The Diversity and Composition of Insect Communities in Urban Forest Fragments near Panama City
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Method
2.3. Data Analysis
3. Results
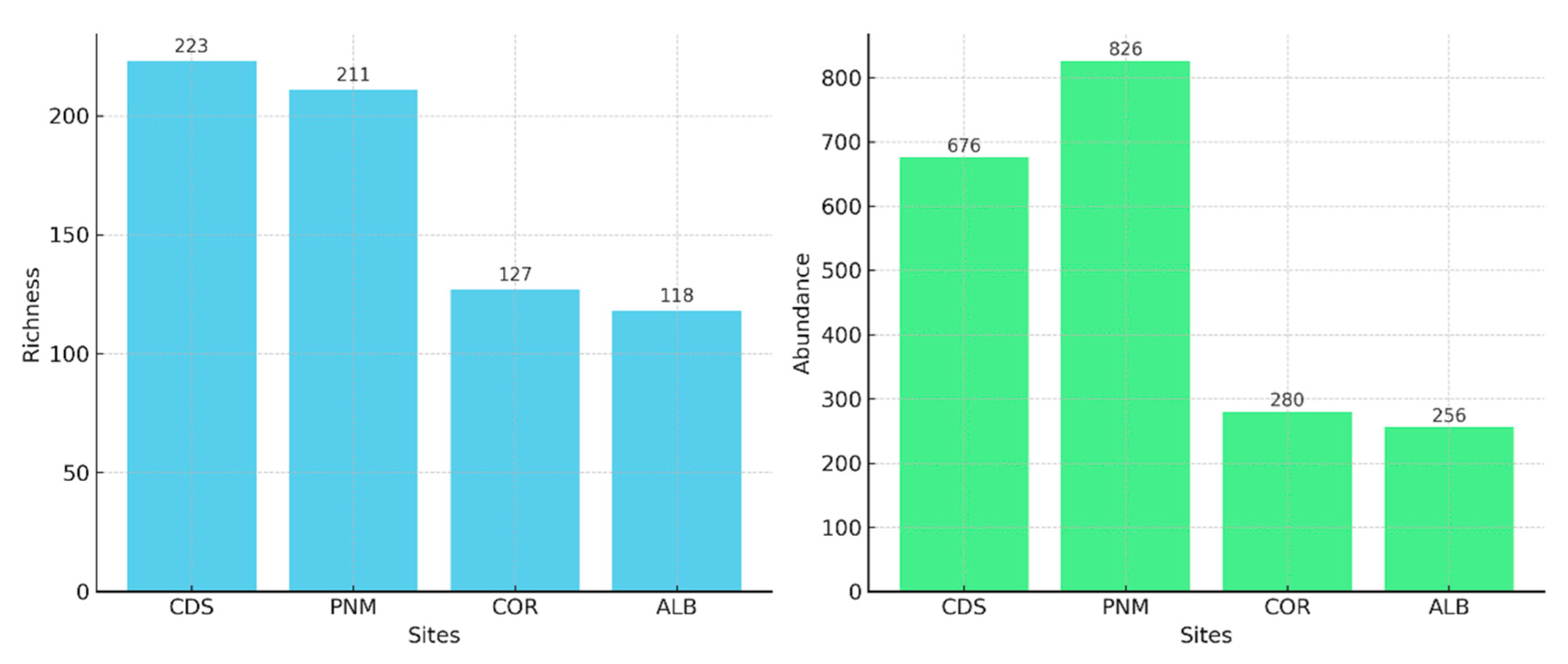
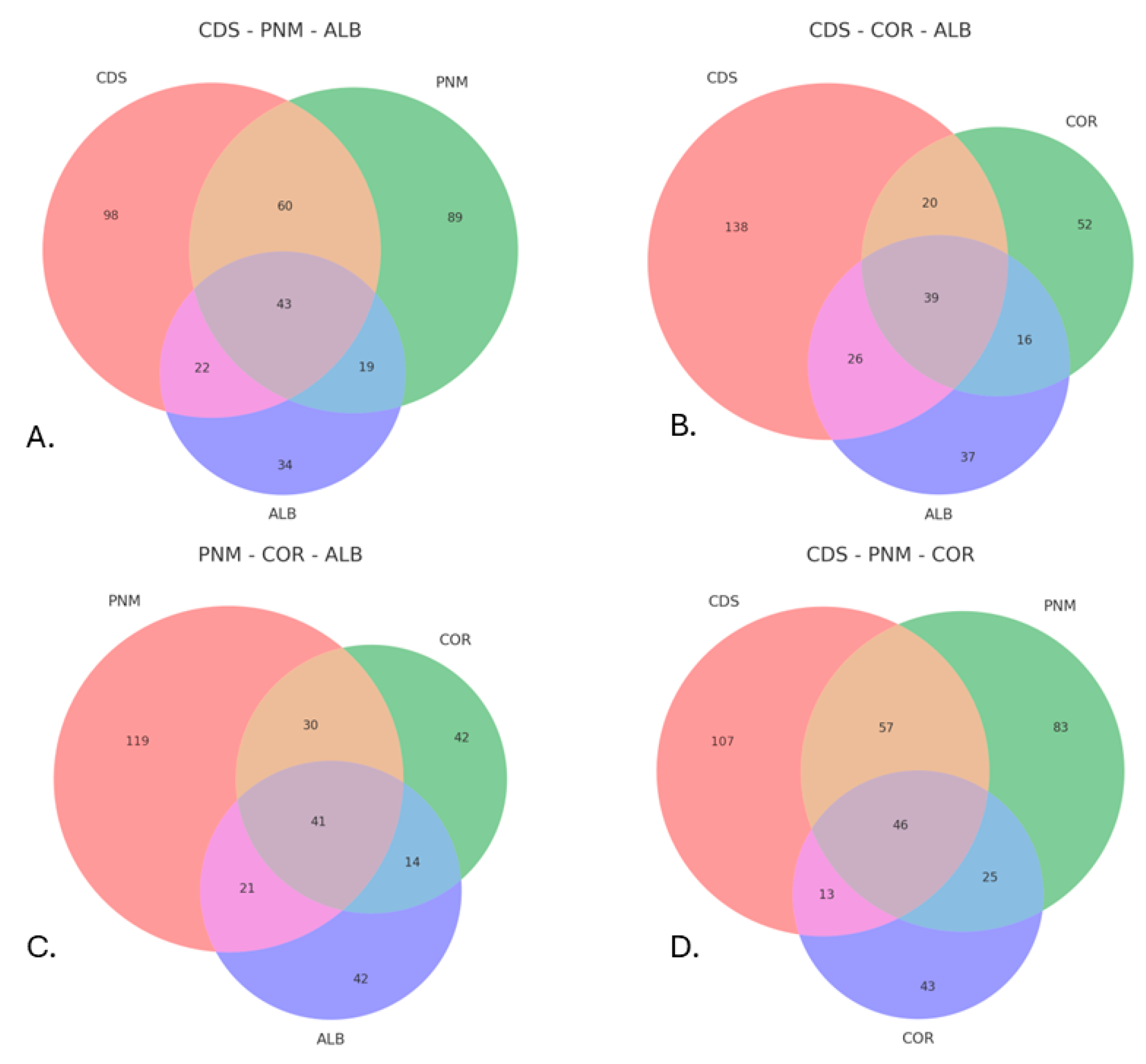
3.1. Diversity and General Abundance
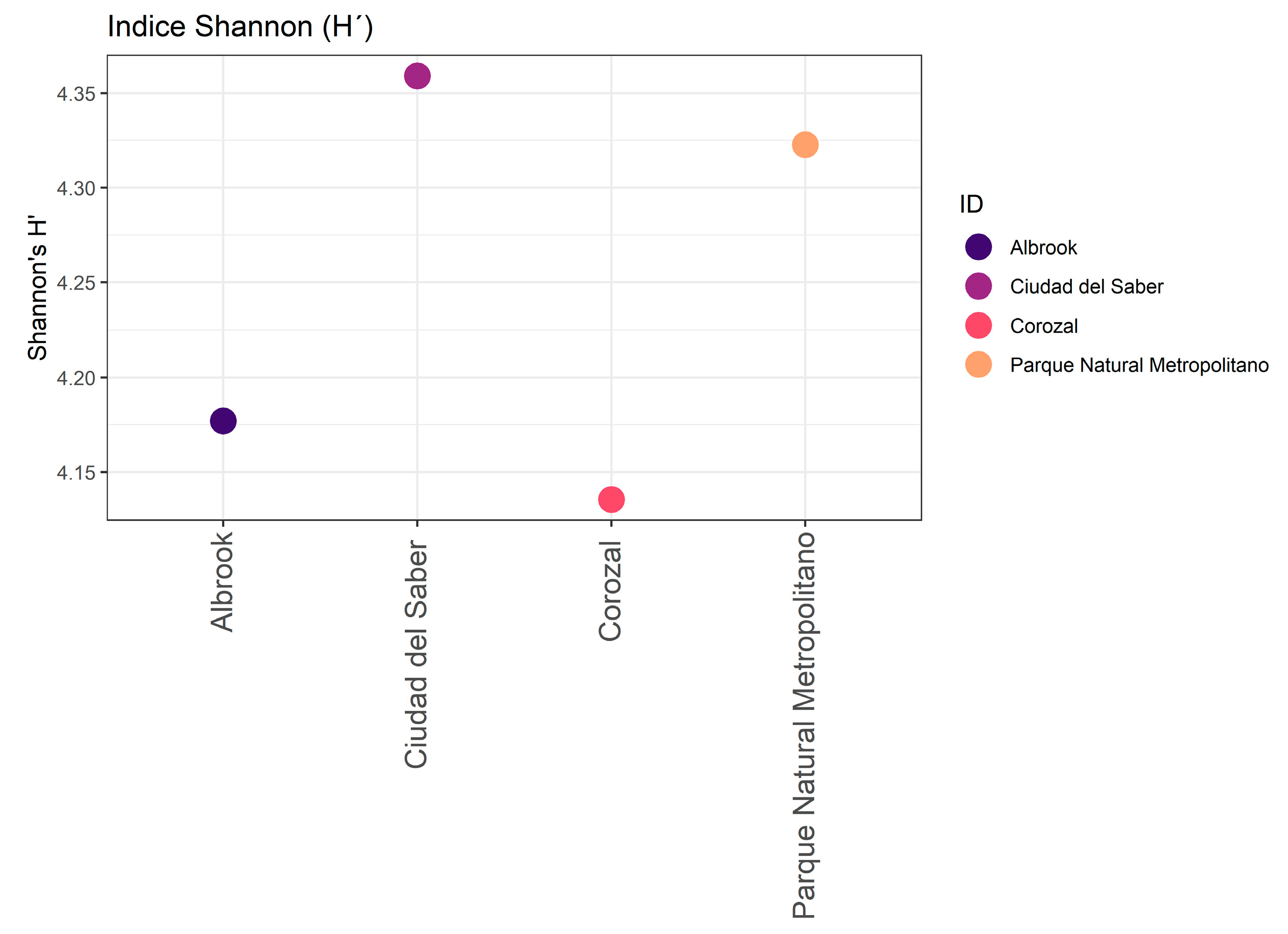
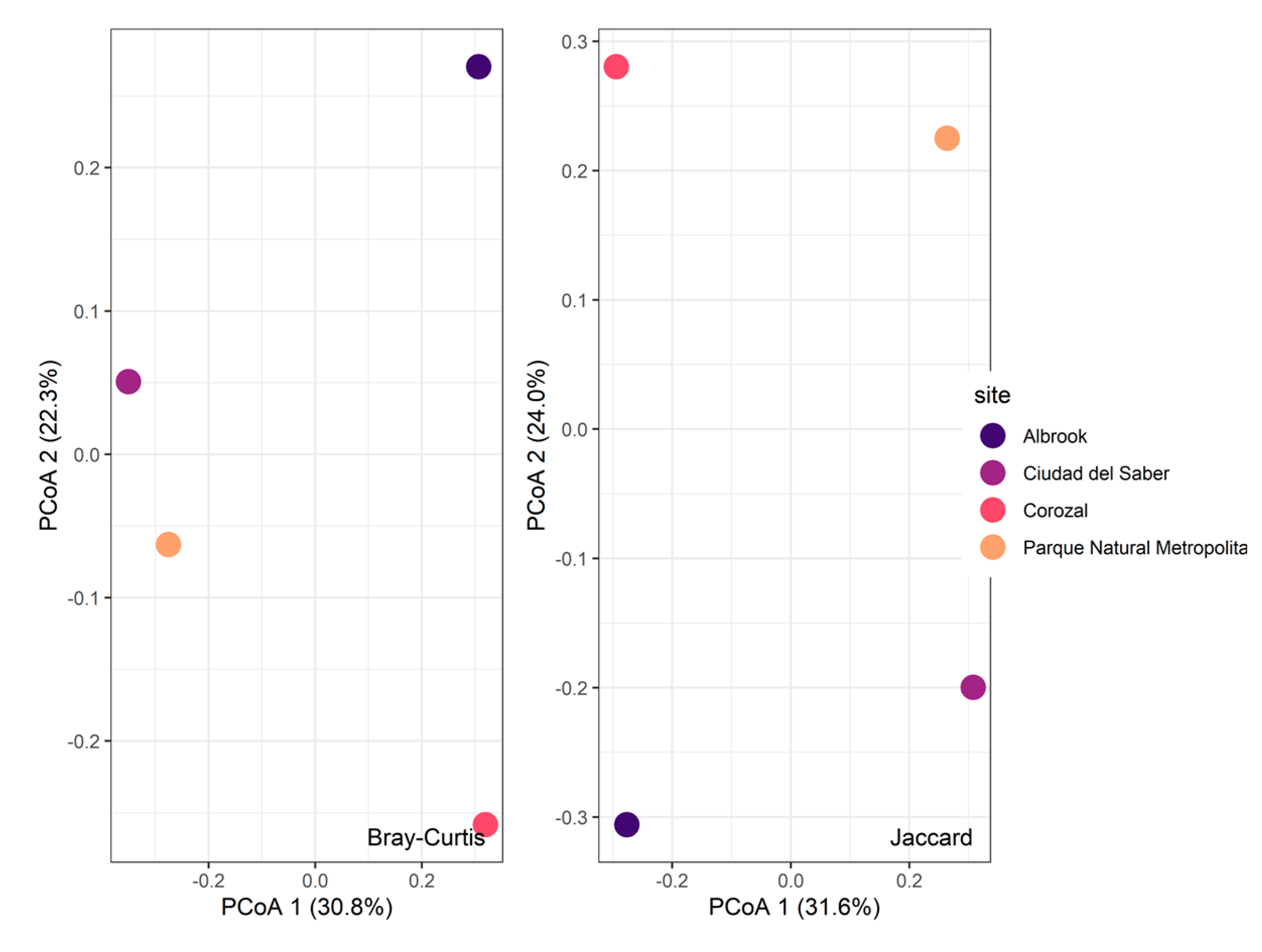
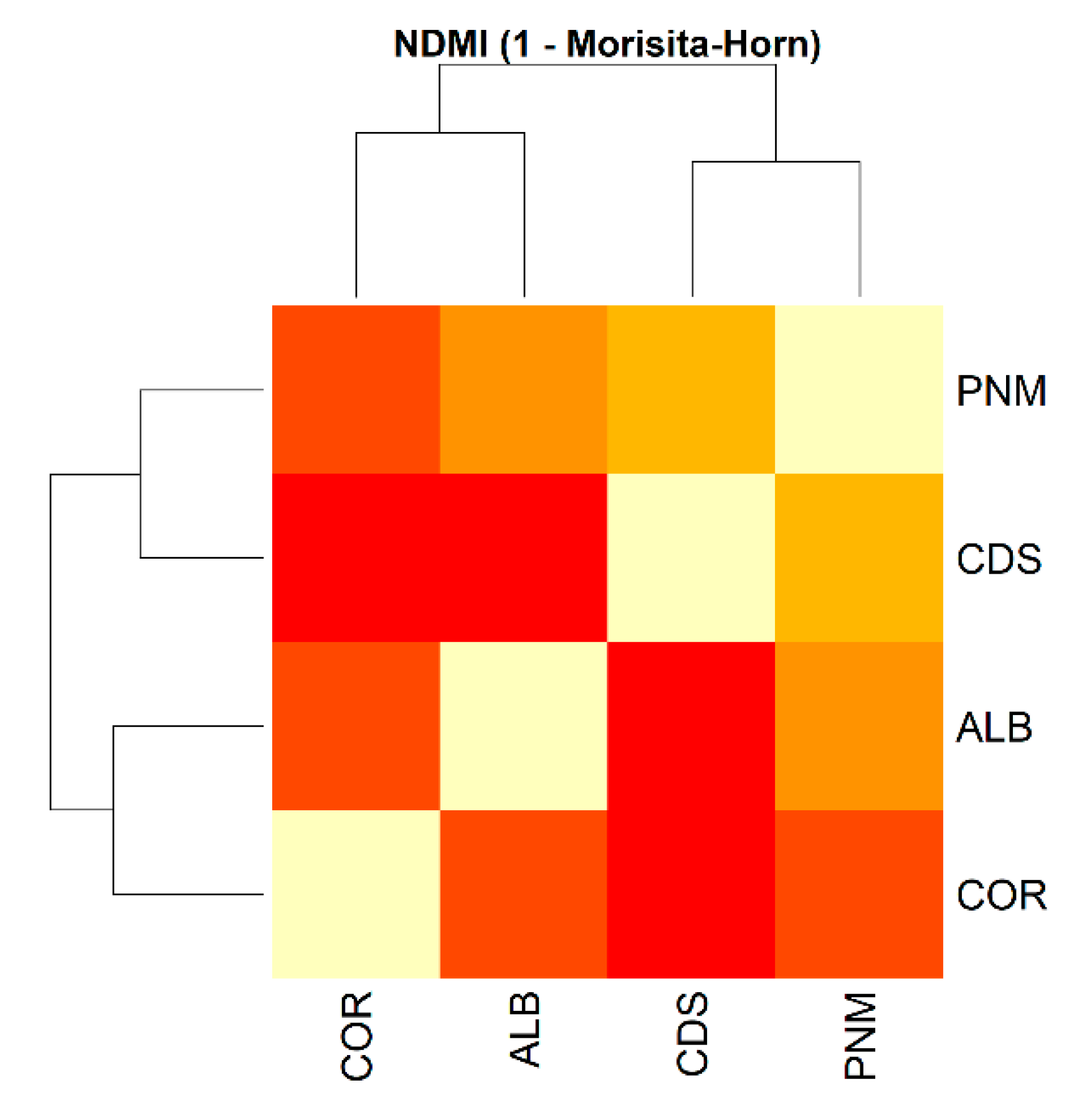
3.2. Alpha and Beta Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, L.A.; Medianero, E. The composition of braconid wasp communities in three forest fragments in a tropical lowland forest of Panama. BMC Ecol. Evol. 2022, 22, 98. [Google Scholar] [CrossRef]
- Medianero, E.; Santos Murgas, A. Insectos Asociados a los Bosques Urbanos de la Ciudad de Panamá; D’McPherson: Panamá, Panama, 2023. [Google Scholar]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Grimm, N.B.; Grove, J.M.; Pickett, S.T.A.; Redman, C.L. Integrated approaches to long-term studies of urban ecological systems. BioScience 2000, 50, 571–584. [Google Scholar] [CrossRef]
- Alberti, M.; Marzluff, J.M.; Shulenberger, E.; Bradley, G.; Ryan, C.; Zumbrunnen, C. Integrating humans into ecology: Opportunities and challenges for studying urban ecosystems. BioScience 2003, 53, 1169–1179. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Didham, R.K.; Hammond, P.M.; Lawton, J.H.; Eggleton, P.; Stork, N.E. Beetle species responses to tropical forest fragmentation. Ecol. Monogr. 1998, 68, 295–323. [Google Scholar] [CrossRef]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Munshi-South, J. Evolution of life in urban environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef]
- Lequerica, T.M.; Faeth, S.H.; Clarke, K.M. Urban form and vegetation influence insect diversity and composition in metropolitan landscapes. Landsc. Urban Plan. 2021, 214, 104238. [Google Scholar] [CrossRef]
- Arjumand, J.; Gaglio, M.; Habel, J.C. Urban green corridors maintain insect diversity and ecological functions in metropolitan areas. J. Nat. Conserv. 2024, 76, 126602. [Google Scholar] [CrossRef]
- Tudor, M.A.; Jones, H.R.; Albrecht, J. Restoration enhances resilience of insect communities in degraded urban ecosystems. Landsc. Ecol. 2023, 38, 1345–1360. [Google Scholar] [CrossRef]
- John, A.; Lim, X.; Prieto, F. Environmental contaminants shape insect community dynamics in tropical urban matrices. Sci. Total Environ. 2025, 906, 179660. [Google Scholar] [CrossRef]
- Laurance, W.F. Comparative responses of five arboreal marsupials to tropical forest fragmentation. J. Mammal. 1990, 71, 641–653. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Pickett, S.T.A. Ecosystem structure and function along urban–rural gradients: An unexploited opportunity for ecology. Ecology 1990, 71, 1232–1237. [Google Scholar] [CrossRef]
- Bergman, K.-O.; Dániel-Ferreira, J.; Milberg, P.; Öckinger, E.; Westerberg, L. Butterflies in Swedish grasslands benefit from forest and respond to landscape composition at different spatial scales. Landsc. Ecol. 2018, 33, 2189–2204. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Liu, M.; Russo, A. Urban green infrastructure: Bridging biodiversity conservation and sustainable development through adaptive management approaches. Front. Ecol. Evol. 2023, 11, 1440477. [Google Scholar]
- Ministerio de Ambiente. Proyecto Corredor Biológico Mesoamericano del Atlántico Panameño, Fase II; Gaceta Oficial Digital No. 28285-A; Ministerio de Ambiente: Panamá, Panama, 2017. Available online: https://www.gacetaoficial.gob.pa/pdfTemp/28285_A/GacetaNo_28285a_20170524.pdf (accessed on 17 February 2025).
- Valdéz, L.; Ortiz, O.; Medianero, E. Diversidad y estructura de las comunidades de mariposas diurnas (Lepidoptera: Rhopalocera) en fragmentos de vegetación urbanas adyacentes a la Ciudad de Panamá. In Insectos Asociados a los Bosques Urbanos de la Ciudad de Panamá; Medianero, E., Santos Murgas, A., Eds.; D’McPherson: Panamá, Panama, 2023; pp. 23–71. [Google Scholar]
- Autoridad Nacional del Ambiente (ANAM). Informe Nacional Sobre el Estado del Ambiente 2010; ANAM: Panamá, Panama, 2010. [Google Scholar]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Parque Natural Metropolitano. Plan de Manejo del Parque Natural Metropolitano; Parque Natural Metropolitano: Panamá, Panama, 1999. [Google Scholar]
- Muirhead-Thomson, R.C. Trap Responses of Flying Insects. The Influence of Trap Design on Capture Efficiency; Academic Press: London, UK, 1991. [Google Scholar]
- Karlsson, D.; Hartop, E.; Forshage, M.; Jaschhof, M.; Ronquist, F. The Swedish Malaise trap project: A 15 year retrospective on a countrywide insect inventory. Biodivers. Data J. 2020, 8, e47255. [Google Scholar] [CrossRef]
- Hanson, P.E.; Gauld, I.D. The Hymenoptera of Costa Rica; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Brown, B.V. Manual of Central American Diptera; NRC Research Press: Ottawa, Canada, 2009. [Google Scholar]
- Evans, H.E. A revision of the genus Apenesia in the Americas (Hymenoptera, Bethylidae). Bull. Mus. Comp. Zool. 1963, 130, 249–359. [Google Scholar]
- Evans, H.E. A synopsis of the American Bethylidae (Hymenoptera, Aculeata). Bull. Mus. Comp. Zool. 1964, 132, 1–222. [Google Scholar]
- Santos Murgas, A.; Gonzales Domínguez, P.E. Biosistemática de la Familia Bethylidae (Insecta: Hymenoptera) en Panamá; Tesis de licenciatura, Universidad de Panamá: Panamá, Panama, 2001. [Google Scholar]
- Aldrete, A.N.G.; Carrejo, N.S.; Mendivil, J.; Calderón, N.; Saenz, O.; Panche, J.; Román, C.; Obando, R.G. Checklist of ‘Psocoptera’ (Psocodea) of Colombia and identification key to the families. Dugesiana 2018, 25, 77–103. [Google Scholar] [CrossRef]
- Hanson, P.E.; Fernández, F. Los Membracidae (Hemiptera: Auchenorrhyncha) de Costa Rica; Instituto Nacional de Biodiversidad (INBio): Santo Domingo de Heredia, Costa Rica, 2006. [Google Scholar]
- Flynn, D.J. Checklist of treehoppers of Panama (Hemiptera: Membracidae) with a list of checklists and keys to the Nearctic and Neotropical fauna. Zootaxa 2012, 3405, 35–63. [Google Scholar] [CrossRef]
- Oliver, I.; Beattie, A.J. Invertebrate morphospecies as surrogates for species: A case study. Conserv. Biol. 1996, 10, 99–109. [Google Scholar] [CrossRef]
- Moreno, C.E.; Sánchez-Rojas, G.; Pineda, E.; Escobar, F. Evaluación de la diversidad en comunidades biológicas. In Capital Natural de México, Vol. I: Conocimiento Actual de la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): México, Mexico, 2006; pp. 21–30. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Hulbert, S.F.; Morrison, S.J.; Klawitter, J.J. Compatibility of porous ceramics with soft tissue; application to tracheal prostheses. J. Biomed. Mater. Res. 1971, 5, 269–279. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Real, R.; Vargas, J.M. The probabilistic basis of Jaccard’s index of similarity. Syst. Biol. 1996, 45, 380–385. [Google Scholar] [CrossRef]
- Diserud, O.H.; Odegaard, F. A multiple-site similarity measure. Biol. Lett. 2007, 3, 20–22. [Google Scholar] [CrossRef]
- Peet, R.K. The measurement of species diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Moreno, C.E.; Rodríguez, P.; Zuria, I. Diversidad alfa, beta y gamma. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Métodos de Estudio; Moreno, C.E., Ed.; Universidad Autónoma del Estado de Hidalgo: Pachuca de Soto, Mexico, 2018; pp. 119–144. [Google Scholar]
- Heidrich, L.; Barlow, J.; Gómez, C.; Lees, A.C.; Nally, R.M.; Schleuning, M. Disentangling direct and indirect effects of forest fragmentation on plant–frugivore interactions. Ecology 2020, 101, e02980. [Google Scholar] [CrossRef]
- Christie, F.J.; Hochuli, D.F. Responses of wasp communities to urbanization: Effects on community resilience and species diversity. J. Insect Conserv. 2009, 13, 213–221. [Google Scholar] [CrossRef]
- Valdés, L. Estructura de las Comunidades de Mariposas Diurnas (Lepidoptera: Rhopalocera) en Fragmentos de Vegetación en La Ciudad de Panamá; Universidad de Panamá: Panamá, Panama, 2018. [Google Scholar]
- Azevedo, C.O. Synopsis of the neotropical Dissomphalus (Hymenoptera, Bethylidae). Zootaxa 2003, 338, 1–74. [Google Scholar] [CrossRef]
- Azevedo, C.O. Characterization of the types of the neotropical Pseudisobrachium (Hymenoptera: Bethylidae), with a key to species. Rev. Bras. Zool. 2008, 25, 737–801. [Google Scholar] [CrossRef]
- Santos Murgas, A.; González, D. Distribución de especies de la subfamilia Epyrinae (Hymenoptera: Bethylidae) en Panamá. Tecnociencia 2006, 8, 37–50. [Google Scholar]
- Grebennikov, V.V.; Newton, A.F. Good-bye Scydmaenidae, or why the ant-like stone beetles should become megadiverse Staphylinidae sensu latissimo (Coleoptera). Eur. J. Entomol. 2009, 106, 275–301. [Google Scholar] [CrossRef]
- Ruiz-Guerra, B.; Renton, K.; Dirzo, R. Consequences of fragmentation of tropical moist forest for birds and their role in predation of herbivorous insects. Biotropica 2012, 44, 228–236. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Z.; Ding, Y. Supporting function of vegetation in urban riparian ecological corridors: A case study in Beijing, China. Sci. Total Environ. 2023, 857, 159377. [Google Scholar] [CrossRef]
- Soanes, K.; Sievers, M.; Chee, Y.E.; Egan, E. Planning for urban biodiversity on shifting social-ecological grounds: A case study of Narrm-Melbourne. Ecol. Solut. Evid. 2023, 4, e70005. [Google Scholar] [CrossRef]
- Magurran, A.E. Species abundance distributions: Pattern or process? Funct. Ecol. 2005, 19, 177–181. [Google Scholar] [CrossRef]
- Moreno, C.E.; Barragán, F.; Pineda, E.; Pavón, N.P. Reanálisis de la diversidad alfa: Alternativas para interpretar y comparar información sobre comunidades ecológicas. Rev. Mex. Biodivers. 2011, 82, 1249–1261. [Google Scholar] [CrossRef]
- European Environment Agency. Green Spaces and Corridors in Urban Areas. Climate-ADAPT. 2023. Available online: https://climate-adapt.eea.europa.eu/en/metadata/adaptation-options/green-spaces-and-corridors-in-urban-areas (accessed on 18 April 2025).
- Mata, L.; Andersen, A.N.; Morán-Ordóñez, A.; Hahs, A.K.; Backstrom, A.; Ives, C.D.; Bickel, D.; Duncan, D.; Palma, E.; Thomas, F.; et al. Indigenous plants promote insect biodiversity in urban greenspaces. Ecol. Appl. 2021, 31, e02309. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhang, H.; Liu, X.; Li, Q. Seasonal variation and driving factors of beta diversity of stream insects in a subtropical urban landscape. Urban Ecosyst. 2023, 26, 123–135. [Google Scholar]
- Lagucki, E.; Burdine, J.D.; McCluney, K.E. Urbanization alters communities of flying arthropods in parks and gardens of a medium-sized city. PeerJ 2017, 5, e3620. [Google Scholar] [CrossRef]
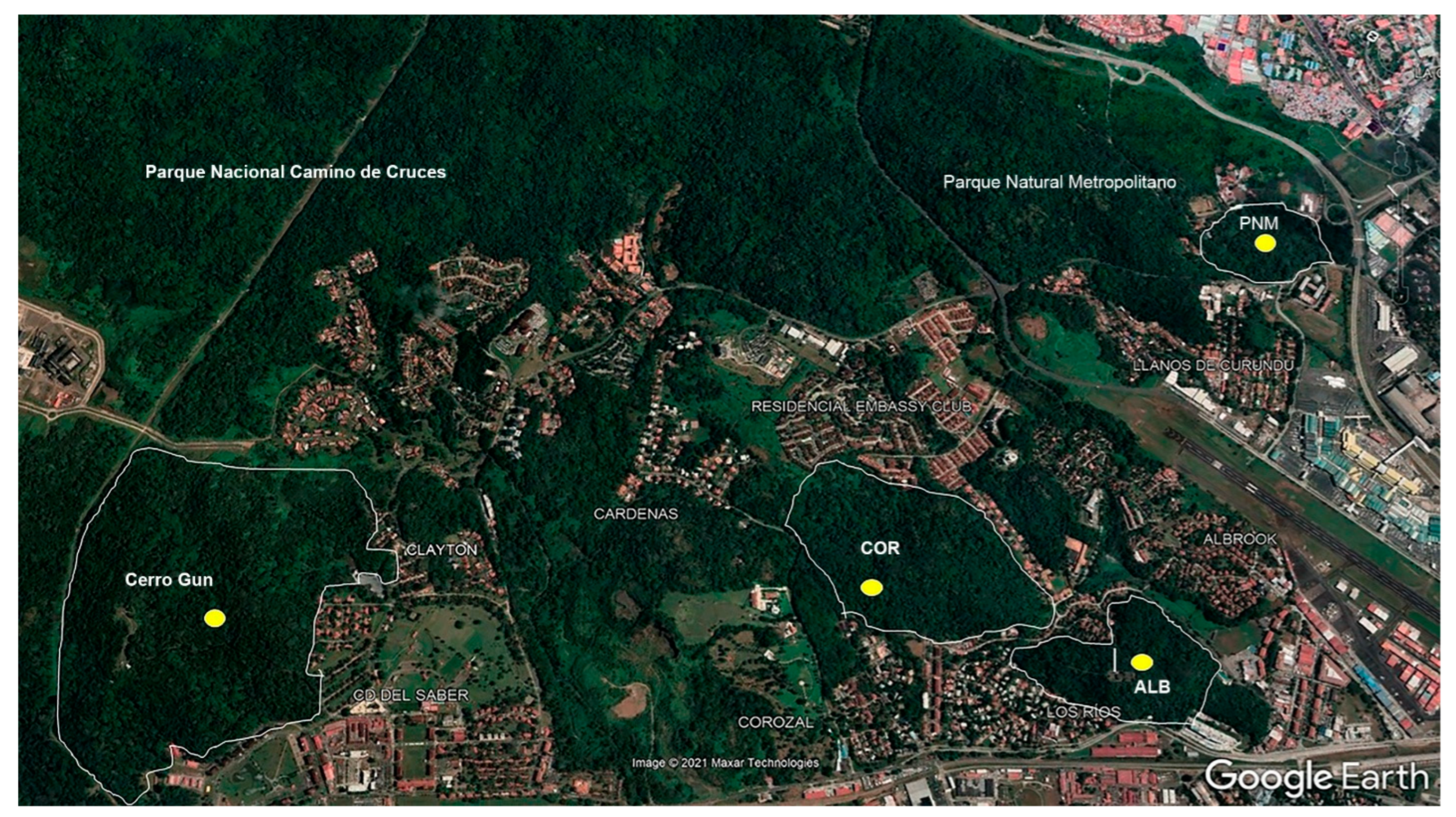
| Sites | Geographical Location | Altitude (Meters) | Vegetation | Annual Temperature (°C) |
|---|---|---|---|---|
| PNM | 8°59′41.55″ N–79°32′35.22″ O | 0–150 | Tropical dry forest | 28 |
| CDS | 9°00′24.3″ N–79°35′05.2″ W | 0–100 | Tropical dry forest | 27.5 |
| COR | 08°59′19.34″ N–079°34′11.83″ W | 30–60 | Overlapping deciduous forest. | 26.5 |
| ALB | 08°58′37.49″ N–079°33′43.82″ W | 20–80 | Overlapping deciduous forest. | 26.5 |
| Albrook | Ciudad del Saber | Corozal | Parque Natural Metropolitano | |
|---|---|---|---|---|
| Taxas | 118 | 223 | 127 | 211 |
| Individuos | 256 | 676 | 280 | 826 |
| Dominancia (D) | 0.02515 | 0.02836 | 0.01946 | 0.02785 |
| Simpson (1-D) | 0.9749 | 0.9716 | 0.9805 | 0.9721 |
| Shannon (H′) | 4.302 | 4.575 | 4.4 | 4.434 |
| Margalef | 21.1 | 34.07 | 22.36 | 31.27 |
| Equidad (J) | 0.9018 | 0.8461 | 0.9083 | 0.8285 |
| Sites | Abundance | True Diversity | |||
|---|---|---|---|---|---|
| Obs. | Est. | Obs. | Est. | ||
| PNM | 826 | 211 | 406.7 (51.88) | 103.307 | 106.810 (96.72) |
| CDS | 676 | 223 | 463.3 (48.13) | 126.970 | 133.482 (95.12) |
| COR | 280 | 127 | 346.0 (36.70) | 119.806 | 118.624 (1.00) |
| ALB | 256 | 118 | 275.5 (42.8%) | 108.86 | 111.97 (97.22%) |
| Sites | Shannon | Simpson | Chao1 |
|---|---|---|---|
| Albrook | 4.30 | 0.975 | 256.8 |
| Ciudad del Saber | 4.58 | 0.972 | 421.2 |
| Corozal | 4.40 | 0.981 | 320.7 |
| Parque Natural Metropolitano | 4.43 | 0.972 | 422.5 |
| Mean ± SD | 4.43 ± 0.12 | 0.975 ± 0.004 | 355.3 ± 78.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrego, J.; Medianero, E. The Diversity and Composition of Insect Communities in Urban Forest Fragments near Panama City. Biology 2025, 14, 721. https://doi.org/10.3390/biology14060721
Abrego J, Medianero E. The Diversity and Composition of Insect Communities in Urban Forest Fragments near Panama City. Biology. 2025; 14(6):721. https://doi.org/10.3390/biology14060721
Chicago/Turabian StyleAbrego, Jeancarlos, and Enrique Medianero. 2025. "The Diversity and Composition of Insect Communities in Urban Forest Fragments near Panama City" Biology 14, no. 6: 721. https://doi.org/10.3390/biology14060721
APA StyleAbrego, J., & Medianero, E. (2025). The Diversity and Composition of Insect Communities in Urban Forest Fragments near Panama City. Biology, 14(6), 721. https://doi.org/10.3390/biology14060721






