1. Introduction
Carotenoids serve multifaceted biological roles in nature, functioning not only as essential regulatory phytohormones, but also as precursors for volatile aromatic compounds and vivid chromophores [
1,
2,
3,
4]. Among plant-derived volatile carotenoid derivatives, β-ionone has garnered particular attention due to its distinctive properties and applications [
5]. Characterized by a 13-carbon monocyclic terpenoid skeleton, β-ionone is ubiquitously distributed across various plant taxa, notably
Osmanthus fragrans,
Vitis vinifera, and
Camellia sinensis [
6]. This compound exhibits an exceptionally low olfactory detection threshold in both aqueous and gaseous phases, presenting characteristic sensory notes of warm woodiness, berry undertones, and violet florals. Regulatory agencies, including China’s National Health Commission (2014), the U.S. Flavor and Extract Manufacturers Association (FEMA), and the Food and Drug Administration (FDA), have approved β-ionone as a safe synthetic flavoring agent [
7]. Beyond alimentary applications, it has extensive utilization in cosmetic formulations (e.g., premium perfumes, shampoos), personal care products, and household cleaning agents. Pharmacological investigations further reveal its broad spectrum of bioactivities, encompassing anticancer, anti-inflammatory, antiviral, antioxidant, antimicrobial, antihelmintic, and hypolipidemic properties [
8].
Although the chemical synthesis of β-ionone remains technically feasible, this approach faces increasing challenges from market demands for natural-sourced alternatives, thereby increasing the demand and commercial value of biologically derived β-ionone [
6,
9]. Conventional synthesis routes involving pseudoionone cyclization require stringent reaction conditions with strong acids, often yielding undesirable byproducts and demonstrating poor selectivity. Subsequent isomerization and oxidation steps necessitate meticulous process control to ensure product purity [
6,
9]. Extraction methodologies, including steam distillation and solvent-based techniques, present inherent limitations, such as energy intensiveness, thermal degradation risks, and reliance on toxic solvents, requiring complex purification [
9]. In light of these challenges, contemporary research has identified alternative biosynthetic pathways by using the oxidative transformation of C40 carotenoids [
10,
11,
12,
13,
14], catalyzing a heightened interest in biotechnological production strategies that leverage enzymatic bioconversion and synthetic biology platforms.
The enzymatic cleavage of carotenoids, mediated by carotenoid cleavage dioxygenases (CCDs), represents an evolutionarily conserved biochemical mechanism spanning the bacterial, fungal, plant, and animal kingdoms [
12]. Nevertheless, efficient β-ionone biosynthesis confronts significant bottlenecks, particularly the catalytic constraints imposed by CCDs [
5,
11,
14,
15]. Phylogenetic analyses categorize CCDs into seven distinct subfamilies: CCD1, CCD2, CCD4, CCD7, CCD8, CCD10, and 9-cis-epoxycarotenoid dioxygenase (NCED) [
16,
17,
18,
19,
20,
21]. Subcellular localization studies reveal the cytoplasmic distribution of CCD1 versus plastid-localized CCD2/4/7/8/NCED, with CCD10 exhibiting dual compartmentalization [
22,
23,
24]. Functional diversification among these isoforms introduces complexity to β-ionone biosynthesis. Current research emphasizes two predominant plant CCD subfamilies (CCD1 and CCD4) capable of catalyzing C9-C10/C10′-C9′ double-bond cleavage in β-carotene to generate β-ionone [
17,
25,
26]. Notably, comparative enzymatic analyses demonstrate substantial variation in substrate specificity and catalytic efficiency across CCD variants. In a comprehensive screening of twelve CCD enzymes (nine CCD4 and three CCD1 isoforms) expressed in
E. coli ε-carotene systems, CCD4 homologs generally exhibited inferior activity relative to CCD1 counterparts, underscoring functional heterogeneity within the CCD superfamily [
15]. Since the seminal identification of
Arabidopsis thaliana CCD1 (
AtCCD1) in 2001 [
27], subsequent studies have achieved significant progress in CCD1 engineering and characterization from diverse sources [
14]. However, substantial inter-species variability in catalytic performance persists among CCD1 orthologs, necessitating the systematic exploration of novel enzymes.
Given the observed functional variability among CCD1 orthologs, we hypothesized that evolutionary differences between woody and herbaceous plants may contribute to distinct catalytic characteristics. To test this, we selected and characterized CCD1 genes from Olea europaea (a perennial woody plant) and Ipomoea nil (a fast-growing herbaceous species), aiming to compare their enzymatic profiles in the context of β-ionone biosynthesis.
To address these limitations, this investigation systematically evaluates two CCD1 homologs—OeCCD1 (from Olea europaea) and InCCD1 (from Ipomoea nil)—using a multidisciplinary approach. Experimental workflows encompassed the following: (1) gene identification and molecular cloning; (2) heterologous expression in E. coli; (3) in vitro/vivo functional validation; (4) expression kinetics and biochemical profiling; and (5) the evaluation of the potential for high-yield β-ionone biosynthesis. Our findings establish these CCD1s as versatile biocatalysts, providing fundamental insights for CCD resource expansion, structure–function elucidation, and synthetic biology applications. This work lays critical groundwork for the engineering of CCD variants and the development of cell factory platforms that are aimed at the high-efficiency production of volatile aroma compounds from carotenoid precursors.
2. Experimental Section
2.1. Chemicals
β-Ionone, β-apo-8′-carotenal, lycopene, β-carotene, zeaxanthin, lutein, astaxanthin, and all-trans-retinal were acquired from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Molecular biology reagents, such as isopropyl-β-D-thiogalactoside (IPTG), chloramphenicol, and ampicillin, were sourced from Sangon Biotech (Shanghai, China). High-performance liquid chromatography (HPLC)-grade solvents, specifically ethyl acetate, methanol, and acetone, were procured from Sigma-Aldrich Co., Ltd. (Shanghai, China). All other reagents used were of analytical grade and were obtained from reputable commercial sources.
2.2. Plasmid Construction and Sequence Analysis of CCD1s
All strains and plasmids used in this study are listed in
Table S1. The carotenoid cleavage dioxygenase gene
OeCCD1 (GenBank accession number XM_023040081.1) from
Olea europaea and
InCCD1 (GenBank accession number XM_019295309.1) from
Ipomoea nil underwent synthetic engineering, incorporating codon optimization tailored for expression in
E. coli. To facilitate molecular cloning, recognition sites for the restriction enzymes
BamH I and
EcoR I were strategically introduced at the 5′ and 3′ termini of the CCD1 coding sequence, respectively. Following enzymatic digestion with
BamH I and
EcoR I, the CCD1 genes were ligated into the bacterial expression vectors pETDuet-1 and pGEX-2T-1, generating the recombinant constructs pETDuet-1-CCD1s and pGEX-2T-CCD1s, respectively.
Phylogenetic relationships were elucidated through the construction of a neighbor-joining (NJ) tree using MEGA 11 software. Multiple sequence alignments were conducted using Clustal W, enabling a comparative genomic analysis of CCD1s with homologous sequences.
2.3. Protein Expression and Purification
The recombinant plasmid pGEX-2T-CCD1s were successfully transformed into E. coli BL21 (DE3) using the heat shock method. For protein expression induction, the transformed E. coli cells were initially cultured under stationary conditions at 37 °C overnight, then inoculated into 50 mL of Luria–Bertani (LB) broth and incubated at 37 °C with continuous agitation at 180 rpm. Upon reaching an optical density (OD600) within the range of 0.6–0.8, expression was induced by adding IPTG to a final concentration of 0.1 mM, followed by further incubation at a reduced temperature of 28 °C for 21 h to optimize protein yield.
In the subsequent phase, culture conditions for E. coli BL21 (DE3) harboring pGEX-2T-CCD1s were refined to maximize expression efficiency. Post-cultivation, cells were collected by centrifugation at 10,000× g for 20 min at 4 °C, resuspended in 5 mL of 1× Phosphate-Buffered Saline (PBS, pH 7.4) containing 5 mM Na-ascorbate to prevent oxidative damage, and subjected to cell lysis via ultrasonication under ice-cold conditions to minimize thermal denaturation. Cellular debris was then removed by additional centrifugation at 10,000× g for 40 min at 4 °C.
Purification of the GST-tagged recombinant protein was achieved through GST affinity chromatography, following the protocols provided by Sangon Biotech (Shanghai, China) [
28]. The integrity of the purified protein was assessed via Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE). Protein concentration was quantified using the Bradford assay, with Bovine Serum Albumin (BSA) as a calibration standard, according to the guidelines provided by Sangon Biotech (Shanghai, China).
2.4. Preparation of Carotenoids for In Vitro Assays
The methodology for carotenoid preparation was adapted from a protocol described in the prior literature, with minor modifications [
29]. Carotenoid substrates were adjusted to a final concentration of 40 μM for experimental procedures. To facilitate carotenoid micelle formation, substrates were first dissolved in 50 μL of dichloromethane, followed by the addition of 50 μL of Triton X-100 in ethanol solution, maintaining a 1:100 molar ratio relative to the carotenoid substrates. This homogenized mixture was then subjected to vacuum centrifugation for desiccation. The desiccated residues were reconstituted in ultrapure water and stored at 4 °C until they were required for subsequent experiments.
2.5. Substrate Specificity Assays In Vitro
Experiments to elucidate the substrate specificity of CCD1s were conducted in accordance with a methodology previously described in the literature, with slight modifications for adaptation to the current study’s objectives. Reaction volumes were standardized to 1 mL, comprising an optimized sodium phosphate buffer, the designated substrate, and 100 μL of the CCD1 enzyme. This reaction mixture was then incubated at a constant temperature of 35 °C for an overnight duration. Subsequent to the incubation period, 0.5 mL of chloroform was introduced to the reaction milieu, functioning dually as an enzymatic inhibitor and as a solvent for the extraction of assay products.
For the analytical determination of C13 compounds, a Trace ISQ-LT Gas Chromatography–Mass Spectrometry (GC-MS) system (Thermo Fisher Scientific, Waltham, MA, USA) was utilized. The system was equipped with a DB-5MS analytical column (dimensions: 30 m × 0.25 mm × 0.25 μm) and integrated with a flame ionization detector. The operational interface temperature was meticulously maintained at 250 °C. Electron impact ionization (EI-MS) methodology was employed, utilizing an ionization energy of 70 eV, and the mass spectrometric analysis was conducted over a range spanning from 50 to 450 m/z. Spectral data acquisition and subsequent analyses were performed using the Xcalibur software, version 1.4, facilitating a comprehensive evaluation of the generated mass spectra.
2.6. Two-Plasmid System for In Vivo Tests on Carotenoid Cleavage Activity
E. coli strains, molecularly engineered to facilitate the biosynthesis of specific carotenoids, namely β-carotene (incorporating plasmid pAC-BETA, Addgene plasmid #53272) and zeaxanthin (utilizing plasmid pAC-ZEAX, Addgene plasmid #53274), were subjected to transformation with either the target expression vector pETDuet-1-CCD1s or the comparative control vector pETDuet-1.
Recombinant protein synthesis in these genetically modified organisms was induced by administering 0.1 mM IPTG. Volatile organic compounds were subsequently collected using Solid Phase Microextraction (SPME) and analytically characterized via GC-MS, following the protocols described in the existing scientific literature.
The extraction of carotenoids from either the bacterial cellular matrices or the cultivation medium was executed according to previously validated methodologies. Analytical quantification and qualitative assessment of the carotenoids were conducted using HPLC (1200 Infinity II, Agilent Technologies, Little Falls, DE, USA). This analysis employed a reversed-phase Zorbax Eclipse Plus C18 column (5 μm particle size, dimensions 1.5 × 4.6 mm, Agilent Technologies, Little Falls, DE, USA). Chromatographic separation was facilitated using a mobile-phase composition of methanol, ethyl acetate, and water in a volumetric ratio of 50%/48%/2% (v/v/v), under isocratic conditions, at a flow rate of 1 mL/min over a period of 20 min at a controlled temperature of 30 °C. The detection of carotenoids was achieved at a Diode Array Detector (DAD) wavelength of 450 nm, enabling the precise identification and quantification of these phytochemicals.
2.7. Enzymatic Activity Assay of Recombinant CCD1
The enzymatic activity of recombinant CCD1 was evaluated using β-apo-8′-carotenal as the substrate. The standard reaction system consisted of 100 mM phosphate buffer (pH 7.0), β-apo-8′-carotenal (40 μM), 200 μL of pure enzyme, and water, and was incubated at 30 °C in a water bath for 20 min.
The enzymatic activity of recombinant CCD1 (U) is defined as the amount of enzyme required to catalyze the formation of 1 nmol of β-ionone from β-apocarotenoid-8′-aldehyde per minute. Enzymatic activity was calculated with reference to a standard curve of β-ionone.
2.8. Study of the Enzyme Properties of Recombinant CCD1
2.8.1. Determination of Optimum Temperature and Temperature Stability
A temperature gradient ranging from 30 °C to 65 °C was established to assess the catalytic activity of the enzyme under different temperatures. The highest measured enzyme activity was defined as 100%, and the relative enzyme activities at various temperatures were calculated accordingly. To evaluate temperature stability, enzyme solutions were incubated at 40 °C, 45 °C, and 50 °C for different durations in phosphate buffer (100 mM, pH 7.0) without a substrate, followed by introduction into the initial reaction system for catalytic reactions to determine the residual activity and temperature stability. The treated enzymes were then introduced into the reaction system to determine the residual activity. The activity of the unheated enzyme was considered to be 100% when calculating the relative enzyme activity.
2.8.2. Determination of Optimum pH and pH Stability
At the optimum temperature, citric acid–sodium hydroxide buffer (pH 4.0–7.0) and glycine–sodium hydroxide buffer (pH 8.0–11.0) were used to adjust the pH of the reaction system. The highest enzyme activity measured was defined as 100%, and the relative enzyme activities at different pH levels were calculated. To assess pH stability, the CCD1 enzyme was incubated in 100 mM citric acid–sodium hydroxide buffer (pH 4.0–7.0) and glycine–sodium hydroxide buffer (pH 8.0–11.0) at 4 °C for 8 h. Subsequently, the treated enzyme was introduced into the reaction system to determine the residual activity. The activity of the untreated enzyme was defined as 100% for calculating the relative enzyme activity.
2.8.3. Influence of Ethanol Concentration and Fe2+ Concentration on CCD1 Enzymatic Activity
Ethanol (0–25% v/v) and Fe2+ (0–1 mM) were added to the reaction system. The enzyme activity in the absence of these additives was used as a control (100% activity), and the relative enzyme activities under various conditions were calculated.
2.8.4. Effects of Metal Ions and EDTA on Enzymatic Activity
The reaction system was supplemented with 1 mM of various metal ions or 5 mM EDTA to determine their effects on enzymatic activity. The enzyme activity in the absence of metal ions and EDTA was used as a control (100% activity), and the relative enzyme activities were calculated accordingly.
2.8.5. Determination of Kinetic Constants
Varying concentrations of substrates were added to the reaction system, and enzyme activity was measured under optimal reaction conditions. The Michaelis–Menten constant (Km) and maximum velocity (Vmax) were calculated using nonlinear regression fitting in GraphPad Prism 9.0 software.
2.8.6. Data Processing and Replicates
All enzyme activity assays and metal ion effect experiments were performed in three independent replicates (n = 3) under identical conditions. The values presented in the figures and tables represent the arithmetic mean of these measurements. Although standard deviations are not shown in some figures for clarity, the variability among replicates was generally within 10% of the mean.
2.9. Culture Conditions of E. coli
Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 1% NaCl) was used for the molecular cloning and preparation of E. coli seed cultures. For β-carotene and β-ionone production, the seed culture was inoculated into 2YT medium (1.6% tryptone, 1% yeast extract, 0.5% NaCl) supplemented with 5 g/L glycerol as the initial carbon source. Cultures were grown in 250 mL shake flasks at 37 °C and 200 rpm. When the optical density at 600 nm (OD600) reached 0.6–0.8, IPTG was added to induce protein expression. Simultaneously, 10% (v/v) dodecane was added to the culture to capture volatile products (β-ionone). The cultures were then incubated at 30 °C and 200 rpm for an additional 48 h. Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 1% NaCl) was utilized for molecular cloning and the preparation of E. coli seed solutions. To produce β-carotene and β-ionone, the seed culture was inoculated into 2YT medium (1.6% tryptone, 1% yeast extract, 0.5% NaCl) with 5 g/L of glycerol as the initial carbon source. This culture was maintained in a 250 mL shake flask at 37 °C and 200 rpm. Upon reaching an optical density at 600 nm (OD600) of 0.6–0.8, appropriate concentrations of IPTG were added. Simultaneously, 10% (v/v) dodecane was introduced to collect volatile products (β-ionone). The cultures were then further incubated at 30 °C and 200 rpm for an additional 48 h.
2.10. Detection of β-Ionone Concentration Using HPLC
β-Ionone concentrations were quantitatively analyzed using an HPLC 1200 system equipped with a C18 column. The mobile phase consisted of distilled water (A) and methanol (B) in a ratio of 15:85. Chromatographic separation was performed for 15 min at a flow rate of 1 mL/min, with the column temperature maintained at 30 °C. Detection was achieved by monitoring absorbance at 300 nm. The optimal temperature, pH, stability, and effects of Fe2+ and ethanol on enzyme activity are expressed as percentages of residual enzymatic activity relative to control conditions.
3. Results and Discussion
3.1. Gene Mining and Screening of CCD1
Carotenoid cleavage dioxygenase (CCD) enzymes are currently classified into seven families based on their functions and characteristics: CCD1, CCD2, CCD4, CCD7, CCD8, CCD10, and NCEDs. Research has shown that the CCD1 enzyme family demonstrates superior specificity for carotenoid catalytic cleavage compared to other CCD enzyme families, which directed our focus towards CCD1 enzymes. Based on the reported highly catalytic PhCCD1 sequence for carotenoids [
27], potential CCD1 genes were identified using BLAST analysis on the NCBI database (BLAST+ version 2.16.0). Two CCD1 genes were selected and synthesized:
OeCCD1 (XM_023040081.1) from olive (
Olea europaea) and
InCCD1 (XM_019295309.1) from morning glory (
Ipomoea nil).
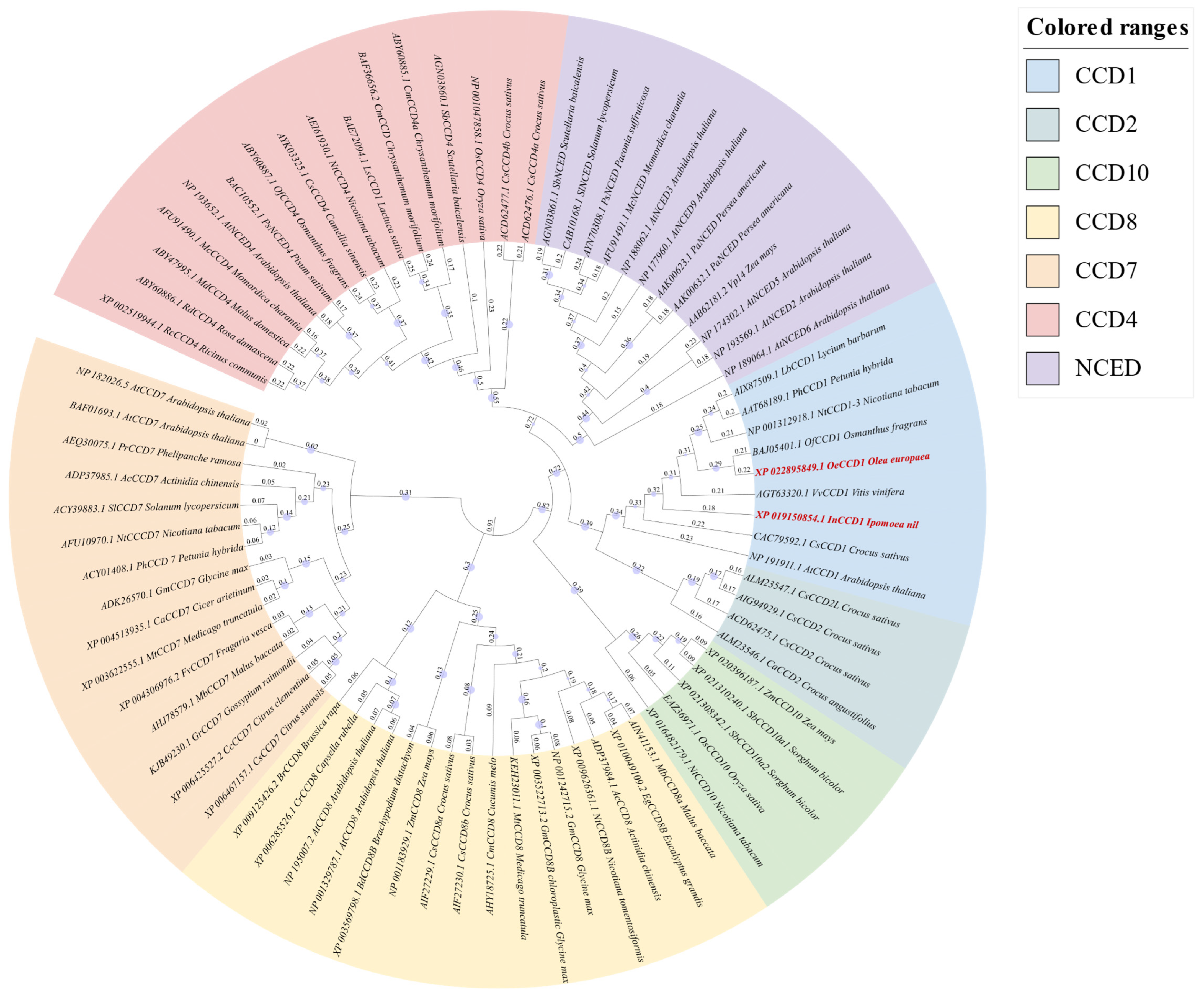
A phylogenetic tree was constructed using MEGA 11.0 software based on the neighbor-joining method, incorporating
OeCCD1,
InCCD1, and other CCD enzymes from various sources (
Figure 1). The analysis confirmed the classification of CCD enzymes into seven families: CCD1, CCD2, CCD4, CCD7, CCD8, CCD10, and NCEDs. The phylogenetic analysis revealed close evolutionary relationships between the selected CCD1 enzymes and previously characterized CCD1 enzymes from other plant species. Specifically,
OeCCD1 was closely related to
OfCCD1 from
Osmanthus grans, and
InCCD1 was closely related to
CsCCD1 from
Crocus sativus.
Sequence analysis revealed that the
OeCCD1 gene is 1629 bp long, encoding 542 amino acids. The
InCCD1 gene is 1704 bp long, encoding 567 amino acids. A multiple sequence alignment was performed comparing these two CCD1 sequences with the well-characterized
PhCCD1 from
Petunia hybrids, which is known for its high catalytic efficiency towards carotenoid substrates (
Figure S1). The analysis demonstrated a high degree of similarity among CCD1 sequences from different plant sources. Specifically,
OeCCD1 showed 79.62% similarity and
InCCD1 showed 75.39% similarity.
3.2. Optimization of CCD1 Expression Conditions
Previous studies have reported challenges in achieving a soluble expression of plant-derived CCD proteins [
27]. To enhance the soluble expression of the synthesized CCD1 proteins, we employed two strategies: (1) the addition of a GST tag to the original gene sequence by assembling both CCD1 genes in the plasmid pGEX-2t-1, a vector containing a t7 promoter, and a GST tag; and (2) the optimization of the expression conditions. Furthermore, considering that CCD1 is a non-heme iron-dependent enzyme [
30] and that its active center may depend on Fe
2+, we investigated the effect of Fe
2+ on enzyme activity. These optimizations were carried out in LB medium.
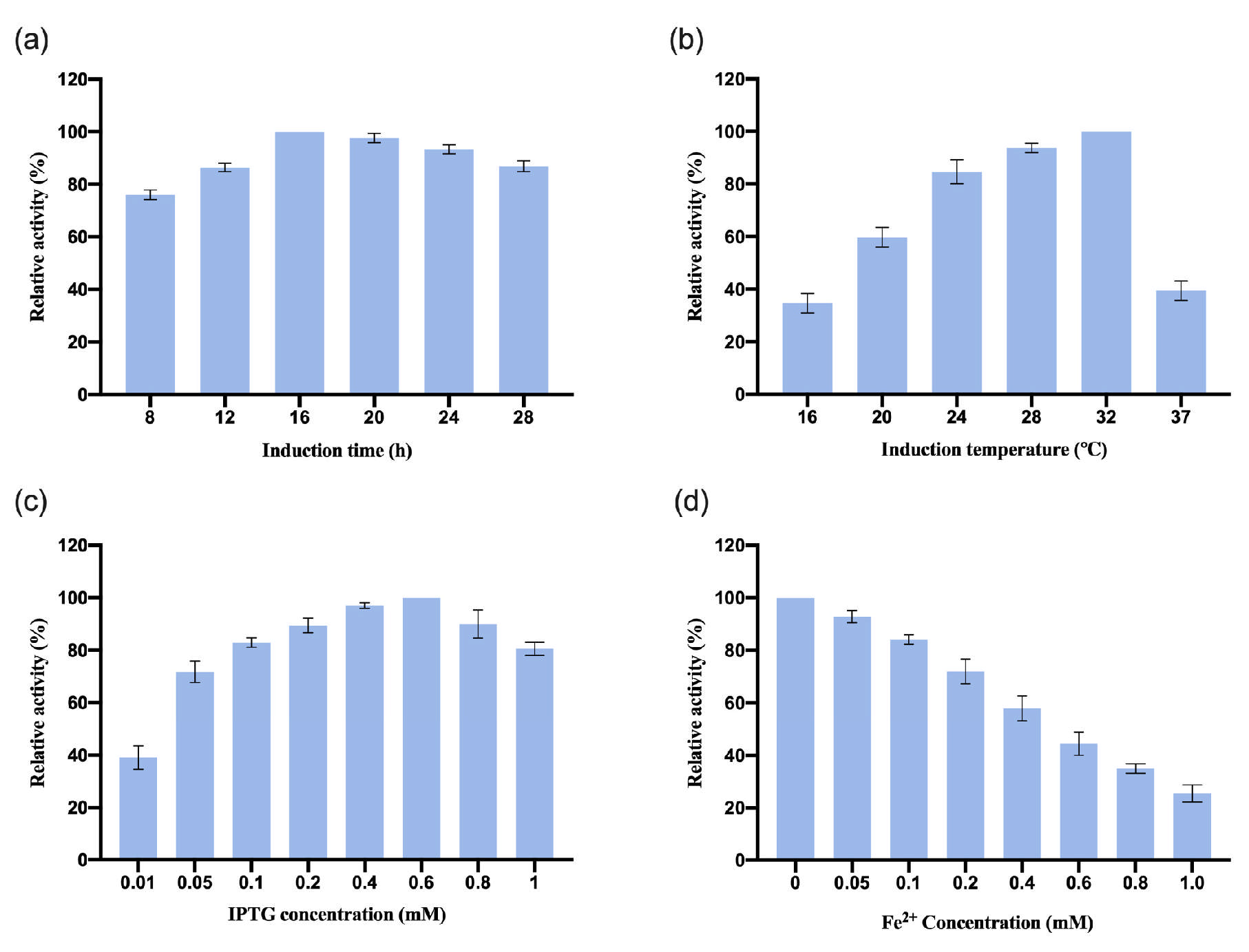
3.2.1. Optimization of OeCCD1 Expression Conditions
After we assembled
OeCCD1 in the plasmid pGEX-2t-1, the recombinant plasmid pGEX-2T-
OeCCD1 was transformed into
E. coli BL21 strains and the induction conditions were optimized. The results are shown in
Figure 2. The results show that the optimal induction time for
OeCCD1 was 24 h. When the induction temperature was below 24 °C, the relative enzyme activity was less than 60%. The relative enzyme activity was highest when the induction temperature reached 28 °C. The expression of
OeCCD1 depended on IPTG, with the highest enzyme activity achieved at 0.1 mM IPTG. The enzyme activity of
OeCCD1 decreased with increasing IPTG concentration, and excessive IPTG significantly reduced the enzyme activity of
OeCCD1. In addition, the concentration of Fe
2+ had a significant inhibitory effect on the enzyme activity of
OeCCD1; at 1 mM Fe
2+ concentration, the enzyme activity of
OeCCD1 was less than 60% of the maximum enzyme activity. While CCD1 has previously been characterized as Fe
2+-dependent [
30], our results reveal a significant reduction in
OeCCD1 activity at 1 mM Fe
2+ concentration (
Figure 2d). This finding suggests that
OeCCD1 does not depend on exogenous Fe
2+ and that endogenous trace-metal ions present in the culture medium may be sufficient for its enzymatic function. The discrepancy with earlier findings could stem from differences in enzyme conformation, expression systems, or metal cofactor incorporation efficiencies.
3.2.2. Optimization of InCCD1 Expression Conditions
The recombinant plasmid pGEX-2T-
InCCD1 was transformed into the
E. coli BL21 strain, and the induction conditions were also optimized. The results are shown in
Figure 3. The results indicate that the optimal induction time for
InCCD1 was 16 h. The relative enzyme activity was highest when the induction temperature reached 32 °C. The expression of
InCCD1 depended on IPTG, with the maximum enzyme activity achieved at 0.6 mM IPTG. The enzyme activity of
InCCD1 decreased with increasing concentrations of IPTG, and excessive IPTG significantly reduced the enzyme activity of
InCCD1. Additionally, the concentration of Fe
2+ had a notable inhibitory effect on
InCCD1 enzyme activity; at a Fe
2+ concentration of 1 mM, the enzyme activity of
InCCD1 was less than 40% of the maximum activity. Similar to
OeCCD1, the enzymatic activity of
InCCD1 was notably suppressed at 1 mM Fe
2+ concentration (
Figure 3d). This observation implies that minimal trace metal availability in
E. coli expression systems may suffice for catalytic function without additional supplementation.
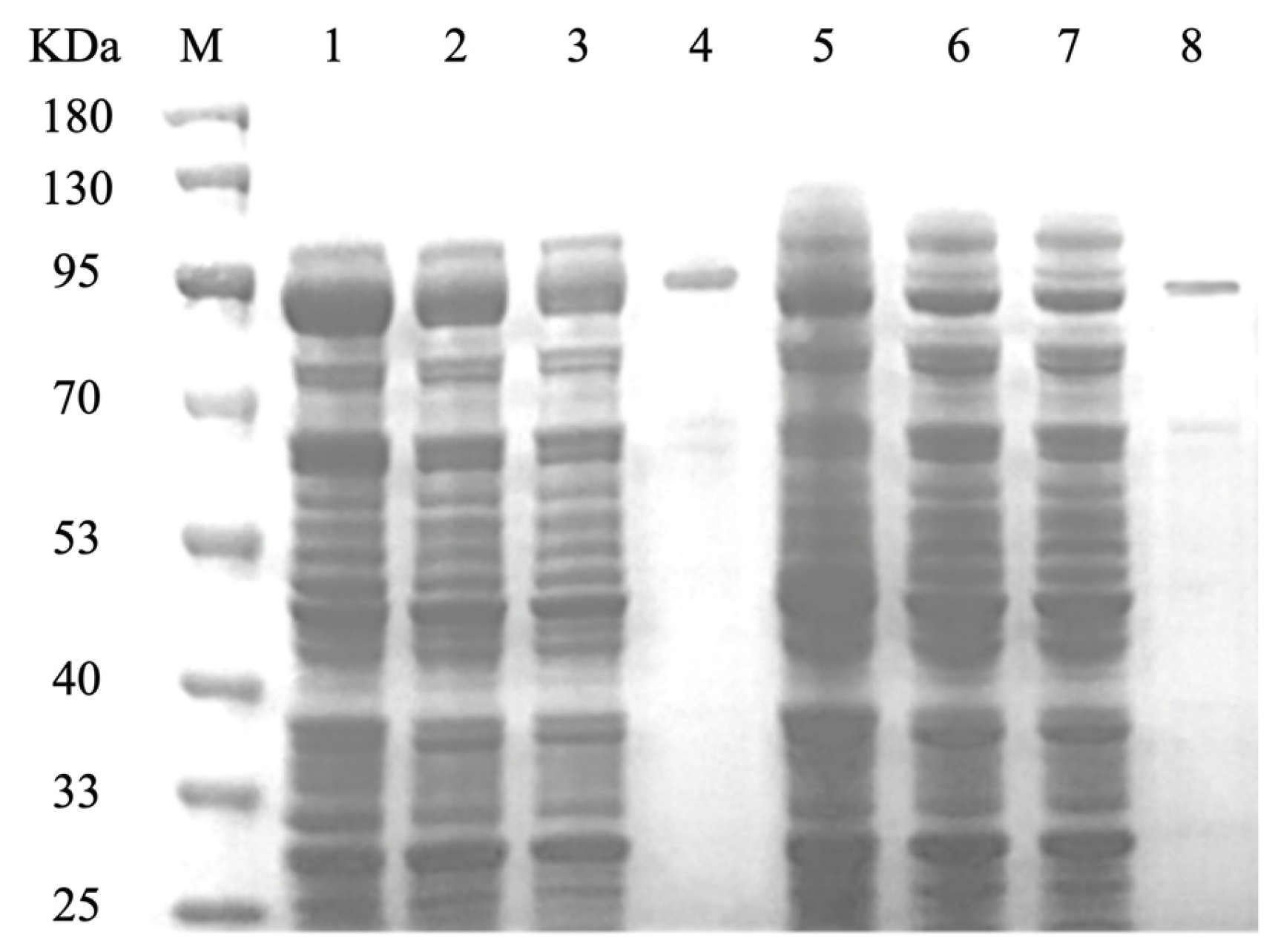
3.3. Purification of CCD1 Protein
The recombinant strains OeCCD1 and InCCD1 were cultured under their respective optimal expression conditions, and the crude enzyme solutions were collected. The crude enzyme solutions were purified using a GST-tagged affinity chromatography column. The collected elution was analyzed for purification results via SDS-PAGE, as shown in
Figure 4. The molecular weights of the protein single bands for OeCCD1 and InCCD1 were approximately 87.43 kDa and 89.55 kDa, respectively, which are consistent with the predicted molecular weights.
3.4. Study of the Enzymatic Properties of CCD1
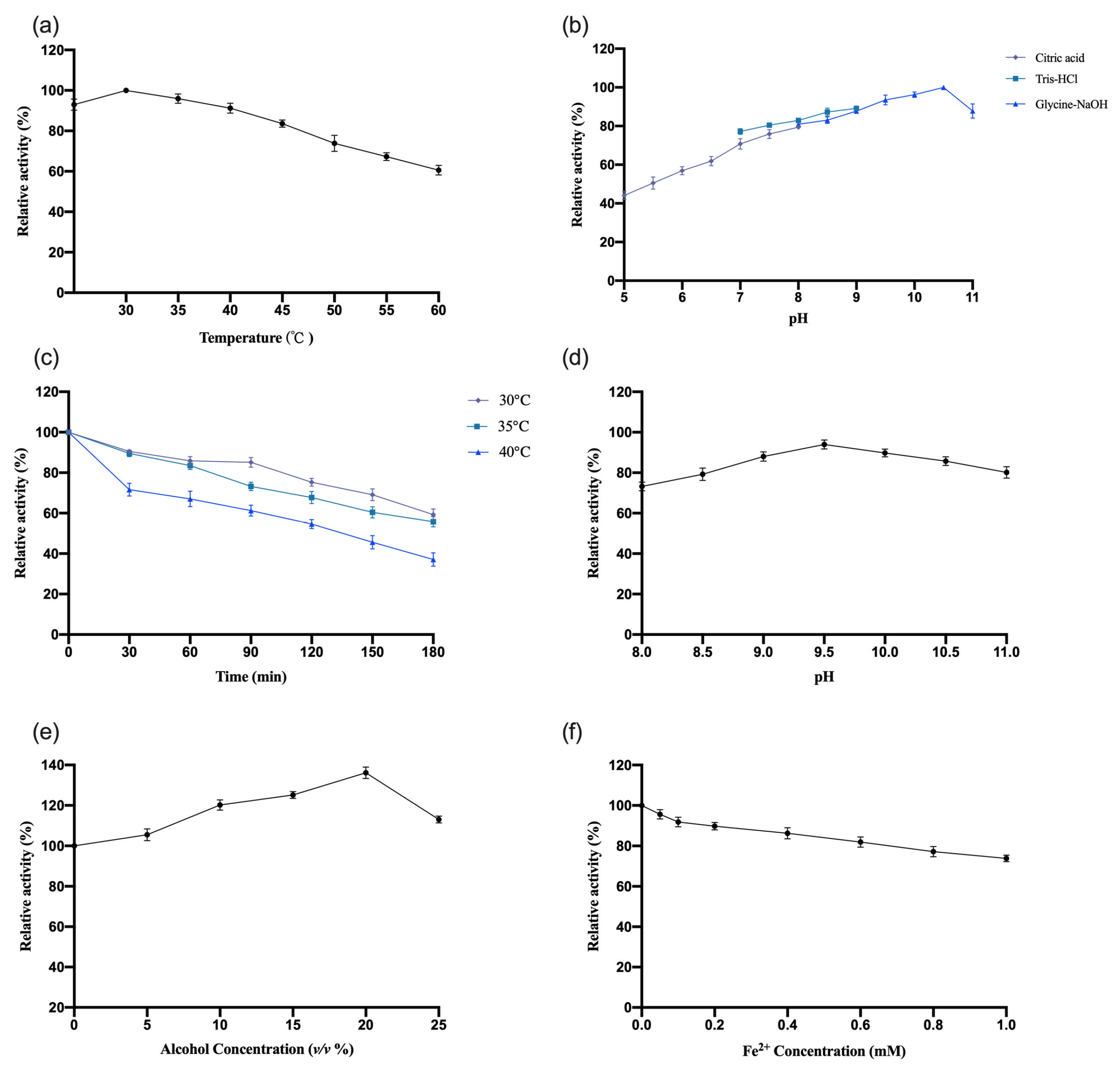
3.4.1. Study of the Enzymatic Properties of OeCCD1
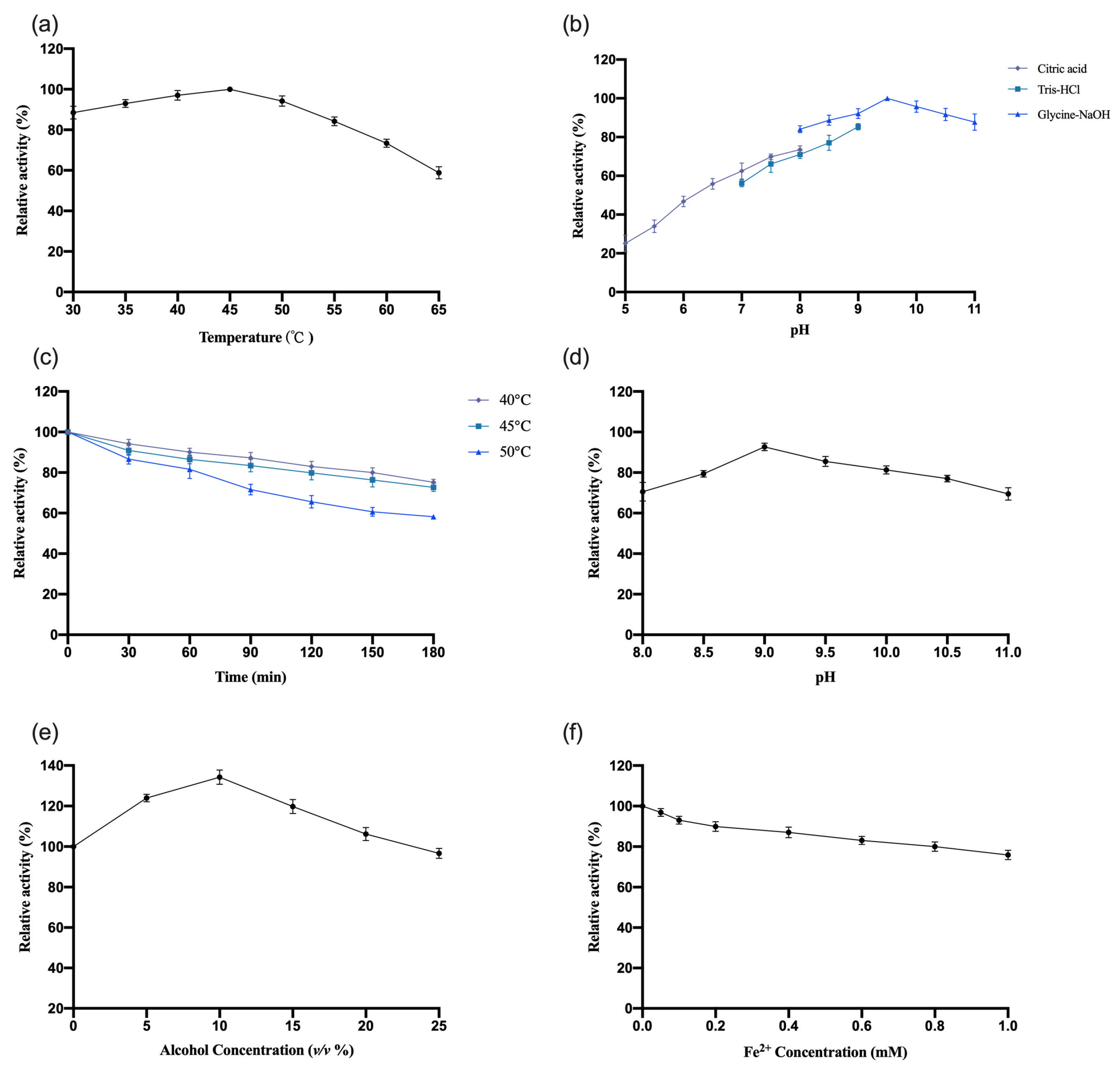
The enzymatic properties of recombinant
OeCCD1 were characterized using the purified enzyme, with β-apo-8′-carotenal as the substrate. The results are shown in
Figure 5. This study indicates that the optimal reaction temperature for the
OeCCD1 enzyme was 45 °C, maintaining over 80% of maximal activity within the temperature range of 30–55 °C. The enzyme activity of
OeCCD1 was measured in buffer solutions with a pH ranging from 5.0 to 11.0 (pH 5.0–8.0: 0.5 M citrate-Na
2HPO
4 buffer; pH 7.0–9.0: 0.5 M Tris-HCl buffer; pH 8.0–11.0: 0.5 M Gly-NaOH buffer). Recombinant
OeCCD1 exhibited higher activity in Gly-NaOH buffer, with an optimal pH of 9.5. Within the pH range of 8.0–11.0, its activity remained above 80% of the maximum enzyme activity. Acidic conditions were unfavorable for the expression of recombinant
OeCCD1, as its activity dropped below 40% of the maximum enzyme activity when the pH was below 6.0. After purification,
OeCCD1 retained more than 60% of its maximum enzyme activity after being incubated at 40 °C, 45 °C, and 50 °C for 3 h without a substrate. The recombinant
OeCCD1 demonstrated good pH stability; after being incubated at 4 °C for 8 h within the pH range of 8.0–11.0, its residual activity remained above 70% of the initial enzyme activity. Additionally, the recombinant
OeCCD1 showed good tolerance to ethanol; the enzyme activity reached its peak with 10% ethanol in the reaction system, and when the ethanol concentration reached 25%, the enzyme activity was similar to that of the enzyme activity without ethanol. The addition of Fe
2+ in the reaction system had a certain inhibitory effect on
OeCCD1 enzyme activity, with the enzyme activity measuring around 80% of that without Fe
2+ when 1 mM Fe
2+ was added. This phenomenon may be due to the trace elements present in the growth medium being sufficient for CCD1 expression, which aligns with the reported enzymatic properties of
HaCCD1 [
31] and
MnCCD1 [
32].
3.4.2. Study of the Enzymatic Properties of InCCD1
The enzymatic properties of recombinant
InCCD1 were investigated using the same methodology as previously described for
OeCCD1. The results are shown in
Figure 6. The recombinant
InCCD1 exhibited higher activity in the Gly-NaOH buffer, with an optimal pH of 10.5. Within the pH range of 8.0–11.0, the activity maintained over 80% of the maximum enzyme activity. After purification, the
InCCD1 enzyme displayed poor thermal stability under conditions without a substrate; after incubation at 30 °C, 35 °C, and 40 °C for 3 h, the remaining activity was less than 80% of the maximum enzyme activity at 30 °C and only about 40% of the maximum enzyme activity after 3 h at 40 °C. The recombinant
InCCD1 enzyme also exhibited good pH stability; following an 8 h incubation at 4 °C within the pH range of 8.0–11.0, the activity consistently remained above 70% of the initial enzyme activity. The recombinant
InCCD1 enzyme also showed good tolerance to ethanol, achieving peak activity at 20% ethanol concentration. When the ethanol concentration reached 25%, the activity of
InCCD1 was comparable to that without ethanol. The addition of Fe
2+ in the reaction system had a certain inhibitory effect on the activity of
InCCD1, with enzyme activity at approximately 80% of the activity when no Fe
2+ was added at a concentration of 1 mM.
3.4.3. Effects of Common Metal Ions and EDTA on the Enzymatic Activities of Recombinant CCD1 Enzymes
The impact of adding metal ions and EDTA at a final concentration of 1 mM on the enzyme activities of
OeCCD1 and
InCCD1 was measured, and the results are shown in
Table 1. In total, 1 mM of Fe
3+, Ca
2+, Ba
2+, Sr
2+, K
+, Ni
2+, Co
2+, Al
3+, Li
+, Mg
2+, NH
4+, and EDTA all slightly promoted the activity of
OeCCD1. However, 1 mM Fe
2+ inhibited the activity of
OeCCD1, with the remaining enzyme activity being less than 80% of the initial activity. 1 mM Co
2+ and Zn
2+ slightly promoted the activity of
InCCD1, while other metal ions inhibited its activity. Among them, 1 mM Fe
2+, Ba
2+, and Mg
2+ strongly inhibited the activity of
InCCD1, leaving less than 80% of its initial activity. Additionally, 1 mM EDTA reduced the activity of
InCCD1 by about 20% compared to its initial activity.
3.4.4. Determination of CCD1 Kinetic Constants
The kinetic constants of
OeCCD1 and
InCCD1 were determined using β-apo-8′-carotenal as the substrate. Kinetic parameters were determined via nonlinear fitting of the Michaelis–Menten equation using GraphPad Prism 9.0. As shown in
Table 2, the K
m for
OeCCD1 was 0.82 mM and the V
max was 2.30 U/mg, with a catalytic efficiency (k
cat/K
m) of 4.09 mM
−1·s
−1; the K
m for
InCCD1 was 0.69 mM, the V
max was 1.22 U/mg, and the k
cat/K
m was 2.64 mM
−1·s
−1.
3.5. Substrate Specificity of CCD1 In Vivo
Due to the poor water solubility of most carotenoids, preparing micellar solutions are challenging. To overcome this limitation, CCD1 plasmids were expressed in E. coli chassis cells engineered to accumulate zeaxanthin and β-carotene. These cells were cultured under optimal expression conditions to assess CCD1 enzyme activity on these three carotenoids in vivo.
The plasmids pETDuet-OeCCD1 and pETDuet-InCCD1 were transformed into E. coli cells accumulating β-carotene and zeaxanthin. E. coli cells transformed with empty plasmids served as controls. Carotenoid retention times were verified against standard substances.
This study reveals that cultures of E. coli chassis cells carrying CCD1 plasmids exhibit noticeably lighter coloration compared to those with empty plasmids for β-carotene and zeaxanthin accumulators. Based on these observations, we hypothesized that OeCCD1 and InCCD1 demonstrate cleavage activity towards β-carotene and zeaxanthin in vivo. To test this hypothesis, a HPLC analysis was employed to detect carotenoid degradation in the fermentation broth.
The experimental results presented in
Figures S2 and S3 show that, compared to cultures of β-carotene and zeaxanthin accumulating chassis cells with empty plasmids, those carrying CCD1 plasmids exhibited significantly reduced carotenoid peak areas.
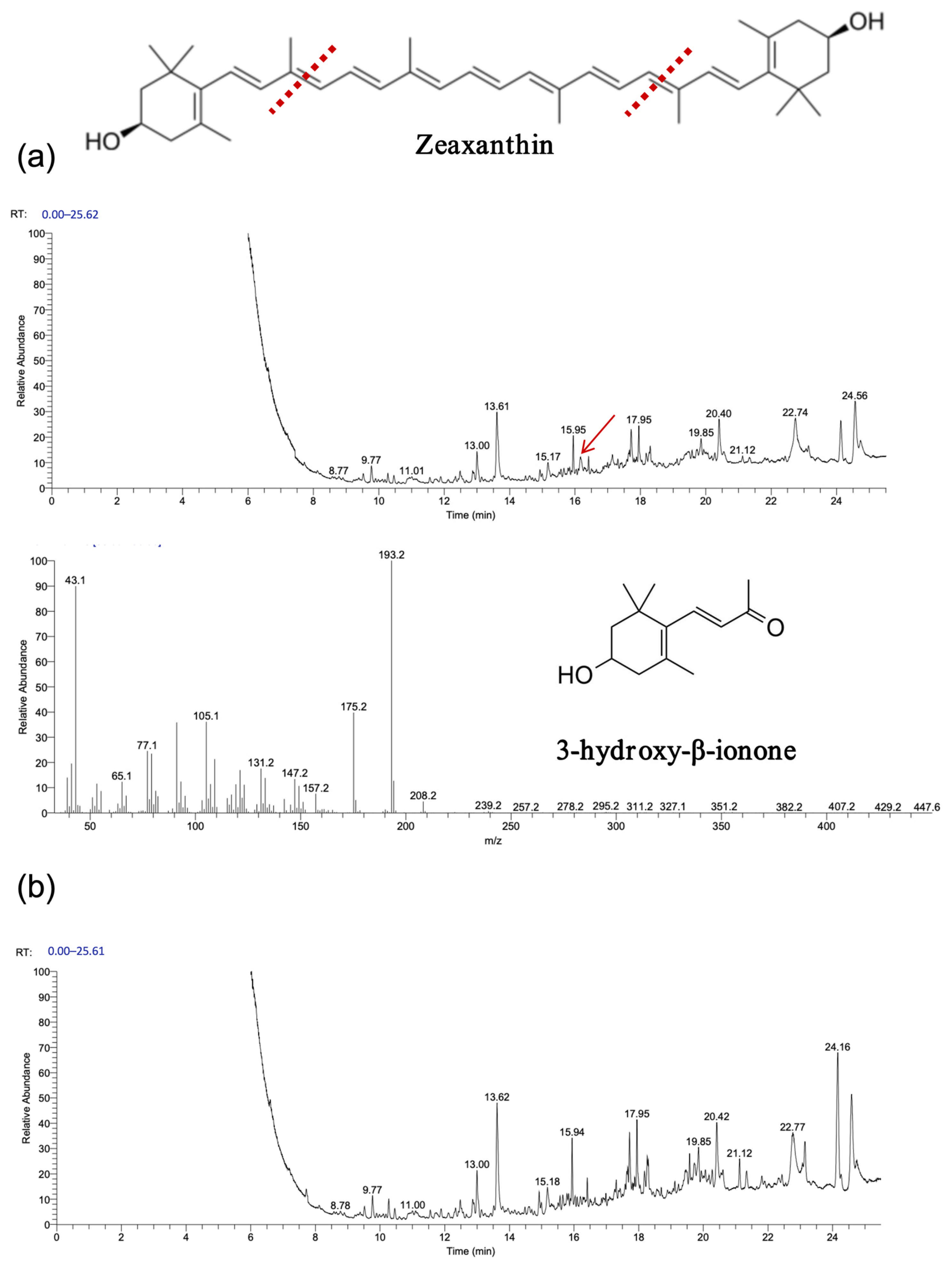
As shown in
Figure 7 and
Figure 8, gas chromatography–mass spectrometry (GC–MS) analysis identified β-ionone as a volatile compound produced in the fermentation broth of chassis cells harboring the CCD1-expressing plasmid. This result is consistent with previous findings [
28,
31,
32]. Interestingly, CCD1 is theoretically capable of cleaving zeaxanthin to generate 3-hydroxy-β-ionone. However, considering that β-carotene is the biosynthetic precursor of zeaxanthin, it is likely that zeaxanthin was not substantially utilized in vivo. Instead, both CCD1 enzymes may preferentially target β-carotene, leading to reduced zeaxanthin accumulation and the predominant production of β-ionone.
3.6. In Vitro Substrate Specificity Study of CCD1
To comprehensively assess CCD1’s substrate specificity, six carotenoids were selected as substrates: β-carotene, zeaxanthin, lycopene, lutein, astaxanthin, and trans-retinal. Following enzymatic reactions, a GC-MS analysis was performed to investigate the cleavage products. The resulting data were compared with standard spectra.
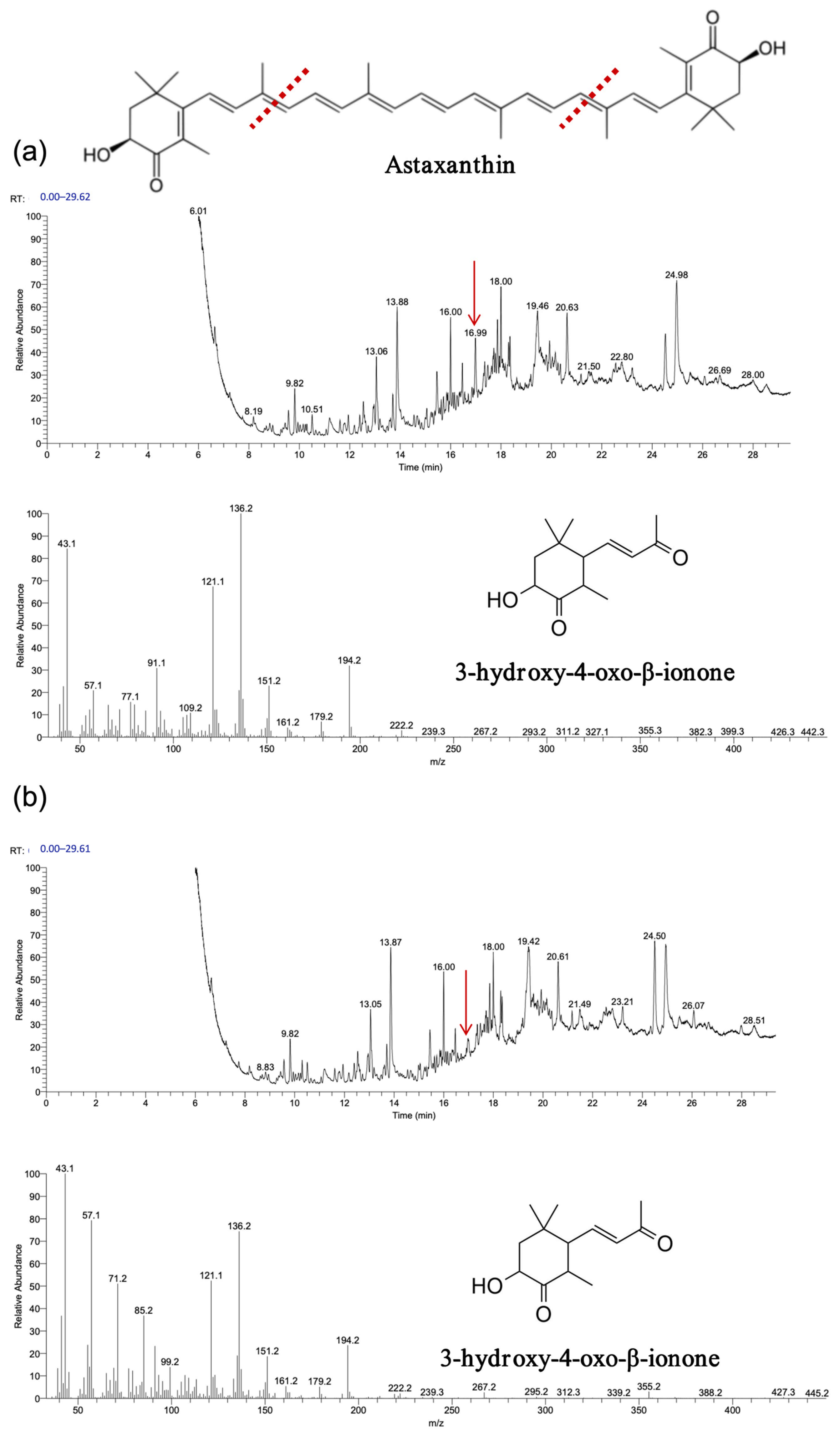
The experimental results, depicted in
Figure 9,
Figure 10 and
Figure 11, demonstrate that recombinant
OeCCD1 and
InCCD1 enzymes catalyzed the cleavage of β-carotene and astaxanthin at the 9,10 (9′,10′) positions, producing β-ionone and 3-hydroxy-4-oxo-β-ionone, respectively. However, no catalytic activity was observed on the 9,10 (9′,10′) positions of lutein or trans-retinal.
Interestingly, recombinant OeCCD1 catalyzed the cleavage of zeaxanthin at the 9,10 (9′,10′) positions to generate 3-hydroxy-β-ionone, while recombinant InCCD1 enzyme did not produce β-ionone or related substances from zeaxanthin. This observation indicates that the recombinant InCCD1 enzyme is unable to oxidatively cleave zeaxanthin in vitro, which further supports the hypothesis proposed in the previous section.
3.7. Evaluation of CCD1 Enzymes for β-Ionone Biosynthesis in Engineered E. coli Under IPTG Induction
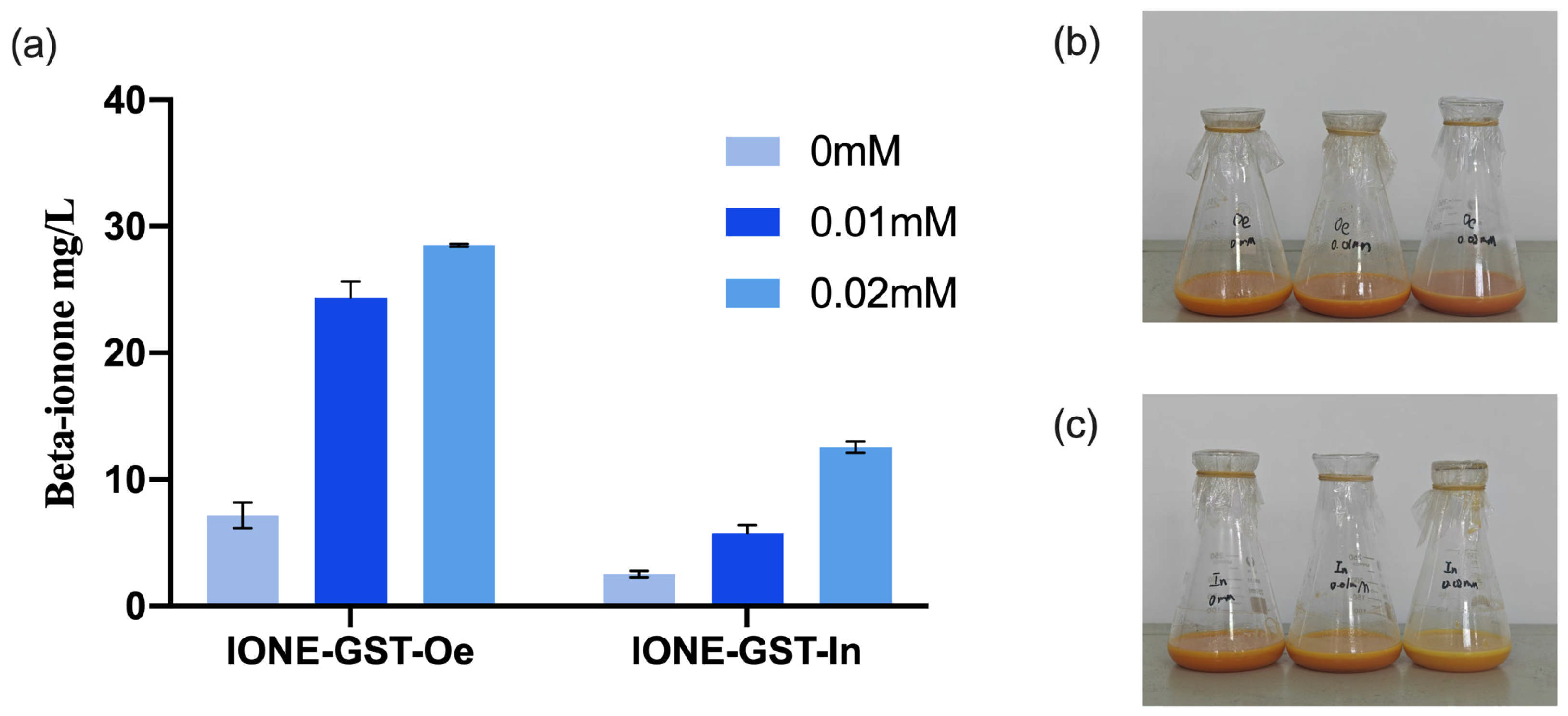
To evaluate the potential applications of CCD1 variants, two types of CCD1 were introduced into the high-yielding β-carotene
E. coli chassis cell MVABETA12 to construct two de novo synthetic β-ionone strains (IONE-GST-Oe, IONE-GST-In) [
33]. Given that previous studies have shown that IPTG concentration significantly influences the production of β-ionone in engineered strains [
28], we investigated three different IPTG concentrations to optimize β-ionone production in these strains. As shown in
Figure 12, IONE-GST-In achieved a β-ionone yield of 12.84 mg/L at an IPTG concentration of 0.02 mM, while IONE-GST-Oe demonstrated superior performance with a yield of 28.37 mg/L under the same IPTG concentration. These results collectively demonstrate that these two CCD1 variants possess application potential for β-ionone production.
4. Conclusions
In this study, we identify and functionally characterize two CCD1 homologs—OeCCD1 from the woody plant Olea europaea, and InCCD1 from the herbaceous plant Ipomoea nil—to explore functional diversity in carotenoid cleavage enzymes. Both enzymes catalyzed the oxidative cleavage of β-carotene at the 9,10 (or 9′,10′) double-bond to produce β-ionone. However, only OeCCD1 exhibited in vitro activity on zeaxanthin, while InCCD1 showed no detectable cleavage of this substrate.
Kinetic analysis using β-apo-8′-carotenal as a substrate revealed that OeCCD1 had a Km of 0.82 mM, a Vmax of 2.30 U/mg, and a catalytic efficiency (kcat/Km) of 4.09 mM−1·s−1, outperforming InCCD1 (Km = 0.69 mM; Vmax = 1.22 U/mg; kcat/Km = 2.64 mM−1·s−1). Under optimized conditions, engineered β-carotene E. coli cell expressing OeCCD1 achieved a β-ionone titer of 28.37 mg/L, more than double that of the InCCD1 strain (12.84 mg/L). Furthermore, OeCCD1 exhibited greater thermal and pH stability, retaining over 80% activity between 30–55 °C and pH 8.0–11.0. These results not only highlight the superior catalytic performance and robustness of OeCCD1, but also suggest that plant lineage (woody vs. herbaceous) may influence CCD1 enzyme properties. This study expands the known repertoire of CCD1 enzymes and highlights OeCCD1 as a promising candidate for constructing microbial cell factories for high-yield β-ionone production.
These findings provide a foundation for the development of efficient microbial cell factories for the production of natural apocarotenoids. Future research should focus on further engineering these enzymes to enhance the production of β-ionone.