Time-of-Day-Dependent Effects of Aerobic Exercise on Carotid Hemodynamics in Sedentary Adults
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
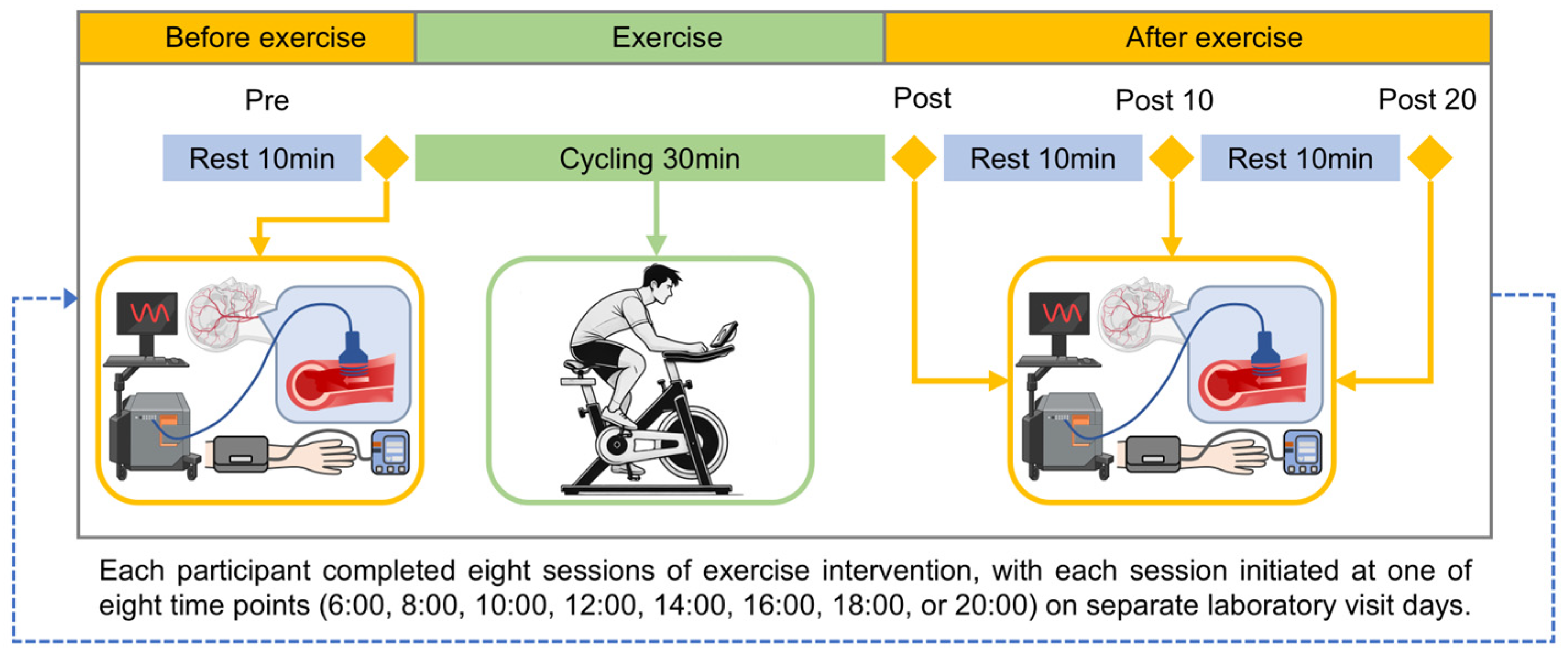
2.2. Experimental Design
2.3. Measurements and Calculations of Local Hemodynamics
- Blood Pressure (P)
- Flow Rate (Q)
- Apparent Elastic Modulus (Ep))
- Wall Shear Stress (τw)
- Oscillatory Shear Index (OSI)
- Peripheral Resistance (PR)
- Pulsatility Index (PI)
2.4. Data Processing and Statistical Analysis
3. Results
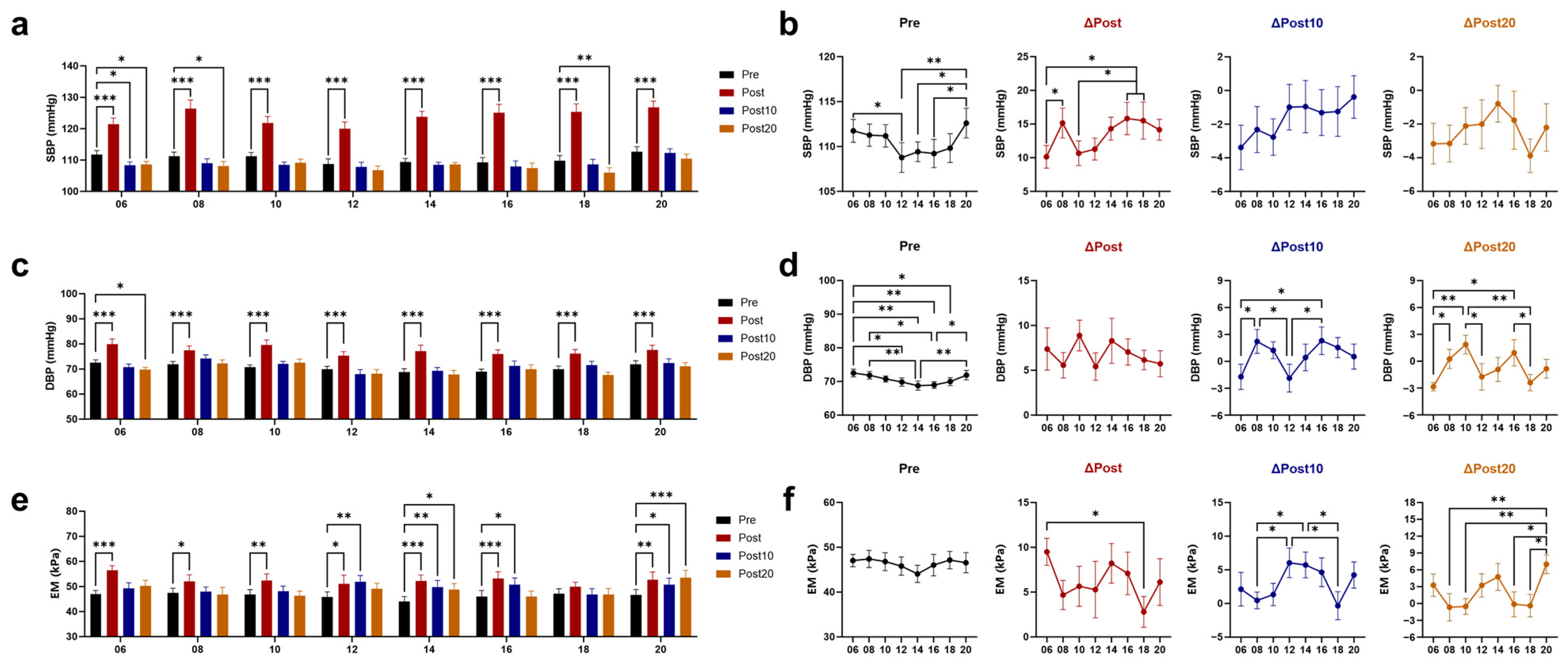
3.1. Effects of Timed AE on Blood Pressure (BP) and Arterial Elasticity
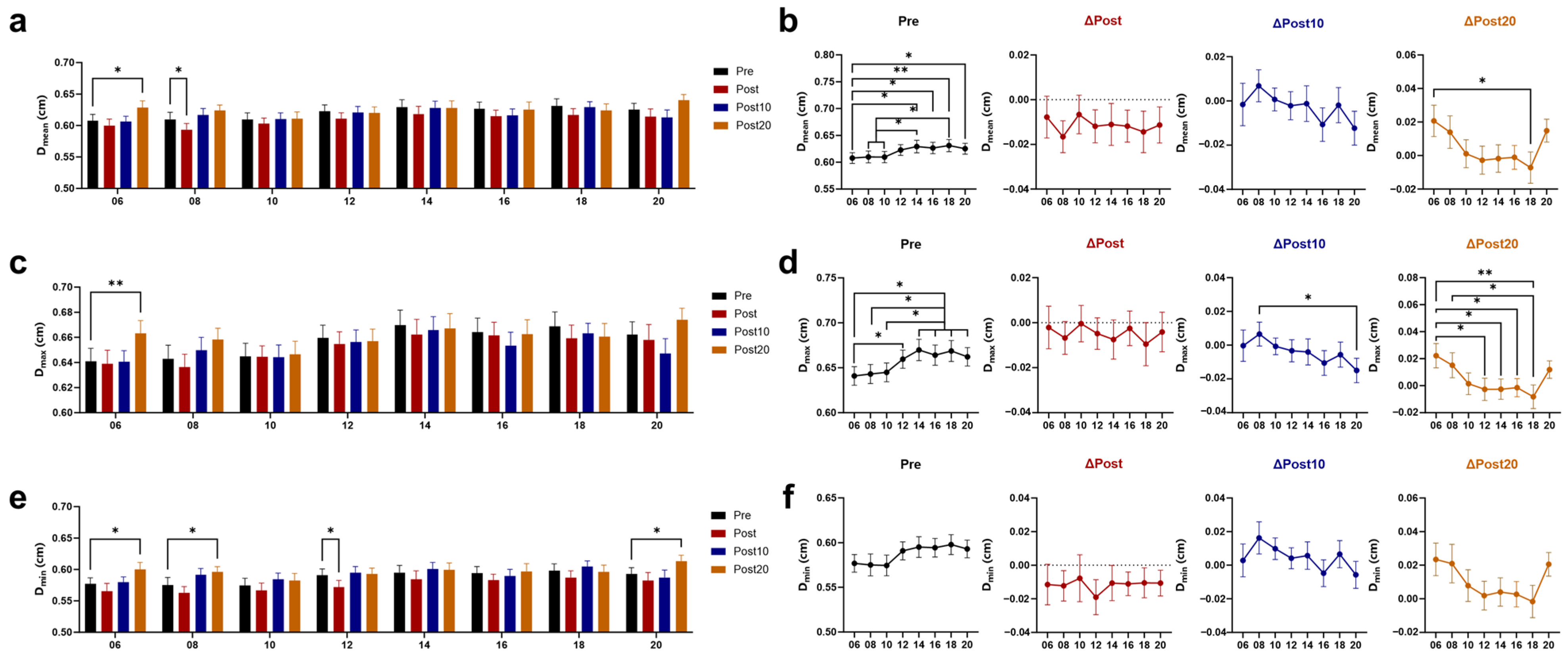
3.2. Effects of Timed AE on Cerebral Blood Supply
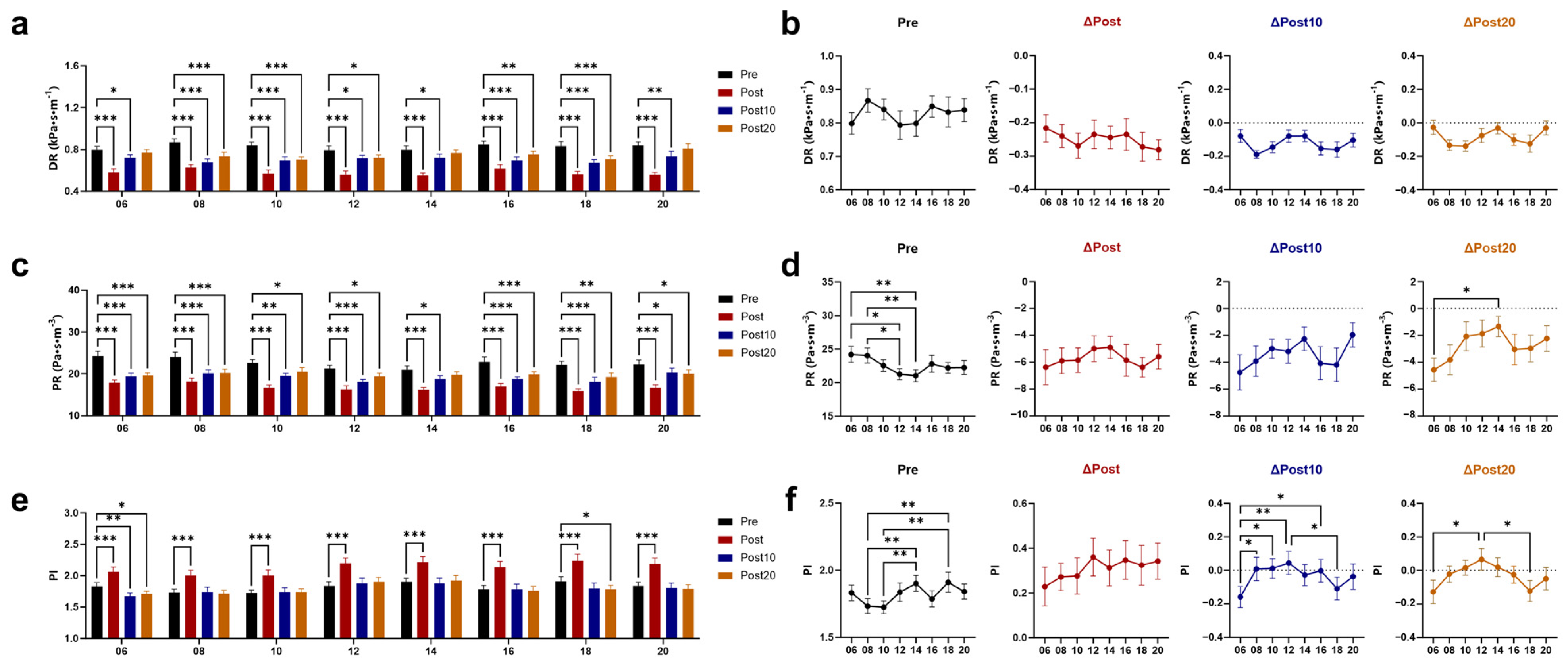
3.3. Effects of Timed AE on Wall Shear Stress (WSS) and Vascular Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; Marin, T.C.; Azevedo, L.; Rosa-Silva, J.M.; Shea, S.A.; Thosar, S.S. Chronobiology of Exercise: Evaluating the Best Time to Exercise for Greater Cardiovascular and Metabolic Benefits. Compr. Physiol. 2022, 12, 3621–3639. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Portaluppi, F.; Hermida, R.C. Circadian and Cyclic Environmental Determinants of Blood Pressure Patterning and Implications for Therapeutic Interventions. In Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics; White, W.B., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 105–127. [Google Scholar]
- Douma, L.G.; Gumz, M.L. Circadian clock-mediated regulation of blood pressure. Free Radic. Biol. Med. 2018, 119, 108–114. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Wu, Z.; Wang, J.; Zhang, X. Effects of Diet and Exercise on Circadian Rhythm: Role of Gut Microbiota in Immune and Metabolic Systems. Nutrients 2023, 15, 2743. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Kaya, Y.; Stenback, V.; Sharma, R.; Sodum, N.; Mutt, S.J.; Gagnon, D.D.; Tulppo, M.; Jarvelin, M.R.; Herzig, K.H.; et al. Effect of Aerobic Exercise and Time-Restricted Feeding on Metabolic Markers and Circadian Rhythm in Mice Fed with the High-Fat Diet. Mol. Nutr. Food Res. 2024, 68, e2300465. [Google Scholar] [CrossRef] [PubMed]
- Tahkamo, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Sevilla-Lorente, R.; Carneiro-Barrera, A.; Molina-Garcia, P.; Ruiz, J.R.; Amaro-Gahete, F.J. Time of the day of exercise impact on cardiovascular disease risk factors in adults: A systematic review and meta-analysis. J. Sci. Med. Sport 2023, 26, 169–179. [Google Scholar] [CrossRef]
- Shen, B.; Ma, C.; Wu, G.; Liu, H.; Chen, L.; Yang, G. Effects of exercise on circadian rhythms in humans. Front. Pharmacol. 2023, 14, 1282357. [Google Scholar] [CrossRef]
- Sabag, A.; Ahmadi, M.N.; Francois, M.E.; Postnova, S.; Cistulli, P.A.; Fontana, L.; Stamatakis, E. Timing of Moderate to Vigorous Physical Activity, Mortality, Cardiovascular Disease, and Microvascular Disease in Adults with Obesity. Diabetes Care 2024, 47, 890–897. [Google Scholar] [CrossRef]
- Jones, H.; Pritchard, C.; George, K.; Edwards, B.; Atkinson, G. The acute post-exercise response of blood pressure varies with time of day. Eur. J. Appl. Physiol. 2008, 104, 481–489. [Google Scholar] [CrossRef]
- Albalak, G.; Stijntjes, M.; van Bodegom, D.; Jukema, J.W.; Atsma, D.E.; van Heemst, D.; Noordam, R. Setting your clock: Associations between timing of objective physical activity and cardiovascular disease risk in the general population. Eur. J. Prev. Cardiol. 2023, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Guo, J.; Li, R.; Zhou, H.; Zhang, X.; Guan, S.; Tian, Y.; Jing, L.; Sun, Q.; Li, G.; et al. Common carotid artery diameter and the risk of cardiovascular disease mortality: A prospective cohort study in northeast China. BMC Public Health 2024, 24, 251. [Google Scholar] [CrossRef] [PubMed]
- Parittotokkaporn, S.; de Castro, D.; Lowe, A.; Pylypchuk, R. Carotid Pulse Wave Analysis: Future Direction of Hemodynamic and Cardiovascular Risk Assessment. JMA J. 2021, 4, 119–128. [Google Scholar] [CrossRef]
- Shen, B.Y.; Liu, H.B.; Cao, L.; Qin, K.R. Acute Effects of Different Intensities of Cycling Acute Exercise on Carotid Arterial Apparent Elasticity and Hemodynamic Variables. Biomed Res. Int. 2020, 2020, 9027560. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, B.; Li, Z.; Xue, C.; Zhao, H.; Pan, X.; Xu, D. Effects of accumulated exercise on the stiffness and hemodynamics of the common carotid artery. Front. Physiol. 2024, 15, 1348811. [Google Scholar] [CrossRef]
- Shi, W.; Chen, J.; He, Y.; Su, P.; Wang, M.; Li, X.; Tang, D. The effects of high-intensity interval training and moderate-intensity continuous training on visceral fat and carotid hemodynamics parameters in obese adults. J. Exerc. Sci. Fit. 2022, 20, 355–365. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Moreira, J.B.N.; Wohlwend, M.; Wisloff, U. Exercise and cardiac health: Physiological and molecular insights. Nat. Metab. 2020, 2, 829–839. [Google Scholar] [CrossRef]
- Bentley, R.F.; Nikolovski, N.; Goodman, J.M. How Does the Dose and Type of Exercise Impact Acute Cardiovascular Function in Healthy Individuals? Can. J. Cardiol. 2025, 41, 398–411. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Liguori, G. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022. [Google Scholar]
- Isath, A.; Koziol, K.J.; Martinez, M.W.; Garber, C.E.; Martinez, M.N.; Emery, M.S.; Baggish, A.L.; Naidu, S.S.; Lavie, C.J.; Arena, R.; et al. Exercise and cardiovascular health: A state-of-the-art review. Prog. Cardiovasc. Dis. 2023, 79, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.B.; da Silva, R.S.N.; de Oliveira, P.C.; da Silva, D.S.; Irigoyen, M.C.C.; Waclawovsky, G.; Schaun, M.I. Effect of aerobic and resistance exercise training on endothelial function in individuals with overweight and obesity: A systematic review with meta-analysis of randomized clinical trials. Sci. Rep. 2023, 13, 11826. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Alley, J.R.; Mazzochi, J.W.; Smith, C.J.; Morris, D.M.; Collier, S.R. Effects of resistance exercise timing on sleep architecture and nocturnal blood pressure. J. Strength. Cond. Res. 2015, 29, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.A.; Midgley, A.W.; Monteiro, W.D.; Farinatti, P.T. Influence of cardiopulmonary exercise testing protocol and resting VO2 assessment on %HRmax, %HRR, %VO2max and %VO2R relationships. Int. J. Sports Med. 2010, 31, 319–326. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Pina, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef]
- Bia, D.; Zocalo, Y.; Sanchez, R.; Torrado, J.F.; Lev, G.; Mendiz, O.; Pessana, F.; Ramirez, A.; Cabrera-Fischer, E.I. Brachial Blood Pressure Invasively and Non-Invasively Obtained Using Oscillometry and Applanation Tonometry: Impact of Mean Blood Pressure Equations and Calibration Schemes on Agreement Levels. J. Cardiovasc. Dev. Dis. 2023, 10, 45. [Google Scholar] [CrossRef]
- Bia, D.; Zocalo, Y.; Sanchez, R.; Lev, G.; Mendiz, O.; Pessana, F.; Ramirez, A.; Cabrera-Fischer, E.I. Aortic systolic and pulse pressure invasively and non-invasively obtained: Comparative analysis of recording techniques, arterial sites of measurement, waveform analysis algorithms and calibration methods. Front. Physiol. 2023, 14, 1113972. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Chen, R.; Liu, X.; Hu, Y.; Ma, Z.; Gao, L.; Jian, W.; Wang, L. Wall shear stress and its role in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1083547. [Google Scholar] [CrossRef]
- Tsubata, H.; Nakanishi, N.; Itatani, K.; Takigami, M.; Matsubara, Y.; Ogo, T.; Fukuda, T.; Matsuda, H.; Matoba, S. Pulmonary artery blood flow dynamics in chronic thromboembolic pulmonary hypertension. Sci. Rep. 2023, 13, 6490. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Gofur, E.M.; Munakomi, S. Anatomy, Head and Neck: Carotid Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Elvsashagen, T.; Mutsaerts, H.J.; Zak, N.; Norbom, L.B.; Quraishi, S.H.; Pedersen, P.O.; Malt, U.F.; Westlye, L.T.; van Someren, E.J.; Bjornerud, A.; et al. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. Neuroimage 2019, 186, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Querido, J.S.; Sheel, A.W. Regulation of cerebral blood flow during exercise. Sports Med. 2007, 37, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Morbiducci, U.; Kok, A.M.; Kwak, B.R.; Stone, P.H.; Steinman, D.A.; Wentzel, J.J. Atherosclerosis at arterial bifurcations: Evidence for the role of haemodynamics and geometry. Thromb. Haemost. 2016, 115, 484–492. [Google Scholar] [CrossRef]
- Malik, J.; Novakova, L.; Valerianova, A.; Chytilova, E.; Lejsek, V.; Buryskova, S.K.; Lambert, L.; Grus, T.; Porizka, M.; Michalek, P. Wall Shear Stress Alteration: A Local Risk Factor of Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 143–151. [Google Scholar] [CrossRef]
- Hartman, E.M.J.; De Nisco, G.; Gijsen, F.J.H.; Korteland, S.A.; van der Steen, A.F.W.; Daemen, J.; Wentzel, J.J. The definition of low wall shear stress and its effect on plaque progression estimation in human coronary arteries. Sci. Rep. 2021, 11, 22086. [Google Scholar] [CrossRef]
- Millar-Craig, M.; Bishop, C.; Raftery, E.B. Circadian variation of blood-pressure. Lancet 1978, 311, 795–797. [Google Scholar] [CrossRef]
- Mancia, G.; Ferrari, A.; Gregorini, L.; Parati, G.; Pomidossi, G.; Bertinieri, G.; Grassi, G.; di Rienzo, M.; Pedotti, A.; Zanchetti, A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ. Res. 1983, 53, 96–104. [Google Scholar] [CrossRef]
- Kawano, Y. Diurnal blood pressure variation and related behavioral factors. Hypertens. Res. 2011, 34, 281–285. [Google Scholar] [CrossRef]
- Jones, H.; George, K.; Edwards, B.; Atkinson, G. Effects of time of day on post-exercise blood pressure: Circadian or sleep-related influences? Chronobiol. Int. 2008, 25, 987–998. [Google Scholar] [CrossRef]
- Jones, H.; Atkinson, G.; Leary, A.; George, K.; Murphy, M.; Waterhouse, J. Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension 2006, 47, 778–784. [Google Scholar] [CrossRef]
- Weiss, S.A.; Blumenthal, R.S.; Sharrett, A.R.; Redberg, R.F.; Mora, S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 2010, 121, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Y.-L.; Wu, Y.-B.; Xu, Y.; Head, G.A.; Barry, M.; Liang, Y.-L. Association between the rate of the morning surge in blood pressure and cardiovascular events and stroke. Chin. Med. J. 2013, 126, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.; Pecanha, T.; Fecchio, R.Y.; Rezende, R.A.; Sousa, P.; Silva-Júnior, N.D.A.; Abreu, A.; Silva, G.; Mion-Junior, D.; Halliwill, J.R.; et al. Morning versus Evening Aerobic Training Effects on Blood Pressure in Treated Hypertension. Med. Sci. Sports Exerc. 2019, 51, 653–662. [Google Scholar] [CrossRef]
- Cardoso, C.G., Jr.; Gomides, R.S.; Queiroz, A.C.; Pinto, L.G.; da Silveira Lobo, F.; Tinucci, T.; Mion, D., Jr.; de Moraes Forjaz, C.L. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics 2010, 65, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Qi, T.; He, S.; Li, Z.; Ou, S.; Zhang, G.; Liu, X.; Huang, Z.; Liang, F. Low Wall Shear Stress Is Associated with Local Aneurysm Wall Enhancement on High-Resolution MR Vessel Wall Imaging. AJNR Am. J. Neuroradiol. 2018, 39, 2082–2087. [Google Scholar] [CrossRef]
- Millon, A.; Sigovan, M.; Boussel, L.; Mathevet, J.L.; Louzier, V.; Paquet, C.; Geloen, A.; Provost, N.; Majd, Z.; Patsouris, D.; et al. Low WSS Induces Intimal Thickening, while Large WSS Variation and Inflammation Induce Medial Thinning, in an Animal Model of Atherosclerosis. PLoS ONE 2015, 10, e0141880. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Farghadan, A.; McConnell, D.R.; Barker, A.J.; Wentzel, J.J.; Budoff, M.J.; Arzani, A. The Story of Wall Shear Stress in Coronary Artery Atherosclerosis: Biochemical Transport and Mechanotransduction. J. Biomech. Eng. 2021, 143, 041002. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Newcomer, S.C.; Bender, S.B. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J. Appl. Physiol. 2008, 104, 588–600. [Google Scholar] [CrossRef]
- Kuchan, M.J.; Frangos, J.A. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am. J. Physiol. 1993, 264, H150–H156. [Google Scholar] [CrossRef]
- Himburg, H.A.; Dowd, S.E.; Friedman, M.H. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H645–H653. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Chappell, D.C.; Ishizaka, N.; Nerem, R.M.; Alexander, R.W.; Griendling, K.K. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide-producing NADH oxidase. Circ. Res. 1998, 82, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Ing, M.H.; Salazar, A.; Lassegue, B.; Griendling, K.; Navab, M.; Sevanian, A.; Hsiai, T.K. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: Implication for native LDL oxidation. Circ. Res. 2003, 93, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef]
- Lin, C.J.; Cocciolone, A.J.; Wagenseil, J.E. Elastin, arterial mechanics, and stenosis. Am. J. Physiol. Cell Physiol. 2022, 322, C875–C886. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Crisafulli, A. Gender Differences in Hemodynamic Regulation and Cardiovascular Adaptations to Dynamic Exercise. Curr. Cardiol. Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef]
- Maruf, F.A.; Ogochukwu, U.N.; Dim, P.A.; Alada, A.R. Absence of sex differences in systolic blood pressure and heart rate responses to exercise in healthy young adults. Niger. J. Physiol. Sci. 2012, 27, 95–100. [Google Scholar]
- Farinatti, P.; Monteiro, W.; Oliveira, R.; Crisafulli, A. Cardiorespiratory responses and myocardial function within incremental exercise in healthy unmedicated older vs. young men and women. Aging Clin. Exp. Res. 2018, 30, 341–349. [Google Scholar] [CrossRef]
- Society, C.N. Eight Key Recommendations from Dietary Guidelines for Chinese Residents. 2022. Available online: https://en.chinacdc.cn/health_topics/nutrition_health/202206/t20220616_259702.html (accessed on 1 February 2025).


| Characteristics | Values |
|---|---|
| Age (years) | 22.05 ± 0.42 |
| Height (cm) | 176.50 ± 1.07 |
| Weight (kg) | 65.95 ± 1.22 |
| BMI (kg/m2) | 21.17 ± 0.32 |
| WHR | 0.89 ± 0.02 |
| HRmax (bpm) | 198.00 ± 0.42 |
| HRrest (bpm) | 66.18 ± 0.65 |
| HRt_mean (bpm) | 151.8 ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.; Liu, H.; Zhang, S.; Chen, L.; Yang, G. Time-of-Day-Dependent Effects of Aerobic Exercise on Carotid Hemodynamics in Sedentary Adults. Biology 2025, 14, 713. https://doi.org/10.3390/biology14060713
Shen B, Liu H, Zhang S, Chen L, Yang G. Time-of-Day-Dependent Effects of Aerobic Exercise on Carotid Hemodynamics in Sedentary Adults. Biology. 2025; 14(6):713. https://doi.org/10.3390/biology14060713
Chicago/Turabian StyleShen, Bingyi, Haibin Liu, Shuying Zhang, Lihong Chen, and Guangrui Yang. 2025. "Time-of-Day-Dependent Effects of Aerobic Exercise on Carotid Hemodynamics in Sedentary Adults" Biology 14, no. 6: 713. https://doi.org/10.3390/biology14060713
APA StyleShen, B., Liu, H., Zhang, S., Chen, L., & Yang, G. (2025). Time-of-Day-Dependent Effects of Aerobic Exercise on Carotid Hemodynamics in Sedentary Adults. Biology, 14(6), 713. https://doi.org/10.3390/biology14060713







