Zhang, J.; Ohgi, K.A.; Grinstein, J.D.; Dorrestein, P.C.; Rosenfeld, M.G. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 2011, 147, 773–788. [Google Scholar] [CrossRef]
- Pinto-Costa, R.; Sousa, S.C.; Leite, S.C.; Nogueira-Rodrigues, J.; Ferreira da Silva, T.; Machado, D.; Marques, J.; Costa, A.C.; Liz, M.A.; Bartolini, F.; et al. Profilin 1 delivery tunes cytoskeletal dynamics toward CNS axon regeneration. J. Clin. Investig. 2020, 130, 2024–2040. [Google Scholar] [CrossRef]
- Hur, E.M.; Saijilafu; Zhou, F.Q. Growing the growth cone: Remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 2012, 35, 164–174. [Google Scholar] [CrossRef]
- Takei, Y.; Teng, J.; Harada, A.; Hirokawa, N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 2000, 150, 989–1000. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Govek, E.E.; Van Aelst, L. Regulators of Rho GTPases in neuronal development. J. Neurosci. 2006, 26, 10633–10635. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Shinoda, M. Role of neuron and non-neuronal cell communication in persistent orofacial pain. J. Dent. Anesth. Pain Med. 2019, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Avraham, O.; Deng, P.Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020, 11, 4891. [Google Scholar] [CrossRef]
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. How Is Peripheral Injury Signaled to Satellite Glial Cells in Sensory Ganglia? Cells 2022, 11, 512. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, Y.; Da, X.; Wang, Y.; Chen, Z.; Xu, C. Satellite glial cells in sensory ganglia play a wider role in chronic pain via multiple mechanisms. Neural Regen. Res. 2024, 19, 1056–1063. [Google Scholar] [CrossRef]
- Retamal, M.A.; Riquelme, M.A.; Stehberg, J.; Alcayaga, J. Connexin43 Hemichannels in Satellite Glial Cells, Can They Influence Sensory Neuron Activity? Front. Mol. Neurosci. 2017, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Shinoda, M.; Honda, K.; Unno, S.; Shimizu, N.; Iwata, K. Connexin 43 contributes to ectopic orofacial pain following inferior alveolar nerve injury. Mol. Pain 2016, 12, 1744806916633704. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Reichling, D.B.; Levine, J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009, 32, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Garraway, S.M. A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain. Neurobiol. Pain 2024, 15, 100151. [Google Scholar] [CrossRef] [PubMed]
- Renthal, W.; Tochitsky, I.; Yang, L.; Cheng, Y.C.; Li, E.; Kawaguchi, R.; Geschwind, D.H.; Woolf, C.J. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020, 108, 128–144.e9. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.Y.; Li, Z.G.; Guan, L.X.; Deng, L.X. Transcriptional and Epigenetic Regulation in Injury-Mediated Neuronal Dendritic Plasticity. Neurosci. Bull. 2017, 33, 85–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, S.L.; Reid, A.J.; Verkhratsky, A.; Magnaghi, V.; Faroni, A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: Roles in inflammation, cell death and nociception. Neural Regen. Res. 2019, 14, 939–947. [Google Scholar]
- Jung, M.; Dourado, M.; Maksymetz, J.; Jacobson, A.; Laufer, B.I.; Baca, M.; Foreman, O.; Hackos, D.H.; Riol-Blanco, L.; Kaminker, J.S. Cross-species transcriptomic atlas of dorsal root ganglia reveals species-specific programs for sensory function. Nat. Commun. 2023, 14, 366. [Google Scholar] [CrossRef]
- Hetman, M.; Pietrzak, M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 2012, 35, 305–314. [Google Scholar] [CrossRef]
- Hetman, M.; Slomnicki, L.P. Ribosomal biogenesis as an emerging target of neurodevelopmental pathologies. J. Neurochem. 2019, 148, 325–347. [Google Scholar] [CrossRef]
- Chen, X.; Tang, S.J. Neural Circuitry Polarization in the Spinal Dorsal Horn (SDH): A Novel Form of Dysregulated Circuitry Plasticity during Pain Pathogenesis. Cells 2024, 13, 398. [Google Scholar] [CrossRef]
- Ackery, A.D.; Norenberg, M.D.; Krassioukov, A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord 2007, 45, 678–686. [Google Scholar] [CrossRef]
- Li, J.; Baccei, M.L. Neonatal tissue damage facilitates nociceptive synaptic input to the developing superficial dorsal horn via NGF-dependent mechanisms. Pain 2011, 152, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Terayama, R.; Uchibe, K. Reorganization of synaptic inputs to spinal dorsal horn neurons in neuropathic pain. Int. J. Neurosci. 2022, 132, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D.; Sankarasubramanian, V.; Lempka, S.F. Dorsal Root Ganglion Stimulation for Chronic Pain: Hypothesized Mechanisms of Action. J. Pain 2022, 23, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.; Moore, S.; Artun, D.; Madhavan, A.; Harte, E.; Torres-Pérez, J.V.; Nagy, I. Transcriptional reprogramming post-peripheral nerve injury: A systematic review. Neurobiol. Dis. 2024, 200, 106624. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Zhu, L.; Jin, H.; Kang, X.; Feng, Z. Single-cell RNA sequencing in the context of neuropathic pain: Progress, challenges, and prospects. Transl. Res. 2023, 251, 96–103. [Google Scholar] [CrossRef]
- Stenz, L.; Carré, J.L.; Luthi, F.; Vuistiner, P.; Burrus, C.; Paoloni-Giacobino, A.; Léger, B. Genome-Wide Epigenomic Analyses in Patients With Nociceptive and Neuropathic Chronic Pain Subtypes Reveals Alterations in Methylation of Genes Involved in the Neuro-Musculoskeletal System. J. Pain 2022, 23, 326–336. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ji, R.R. Gene therapy for chronic pain management. Cell Rep. Med. 2024, 5, 101756. [Google Scholar] [CrossRef]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Mika, J. Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain. Molecules 2023, 28, 5766. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Vasilchuk, A.G.; Voronina, T.A. Protein Kinase A Inhibitor Attenuates the Antinociceptive Effect of NMDA-Receptor Channel Antagonists in the Capsaicin Test in Mice. Bull. Exp. Biol. Med. 2024, 177, 231–234. [Google Scholar] [CrossRef]
- Géranton, S.M. Targeting epigenetic mechanisms for pain relief. Curr. Opin. Pharmacol. 2012, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Ligon, C.O.; Johnson, A.C.; Greenwood-Van Meerveld, B. Targeting epigenetic mechanisms for chronic visceral pain: A valid approach for the development of novel therapeutics. Neurogastroenterol. Motil. 2019, 31, e13500. [Google Scholar] [CrossRef]
- Wolfe, D.; Mata, M.; Fink, D.J. Targeted drug delivery to the peripheral nervous system using gene therapy. Neurosci. Lett. 2012, 527, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Jayakar, S.; Shim, J.; Jo, S.; Bean, B.P.; Singeç, I.; Woolf, C.J. Developing nociceptor-selective treatments for acute and chronic pain. Sci. Transl. Med. 2021, 13, eabj9837. [Google Scholar] [CrossRef]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal. Transduct. Target Ther. 2024, 9, 155. [Google Scholar] [PubMed]
- Rijsdijk, M.; Smits, H.M.; Azizoglu, H.R.; Brugman, S.; van de Burgt, Y.; van Charldorp, T.C.; van Gelder, D.J.; de Grauw, J.C.; van Lange, E.A.; Meye, F.J.; et al. Identifying patient subgroups in the heterogeneous chronic pain population using cluster analysis. J. Pain 2025, 28, 104792. [Google Scholar] [CrossRef]
- Tracey, I.; Woolf, C.J.; Andrews, N.A. Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron 2019, 101, 783–800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. Injury-Driven Structural and Molecular Modifications in Nociceptors. Biology 2025, 14, 788. https://doi.org/10.3390/biology14070788
García-Domínguez M. Injury-Driven Structural and Molecular Modifications in Nociceptors. Biology. 2025; 14(7):788. https://doi.org/10.3390/biology14070788
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "Injury-Driven Structural and Molecular Modifications in Nociceptors" Biology 14, no. 7: 788. https://doi.org/10.3390/biology14070788
APA StyleGarcía-Domínguez, M. (2025). Injury-Driven Structural and Molecular Modifications in Nociceptors. Biology, 14(7), 788. https://doi.org/10.3390/biology14070788



