Simple Summary
The Alpine Arc has acted as a diversification centre for many taxa of plants, and today it hosts an impressively rich biodiversity. However, the number of genera or subgeneric lineages containing species endemic to the Alps is very low. The reasons for such few and species-poor local diversification events are to be found in Quaternary climatic oscillations, and particularly glaciations, which prevented the evolution of species-rich lineages. The genus Fritillaria includes more than 140 species worldwide, but only a few are endemic to the Alps. A correct delimitation of the different species distributed on the Alps would help to assign correct taxonomic ranks to these endemics when their DNA is used in phylogenies based on a large dataset. This study clarifies the taxonomic status of Fritillaria taxa endemic to the Alps, only marginally studied so far on phylogenetic grounds. Based on our results, we recognise F. tubaeformis, F. moggridgei, and F. burnatii at species rank.
Abstract
The number of Fritillaria species native to the Alps has long been debated, and observational biases due to the short flowering periods and the scattered distributions of endemic Fritillaria populations along the mountain range have probably made the task of botanists more complicated. Moreover, previous phylogenetic studies in Fritillaria have considered alpine taxa only marginally. To test species boundaries within the F. tubaeformis species complex and to study their phylogenetic relationships, intra- and inter-specific genetic variability of sixteen samples belonging to four Fritillaria species was carried out in different localities of the Maritime and Ligurian Alps, with extensions to the rest of the Alpine arc. The combined use of five plastid DNA markers (matK, ndhF, rpl16, rpoC1, and petA-psbJ) and nrITS showed that F. tubaeformis and F. burnatii are phylogenetically independent taxa, fully confirming morphological and morphometric divergences and, that F. burnatii is not related phylogenetically to the central European F. meleagris. Our phylogenetic study also supports the separation of F. tubaeformis from F. moggridgei, pointing to environment/ecological constraints or reproductive barriers as possible causes of their distinct evolutionary status. Our analysis also showed that the mountain endemic F. involucrata is not closely related to F. tubaeformis, contrasting with previous studies. The phylogenetic analysis of the nrITS region supports a close relationship between F. burnatii and F. moggridgei, but with low statistical support.
1. Introduction
The Alps are the highest mountains in Europe and are centres of diversity and endemism for many species [1,2,3]. The large climatic oscillations and physiographic heterogeneity typical of the alpine habitats have been key drivers of diversification and speciation in many alpine plants [3,4]. Located at the southern edge of the alpine arc, the Maritime and Ligurian Alps lie at the intersection of temperate and Mediterranean climates, making them particularly important for the study of plant evolution. These areas acted as key refugia during the last glacial maximum [5], sheltering numerous endemic species [6]. During Pleistocene glaciations, many species were pushed toward ice-free peripheral ranges, with the south-western and southern Alps serving as major refugia [7,8].
Here, we focus on the genus Fritillaria. It is the largest genus of the tribe Lilieae (Liliaceae) and includes about 140 bulbous perennial species occurring over a wide range of climates and habitats in the temperate regions of the Northern Hemisphere [9,10]. The Mediterranean area is generally considered the modern abundance centre of Fritillaria [11] and more specifically, a secondary diversification area of species distributed across Europe (mainly Greece), the Middle East, and North Africa (F. subg. Fritillaria, clade A sensu [12,13]). During the Late Pliocene, the onset of a seasonal climate in the Mediterranean Basin [2,14] promoted species diversification in this group of plants and several Fritillaria species further dispersed into Europe [12,13,15].
However, when we look at the number of Fritillaria species endemic to the Alps, we find a situation significantly different from the rest of the Mediterranean basin. Although hosting one of the most species-rich floras of the world, the Alps witnessed a rather limited number of local diversification events. This has resulted in a rather low number of endemic lineages compared to the overall number of endemic species in this mountain range [16]. It has been estimated that no more than 9% of alpine endemics have originated or diversified in situ (species diversification sensu Kadereit [16]). In this geographical context, the genus Fritillaria is exemplary of this paradigm, since only four species are endemic to the Alps.
Within the Alpine boundary, Fritillaria involucrata All. is endemic to south-eastern France and north-western Italy. It can be found on the Prealps, Maritime Alps [17,18,19], and Ligurian Alps [19,20,21,22]. This species is found on fresh and moderately fertile soils, in rocky open woods or fringes between 400 and 1500 m a.s.l. [19]. In the Ligurian Alps, it grows in open woods of Ostrya carpinifolia Scop. and in woodlands of beech forests or in clearings on calcareous soils (U. Ferrando, pers. comm.). According to the phylogenetic analysis published by Day et al. [12], which was based on a combined plastid dataset, F. involucrata would be paraphyletic with respect to F. tubaeformis. No further information is currently available on the phylogenetic position of this very rare species.
In the literature, taxa of the F. tubaeformis complex have been variously treated (see Mucciarelli et al. [23] for details): a single variable species with no infraspecific taxa [24,25,26], a single species with two infraspecific taxa, F. tubaeformis Gren. & Godr. subsp. tubaeformis and F. tubaeformis subsp. moggridgei (Boiss. & Reut. ex Planch.) Rix [27,28], three distinct taxa belonging to two different species: F. tubaeformis subsp. tubaeformis, F. tubaeformis subsp. moggridgei, F. meleagris subsp. burnatii (Planch.) Rix for Rix [29,30], or more recently as F. tubaeformis subsp. tubaeformis, F. tubaeformis subsp. moggridgei and F. burnatii (Planch.) Backh. [18,19]. Finally, the three taxa were treated as different species by Tison and de Foucault [31]: F. tubaeformis Gren. and Godr., F. moggridgei (Boiss. & Reut. ex Planch.) L.A.Cusin and F. burnatii (Planch.) Backh.
In line with the results presented in this study, we adopted the nomenclatural approach proposed by Tison and de Foucault [31] who recognised the species status for the three taxa of the F. tubaeformis complex. In accordance with the previous literature based on a morphological approach [19,31], Mucciarelli et al. [23] successfully distinguished the three taxa on morphometric grounds. In particular, the perigone is sub-rectangular in both F. tubaeformis and F. moggridgei, while it is typically rounded (U-shaped) in F. burnatii [23]. However, a comprehensive phylogenetic appraisal of this complex of species is presently lacking.
Most analyses to date have focused mainly on the genetic differentiation among the populations of F. moggridgei and F. burnatii [32,33], leaving their phylogenetic relationships—including those with respect to F. tubaeformis s. str.—unresolved.
To further test species boundaries within the three taxa, an intra- and inter-specific geographically oriented sampling was carried out, according to the most recent taxonomic and biogeographic revisions [23,34]. A multilocus dataset, combining plastid markers (including matK, ndhF, rpl16, rpoC1, and petA-psbJ) with the nuclear ITS region, was used to assess variability and illustrate phylogenetic relationships among alpine Fritillaria taxa.
The most recent taxonomic hypothesis to be tested sees F. tubaeformis and F. burnatii as genetically other than morphologically independent taxa, and the former species further split into two subspecies [23]. A plastid and nuclear DNA phylogenetic approach was applied to examine:
- (a)
- The phylogenetic relationships between F. tubaeformis and F. burnatii.
- (b)
- The phylogenetic placement of F. tubaeformis and F. burnatii with respect to the central European F. meleagris and to the SW Alpine endemic F. involucrata.
- (c)
- The taxonomic status of the two putative subspecies within F. tubaeformis.
2. Materials and Methods
2.1. DNA Extraction and PCR Amplification
Genomic DNA extraction was performed following two protocols [32,35]. The matK partial region, rpl16 intron, rpoC1 intron, petA-psbJ intergenic spacer were amplified using primers and conditions summarised by Mucciarelli and Fay [32]. For matK partial region in addiction BF and CR primers [36] were used. The ndhF region was amplified with primers 1318 and 2110 [37] following the conditions summarised by Marques et al. [38]. The ITS region was amplified using the primers ITS1/ITS4 or ITS-p5/ITS-u4 following the protocol provided by Cheng et al., 2016 [39].
2.2. Species Distribution Ranges in the F. tubaeformis Species Complex
In order to show the distribution of F. burnatii, F. moggridgei, and F. tubaeformis, distribution maps were produced using ConR package implemented in R package (version 4.1.1) [40]. Occurrence data were retrieved using online resources [41,42,43].
2.3. Sequence Alignment, Dataset Assembly, and Phylogenetic Analysis
Sequences were assembled and edited in Geneious v. 11.1.5 [44] and then submitted to GenBank® (www.ncbi.nlm.nih.gov/genbank/ (accessed on 25 March 2023)). In cpDNA analysis, a total of eighteen accessions of Fritillaria were investigated in this work, while two accessions of Lilium from GenBank were used as outgroups. Table 1, Table 2 and Table 3 list accession names, DNA/herbarium voucher codes, and sequences used in the phylogenetic analysis, together with information on their origin. The accessions investigated in this study accounted for 74 new sequences belonging to five different taxa. Four taxa were sampled in the alpine region according to our previous studies [23,34]. Sequences of Lilium superbum and Lilium bakerianum were chosen as the outgroup taxa. Sequences of matK region, ndhF, rpl16 intron, rpoC1 intron, petA-psbJ intergenic spacer, and nrITS region were separately aligned with MAFFT v. 7.017 [45] and then manually adjusted using Geneious v. R 11.1.4. For all independent datasets, five separate maximum likelihood (ML) analyses were run using W-IQ-TREE (version 1.6.12) [46]. The SH-like approximate likelihood ratio test (with 1000 replicates) and ultrafast bootstrap approximation (UFB) (1000 replicates) [47] were used to evaluate the reliability of clades (data available in Supplementary). No topological conflict was detected in the phylogenetic analyses of the matK and ndhF regions, rpl16 and rpoC1 introns, and petA-psbJ intergenic spacer datasets (Figure S1a–e), so a combined dataset was created by concatenating the matK, ndhF, rpl16, rpoC1, and petA-psbJ alignments using Geneious v. R 11.1.4.

Table 1.
Sampled plant materials and voucher codes for the Fritillaria plastid regions.
For the analysis of the nrITS region, a dataset was used consisting of nine new sequences generated in this study: three of F. burnatii, one of F. involucrata, three of F. moggridgei and two of F. tubaeformis. Additionally, two sequences from F. meleagris were included, along with Lilium bakerianum and Lilium superbum, which were used as outgroups (Table 3). Partition Finder 2 [48] was used to estimate the best partitioning schemes and evolution models for each subset with MrBayes option. The concatenate cpDNA and nrITS datasets were analysed using Bayesian inference (BI) and maximum likelihood (ML) criteria. BI was performed with MrBayes v.3.2 [49] with four incrementally heated simultaneous Monte Carlo Markov Chains (MCMC) run for 10 million generations for cpDNA and nrITS alignment. Trees were sampled every 1000 generations, and the first 2500 trees were discarded as “burn-in” (25%). For the remaining trees, a majority rule consensus tree showing all compatible partitions was computed to obtain estimates for Bayesian Posterior Probabilities (BPP). The ML for the concatenated dataset was performed with IQ-TREE 2 [50]. The best models were selected using ModelFinder implemented in IQ-TREE 2 [51].

Table 2.
Fritillaria samples used in cpDNA phylogenetic analyses together with GenBank accession numbers.
Table 2.
Fritillaria samples used in cpDNA phylogenetic analyses together with GenBank accession numbers.
| Taxa | References | matK | ndhF | rpl16 | rpoC1 | petA-psbJ |
|---|---|---|---|---|---|---|
| F. burnatii (TEN1-20p2017) | this study | MN917987 | MN917996 | MN918014 | - | MN918005 |
| F. burnatii (TEN2-04p2013) | this study | - | MN917995 | MN918015 | - | MN918004 |
| F. burnatii (SER-05p2013) | this study | MN917986 | - | MN918013 | MN918023 | MN918003 |
| F. burnatii (TN01) | this study | - | OP021726 | - | OP021738 | OP021733 |
| F. burnatii (TN02) | this study | - | OP021727 | - | OP021739 | OP021732 |
| F. burnatii (TN03) | this study | - | OP021728 | - | OP021740 | OP021734 |
| F. burnatii (TN04) | this study | - | OP021729 | - | OP021741 | OP021735 |
| F. involucrata (MEN-14p2016) | this study | MN917988 | MN917997 | MN918016 | - | - |
| F. involucrata (HYE-CB976) | this study | - | OP021730 | OP021736 | OP021742 | - |
| F. meleagris (GER-11p2015) | this study | MN917989 | MN917998 | MN918020 | MN918025 | MN918008 |
| F. meleagris (Chase 2566 (K), Kew 1990-3088) | [11] | AY624445 | AY624393 | |||
| F. moggridgei (MAR-07p2014) | this study | MN917992 | MN917999 | PV832077 | - | MN918012 |
| F. moggridgei (CRA-12p2014) | this study | MN917990 | PV832078 | MN918017 | - | MN918009 |
| F. moggridgei (FRO-00p2013) | this study | MN917991 | - | MN918021 | MN918027 | MN918010 |
| F. moggridgei (PLU-08p2014) | this study | PV832076 | MN918000 | MN918022 | MN918026 | MN918011 |
| F. moggridgei (HYE-CB6181) | this study | - | OP021731 | OP021737 | - | - |
| F. tubaeformis (GLE-10p2013) | this study | MN917993 | MN918001 | MN918018 | MN918028 | MN918006 |
| F. tubaeformis (GLE-09p2015) | this study | MN917994 | MN918002 | MN918019 | MN918024 | MN918007 |
| L. superbum | [52] | KP462883 | KP462883 | KP462883 | KP462883 | KP462883 |
| L. bakerianum | [53] | KY748301 | KY748301 | KY748301 | KY748301 | KY748301 |

Table 3.
Fritillaria samples used in nrITS phylogenetic analyses together with GenBank accession numbers.
Table 3.
Fritillaria samples used in nrITS phylogenetic analyses together with GenBank accession numbers.
| Taxa | References | ITS |
|---|---|---|
| F. burnatii (TEN1-20p2017) | this study | MT522874 |
| F. burnatii (TEN2-04p2013) | this study | MT522877 |
| F. burnatii (SER-05p2013) | this study | PV816804 |
| F. involucrata (MEN-14p2016) | this study | MT522876 |
| F. meleagris (Chase 2566 (K)) | [11] | AY616730 |
| F. meleagris (K:DNA:MWC12064) | [54] | HE656029 |
| F. moggridgei (MAR-07p2014) | this study | PV816806 |
| F. moggridgei (CRA-12p2014) | this study | MT522875 |
| F. moggridgei (PLU-08p2014) | this study | PV816805 |
| F. tubaeformis (GLE-10p2013) | this study | MT522878 |
| F. tubaeformis (GLE-09p2015) | this study | MT522879 |
| L. superbum | [55] | JF829386 |
| L. bakerianum (G2013001) | [56] | KF851093 |
The SH-like approximate likelihood ratio test (with 1000 replicates) and bootstrap (1000 replicates) were used to evaluate the reliability of clades. Significant support is considered to be ≥80% for the SH-aLRT test, ≥70% for bootstrap support in the ML analysis (MLB), and ≥0.95 for BPP values in the Bayesian analysis (Figure 1 and Figure 2). The list of sequences used in our datasets are given in Table 2 and Table 3.
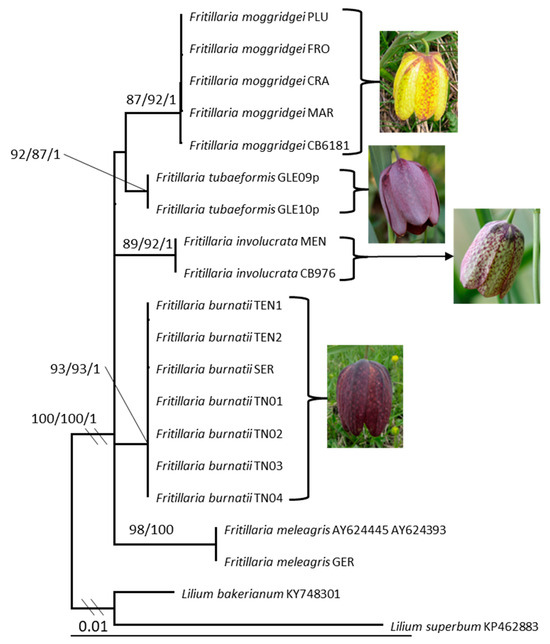
Figure 1.
Maximum likelihood phylogram obtained from combined matK, ndhF, rpl16, rpoC1, and petA-psbJ sequence alignment of Fritillaria. Values above or below branches indicate SH-aLRT support ≥ 80%/bootstrap support ≥ 70%/Bayesian posterior probabilities ≥ 0.95.
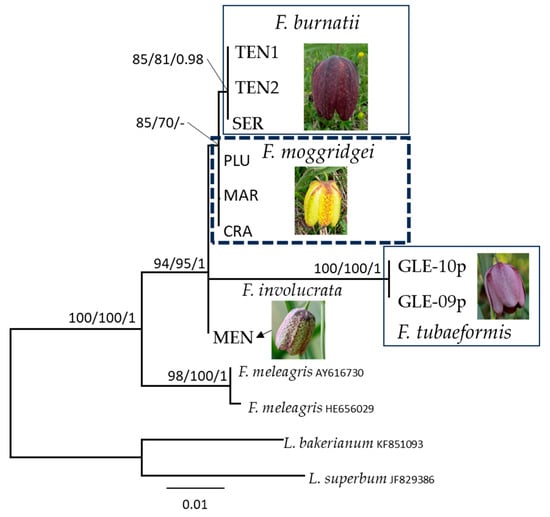
Figure 2.
Maximum likelihood phylogram obtained from nrITS sequence alignment of Fritillaria. Only BPP values ≥ 0.90 and MLB values ≥ 70% are given above the clade branches. Values above or below branches indicate SH-aLRT support ≥ 80%/ML bootstrap support ≥ 70%/Bayesian posterior probabilities ≥ 0.95.
3. Results
Molecular and Phylogenetic Analysis
Ten, fifteen, thirteen, eleven, and fourteen sequences were newly generated for matK, ndhF, rpl16, rpoC1, petA-psbJ (Table 2), and nine sequences for nrITS (Table 3).
Both Bayesian and Maximum Likelihood analyses produced the same topology for both alignments and therefore, only the Maximum Likelihood tree with SH-aLRT test, MLB and BPP values is presented here (Figure 1 and Figure 2). The combined matK/ndhF/rpl16/rpoC1/petA-psbJ data matrix comprises 20 terminal labels and contains 3105 total nucleotide sites. In the cpDNA phylogenetic analysis, the various taxa are consistently resolved into well-supported, distinct clades (see Figure 1). Fritillaria burnatii, F. moggridgei, F. tubaeformis, F. involucrata, and F. meleagris group in a highly supported clade (SH-aLRT = 100, MLB = 100 and BPP = 1; Figure 1). F. moggridgei from the Ligurian Alps, together with the accession from Maritime Alps, Tende (France) (Table 1) form a supported clade (SH-aLRT = 87, MLB = 92 and BPP = 1). All the accessions of F. burnatii from Ligurian and Maritime Alps (3), and from Trentino-Alto Adige (4) (Table 1) form a distinct lineage with a strong support (SH-aLRT = 93, MLB = 93 and BPP = 1; Figure 1). F. burnatii shows two characteristic deletions in petA-psbJ intergenic spacer (TTCGG and AAAGAAT) and one deletion in rpoC1 intron region; the latter deletion is also shared with F. involucrata (GTAAA). Sequences of the French endemic F. tubaeformis form a supported clade (SH-aLRT = 92, MLB = 87 and BPP = 1; Figure 1) and show a 7 bp characteristic long indel (CTACATT) in the rpl16 intron.
The sequences of F. involucrata from the Ligurian and Maritime Alps (Table 1) group together in a highly supported clade (SH-aLRT = 89, MLB = 92 and BPP = 1) and show an exclusive insertion in the rpoC1 intron region (TTTTAGTT).
F. meleagris sequences grouped in a well-supported clade (SH-aLRT = 98, MLB = 100 and BPP = 1; Figure 1).
The nrITS alignment includes thirteen terminal labels and contains 622 sites. Fritillaria burnatii, F. involucrata, F. moggridgei, and F. tubaeformis form a well-supported clade (SH-aLRT = 94, MLB = 95 and BPP = 1; Figure 2) independent of F. meleagris interdigitated. Within this clade, F. burnatii is sister to F. moggridgei, but with weak support (SH-aLRT = 85, MLB = 70 and BPP = -), and they exhibit a sequence identity of 629/632 bp (99%) with 1 gap. The nrITS sequence identity between F. burnatii and F. involucrata is 469/475 bp (99%) with 1 gap. Within the alpine clade, F. tubaeformis diverges the most, showing an identity of 95% to 96% with the other species. In the Supplementary Materials (Figure S1a–e), five phylogenetic trees corresponding to the analysed plastid regions are presented to illustrate the capacity of each region to distinguish between different taxa. The central portion of the matK region used in the phylogenetic analysis (amplified with primers BF and CR, 446 bp) successfully distinguishes two species. The rpl16 region separates three species, while the ndhF region effectively discriminates all five species under consideration. The petA region distinguishes four species; however, the sample of F. moggridgei (FRO) is positioned ambiguously in the tree due to an incomplete sequence (210 bp out of a total of 691 bp). Finally, the rpoC1 region is capable of resolving all five Fritillaria species examined.
4. Discussion
In our study of the Fritillaria tubaeformis complex, previously investigated through a morphological approach [23], we aimed to refine species boundaries using molecular methods. Our results revealed that the alpine Fritillaria taxa represent distinct phylogenetic species, as supported by both chloroplast (cpDNA) and nuclear (nrITS) DNA analyses. The phylogenetic species concept—which emphasises evolutionary history and relies on genetic data to group individuals into monophyletic units—proved effective in achieving our research objectives. However, it is important to note that this approach to the species does not hold absolute value, as it remains a complex and widely debated framework in systematic biology.
In support of our results, all species analysed across the Alpine arc exhibit species-specific indels, which likely represent putative apomorphies and can be used as genetic markers for species circumscription. These indels, extensively documented in the results section, were not computed in our phylogenetic analyses and thus provide additional support for the recognition of F. burnatii, F. involucrata, F. tubaeformis, and F. moggridgei as distinct species.
The plastid DNA analysis apparently supports a close relationship between F. moggridgei and F. tubaeformis but without statistical support, whereas the ML analysis of the nrITS region suggests a closer affinity between F. moggridgei and F. burnatii. This latter relationship, however, is not supported by the Bayesian analysis. Species delimitation is pivotal to understanding biological and biogeographical processes governing dynamics of plant communities and for the conservation of their population’s diversity. For the regions studied, no intraspecific variation was detected, except for the presence of some heterozygosity. In the nuclear nrITS sequences, different heterozygous sites were observed within sequences belonging to the same species. Particularly, our results supported the separation of F. burnatii (Planch.) Backh. from the Liguro-provençal microendemic F. moggridgei (Boiss. & Reut. ex Planch.) L.A.Cusin (Figure 3B) and from F. tubaeformis Gren. & Godr. The former has a range centred in Italy in the Ligurian and Maritime Alps, extending to Lombardy Prealps and Trentino (Figure 3A) [18,23,34]. Conversely, F. tubaeformis occurs throughout the French Préalpes, Haute-Alpes, Drôme, Basses-Alpes with a few populations on the Maritime Alps (Figure 3C). Until recently, F. burnatii and F. tubaeformis have often been treated as the same species by several European authors, likely due to their similar purple-brown flowers (Figure 4a–d), and influenced by earlier classifications such as that proposed by Rix [29]. As already noticed, observation bias could have originated also from their close and, somehow, interdigitated (albeit never overlapping) distributions (Figure 3). However, in F. burnatii, the perigone is campanulate with a typical U profile. Tepals are sombre-purple or wine-purple with evident white or cream-yellow tessellation and they are shorter and narrower than in F. tubaeformis and in F. moggridgei. Differently, here perigones are large and with a sub-rectangular profile being flower elements angled at the point of inflexion. Moreover, tepals are uniformly purple, glaucous, and covered with pruina in F. tubaeformis; they are yellow ochre with a typical tessellation of purple lines, spots, and green veins in F. moggridgei.
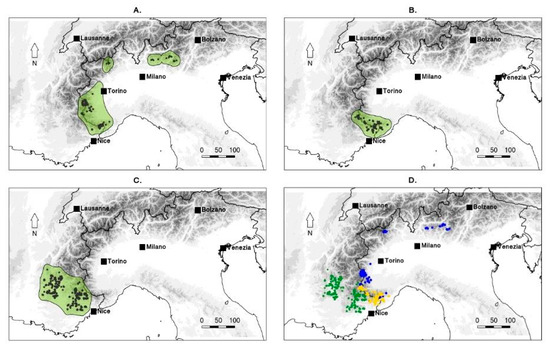
Figure 3.
Geographical distributions of the three endemic taxa of Fritillaria based on available occurrence data. (A) F. burnatii (Planch.) Backh.; (B) F. moggridgei (Boiss. & Reut. ex Planch.) L.A. Cusin; (C) F. tubaeformis Gren. and Godr.; (D) Distributions of the three taxa have been overlaid in the map, representing population occurrences with dots of different colours: F. burnatii (blue dots), F. moggridgei (yellow dots), F. tubaeformis (green dots).

Figure 4.
The Fritillaria taxa endemic of south-western Alps in their natural habitats: (a) F. burnatii (TEN1-20p2017); (b) F. burnatii, a close up of the individual bearing a discoloured perigone (TEN2-204p2013); (c) the same plant in the wild (left) and nearby individuals of F. burnatii with normal purple flowers (the image on the right has been cropped to put plants closer, same magnification; see text for details); (d) F. tubaeformis (GLE-10p2013); (e) F. moggridgei (FRO-00p2013); (f) F. involucrata (MEN-14p2016). In brackets, voucher codes of the sampled populations as in Table 1.
Our phylogenetic analysis, based on four plastid regions (ndhF, rpl16, rpoC1, petA-psbJ) and the nuclear ITS region, support the morphological distinction within the F. tubaeformis group.
4.1. Fritillaria meleagris
Fritillaria meleagris shows a wide Eurasian distribution [57,58]. This species is native to south-eastern Europe with a scattered range extending to 52–53° N [58], from where it probably spread into north-western parts of Europe after forest grazing and clearance of woodlands. It was repeatedly considered as closely related to or identical with F. burnatii, also known as F. meleagris subsp. burnatii (Planch.) Rix [29]. However, F. meleagris is distinguished by the former for its nodding, bell-shaped flowers with a distinctive checkerboard pattern in shades of purple and white. Tepals are broad but markedly acute at the apex, and not pruinose, two traits rarely found in flowers of the F. tubaeformis complex. The occurrence of F. meleagris s.str. in French and Italian Alps was erroneously reported in the past [21,25] and even more recently [59]. Its occurrence in the Alps is limited to the easternmost border of the mountain chain [26], namely in Austria and Slovenia. In our nrITS phylogenetic analysis, F. meleagris was strongly supported as sister to the alpine Fritillaria included in this study (SH-aLRT = 100, MLB = 100 and BPP = 1; Figure 2), providing strong evidence that this is a well-circumscribed species.
4.2. Fritillaria burnatii
Fritillaria burnatii (Planch.) Backh. has its type locality in the Maritime Alps of Piedmont (Italy), at the very boundary with France, where it shows a well-defined range [18] (Figure 3A). In France, this taxon has remained long unknown and sometimes confused with Fritillaria tubaeformis s.str. Only more recently it has been recognised in the French floras [19,31]. Many Italian botanists have long misidentified this taxon with F. meleagris or F. involucrata [34], or even fully synonymised with F. tubaeformis (≡ F. delphinensis) [24,25,27,60]. When Bartolucci and Peruzzi [34] designated the lectotype based on a specimen collected by Planchon and conserved at G-BU, they argued its independent taxonomic status from F. tubaeformis, and provisionally maintained the validity of the taxonomic setting proposed by Rix [29], namely Fritillaria meleagris subsp. burnatii (Planch.) Rix. Based on morphometric data, Mucciarelli et al. [23] have clearly shown the separation between the two taxa. Specifically, it was shown that F. burnatii has narrower, markedly canaliculate and ± glaucous leaves, acute at apex and inward folding after drying. These in F. tubaeformis are only briefly canaliculate, the lower ones slightly obtuse, the upper ones acute at apex and never folding as in the previous species. In addition to differences in flower morphologies, previously highlighted in the text, we also observed significantly shorter ovaries and styles than in F. tubaeformis s.l. [23]. Therefore, also in accordance with the most recent floras of France [18] and the Italian checklist of vascular flora [21], all records of F. tubaeformis subsp. tubaeformis from Italy (ref. [28] have been referred to as F. burnatii [23,34,61]. Indeed, in this study, we have shown that F. burnatii and F. tubaeformis belong to independent lineages, confirming previous results [32]. In addition, F. burnatii is not closely related to F. meleagris. In our phylogenetic analysis, the accession collected in Valle Pesio (SER-05p2013), the two accessions collected in the type locality (Cima di Forte Pernante, Mont Piernaud, Cuneo), together with samples from Trentino clustered together in a well-supported clade (SH-aLRT = 93, MLB = 93 and BPP = 1; Figure 1). The analysis of the nrITS region also supports the placement of F. burnatii as an independent lineage (SH-aLRT = 85, MLB = 81, and BPP = 0.98; Figure 2), seemingly related to F. moggridgei but with low statistical support. The sample here labelled as TEN2-04p2013 corresponds to a single individual observed at Cima di Forte Pernante in spring 2013, occurring alongside typical F. burnatii individuals. This single individual showed a creamy yellow U-shaped perigone with pale greenish venations (Figure 4b,c), sensibly differing from the perigone of F. moggridgei, which is usually of a much darker yellow with green or purple tessellations (Figure 4e). Based on morphometric measurements made at Cima di Forte Pernante, discriminant analysis assigned this individual to F. burnatii with a 99% probability.
The occurrence of yellow or whitish perigones within Fritillaria populations usually showing purple flowers is already documented in the literature [62], and the possibility exists that albino variants occur in populations of these plants. Interestingly, the occurrence of a distinct “ivory whitish variant” of flower in F. tubaeformis s.str. (≡ F. delphinensis) was documented in 1800s [63] and also observed recently by one of us (VN).
4.3. Fritillaria tubaeformis and F. moggridgei
Our results demonstrated that the sampled populations of Fritillaria moggridgei are distinct from F. tubaeformis. The former has been treated, in the past, either as a subspecies, a variety or a full synonym of F. tubaeformis [34]. As mentioned before, Fritillaria tubaeformis shows uniformly dark purple perigones with no apparent tessellation (Figure 4d). In contrast, F. moggridgei shows yellow perigones with a green or purple tessellation (Figure 4e). Indeed, the shape of the perigone is very similar between the two taxa. This occurrence, combined with the fact that F. moggridgei occasionally co-occurs not with F. tubaeformis but rather with F. burnatii (yellow-blue dots, Figure 4d) increases the likelihood of misidentification, and may also erroneously suggest a genetic relatedness among the two taxa. The occasional occurrence in France of individuals of F. burnatii growing just a few metres from F. moggridgei has been reported by one of us (VN). In the past, the co-occurrence of F. moggridgei and F. burnatii in Italy was documented by Planchon [57], who, referring to a letter of Burnat, recorded a note of F. burnatii being interspersed to F. moggridgei at the boundary between the Pesio and Vermenagna Valleys (Colle del Carbone, Cuneo).
Based on the narrower outer tepals and longer outer nectaries, ovaries, stigma lobes and anthers, we were able to separate F. moggridgei from F. tubaeformis on morphological grounds [23]. However, acknowledging the general morphological overlap occurring between these two taxa, we firstly had deemed more opportune to recognise them at subspecies level, also considering that they show contiguous, partially interdigitated, but never overlapping ranges [18] (yellow-green dots, Figure 3D). Our results, however, point to a clear phylogenetic separation between the two taxa, whose sister relationship is not statistically supported. Genetic isolation by environment or ecology might have occurred between F. tubaeformis and F. moggridgei, as outlined by Dagnino et al. [64]. These authors found a relatively high degree of niche differentiation among the two taxa. Based on this observation, they inferred that F. moggridgei is a derivative taxon with a smaller range [64].
4.4. Fritillaria involucrata
Fritillaria involucrata has been included so far only in two large phylogenetic analyses at genus level [12,13] based on combined cpDNA datasets (matK + rbcL + rpl16). Both papers agreed in placing F. involucrata (two accessions in Day et al. [12] and one accession in Huang et al. [13]) in a clade with F. tubaeformis. Our results do not support a close phylogenetic relationship of the two taxa and place F. involucrata in a strongly supported polytomy with the remaining alpine species (Figure 1 and Figure 2). The sequence of F. tubaeformis used by Day et al. [12] and by Huang et al. [13] correspond to a living accession at Kew (voucher Chase 2558) [11,12]. Images of the living specimen were not available at the time of publication [12,13,65] but it is still possible to determine the taxonomic identity of this material and distinguish it based on morphological traits. F. involucrata, like the taxa of the F. tubaeformis complex, has campanulate flowers. Yet, its tepals often display a distinctive mix of reddish-purple hues with yellow or greenish markings (Figure 4f). Both the background colour and tessellation pattern are highly variable in this species, a feature that may have caused some confusion or misidentification. However, a notable feature of F. involucrata is that the upper leaves are typically fused, forming a whorl of three leaves that resemble a false involucre.
5. Conclusions
Based on the morphological differences between the analysed samples outlined throughout the paper (see, for example, differences in morphology of tepals and leaves within the endemic species) along with the support received from phylogenetic analyses and the presence of characteristic indels, we believe that the specific status of these taxa was consistently confirmed. Despite the marked morphological variability, a clear congruence between morphological traits and phylogenetic species boundaries was demonstrated within the F. tubaeformis complex.
The scattered distribution pattern of these taxa has probably favoured the origin and maintenance of this diversity. Moreover, clonal propagation by bulbs, in addition to isolation in scattered refugial areas during the climatic oscillations of the Quaternary, might have contributed to the maintenance of relatively high levels of population differentiation as previously found [32,33]. Our phylogeny shed light on the taxonomic status of a group of Fritillaria species with a relatively large range in the Alps. Although the analysis does not fully resolve the relationships within the Fritillaria tubaeformis complex, it supports the phylogenetic independence of the three taxa, by providing additional insights on the high conservation value of the Alpine populations. Molecular evidence aligns well with key diagnostic traits, reinforcing the recognition of F. tubaeformis, F. moggridgei, and F. burnatii as three distinct species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14070785/s1, Figure S1a–e. Maximum Likelihood phylogenetic trees for each plastid region considered (a: matK, b: ndhF, c: rpl16, d: rpoC1 and e: petA-psbJ).
Author Contributions
Conceptualization, M.M. and F.D.; formal analysis, F.D.; molecular laboratory analyses, F.D. and M.A. field work, M.M., V.N. and C.B.; occurrence data curation, M.A.; writing—original draft preparation, F.D. and M.M.; writing—review and editing, F.D., L.P., V.N. and M.M.; supervision, L.P., V.N. and M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported with funds from Ricerca Locale, University of Torino (Mucciarelli, MUCM-RILO 17/20-01). MM and MA acknowledge the support of National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP D13C22001350001, Project title “National Biodiversity Future Center—NBFC”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences employed in this study are openly available at www.ncbi.nlm.nih.gov/genbank/ (accessed on 25 June 2025). All herbarium specimens used in this study are kept in the collections of different institutions (see Table 1 for details).
Acknowledgments
The authors are particularly grateful to Bruno Gallino at the Centro Biodiversità Vegetale of the Ente di gestione Aree Protette delle Alpi Marittime (Chiusa di Pesio, Cuneo) for helping in plant collection and for providing helpful information on population location and dates of flowering. MM is grateful to his wife Wanda for her helpful assistance and patience during the countless hikes in the mountains.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tekşen, M.; Aytaç, Z. The revision of the genus Fritillaria L. (Liliaceae) in the Mediterranean region (Turkey). Turk J. Bot. 2011, 35, 447–478. [Google Scholar] [CrossRef]
- Givnish, T.J.; Zuluaga, A.; Marques, I.; Lam, V.K.; Gomez, M.S.; Iles, W.J.; Ames, M.; Spalink, D.; Moeller, J.R.; Briggs, B.G. Phylogenomics and historical biogeography of the monocot order Liliales: Out of Australia and through Antarctica. Cladistics 2016, 32, 581–605. [Google Scholar] [CrossRef]
- Smyčka, J.; Roquet, C.; Renaud, J.; Thuiller, W.; Zimmermann, N.E.; Lavergne, S. Disentangling drivers of plant endemism and diversification in the European Alps—A phylogenetic and spatially explicit approach. Perspect. Plant Ecol. Evol. Syst. 2017, 28, 9–27. [Google Scholar] [CrossRef]
- Ebersbach, J.; Schnitzler, J.; Favre, A.; Muellner-Riehl, A.N. Evolutionary radiations in the species-rich mountain genus Saxifraga L. BMC Evol. Biol. 2017, 17, 119. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biog. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Casazza, G.; Barberis, G.; Guerrina, M.; Zappa, E.; Mariotti, M.; Minuto, L. The plant endemism in the Maritime and Ligurian Alps. Biogeographia 2016, 31, 73–88. [Google Scholar] [CrossRef]
- Schönswetter, P.; Stehlik, I.; Holderegger, R.; Tribsch, A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol. Ecol. 2005, 14, 3547–3555. [Google Scholar] [CrossRef]
- Holderegger, R.; Thiel-Egenter, C. A discussion of different types of glacial refugia used in mountain biogeography and phylogeography. J. Biogeogr. 2009, 36, 476–480. [Google Scholar] [CrossRef]
- Rix, E.M. Fritillaria. A Revised Classification Together with an Updated List of Species; The Fritillaria Group of the Alpine Garden Society: Edinburgh, UK, 2001; pp. 1–14. [Google Scholar]
- Peruzzi, L. A new infrafamilial taxonomic setting for Liliaceae, with a key to genera and tribes. Plant Biosyst. 2016, 150, 1341–1347. [Google Scholar] [CrossRef]
- Rønsted, N.; Law, S.; Thornton, H.; Fay, M.F.; Chase, M.W. Molecular phylogenetic evidence for the monophyly of Fritillaria and Lilium (Liliaceae; Liliales) and the infrageneric classification of Fritillaria. Molec. Phyl. Evol. 2005, 35, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Day, P.D.; Berger, M.; Hill, L.; Fay, M.F.; Leitch, A.R.; Leitch, I.J.; Kelly, L.J. Evolutionary relationships in the medicinally important genus Fritillaria L. (Liliaceae). Molec. Phyl. Evol. 2014, 80, 11–19. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.Q.; Yu, Y.; Liu, Y.M.; Xie, D.F.; Li, J.; He, X.J.; Zhou, S.D. Molecular phylogenetics and historical biogeography of the tribe Lilieae (Liliaceae): Bi-directional dispersal between biodiversity hotspots in Eurasia. Ann. Bot. 2018, 122, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: Oxford, UK, 2005; ISBN 9780198515340. [Google Scholar]
- Huang, J.; Yu, Y.; Liu, Y.M.; Xie, D.F.; He, X.J.; Zhou, S.D. Comparative chloroplast genomics of Fritillaria (Liliaceae), inferences for phylogenetic relationships between Fritillaria and Lilium and plastome evolution. Plants 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Kadereit, J.W. The role of in situ species diversification for the evolution of high vascular plant species diversity in the European Alps—A review and interpretation of phylogenetic studies of the endemic flora of the Alps. Perspect. Plant Ecol. Evol. Syst. 2017, 26, 28–38. [Google Scholar] [CrossRef]
- Rix, E.M. Fritillaria involucrata: Liliaceae. Curtis’s Bot. Mag. 2000, 17, 179–181. [Google Scholar] [CrossRef]
- Noble, V.; Diadema, K. La Flore des Alpes-Maritimes et de la Principauté de Monaco. Originalité et Diversité; Naturalia Publications: Turriers, France, 2011; p. 504. [Google Scholar]
- Tison, J.M.; Jauzein, P.J.; Michaud, H. Flore de la France Méditerranèenne Continentale; Naturalia Publications: Turriers, France, 2014; pp. 1–2080. ISBN 9782909717906. [Google Scholar]
- Selvaggi, A.; Ebone, A.; Bombonati, D.; Gallino, B. Fritillaria involucrata All. (Liliaceae). In: Selvaggi, A.; Soldano, A.; Pascale, M.; Pascal, R. (eds.). Note Floristiche Piemontesi n. 176-245. Riv. Piem. St. Nat. 2009, 30, 313–340. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E. A second update to the checklist of the vascular flora native to Italy. Plant Biosyst. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Available online: https://www.gbif.org/occurrence/search?/ (accessed on 8 April 2022).
- Mucciarelli, M.; Rosso, P.; Noble, V.; Bartolucci, F.; Peruzzi, L. A morphometric study and taxonomic revision of Fritillaria tubaeformis species complex (Liliaceae). Plant Syst. Evol. 2016, 302, 1329–1343. [Google Scholar] [CrossRef]
- Fiori, A. Nuova Flora Analitica d’Italia; Tipografia di M. Ricci: Firenze, Italy, 1923. [Google Scholar]
- Pignatti, S. Flora d’Italia 1; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.-P. Flora Alpina 1; Zanichelli: Bologna, Italy, 2004. [Google Scholar]
- Zangheri, P. Flora Italica; Cedam: Padova, Italy, 1976. [Google Scholar]
- Conti, F.; Abbate, G.; Alessandrini, A.; Blasi, C. An Annotated Checklist of the Italian Vascular Flora; Palombi Editori: Roma, Italy, 2005; pp. 1–428. [Google Scholar]
- Rix, E.M. Short notes (Liliaceae). Bot. J. Linn. Soc. 1978, 76, 356. [Google Scholar]
- Rix, E.M. Fritillaria L. In Flora Europaea; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 31–34. [Google Scholar]
- Tison, J.M.; de Foucault, B. Flora Gallica: Flore de France; Biotope Èditions: Mèze, France, 2014; pp. 1–1196. [Google Scholar]
- Mucciarelli, M.; Fay, M.F. Plastid DNA fingerprinting of the rare Fritillaria moggridgei (Liliaceae) reveals population differentiation and genetic isolation within the Fritillaria tubiformis complex. Phytotaxa 2013, 91, 1–23. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Ferrazzini, D.; Belletti, P. Genetic variability and population divergence in the rare Fritillaria tubiformis subsp. moggridgei Rix (Liliaceae) as revealed by RAPD analysis. PLoS ONE 2014, 9, e101967. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L. Typification of Fritillaria tubiformis Gren. & Godr., Fritillaria delphinensis f. moggridgei Planch. and Fritillaria delphinensis var. burnatii Planch (Liliaceae) from SW Europe. Candollea 2012, 67, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Qi, M.; Cutler, A.J. A simple method of preparing plant samples for PCR. Nuc. Acids Res. 1993, 21, 4153–4154. [Google Scholar] [CrossRef]
- Ito, M.A.; Kawamoto, Y.; Yukawa, K.T.; Kurita, S. Phylogenetic relationships of Amaryllidaceae based on matK sequence data. J. Plant Res. 1999, 112, 207–216. [Google Scholar] [CrossRef]
- Olmstead, R.G.; Sweere, J.A. Combining data in phylogenetic systematics: An empirical approach using three molecular data sets in the Solanaceae. Syst. Biol. 1994, 43, 67–481. [Google Scholar] [CrossRef]
- Marques, I.; Nieto Feliner, G.; Draper, D.; Martins-Loução, M.A.; Fuertes Aguilar, J. Unravelling cryptic reticulate relationships and the origin of orphan hybrid disjunct populations in Narcissus. Evolution 2010, 64, 2353–2368. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, C.; Lei, L.; Li, C.; Zhang, Y.; Zhou, S. Barcoding the kingdom Plantae: New PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Resour. 2016, 16, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef]
- GBIF.org GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0252572-210914110416597 (accessed on 28 April 2022). [CrossRef]
- GBIF.org GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0252573-210914110416597 (accessed on 28 April 2022). [CrossRef]
- Argagnon, A.; De Barros, G.; Noble, V. SIMETHIS-Flore-CBNMed-Database of Southeastern France vegetation. Veg. Classif. Surv. 2022, 3, 119–120. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Le Vinh, S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological hylogenetic analyses. Molec. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.F.K.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Mennes, C.B.; Lam, V.K.Y.; Rudall, P.J.; Lyon, S.P.; Graham, S.W.; Smets, E.F.; Merckx, V.S.F.T. Ancient Gondwana break-up explains the distribution of the mycoheterotrophic family Corsiaceae (Liliales). J. Biogeogr. 2015, 42, 1123–1136. [Google Scholar] [CrossRef]
- Du, Y.P.; Bi, Y.; Yang, F.P.; Zhang, M.F.; Chen, X.Q.; Xue, J.; Zhang, X.H. Complete chloroplast genome sequences of Lilium: Insights into evolutionary dynamics and phylogenetic analyses. Sci. Rep. 2017, 7, 5751. [Google Scholar] [CrossRef]
- Clennett, J.C.B.; Chase, M.W.; Forest, F.; Maurin, O.; Wilkin, P. Phylogenetic systematics of Erythronium (Liliaceae): Morphological and molecular analyses. Bot. J. Linn. Soc. 2012, 170, 504–528. [Google Scholar] [CrossRef]
- Douglas, N.A.; Wall, W.A.; Xiang, Q.Y.; Hoffmann, W.A.; Wentworth, T.R.; Gray, J.B.; Hohmann, M.G. Recent Vicariance and the Origin of the Rare, Edaphically Specialized Sandhills Lily, Lilium pyrophilum (Liliaceae): Evidence from Phylogenetic and Coalescent Analyses. Mol. Ecol. 2021, 20, 2901–2915. [Google Scholar] [CrossRef]
- Gao, Y.D.; Harris, A.; He, X.J. Morphological and ecological divergence of Lilium and Nomocharis within the Hengduan Mountains and Qinghai-Tibetan Plateau may result from habitat specialization and hybridization. BMC Evol. Biol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Day, P. Studies in the genus Fritillaria L. (Liliaceae). Ph.D. Thesis, Queen Mary University, London, UK, 2017. [Google Scholar]
- Turrill, W.B. The Snake’s Head (Fritillaria meleagris). In The Lily Year Book 1951-2 (Number Fifteen); Synge, P.M., Ed.; Royal Horticultural Society: London, UK, 1951; pp. 108–116. [Google Scholar]
- Tatarenko, I.; Walker, K.; Dyson, M. Biological Flora of Britain and Ireland: Fritillaria meleagris. J. Ecol. 2022, 299, 1704–1726. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 2; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Bovio, M. Flora vascolare della Valle d’Aosta [Vascular flora of Valle d’Aosta]. Testolin Editore: Aosta, Italy, 2014; p. 335. ISBN 978-88-909-4664-6. [Google Scholar]
- Kamari, G.; Zahos, A.; Siagou, I. A new yellow-flowered Fritillaria species (Liliaceae) from Mt. Tisseon, continental Greece and its taxonomic relationships. Phytotaxa 2017, 328, 227–242. [Google Scholar] [CrossRef]
- Planchon, J.E. Sur les espèces de fritillaires de France, à propos des Icones et d’un manuscrit inédit de Pierre de Belleval. Bull. Soc. Bot. France 1873, 20, 96–124. [Google Scholar] [CrossRef]
- Dagnino, D.; Minuto, L.; Casazza, G. Niche divergence between putative taxa: Ecological niche models in taxonomic researches. Plant Biol. 2017, 19, 1003–1011. [Google Scholar] [CrossRef]
- Kelly, L.; Renny-Byfield, S.; Pellicer, J.; Macas, J.; Novak, P.; Neumann, P.; Lysak, M.A.; Day, P.D.; Berger, M.; Fay, M.F.; et al. Analysis of the giant genomes of Fritillaria (Liliaceae) indicates that a lack of DNA removal characterises extreme expansions in genome size. New Phytol. 2015, 208, 596–607. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).