The Role of TGF-β Signaling Pathway in Determining Small Ruminant Litter Size
Simple Summary
Abstract
1. Introduction
2. Methodology for Literature Search
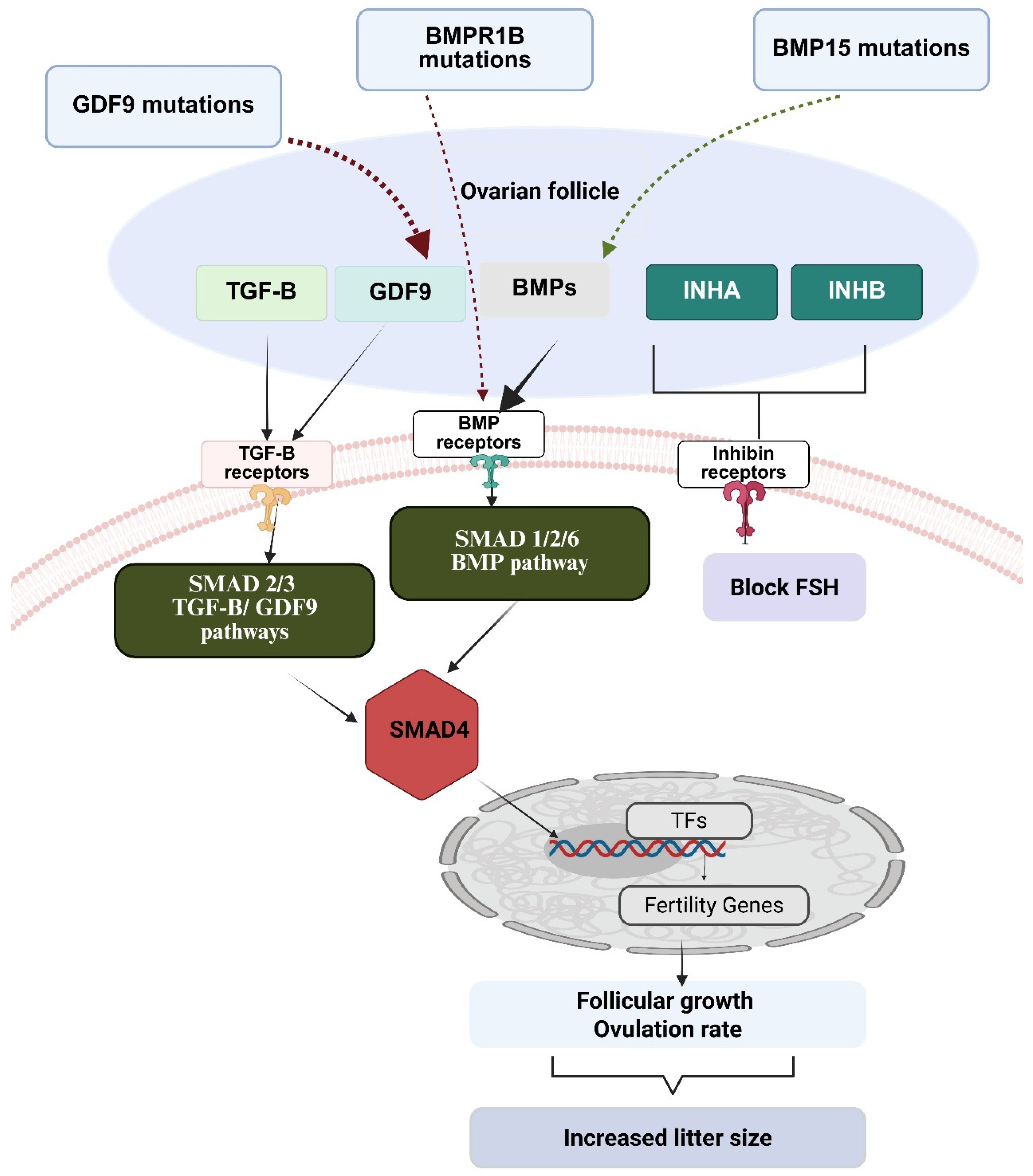
3. The TGF-β Superfamily Genes Role in Sheep and Goats Litter Size
3.1. Association of BMP Family Genes and GDF9 with Litter Size in Goats
3.2. Role of BMP Family Genes and GDF9 in Litter Size in Sheep
3.3. Effect of Inhibins, SMAD Family Genes, and AMH on Litter Size in Goats and Sheep
3.3.1. The AMH and Reproductive Performance
3.3.2. Role of SMAD Family Genes in Reproductive Regulation
3.3.3. Inhibins’ Role in Follicular Development and Litter Size
| Gene | SNP/Variant | Effect | Breed | Country | References |
|---|---|---|---|---|---|
| SMAD1 | g.10729C>T | CC genotype produced significantly higher litter sizes than CT genotype | Tibetan sheep | China | [7] |
| SMAD3 | g.21447C>T | TT genotype showed higher litter sizes than CC and CT genotypes | Tibetan sheep | China | [7] |
| SMAD1 | rs406357666 | Higher expression in high-prolificacy sheep | Hu sheep | China | [10] |
| AMH | g.89172108T>G | TT genotype exhibited higher litter size compared to TG and GG genotypes | Chuanzhong black goats | China | [75] |
| AMH | g.89172108A>C | CC genotype associated with higher litter size compared to AC genotype | Dazu black goats | China | [77] |
| INHB | rs412280524rs429836421 | Associated with variation in litter size | Icelandic and Finn sheep | China | [86] |
| SMAD1 | Activates BMP signaling pathway associated with accelerated ovulation | Malpura sheep | India | [87] | |
| SMAD2 and SMAD1 | Associated with litter size | Shaanbei white cashmere | China | [91,92,93] | |
| INHA | g.28317663A>C | AC genotype associated with significantly higher litter size than AA genotype | Hainan black goats | China | [101] |
| INHA | g.3234C>T | CT genotype associated with significantly larger litter size compared to CC genotype | West African dwarf goats | Nigeria | [103] |
| INHA | Target of miR-134-3p regulation affecting follicular development through TGF- TGF-β/PI3K/AKT/mTOR pathway in GCs | Sheep granulosa cells (GCs) | China | [107] | |
| INHA | g.236311367G>A | GA genotype had significantly higher litter size than AA or GG genotype | Thin-tailed sheep | Indonesia | [108] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abuzahra, M.; Wijayanti, D.; Effendi, M.H.; Mustofa, I.; Munyaneza, J.P.; Moses, I.B. Improved Litter Size in Thin-Tailed Indonesian Sheep Through Analysis of TGIF1 Gene Polymorphisms. Vet. Med. Int. 2025, 2025, 7778088. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Han, Y.; Chen, Y.; Liu, X.; Liang, H.; Wang, C.; Khan, M.Z. Potential Candidate Genes Associated with Litter Size in Goats: A Review. Animals 2025, 15, 82. [Google Scholar] [CrossRef]
- Tao, L.; He, X.; Wang, X.; Di, R.; Chu, M. Litter size of sheep (Ovis aries): Inbreeding depression and homozygous regions. Genes 2021, 12, 109. [Google Scholar] [CrossRef]
- Wang, W.; La, Y.; Zhou, X.; Zhang, X.; Li, F.; Liu, B. The genetic polymorphisms of TGFβ superfamily genes are associated with litter size in a Chinese indigenous sheep breed (Hu sheep). Anim. Reprod. Sci. 2018, 189, 19–29. [Google Scholar] [CrossRef]
- Ren, S.; Liu, Y.; Guo, Y.; Zhao, Z.; Cui, J.; Li, M.; Wang, J. TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep. Int. J. Mol. Sci. 2025, 26, 1961. [Google Scholar] [CrossRef] [PubMed]
- Fountas, S.; Petinaki, E.; Bolaris, S.; Kargakou, M.; Dafopoulos, S.; Zikopoulos, A.; Moustakli, E.; Sotiriou, S.; Dafopoulos, K. The Roles of GDF-9, BMP-15, BMP-4, and EMMPRIN in Folliculogenesis and In Vitro Fertilization. J. Clin. Med. 2024, 13, 3775. [Google Scholar] [CrossRef]
- Li, M.; He, N.; Sun, R.; Deng, Y.; Wen, X.; Zhang, J. Expression and polymorphisms of SMAD1, SMAD2 and SMAD3 genes and their association with litter size in Tibetan sheep (Ovis aries). Genes 2022, 13, 2307. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rajput, P.K.; Bahire, S.V.; Jyotsana, B.; Kumar, V.; Kumar, D. Differential expression of BMP/SMAD signaling and ovarian-associated genes in the granulosa cells of FecB introgressed GMM sheep. Syst. Biol. Reprod. Med. 2020, 66, 185–201. [Google Scholar] [CrossRef]
- Ahlawat, S.; Sharma, R.; Roy, M.; Tantia, M.S.; Prakash, V. Association Analysis of Novel SNPs in BMPR1B, BMP15 and GDF9 Genes with Reproductive Traits in Black Bengal Goats. Small Rumin. Res. 2015, 132, 92–98. [Google Scholar] [CrossRef]
- Xu, Y.; Li, E.; Han, Y.; Chen, L.; Xie, Z. Differential expression of mRNAs encoding BMP/Smad pathway molecules in antral follicles of high-and low-fecundity Hu sheep. Anim. Reprod. Sci. 2010, 120, 47–55. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Liu, Q.; Tang, J.; Di, R.; Chu, M. TGIF1 and SF1 polymorphisms are associated with litter size in Small Tail Han sheep. Reprod. Domest. Anim. 2020, 55, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, L.; He, X.; Di, R.; Wang, X.; Chu, M. Single-nucleotide polymorphisms in FLT3, NLRP5, and TGIF1 are associated with litter size in Small-tailed Han sheep. Arch. Anim. Breed. 2021, 64, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Sun, R.; He, N.; Wen, X.; Han, X.; Luo, Z. Molecular characterization and expression of TGFβRI and TGFβRII and its association with litter size in Tibetan sheep. Vet. Med. Sci. 2023, 9, 934–944. [Google Scholar] [CrossRef]
- Belli, M.; Shimasaki, S. Molecular Aspects and Clinical Relevance of GDF9 and BMP15 in Ovarian Function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar] [PubMed]
- Sharma, R.; Ahlawat, S.; Maitra, A.; Roy, M.; Mandakmale, S.; Tantia, M.S. Polymorphism of BMP4 Gene in Indian Goat Breeds Differing in Prolificacy. Gene. 2013, 532, 140–145. [Google Scholar] [CrossRef]
- Christoforou, E.R.; Pitman, J.L. Intrafollicular Growth Differentiation Factor 9: Bone Morphogenetic Protein 15 Ratio Determines Litter Size in Mammals. Biol. Reprod. 2019, 100, 1333–1343. [Google Scholar] [CrossRef]
- Paulini, F.; Melo, E.O. The Role of Oocyte-Secreted Factors GDF9 and BMP15 in Follicular Development and Oogenesis. Reprod. Domest. Anim. 2011, 46, 354–361. [Google Scholar] [CrossRef]
- Spicer, L.J.; Aad, P.Y.; Allen, D.T.; Mazerbourg, S.; Payne, A.H.; Hsueh, A.J. Growth Differentiation Factor 9 (GDF9) Stimulates Proliferation and Inhibits Steroidogenesis by Bovine Theca Cells: Influence of Follicle Size on Responses to GDF9. Biol. Reprod. 2008, 78, 243–253. [Google Scholar] [CrossRef]
- Kumar, A.; Nanda, R.; Samad, H.A.; Maurya, V.P.; Singh, G.; Chouhan, V.S. Transcriptional Profiling of GDF9 and ITS Signaling Receptors in Goat Granulosa and Theca Cells. Indian J. Small Rumin. 2024, 30, 41–45. [Google Scholar]
- Chu, M.X.; Lu, L.; Feng, T.; Di, R.; Cao, G.L.; Wang, P.Q.; Fang, L.; Ma, Y.H.; Li, K. Polymorphism of Bone Morphogenetic Protein 4 Gene and Its Relationship with Litter Size of Jining Grey Goats. Mol. Biol. Rep. 2011, 38, 4315–4320. [Google Scholar] [CrossRef]
- Abuzahra, M.; Abu Eid, L.; Effendi, M.H.; Mustofa, I.; Lamid, M.; Rehman, S. Polymorphism Studies and Candidate Genes Associated with Litter Size Traits in Indonesian Goats: A Systematic Review. F1000Research 2023, 12, 61. [Google Scholar] [CrossRef]
- Zergani, E.; Rashidi, A.; Rostamzadeh, J.; Razmkabir, M.; Tetens, J. Meta-Analysis of Association between c.963A>G Single-Nucleotide Polymorphism on BMP15 Gene and Litter Size in Goats. Arch. Anim. Breed. 2022, 65, 309–318. [Google Scholar] [CrossRef]
- Das, A.; Shaha, M.; Gupta, M.D.; Dutta, A.; Miazi, O.F. Polymorphism of Fecundity Genes (BMP15 and GDF9) and Their Association with Litter Size in Bangladeshi Prolific Black Bengal Goat. Trop. Anim. Health Prod. 2021, 53, 230. [Google Scholar] [CrossRef] [PubMed]
- Liandris, E.; Kominakis, A.; Andreadou, M.; Kapeoldassi, K.; Chadio, S.; Tsiligianni, T.; Gazouli, M.; Ikonomopoulos, I. Associations between Single Nucleotide Polymorphisms of GDF9 and BMP15 Genes and Litter Size in Two Dairy Sheep Breeds of Greece. Small Rumin. Res. 2012, 107, 16–21. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, N.; Wang, Z.; Bai, J. Polymorphism of Exon 2 of BMP15 Gene and Its Relationship with Litter Size of Two Chinese Goats. Asian-Australas. J. Anim. Sci. 2011, 24, 905–911. [Google Scholar] [CrossRef]
- Liang, C.; Han, M.; Zhou, Z.; Liu, Y.; He, X.; Jiang, Y.; Ouyang, Y.; Hong, Q.; Chu, M. Hypothalamic Transcriptome Analysis Reveals the Crucial MicroRNAs and mRNAs Affecting Litter Size in Goats. Front. Vet. Sci. 2021, 8, 747100. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Bai, W.L.; Zheng, Y.Y.; Hui, T.Y.; Sun, J.M.; Guo, D.; Guo, S.L.; Wang, Z.Y. Correlation Analysis of Candidate Gene SNP for High-Yield in Liaoning Cashmere Goats with Litter Size and Cashmere Performance. Anim. Biotechnol. 2021, 32, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, B.; Morammazi, S. Polymorphism of GDF9 and BMPR1B Genes and Their Association with Litter Size in Markhoz Goats. Reprod. Domest. Anim. 2018, 53, 971–978. [Google Scholar] [CrossRef]
- Ahlawat, S.; Sharma, R.; Roy, M.; Mandakmale, S.; Prakash, V.; Tantia, M.S. Genotyping of Novel SNPs in BMPR1B, BMP15, and GDF9 Genes for Association with Prolificacy in Seven Indian Goat Breeds. Anim. Biotechnol. 2016, 27, 199–207. [Google Scholar] [CrossRef]
- An, X.P.; Hou, J.X.; Zhao, H.B.; Li, G.; Bai, L.; Peng, J.Y.; Yan, M.Q.; Song, Y.X.; Wang, J.G.; Cao, B.Y. Polymorphism Identification in Goat GNRH1 and GDF9 Genes and Their Association Analysis with Litter Size. Anim. Genet. 2013, 44, 234–238. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Wang, K.; Yan, H.; Pan, C.; Chen, H.; Liu, J.; Zhu, H.; Qu, L.; Lan, X. Two Strongly Linked Single Nucleotide Polymorphisms (Q320P and V397I) in GDF9 Gene Are Associated with Litter Size in Cashmere Goats. Theriogenology 2019, 125, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Shaha, M.; Miah, G.; Lima, A.; Miazi, O.F.; Gupta, M.D.; Das, A. Identification of Polymorphisms in GDF9 and BMP15 Genes in Jamunapari and Crossbred Goats in Bangladesh. Trop. Anim. Health Prod. 2022, 54, 350. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, P.; Rashidi, A.; Rostamzadeh, J.; Razmkabir, M. Association between c.1189G>A Single Nucleotide Polymorphism of GDF9 Gene and Litter Size in Goats: A Meta-Analysis. Anim. Reprod. Sci. 2019, 209, 106140. [Google Scholar] [CrossRef] [PubMed]
- Arefnejad, B.; Mehdizadeh, Y.; Javanmard, A.; Zamiri, M.J.; Niazi, A. Novel Single Nucleotide Polymorphisms (SNPs) in Two Oogenesis-Specific Genes (BMP15, GDF9) and Their Association with Litter Size in Markhoz Goat (Iranian Angora) Iran. J. Appl. Anim. Sci. 2018, 8, 91–99. [Google Scholar]
- Chu, M.X.; Wu, Z.H.; Feng, T.; Cao, G.L.; Fang, L.; Di, R.; Huang, D.W.; Li, X.W.; Li, N. Polymorphism of GDF9 Gene and Its Association with Litter Size in Goats. Vet. Res. Commun. 2011, 35, 329–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, T.; Zhang, N.; Chen, J.; Yang, H.; Peng, S.; Ma, R.; Wang, D.; Liu, Q.; et al. Association of BMP15 and GDF9 Gene Polymorphisms with Litter Size in Hu Sheep. Genes 2025, 16, 168. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Jiang, H.; Sun, L.; Guo, Z. Regulation of litter size in sheep (Ovis aries) by the GDF9 and BMP15 genes. Ann. Agric. Sci. 2023, 68, 148–158. [Google Scholar] [CrossRef]
- Tang, J.; Hu, W.; Di, R.; Liu, Q.; Wang, X.; Zhang, X.; Zhang, J.; Chu, M. Expression analysis of the prolific candidate genes, BMPR1B, BMP15, and GDF9 in Small Tail Han ewes with three fecundity (FecB gene) genotypes. Animals 2018, 8, 166. [Google Scholar] [CrossRef]
- Ji, X.; Cao, Z.; Hao, Q.; He, M.; Cang, M.; Yu, H.; Ma, Q.; Li, X.; Bao, S.; Wang, J.; et al. Effects of New Mutations in BMPRIB, GDF9, BMP15, LEPR, and B4GALNT2 Genes on Litter Size in Sheep. Vet. Sci. 2023, 10, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, Z.; Jia, S.; Zhao, S.; Cao, G.; Purev, C.; Cang, M.; Yu, H.; Li, X.; Bao, S.; et al. Effects of novel variants in BMP15 gene on litter size in Mongolia and Ujimqin sheep breeds. Theriogenology 2023, 198, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Liu, G.; Deng, M.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. Polymorphisms in BMPR-IB gene and their association with litter size trait in Chinese Hu sheep. Anim. Biotechnol. 2022, 33, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Kirikçi, K. Investigation of BMP15 and GDF9 gene polymorphisms and their effects on litter size in Anatolian sheep breed Akkaraman. Turk. J. Vet. Anim. Sci. 2023, 47, 248–254. [Google Scholar] [CrossRef]
- Akhatayeva, Z.; Cao, C.; Huang, Y.; Zhou, Q.; Zhang, Q.; Guo, Z.; Tan, S.; Yue, X.; Xu, H.; Li, R.; et al. Newly reported 90-bp deletion within the ovine BMPRIB gene: Does it widely distribute, link to the famous FecB (p. Q249R) mutation, and affect litter size? Theriogenology 2022, 189, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Guo, S.; Di, R.; Wang, X.; He, X.; Chu, M. Polymorphisms of the BMPR1B, BMP15 and GDF9 fecundity genes in four Chinese sheep breeds. Arch. Anim. Breed. 2024, 67, 51–60. [Google Scholar] [CrossRef]
- Cui, W.; Wang, H.; Li, J.; Lv, D.; Xu, J.; Liu, M.; Yin, G. Sheep litter size heredity basis using genome-wide selective analysis. Reprod. Domest. Anim. 2024, 59, e14689. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S.; Wu, K.; Zhang, M.; Alatan, S.; Cang, M.; Cao, G.; Jin, H.; Li, C.; Tong, B. The Impact of Novel BMPR1B Mutations on Litter Size in Short-Tailed Gobi Sheep and Larger-Tailed Ujimqin Sheep. Vet. Sci. 2024, 11, 297. [Google Scholar] [CrossRef]
- Li, Y.; Jin, W.; Wang, Y.; Zhang, J.; Meng, C.; Wang, H.; Qian, Y.; Li, Q.; Cao, S. Three complete linkage SNPs of GDF9 gene affect the litter size probably mediated by OCT1 in Hu sheep. DNA Cell Biol. 2020, 39, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, A.Z.; Musa, L.M.; Jawasreh, K.I.; Saleem, A.O.; El-Hag, F.; Ahmed, M.K. G1 point mutation in growth differentiation factor 9 gene affects litter size in Sudanese desert sheep. Vet. World 2021, 14, 104. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Q.; Gui, L.; Jin, J.; Li, Y.; Ru, Q.; Hou, S. Association of polymorphisms in bone morphogenetic protein receptor-1B gene exon-9 with litter size in Dorset, Mongolian, and Small Tail Han ewes. Asian-Australas. J. Anim. Sci. 2019, 32, 949. [Google Scholar] [CrossRef]
- Tang, J.S.; Hu, W.P.; Di, R.; Wang, X.Y.; Zhang, X.S.; Zhang, J.L.; Liu, Q.Y.; Chu, M.X. Expression analysis of BMPR1B, BMP15, and GDF9 in prolific and non-prolific sheep breeds during the follicular phase. Czech J. Anim. Sci. 2019, 64, 439–447. [Google Scholar] [CrossRef]
- Gorlov, I.F.; Kolosov, Y.A.; Shirokova, N.V.; Getmantseva, L.V.; Slozhenkina, M.I.; Mosolova, N.I.; Bakoev, N.F.; Leonova, M.A.; Kolosov, A.Y.; Zlobina, E.Y. GDF9 gene polymorphism and its association with litter size in two Russian sheep breeds. Rendiconti Lincei. Sci. Fis. E Nat. 2018, 29, 61–66. [Google Scholar]
- El-Halawany, N.; Kandil, O.M.; Abd-El-Monsif, A.S.; Al-Tohamy, A.F.; El-Sayd, Y.A.; Abdel-Shafy, H.; Abou-Fandoud, E.S.; Abdel-Azeem, S.N.; Abd El-Rahim, A.H.; Abdoon, A.S.; et al. Investigating the effect of GDF9, BMP15, BMP6 and BMPR1B polymorphisms on Egyptian sheep fecundity and their transcripts expression in ovarian cells. Small Rumin. Res. 2018, 165, 34–40. [Google Scholar] [CrossRef]
- Amini, H.R.; Ajaki, A.; Farahi, M.; Heidari, M.; Pirali, A.; Forouzanfar, M.; Eghbalsaied, S. The novel T755C mutation in BMP15 is associated with the litter size of Iranian Afshari, Ghezel, and Shal breeds. Arch. Anim. Breed. 2018, 61, 153–160. [Google Scholar] [CrossRef]
- Wang, S.; Bai, Y.; Wang, D.; Zhang, M.; Alatan, S.; Cang, M.; Jin, H.; Li, C.; Du, G.; Cao, G.; et al. Variants in BMP15 Gene Affect Promoter Activity and Litter Size in Gobi Short Tail and Ujimqin Sheep. Vet. Sci. 2025, 12, 222. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, Q.; Kang, Y.; Akhatayeva, Z.; Liu, P.; Bai, Y.; Li, R.; Jiang, Y.; Zhang, Q.; Lan, X.; et al. A repertoire of single nucleotide polymorphisms (SNPs) of major fecundity BMPR1B gene among 75 sheep breeds worldwide. Theriogenology 2024, 219, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, I.; Stancheva, N.; Bozhilova-Sakova, M.; Tzonev, T. Polymorphism in SNP G1 of the GDF9 gene associated with reproductive traits in Bulgarian dairy sheep. Biotechnol. Biotechnol. Equip. 2024, 38, 2399939. [Google Scholar] [CrossRef]
- Akhatayeva, Z.; Bi, Y.; He, Y.; Khan, R.; Li, J.; Li, H.; Pan, C.; Lan, X. Survey of the relationship between polymorphisms within the BMPR1B gene and sheep reproductive traits. Anim. Biotechnol. 2023, 34, 718–727. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Wang, Y.; Chen, X.; Li, F.; Yang, L.; Cui, J.; Li, R.; Cao, B.; An, X.; et al. FecB mutation and litter size are associated with a 90-base pair deletion in BMPR1B in East Friesian and Hu crossbred sheep. Anim. Biotechnol. 2023, 34, 1314–1323. [Google Scholar] [CrossRef]
- Mo, F.; Sun, W.; Zhang, L.; Zhang, X.; La, Y.; Xiao, F.; Jia, J.; Jin, J. Polymorphisms in BMPRIB gene affect litter size in Chinese indigenous sheep breed. Anim. Biotechnol. 2023, 34, 538–545. [Google Scholar] [CrossRef]
- Chong, Y.; Jiang, X.; Liu, G. An ancient positively selected BMPRIB missense variant increases litter size of Mongolian sheep populations following latitudinal gradient. Mol. Genet. Genom. 2022, 297, 155–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, Q.; Song, Y.; Zhang, H.; Jia, J. Evaluation of the BMPR-1B gene functional polymorphisms and their association with litter size in Qinghai Tibetan sheep. Small Rumin. Res. 2022, 216, 106816. [Google Scholar] [CrossRef]
- Çelikeloğlu, K.; Tekerli, M.; Erdoğan, M.; Koçak, S.; Hacan, Ö.; Bozkurt, Z. An investigation of the effects of BMPR1B, BMP15, and GDF9 genes on litter size in Ramlıç and Dağlıç sheep. Arch. Anim. Breed. 2021, 64, 223–230. [Google Scholar] [CrossRef]
- Di, R.; Wang, F.; Yu, P.; Wang, X.; He, X.; Mwacharo, J.M.; Pan, L.; Chu, M. Detection of novel variations related to litter size in BMP15 gene of luzhong mutton sheep (Ovis aries). Animals 2021, 11, 3528. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chu, M.; Pan, L.; Wang, X.; He, X.; Zhang, R.; Tao, L.; La, Y.; Ma, L.; Di, R. Polymorphism detection of GDF9 gene and its association with litter size in Luzhong mutton sheep (Ovis aries). Animals 2021, 11, 571. [Google Scholar] [CrossRef]
- Drobik-Czwarno, W.; Martyniuk, E.; Nowak-Życzyńska, Z.; Kaczor, U.; Kucharski, M. Frequency of BMP15 and GDF9 mutations increasing litter size and their phenotypic effects in Olkuska sheep population. Ann. Anim. Sci. 2021, 21, 89–108. [Google Scholar] [CrossRef]
- Najafabadi, H.A.; Khansefid, M.; Mahmoud, G.G.; Haruna, I.L.; Zhou, H.; Hickford, J.G. Identification of sequence variation in the oocyte-derived bone morphogenetic protein 15 (BMP15) gene (BMP15) associated with litter size in New Zealand sheep (Ovis aries) breeds. Mol. Biol. Rep. 2021, 48, 6335–6342. [Google Scholar] [CrossRef]
- Niu, Z.G.; Qin, J.; Jiang, Y.; Ding, X.D.; Ding, Y.G.; Tang, S.; Shi, H.C. The identification of mutation in BMP15 gene associated with litter size in Xinjiang cele black sheep. Animals 2021, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, Q.; Cang, M.; Wang, J.; Yu, H.; Liu, Y.; Zhang, W.; Tong, B. Association between novel variants in BMPR1B gene and litter size in Mongolia and Ujimqin sheep breeds. Reprod. Domest. Anim. 2021, 56, 1562–1571. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Akhatayeva, Z.; Liu, H.; Lin, C.; Han, X.; Lu, X.; Lan, X.; Zhang, Q.; Pan, C. Novel indel variations of the sheep FecB gene and their effects on litter size. Gene 2021, 767, 145176. [Google Scholar] [CrossRef]
- Wen, Y.L.; Guo, X.F.; Ma, L.; Zhang, X.S.; Zhang, J.L.; Zhao, S.G.; Chu, M.X. The expression and mutation of BMPR1B and its association with litter size in small-tail Han sheep (Ovis aries). Arch. Anim. Breed. 2021, 64, 211–221. [Google Scholar] [CrossRef]
- Tong, B.; Wang, J.; Cheng, Z.; Liu, J.; Wu, Y.; Li, Y.; Bai, C.; Zhao, S.; Yu, H.; Li, G. Novel variants in GDF9 gene affect promoter activity and litter size in Mongolia sheep. Genes 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Hammoud, M.H.; Dabour, N.A.; Hafez, E.E.; Sharaby, M.A. BMPR-1B, BMP-15 and GDF-9 genes structure and their relationship with litter size in six sheep breeds reared in Egypt. BMC Res. Notes 2020, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Amirpour Najafabadi, H.; Khansefid, M.; Mahmoud, G.G.; Zhou, H.; Hickford, J.G. Identification of polymorphisms in the oocyte-derived growth differentiation growth factor 9 (GDF9) gene associated with litter size in New Zealand sheep (Ovis aries) breeds. Reprod. Domest. Anim. 2020, 55, 1585–1591. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Di, R.; Hu, W.; Wang, X.; He, X.; Ma, L.; Chu, M. Single nucleotide polymorphisms in BMP2 and BMP7 and the association with litter size in Small Tail Han sheep. Anim. Reprod. Sci. 2019, 204, 183–192. [Google Scholar] [CrossRef]
- Guo, C.; Ye, J.; Liu, J.; Li, Z.; Deng, M.; Guo, Y.; Liu, G.; Sun, B.; Li, Y.; Liu, D. Whole-Genome Sequencing Identified Candidate Genes Associated with High and Low Litter Size in Chuanzhong Black Goats. Front. Vet. Sci. 2024, 11, 1420164. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. TGF-β Superfamily Members and Ovarian Follicle Development. Reproduction. 2006, 132, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Zeng, Y.; Huang, Y.F.; Huang, D.L.; Peng, P.; Na, R.S. A Nonsynonymous SNP within the AMH Gene Is Associated with Litter Size in Dazu Black Goats. Anim. Biotechnol. 2021, 33, 992–996. [Google Scholar] [CrossRef]
- Kharayat, N.S.; Parameshwarappa, M.A.; Ramdas, G.A.; Bisht, D.; Gautam, S.; Singh, S.K.; Krishnaswamy, N.; Chand, K.; Rialch, A.; Chandra, P.; et al. Anti-Müllerian Hormone Profile and Its Association with Ovarian Parameters in the Chaugarkha Goat. Small Rumin. Res. 2024, 230, 107165. [Google Scholar] [CrossRef]
- Monniaux, D.; Baril, G.; Laine, A.L.; Jarrier, P.; Poulin, N.; Cognié, J.; Fabre, S. Anti-Müllerian Hormone as a Predictive Endocrine Marker for Embryo Production in the Goat. Reproduction. 2011, 142, 845–854. [Google Scholar] [CrossRef]
- Turgut, A.O.; Koca, D. Anti-Müllerian Hormone as a Promising Novel Biomarker for Litter Size in Romanov Sheep. Reprod. Domest. Anim. 2024, 59, e14692. [Google Scholar] [CrossRef]
- Saneyasu, T.; Honda, K.; Kamisoyama, H. Myostatin Increases Smad2 Phosphorylation and Atrogin-1 Expression in Chick Embryonic Myotubes. J. Poult. Sci. 2019, 56, 224–230. [Google Scholar] [CrossRef]
- Villacorte, M.; Delmarcelle, A.-S.; Lernoux, M.; Bouquet, M.; Lemoine, P.; Bolsee, J.; Umans, L.; Lopes, S.C.d.S.; Van Der Smissen, P.; Sasaki, T.; et al. Thyroid Follicle Development Requires Smad1/Smad5- and Endothelial-Dependent Basement Membrane Assembly. Development. 2016, 134, 171. [Google Scholar]
- Weiss, A.; Attisano, L. The TGFβ Superfamily Signaling Pathway. WIREs Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, L.J.; Gupta, S.; Figueroa, M.E.; Mullins, M.C.; Evans, T. Smad1 and Smad5 Differentially Regulate Embryonic Hematopoiesis. Blood. 2007, 110, 3881–3890. [Google Scholar] [CrossRef]
- Kaivo-Oja, N.; Jeffery, L.A.; Ritvos, O.; Mottershead, D.G. Smad Signaling in the Ovary. Reprod. Biol. Endocrinol. 2006, 4, 21. [Google Scholar] [CrossRef]
- Xu, S.-S.; Gao, L.; Xie, X.-L.; Ren, Y.-L.; Shen, Z.-Q.; Wang, F.; Shen, M.; Eyϸórsdóttir, E.; Hallsson, J.H.; Kiseleva, T.; et al. Genome-Wide Association Analyses Highlight the Potential for Different Genetic Mechanisms for Litter Size Among Sheep Breeds. Front. Genet. 2018, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Bahire, S.V.; Rajput, P.K.; Kumar, V.; Kumar, D.; Kataria, M.; Kumar, S. Quantitative Expression of mRNA Encoding BMP/SMAD Signaling Genes in the Ovaries of Booroola Carrier and Non-Carrier GMM Sheep. Reprod. Domest. Anim. 2019, 54, 1375–1383. [Google Scholar] [CrossRef]
- Arnold, S.J.; Maretto, S.; Islam, A.; Bikoff, E.K.; Robertson, E.J. Dose-Dependent Smad1, Smad5, and Smad8 Signaling in the Early Mouse Embryo. Dev. Biol. 2006, 296, 104–118. [Google Scholar] [CrossRef]
- Sun, R.; Li, M.; He, N.; Wen, X.; Zhang, J. Molecular characterization, expression profiles of SMAD4, SMAD5 and SMAD7 genes and lack of association with litter size in Tibetan sheep. Animals 2022, 12, 2232. [Google Scholar] [CrossRef]
- Li, Q.; Pangas, S.A.; Jorgez, C.J.; Graff, J.M.; Weinstein, M.; Matzuk, M.M. Redundant Roles of SMAD2 and SMAD3 in Ovarian Granulosa Cells In Vivo. Mol. Cell. Biol. 2008, 28, 7001–7011. [Google Scholar] [CrossRef]
- Wijayanti, D.; Luo, Y.; Bai, Y.; Pan, C.; Qu, L.; Guo, Z.; Lan, X. New Insight into Copy Number Variations of Goat SMAD2 Gene and Their Associations with Litter Size and Semen Quality. Theriogenology 2023, 206, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, D.; Zhang, S.; Bai, Y.; Pan, C.; Chen, H.; Qu, L.; Guo, Z.; Lan, X. Investigation on mRNA Expression and Genetic Variation within Goat SMAD2 Gene and Its Association with Litter Size. Anim. Biotechnol. 2023, 34, 2111–2119. [Google Scholar] [CrossRef]
- Wijayanti, D.; Zhang, S.; Yang, Y.; Bai, Y.; Akhatayeva, Z.; Pan, C.; Zhu, H.; Qu, L.; Lan, X. Goat SMAD Family Member 1 (SMAD1): mRNA Expression, Genetic Variants, and Their Associations with Litter Size. Theriogenology 2022, 193, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Qi, T.; Hui, Y.; Yan, H.; Qu, L.; Lan, X.; Pan, C. Whole-Genome Sequencing to Identify Candidate Genes for Litter Size and to Uncover the Variant Function in Goats (Capra hircus). Genomics 2021, 113, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.N.; Zhai, H.L.; Cheng, M.; Ma, J.Y.; Cheng, S.F.; Ge, W.; Zhang, G.L.; Wang, J.J.; Zhang, R.Q.; Wang, X.; et al. Whole-Genome Scanning for the Litter Size Trait Associated Genes and SNPs under Selection in Dairy Goat (Capra hircus). Sci. Rep. 2016, 6, 38096. [Google Scholar] [CrossRef]
- Kempisty, B.; Jackowska, M.; Woźna, M.; Antosik, P.; Piotrowska, H.; Zawierucha, P.; Bukowska, D.; Jaśkowski, J.M.; Nowicki, M.; Brüssow, K.P. Expression and Cellular Distribution of INHA and INHB Before and After In Vitro Cultivation of Porcine Oocytes Isolated from Follicles of Different Size. BioMed Res. Int. 2012, 2012, 742829. [Google Scholar]
- Andreone, L.; Velásquez, E.V.; Abramovich, D.; Ambao, V.; Loreti, N.; Croxatto, H.B.; Parborell, F.; Tesone, M.; Campo, S. Regulation of Inhibin/Activin Expression in Rat Early Antral Follicles. Mol. Cell. Endocrinol. 2009, 309, 48–54. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, T.; Shi, J.A.; Liu, A.; Liu, L. Role and regulatory mechanism of inhibin in animal reproductive system. Therogenology 2023, 202, 10–20. [Google Scholar] [CrossRef]
- Walton, K.L.; Goney, M.P.; Peppas, Z.; Stringer, J.M.; Winship, A.; Hutt, K.; Goodchild, G.; Maskey, S.; Chan, K.L.; Brûlé, E.; et al. Inhibin inactivation in female mice leads to elevated FSH levels, ovarian overstimulation, and pregnancy loss. Endocrinology 2022, 163, bqac025. [Google Scholar] [CrossRef]
- Abuzahra, M.; Wijayanti, D.; Effendi, M.H.; Mustofa, I.; Munyaneza, J.P.; Eid, L.A.; Ugbo, E.N. Association of INHA Gene Polymorphisms with Litter Size Trait in Indonesian Thin-Tailed Sheep. Trop. Anim. Sci. J. 2024, 47, 3. [Google Scholar] [CrossRef]
- Bian, Z.; Li, K.; Chen, S.; Man, C.; Wang, F.; Li, L. Association between INHA Gene Polymorphisms and Litter Size in Hainan Black Goats. PeerJ. 2023, 11, e15381. [Google Scholar] [CrossRef]
- Wang, S.Z.; E, G.X.; Zeng, Y.; Han, Y.G.; Huang, Y.F.; Na, R.S. Three SNPs within Exons of INHA and ACVR2B Genes Are Significantly Associated with Litter Size in Dazu Black Goats. Reprod. Domest. Anim. 2021, 56, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Isa, A.M.; Bemji, M.N.; Wheto, M.; Williams, T.J.; Ibeagha-Awemu, E.M. Mutations in Inhibin Alpha Gene and Their Association with Litter Size in Kalahari Red and Nigerian Goats. Livest. Sci. 2017, 203, 106–109. [Google Scholar] [CrossRef]
- Liu, Q.Y.; He, Y.Q.; Ge, Y.; Chu, M.X.; Jin, M.; Zhang, Y.J.; Wang, J.Y.; Ma, X.K.; Di, R.; Huang, D.W.; et al. Polymorphism of Inhibin A Gene and Its Relationship with Litter Size in Chinese Indigenous Goat. J. Anim. Plant Sci. 2017, 27, 1488–1495. [Google Scholar]
- Zoheir, K.M.; El-Halawany, N.; Harisa, G.I.; Yang, L. Preliminary Investigation on Activin-A as a Candidate Gene Affecting Litter Size in Goats. Pak. J. Zool. 2015, 47, 3. [Google Scholar]
- Wu, W.; Hua, G.; Yang, L.; Wen, Q.; Zhang, C.; Zoheir, K.M.; Chen, S. Association Analysis of the INHA Gene with Litter Size in Boer Goats. Small Rumin. Res. 2009, 82, 139–143. [Google Scholar] [CrossRef]
- Huang, X.; Bao, Y.; Yang, F.; Li, X.; Wang, F.; Zhang, C. miR-134-3p Regulates Cell Proliferation and Apoptosis by Targeting INHBA via Inhibiting the TGF-β/PI3K/AKT Pathway in Sheep Granulosa Cells. Biology 2024, 14, 24. [Google Scholar] [CrossRef]
- Abuzahra, M.; Wijayanti, D.; Effendi, M.H.; Mustofa, I.; Munyaneza, J.P.; Eid, L.A.; Ugbo, E.N. Association of a Synonymous SNP of INHA Gene with Litter Size Trait in Indonesian Thin-Tailed Sheep. Trop. Anim. Sci. J. 2024, 47, 273–279. [Google Scholar]
- Tang, Y.; Lu, S.; Wei, J.; Xu, R.; Zhang, H.; Wei, Q.; Han, B.; Gao, Y.; Zhao, X.; Peng, S.; et al. Growth differentiation factor 9 regulates the expression of estrogen receptors via Smad2/3 signaling in goat cumulus cells. Theriogenology 2024, 219, 65–74. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef]
- Paradis, F.; Novak, S.; Murdoch, G.K.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 2009, 138, 115. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Orisaka, S.; Jiang, J.Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Getmantseva, L.; Bakoev, N.; Shirokova, N.; Kolosova, M.; Bakoev, S.; Kolosov, A.; Usatov, A.; Shevtsova, V.; Yuri, K. Effect of the GDF9 gene on the weight of lambs at birth. Bulg. J. Agric. Sci. 2019, 25, 153–157. [Google Scholar]
- de Castro, F.C.; Cruz, M.H.; Leal, C.L.V. Role of growth differentiation factor 9 and bone morphogenetic protein 15 in ovarian function and their importance in mammalian female fertility—A review. Asian-Australas. J. Anim. Sci. 2015, 29, 1065. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.K.; Otsuka, F.; Shimasaki, S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J. Biol. Chem. 2003, 278, 304–310. [Google Scholar] [CrossRef]
- Pangas, S.A. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol. Cell. Endocrinol. 2012, 356, 40–47. [Google Scholar] [CrossRef]
- Lu, C.; Yang, W.; Chen, M.; Liu, T.; Yang, J.; Tan, P.; Li, L.; Hu, X.; Fan, C.; Hu, Z.; et al. Inhibin A inhibits follicle-stimulating hormone (FSH) action by suppressing its receptor expression in cultured rat granulosa cells. Mol. Cell. Endocrinol. 2009, 298, 48–56. [Google Scholar] [CrossRef]
- Knight, P.G.; Satchell, L.; Glister, C. Intra-ovarian roles of activins and inhibins. Mol. Cell. Endocrinol. 2012, 359, 53–65. [Google Scholar] [CrossRef]
- Yu, X.; Qiao, T.; Hua, L.; Liu, S.; Zhao, X.; Lv, C.; Zhao, X.; Wang, J.; Han, L.; Yang, L.; et al. Synergistic regulatory effect of inhibin and anti-Müllerian hormone on fertility of mice. Front. Vet. Sci. 2021, 8, 747619. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, Y.; Guo, J.; Jiang, X.; Liu, X.; Chen, Y.; Cong, P.; He, Z. FecB mutation enhances follicle stimulating hormone sensitivity of granulosa cells by up-regulating the SMAD1/5–USF1–FSHR signaling pathway. Int. J. Biol. Macromol. 2024, 280, 135697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; He, X.; Liu, Q.; Di, R.; Hu, W.; Cao, X.; Zhang, X.; Zhang, J.; Chu, M. Effects of FecB mutation on estrus, ovulation, and endocrine characteristics in small tail Han sheep. Front. Vet. Sci. 2021, 8, 709737. [Google Scholar] [CrossRef] [PubMed]
- Sasi, R.; Kanakkaparambil, R.; Thazhathuveettil, A. Polymorphism of fecundity genes, BMPR1B, BMP15 and GDF9, in tropical goat breeds of Kerala. Gene Rep. 2020, 21, 100944. [Google Scholar] [CrossRef]
- Qi, M.Y.; Xu, L.Q.; Zhang, J.N.; Li, M.O.; Lu, M.H.; Yao, Y.C. Effect of the Booroola fecundity (FecB) gene on the reproductive performance of ewes under assisted reproduction. Theriogenology 2020, 142, 246–250. [Google Scholar] [CrossRef]
- Juengel, J.L.; Davis, G.H.; McNatty, K.P. Using sheep lines with mutations in single genes to better understand ovarian function. Reproduction 2013, 146, R111–R123. [Google Scholar] [CrossRef]
- Guo, X.; Fang, Y.; Liang, R.; Wang, X.; Zhang, J.; Dong, C.; Wang, B.; Liu, Y.; Chu, M.; Zhang, X.; et al. Single-cell RNA-seq reveals the effects of the FecB mutation on the transcriptome profile in ovine cumulus cells. Sci. Rep. 2024, 14, 13087. [Google Scholar] [CrossRef]
- Zhong, T.; Hou, D.; Zhao, Q.; Zhan, S.; Wang, L.; Li, L.; Zhang, H.; Zhao, W.; Yang, S.; Niu, L. Comparative whole-genome resequencing to uncover selection signatures linked to litter size in Hu Sheep and five other breeds. BMC Genom. 2024, 25, 480. [Google Scholar] [CrossRef] [PubMed]

| Genes | Polymorphism | Female Reproductive Traits | Breeds | Country | References |
|---|---|---|---|---|---|
| BMP15 GDF9 | c.31_33CTT | Litter size | Hu Sheep | China | [36] |
| GDF9 BMP15 | S395F and S427R | Increase the ovulation rate and litter size | Sheep | China | [37] |
| BMPR1B BMP15 GDF9 | c.746A>G c.31_33CTTinsdel c.994A>G | Litter size | Ujimqin, Dorper × Ujimqin crossbred Ujimqin Suffolk × Ujimqin crossbred | China | [39] |
| BMP15 | g.50985975 G>A and c.755 T>C g.50988478C>A and g.50987863G>A | Litter size | Mongolia sheep Ujimqin sheep | China | [40] |
| BMPRIB | g.29362047T > C, g.29427689G > A | Litter size | Hu sheep | China | [41] |
| BMP15 GDF9 | Litter size | Akkaraman | Turkey | [42] | |
| BMPRIB | p.Q249R | Litter size | Australian white, small-tailed Han, Guiqian semi-fine wool sheep | China | [43] |
| BMPR1B, BMP15, GDF9 | Prolificacy | Han, Hu, Wadi, Tan sheep | China | [44] | |
| BMPR1B, BMP2 | Litter size | Chinese indigenous sheep | China | [45] | |
| BMPR1B | c.687G>A g.30058882_30058873GCAGATTAAAIndel | Litter size | Gobi short-tailed sheep | China | [46] |
| GDF9 | Litter size | Sudanese desert sheep | China | [48] | |
| BMPR1B | C864T | Litter size | Dorset, Mongolian, small-tailed Han | China | [49] |
| BMP15 | g.54285159_54285161TTA indel g.54291460G>A, g.54288671C>T, g.54285159_54285161TTA indel | Litter size | Gobi short-tailed sheep Ujimqin sheep | China | [54] |
| BMPR1B | g.30050773C>T | Fecundity | Duolang sheep | China | [55] |
| GDF9 | Litter size | Bulgarian sheep | Bulgaria | [56] | |
| BMPR1B | g.746A>G, g.29362047T>C, g.29427689G>A, g.29382337G>A, g.29382340G>A, g.29380965A>G | Litter size | Sheep | China | [57] |
| BMPR1B | c.746A > G | Litter size | Hu, East Friesian/Hu crossbred sheep | China | [58] |
| BMPRIB | rs427897187 G>A rs403555643 A>G | Litter size | Oula sheep | China | [59] |
| BMPRIB | Litter size | Mongolian sheep | China | [60] | |
| BMPRIB | A746G, T864C, A1354G | Litter size | Qinghai Tibetan sheep | China | [61] |
| BMPR1B, BMP15, GDF9 | Litter size | Dağlıç sheep | China | [62] | |
| BMP15, GDF9 | Litter size | Luzhong mutton sheep | China | [63,64] | |
| BMP15, GDF9 | Ovulation rate and litter size | Olkuska sheep | Poland | [65] | |
| BMP15 | c.31_33del | Litter size | Finnish Landrace sheep, Finnish, Landrace × Texel-cross sheep, composite sheep | New Zealand | [66] |
| BMP15 | p.L252P | Fecundity | Cele black Sheep | China | [67] |
| BMPR1B | g.29346567C>T | Litter size | Mongolia, Ujimqin sheep | China | [68] |
| BMPR1B | Litter size | Chinese Australian white sheep | China | [69] | |
| BMPR1B | g.29380965A>G | Litter size | Small-tailed Han sheep | China | [70] |
| GDF9 | Litter size | Hu sheep, Mongolian sheep | China | [47,71] | |
| BMPR1B, BMP15, GDF9 | Litter size | Rahmani, Rahmani × Barki cross | Egypt | [72] | |
| GDF9 | c.1111A | Litter size | Purebred Finnish Landrace sheep, Finnish Landrace × Texel-cross sheep, composite sheep | New Zealand | [73] |
| BMP2 BMP7 | g.48462350C>T g.58171856C>G and g.58171886A>C | Litter size | Small-tailed Han sheep | China | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Cao, G.; Chen, W.; Wang, C.; Khan, M.Z. The Role of TGF-β Signaling Pathway in Determining Small Ruminant Litter Size. Biology 2025, 14, 786. https://doi.org/10.3390/biology14070786
Han Y, Cao G, Chen W, Wang C, Khan MZ. The Role of TGF-β Signaling Pathway in Determining Small Ruminant Litter Size. Biology. 2025; 14(7):786. https://doi.org/10.3390/biology14070786
Chicago/Turabian StyleHan, Ying, Guiling Cao, Wenting Chen, Changfa Wang, and Muhammad Zahoor Khan. 2025. "The Role of TGF-β Signaling Pathway in Determining Small Ruminant Litter Size" Biology 14, no. 7: 786. https://doi.org/10.3390/biology14070786
APA StyleHan, Y., Cao, G., Chen, W., Wang, C., & Khan, M. Z. (2025). The Role of TGF-β Signaling Pathway in Determining Small Ruminant Litter Size. Biology, 14(7), 786. https://doi.org/10.3390/biology14070786







