Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
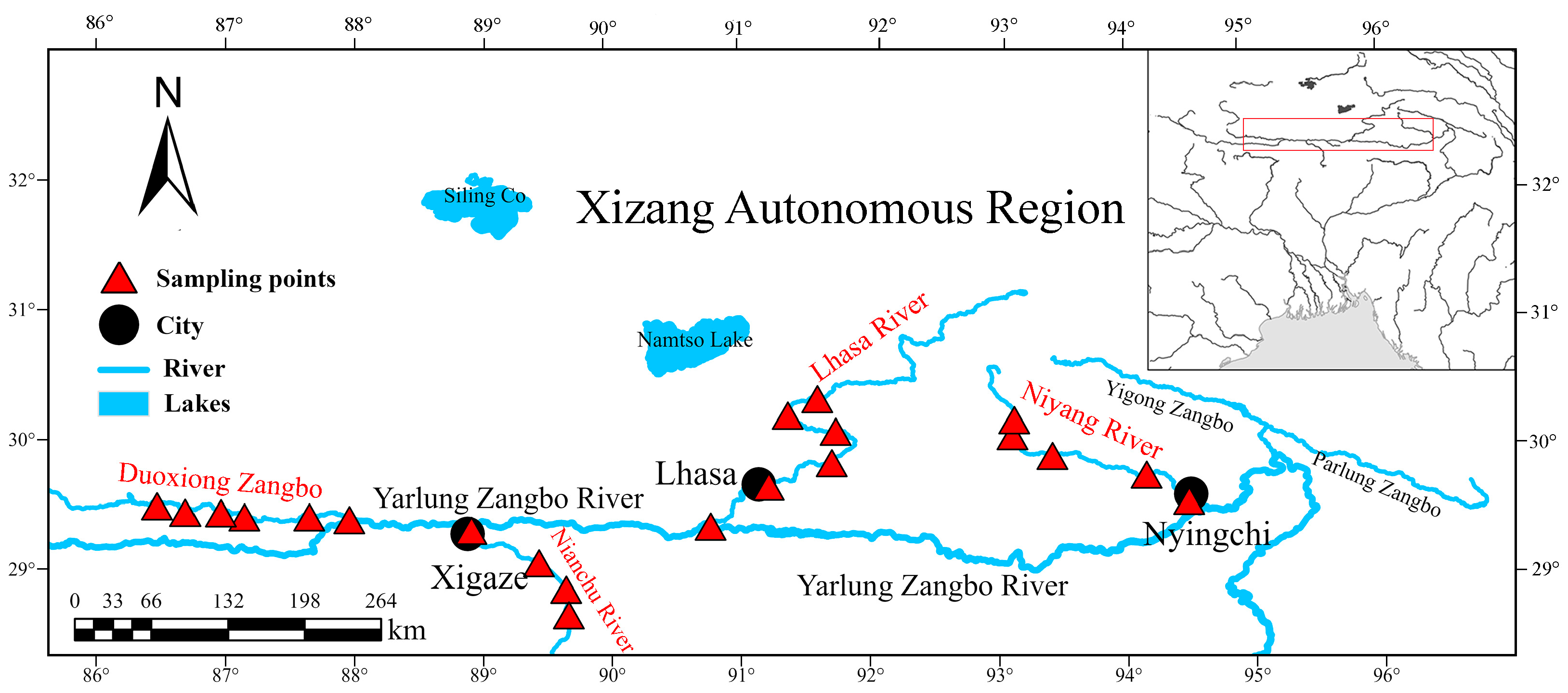
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Lapillus Treatment and Age Determination
2.4. Data Statistics and Analysis Methods
2.4.1. Body Length and Weight Relationship
2.4.2. Growth Equation, Growth Rate Equation, and Growth Acceleration Equation
- Body length growth equation: Lt = L∞ (1 − e−k(t−t0));
- Body weight growth equation: Wt = W∞ (1 − e−k(t−t0));
- Body length growth rate equation: dL/dt = L∞ k e−k(t−t0);
- Body weight growth rate equation: dW/dt = b W∞ k e−k(t−t0) (1 − e−k(t−t0))b−1;
- Body length growth acceleration equation: d2L/dt2 = −L∞ k2e−k(t−t0);
- Body weight Growth Acceleration Equation: d2W/dt2 = bW∞k2e − k (t − t0) (1 − e−k(t−t0))b−2 (be−k(t−t0) − 1);
- Inflection point age: ti = ln b/k + t0;
- Critical age: tc = [Kt0 − lnM + ln (bK + M)]/K;
- Growth performance index: φ = lgk + 2 lgL∞.
2.4.3. Mortality Characteristics and Exploitation Rate
2.4.4. Relative Units Replenish Catches and Biomass
3. Results
3.1. Group Structure
3.2. Body Length and Weight Relationship
3.3. Growth Equation, Growth Velocity Equation, and Growth Acceleration Equation
3.4. Mortality Characteristics and Exploitation Rate
3.5. Relative Units Replenish Catches and Biomass
4. Discussion
4.1. Selection of Age Identification Materials and Growth Models for S. younghusbandi
4.2. Growth Characteristics of Lhasa Bare Schizori
4.3. Current Status and Protection Measures of Bare Rift Resources in Lhasa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.P.; Zhang, Y.Q.; Xu, Z.X.; Teng, J.; Liu, C.M.; Liu, W.F.; Mpelasoka, F. The impact of climate change on runoff in the southeastern Tibetan Plateau. J. Hydrol. 2013, 505, 188–201. [Google Scholar] [CrossRef]
- Zhang, J.W.; Yan, Y.N.; Zhao, Z.Q.; Liu, X.M.; Li, X.D.; Zhang, D.; Ding, H.; Meng, J.L.; Liu, C.Q. Spatiotemporal variation of Li isotopes in the Yarlung Zangbo River basin (upper reaches of the Brahmaputra River): Source and process. Earth Planet. Sci. Lett. 2022, 600, 117875. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Li, X.D.; Yang, S.X.; Chao, X.; Liu, H.Q.; Ba, S. Distribution patterns and community assembly processes of eukaryotic microorganisms along an altitudinal gradient in the middle reaches of the Yarlung Zangbo River. Water Res. 2023, 239, 120047. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Hao, J.S.; Zhang, G.T.; Fang, H.Y.; Wang, Y.; Lu, H.J. Runoff variations affected by climate change and human activities in Yarlung Zangbo River, southeastern Tibetan Plateau. Catena 2023, 230, 107184. [Google Scholar] [CrossRef]
- Sun, W.C.; Wang, Y.Y.; Fu, Y.S.H.; Xue, B.L.; Wang, G.Q.; Yu, J.S.; Zuo, D.P.; Xu, Z.X. Spatial heterogeneity of changes in vegetation growth and their driving forces based on satellite observations of the Yarlung Zangbo River Basin in the Tibetan Plateau. J. Hydrol. 2019, 574, 324–332. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.H.; Zhao, L.; Liu, Y.; Gao, F.Y.; Tang, J.M.; Wang, H.Y.; Chen, Z.X.; Wang, S.Y.; Li, G.H.; et al. Aeolian activity in the Yarlung Zangbo River Basin, southern Tibetan Plateau, began at 584 ka: Implications for the glaciation of the Tibetan Plateau. Quat. Sci. Rev. 2024, 337, 108799. [Google Scholar] [CrossRef]
- Li, F.P.; Xu, Z.X.; Feng, Y.C.; Liu, M.; Liu, W.F. Changes of land cover in the Yarlung Zangbo River basin from1985 to 2005. Environ. Earth Sci. 2013, 68, 181–188. [Google Scholar] [CrossRef]
- Liu, C.C.; Witonsky, D.; Gosling, A.; Hyeon, L.J.; Ringbauer, H.; Hagan, R.; Patel, N.; Stahl, R.; Novembre, J.; Aldenderfer, M.; et al. Ancient genomes from the Himalayas illuminate the genetic history of Tibetans and their Tibeto-Burman speaking neighbors. Nat. Commun. 2022, 13, 1203. [Google Scholar] [CrossRef]
- Duan, Y.J.; Xie, C.X.; Zhou, X.; Ma, B.S.; Huo, B. Age and growth characteristics of Schizopygopsis younghusbandi Regan, 1905 in the Yarlung Zangbo River in Tibet, China. J. Appl. Ichthyol. 2014, 30, 948–954. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.J.; Zhang, D.F.; Giorgi, F. Climate change over the Yarlung Zangbo-Brahmaputra River Basin in the 21st century as simulated by a high resolution regional climate model. Quat. Int. 2011, 244, 159–168. [Google Scholar] [CrossRef]
- Yi, X.; Lai, Q.; Shi, J.; Gao, P.; Zhou, K.; Qi, H.; Wang, H.; Me, Z. Nitrogenous waste excretion and gene expression of nitrogen transporter in Gymnocypris przewalskii in high alkaline environment. J. Fish. Sci. China 2017, 24, 681–689. [Google Scholar]
- Huo, B.; Xie, C.X.; Ma, B.S.; Yang, X.F.; Huang, H.P. Age and growth of Oxygymnocypris stewartii in the Yarlung Zangbo River, Tibet, China. Zool. Stud. 2012, 51, 185–194. [Google Scholar]
- Ma, B.S.; Xie, C.X.; Huo, B.; Yang, X.F.; Chen, S.S. Reproductive biology of Schizothorax o’connori (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River, Tibet. Zool. Stud. 2012, 51, 1066–1076. [Google Scholar]
- Zhou, X.J.; Xie, C.X.; Huo, B.; Duan, Y.J.; Yang, X.; Ma, B.S. Reproductive biology of Schizothorax waltoni (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fishes 2015, 98, 597–609. [Google Scholar] [CrossRef]
- Feng, X.; Jia, Y.T.; Zhu, R.; Chen, K.; Chen, Y.F. Characterization and analysis of the transcriptome in Gymnocypris selincuoensis on the Qinghai-Tibetan Plateau using single-molecule long-read sequencing and RNA-seq. DNA Res. 2019, 26, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, M.Z.; Wang, J.; Gong, Z.; Liu, M.; Liu, H.Z.; Lin, P.C. Species composition and longitudinal patterns of fish assemblages in the middle and lower Yarlung Zangbo River, Tibetan Plateau, China. Ecol. Indic. 2021, 125, 107542. [Google Scholar] [CrossRef]
- Li, L.; Ma, B.; Jin, X.; Wang, P.; Chen, Z.; Wang, N.; Wu, S.; Zhang, C.; Gong, J. Quantitative assessment of the priority conservation of Schizothoracinae fishes in the middle Yarlung Zangbo River. J. Fish. Sci. China 2019, 26, 914–924. [Google Scholar]
- Chen, Y.F.; He, D.K.; Cao, W.X.; Duan, Z.H. Growth of Selincuo schizothoracini (Gymnocypris selincuoensis) in Selincuo Lake. Tibet Platean. Acta Zool. Sin. 2002, 48, 667–676. [Google Scholar]
- Shen, W.S.; Li, H.D.; Sun, M.; Jiang, J. Dynamics of aeolian sandy land in the Yarlung Zangbo River basin of Tibet, China from 1975 to 2008. Glob. Planet. Change 2012, 86, 37–44. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, J.; Qiao, N.Q.; An, R.Z.; Da, Z.; Miao, W.; Ba, S. Spatiotemporal distribution of protists in the Yarlung Zangbo River, Tibetan Plateau. Water Biol. Secur. 2022, 1, 100064. [Google Scholar] [CrossRef]
- Tian, P.P.; Lu, H.W.; Feng, W.; Guan, Y.L.; Xue, Y.X. Large decrease in streamflow and sediment load of Qinghai-Tibetan Plateau driven by future climate change: A case study in Lhasa River Basin. Catena 2020, 187, 104340. [Google Scholar] [CrossRef]
- Yao, Z.J.; Liu, J.; Huang, H.Q.; Song, X.F.; Dong, X.H.; Liu, X. Characteristics of isotope in precipitation, river water and lake water in the Manasarovar basin of Qinghai-Tibet Plateau. Environ. Geol. 2009, 57, 551–556. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhong, X.H.; Liu, S.Z.; Liu, J.G.; Wang, Z.Y.; Li, M.H. Regional assessment of environmental vulnerability in the Tibetan Plateau. Development and application of a new method. J. Arid. Environ. 2008, 72, 1929–1939. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, Z.; Huang, H.; Wu, S.; Liu, G. Land use and climate changes and their impacts on runoff in the Yarlung Zangbo River basin, China. Land Degrad. Dev. 2012, 25, 203–215. [Google Scholar] [CrossRef]
- He, D.K.; Sui, X.Y.; Sun, H.Y.; Tao, J.; Ding, C.Z.; Chen, Y.F.; Chen, Y.Y. Diversity, pattern and ecological drivers of freshwater fish in China and adjacent areas. Rev. Fish Biol. Fish. 2020, 30, 387–404. [Google Scholar] [CrossRef]
- Zheng, G.; Zhai, D.D.; Chen, J.; Liu, B.; Zhu, T.S. Landscape determinants of genetic structure for Schizopygopsis younghusbandi in the Yarlung Zangbo River drainage, Tibetan Plateau. Ecol. Indic. 2023, 151, 110309. [Google Scholar]
- Foubert, A.; Lecomte, F.; Legendre, P.; Cusson, M. Spatial organization of fish communities in the St. Lawrence River: A test for longitudinal gradients and spatial heterogeneities in a large river system. Hydrobiologia 2018, 809, 155–173. [Google Scholar] [CrossRef]
- Murua, H.; Rodriguez-Marin, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus slow growing tuna species: Age, growth, and implications for population dynamics and fisheries management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Denechaud, C.; Smoliński, S.; Geffen, A.J.; Godiksen, J.A.; Campana, S.E. A century of fish growth in relation to climate change, population dynamics and exploitation. Glob. Change Biol. 2020, 26, 5661–5678. [Google Scholar] [CrossRef]
- Shao, J.; Ma, B.S.; Duan, Y.J.; Xie, C.X.; Lin, S.Q.; Zhou, X.J.; Huo, B. Population resources and fishery management policies of Schizopygopsis younghusbandi in the Yarlung Zangbo River, China. J. Appl. Ecol. 2019, 30, 2437–2446. [Google Scholar]
- Zhou, C.W.; Zhou, Y.; Xu, L.H.; Liu, F.; Lei, L.; Gao, H.; Li, J.T.; Fu, S.X.; Duan, Y.T.; Tan, Y.G.; et al. Chromosome-level genome assembly and population genomic analysis provide insights into the genetic diversity and adaption of Schizopygopsis younghusbandi on the Tibetan Plateau. Integr. Zool. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.F.; He, D.K. Age and growth of Schizopygopsis younghusbandi younghusbandi in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fishes 2008, 86, 155–162. [Google Scholar] [CrossRef]
- Duan, Y.J.; Huo, B.; Ma, B.S.; Yang, X.; Xie, C.X. Reproductive biology of Schizopygopsis younghusbandi Regan 1905 (Cyprinidae: Schizothoracinae) in the middle reaches of Yarlung Zangbo River, China. J. Oceanol. Limnol. 2018, 36, 1825–1834. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Li, J.; An, R.D.; Li, Y. Ecohydraulogical Characteristic Index System of Schizopygopsis younghusbandi during Spawning Periods in the Yarlung Zangbo River. Int. J. Environ. Res. Public Health 2018, 15, 1949. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Cheng, Q.Q.; Chen, Y.; Ren, G.J. The complete mitochondrial genome sequence of Schizopygopsis younghusbandi (Cypriniformes: Cyprinidae). Mitochondrial DNA 2013, 24, 388–390. [Google Scholar] [CrossRef]
- Li, M.; Shi, X.T.; Jin, Z.J.; Ke, S.F.; Liu, C.Y.; An, R.D.; Li, J.; Katopodis, C. Behaviour and ability of a cyprinid (Schizopygopsis younghusbandi) to cope with accelerating flows when migrating downstream. River Res. Appl. 2020, 37, 1168–1179. [Google Scholar] [CrossRef]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organicgrowth (inquiries on growth lawsⅡ). Human Biol. 1938, 10, 181–213. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality growth parameters and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; ICLARM: Makati City, Philippines, 1984; 325p. [Google Scholar]
- Pauly, D. Length converted catch curves and the sea-sonal growth of fishes. Fishbyte 1990, 8, 33. [Google Scholar]
- Campana, S. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2021, 59, 197–242. [Google Scholar] [CrossRef]
- Campana, S.; Thorrold, S. Otoliths, increments, andelements: Keys to a comprehensive understanding of fish populations. Canadian. Fish. Aquat. Sci. 2021, 58, 30–38. [Google Scholar] [CrossRef]
- Zymonas, N.D.; McMahon, T.E. Comparison of pelvic fin rays, scales and otoliths for estimating age and growth of bull trout, Salvelinus confluentus. Fish. Manag. Ecol. 2009, 16, 155–164. [Google Scholar] [CrossRef]
- Jin, H.Y.; Li, L.; Wu, S.; Wang, N.M.; Chen, Z.X.; Tang, S.Z.; Jin, X.; Ma, B. Age Structure and Growth Characteristics of Garra sp. Population in Motuo Section of Yarlung Zangbo River. Wetl. Sci. 2022, 20, 785–792. [Google Scholar]
- Gamito, S. Growth models and their use in ecological modelling: An application to a fish population. Ecol. Model. 1998, 113, 83–94. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, Y.F. Age and growth of Schizothorax waltoni in the Yarlung Zangbo River in Tibet, China. Ichthyol. Res. 2009, 56, 260–265. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Maravelias, C.D. Modelling fish growth: Multi-model inference as a better alternative to a priori using von Bertalanffy equation. Fish Fish. 2008, 9, 178–187. [Google Scholar] [CrossRef]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Kallianiotis, A. Linking habitat conditions and growth in the European anchovy (Engraulis encrasicolus). Fish. Res. 2004, 68, 9–19. [Google Scholar] [CrossRef]
- Rochet, M.; Trenkel, V. Which community indicators can measure the impact of fishing? A review and proposals. Can. J. Fish. Aquat. Sci. 2003, 60, 86–99. [Google Scholar] [CrossRef]
- Mercier, L.; Panfili, J.; Paillin, C.; Ndiaye, A.; Mouillot, D.; Darnaude, A. Otolith reading and multi-model inference for improved estimation of age and growth in the gilthead seabream Sparus aurata (L.). Estuar. Coast. Shelf Sci. 2011, 92, 534–545. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Coulsin, P.G.; Meeuwig, J.J.; Meekan, M. Water temperature and fish growth: Otoliths predict growth patterns of a marine fish in a changing climate. Glob. Change Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Musick, J.A. Ecology and conservation of long-lived marine animals. Am. Fish. Soc. Symp. 1999, 23, 1–10. [Google Scholar]
- Branstetter, S. Age and growth estimates for blacktip, Carcharhinus limbatus and spinner C. brevipinna, sharks from the northwestern Gulf of Mexico. Copeia 1987, 4, 964–974. [Google Scholar] [CrossRef]
- Brown, C.J.; Broadley, A.; Adame, M.F.; Branch, T.A.; Turschwell, M.P.; Connolly, M.R. The assessment of fishery status depends on fish habitats. Fish Fish. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; González-Wangüemert, M.; Lenfant, P.; Marcos, C.; García-Charton, J.A. Effects of fishing protection on the genetic structure of fish populations. Biol. Conserv. 2006, 129, 244–255. [Google Scholar] [CrossRef]
- You, Q.L.; Kang, S.C.; Wu, Y.H.; Yan, Y.P. Climate change over the Yarlung Zangbo River Basin during 1961–2005. J. Geogr. Sci. 2007, 17, 409–420. [Google Scholar] [CrossRef]
- Rochet, M.J. A comparative approach to life-history strategies and tactics among four orders of teleost fish. ICES J. Mar. Sci. 2000, 27, 228–239. [Google Scholar] [CrossRef]
- Margarida, C.M.; Lawing, W. A study of sampling strategies for estimating growth parameters in fish populations. Fish. Res. 1995, 22, 59–75. [Google Scholar]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Schindler, D.E.; Geib, S.I.; Willians, M.R. Patterns of Fish Growth along a Residential Development Gradient in North Temperate Lakes. Ecosystems 2000, 3, 229–237. [Google Scholar] [CrossRef]
- Pecuchet, L.; Lindegren, M.; Hidalgo, M.; Delgado, M.; Esteban, A.; Fock, H.O.; Salo, L.G.D.; Punzón, A.; Sólmundsson, J.; Payne, M.R. From traits to life-history strategies: Deconstructing fish community composition across European seas. Glob. Ecol. Biogeogr. 2017, 26, 812–822. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.S.; Wang, X.A.; Wang, M.J.; He, R.H.; Wang, M. Distribution Pattern of Fish Richness in the Yarlung Zangbo River Basin. Diversity 2022, 14, 1142. [Google Scholar] [CrossRef]
- Bai, Q.Q.; Wang, L.; Cidang, Y.Z. Spatial and Temporal Variability of Rainfall Erosivity in the Niyang River Basin. Atmosphere 2024, 15, 1032. [Google Scholar] [CrossRef]
- Zhang, M.F.; Ren, Q.S.; Wei, X.H.; Wang, J.S.; Yang, X.L.; Jiang, Z.S. Climate change, glacier melting and streamflow in the Niyang River Basin, Southeast Tibet, China. Ecohydrology 2011, 4, 288–298. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yan, N.; Wu, G.J.; Xu, T.L.; Li, F. Chemical weathering in the upstream and midstream reaches of the Yarlung Zangbo basin, southern Tibetan Plateau. Chem. Geol. 2021, 559, 119906. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Liu, K.F.; Dan, Z.; Gao, Q.Z.; Mima, C.; Lu, C.H. Runoff spatiotemporal variability driven by climate change and human activity for the Nianchu River Basin in Southwestern Tibet. J. Hydrol. Reg. Stud. 2025, 58, 102301. [Google Scholar] [CrossRef]
- Li, J.T.; Chiu, M.C.; Lin, X.W.; Liu, C.; Tian, Z.; Cai, Q.H.; Resh, V.H. Environmental Factors Shape the Differences in Biodiversity-Area Relationships in Riverine Macroinvertebrates of Two Rivers in the Tibetan Plateau in China. Water 2024, 16, 882. [Google Scholar] [CrossRef]
- Chen, T.D.; Jiao, J.Y.; Zhang, Z.Q.; Lin, H.; Zhao, C.J.; Wang, H.L. Soil quality evaluation of the alluvial fan in the Lhasa River Basin, Qinghai-Tibet Plateau. Catena 2022, 209, 105829. [Google Scholar] [CrossRef]
- Ama-Abasi, D.; Holzloehner, S.; Enin, U. The dynamics of the exploited population of Ethmalosa fimbriata (Bowdich, 1825, Clupeidae) in the Cross River Estuary and adjacent Gulf of Guinea. Fish. Res. 2004, 68, 225–235. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhu, F.Y.; Liu, M.D.; Duan, X.B.; Liu, S.P.; Chen, D.Q.; Wang, D.Q. AgeStructure and Growth Characteristics of Schizopygopsis thermalis in the Upper Nujiang River. J. Hydrol. 2022, 43, 111–118. [Google Scholar]
- Yang, X.; Huo, B.; Duan, Y.J.; Ma, B.S.; Xie, C.X. Age structure and growth characteristics of Ptychobarbus dipogon in the Yarlung Zangbo River, Tibet. J. Fish. Sci. China 2015, 22, 1085–1094. [Google Scholar]
- Yao, J.L.; Chen, Y.F.; Chen, F.; He, D.K. Age and growth of an endemic Tibetan fish, Schizothorax o’connori, in the Yarlung Zangbo River. J. Freshw. Ecol. 2009, 24, 343–345. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.B.; Hu, H.M.; Gong, Z.; Cao, W.X.; Lin, P.C. Characteristics of age and growth of Schizothorax curcilabiatus in the lower reaches of the Yarlung Zangbo River. Acta Hydrobiol. Sin. 2022, 46, 1770–1779. [Google Scholar]
- Jia, Y.T.; Chen, Y.F. Age Structure and Growth Characteristics of the Endemic Fish Oxygymnocypris stewartii (Cypriniformes: Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River, Tibet. Zool. Stud. 2011, 50, 69–75. [Google Scholar]
- Tan, B.Z.; Yang, X.F.; Yang, R.B. Age structure and growth characteristics of Gymnocypris waddelli in the Zhegu Lake, Tibet. J. Fish. Sci. China 2020, 27, 879–885. [Google Scholar]
- Zhu, Q.G.; Tang, H.Y.; Lin, H.; Gong, Y.; Li, X.N.; Yang, Z. Age Structure, Growth Characteristics and Population Dynamic of Schizothorax chongi in Middle and Lower Jinsha River. J. Hydro. 2021, 42, 56–63. [Google Scholar]
- Li, X.F.; Jiang, R.J.; Bing, Y.; Wang, Y.L.; Sun, H.Q.; Yin, R.; Li, K.; Hu, Z.J. Growth, Mortaily and optimum catahable size of Coilia mystus in Oujiang river estuary. Acta Hydrobiol. Sin. 2022, 46, 1393–1401. [Google Scholar]
- Gulland, J.A. Fish stock assessment: A manual of basic methods. J. Mar. Biol. Assoc. U. K. 1984, 64, 249. [Google Scholar]
- Mehanna, S.F. Stock assessment and management of the Egyptian sole Solea aegyptiaca Chabanaud, 1927(Osteichthyes: Soleidae), in the Southeastern Mediterranean, Egypt. Turk. J. Zool. 2007, 31, 379–388. [Google Scholar]
- Su, L.; Zhang, K.; Xu, Y.W.; Chen, Z.Z. Variations in the fish community of the Beibu Gulf (South China Sea) following fishery resources protection measures. Fish. Res. 2025, 283, 107293. [Google Scholar] [CrossRef]
- Halliday, R.G.; Pinhorn, A.T. A review of the scientific and technical bases for policies on the capture of small fish in North Atlantic groundfish fisheries. Fish. Res. 2002, 57, 211–222. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, M.; Shen, L.; Wu, J.M.; Li, J.Y.; Du, H.; Wang, C.Y.; Yang, H.L.; Zhou, Q.; Liu, Z.G.; et al. Rapid change in Yangtze fisheries and its implications for global freshwater ecosystem management. Fish Fish. 2020, 21, 601–620. [Google Scholar] [CrossRef]
- Alma, V.; Romagnoni, G.; Wolff, M. Exploration of fisheries management policies in the Gulf of Nicoya (Costa Rica) using ecosystem modelling. Ocean Coast. Manag. 2022, 230, 106349. [Google Scholar]
- Comdie, H.M.; Grant, A.; Catchpole, T.L. Incentivising selective fishing under a policy to ban discards; lessons from European and global fisheries. Mar. Policy 2014, 45, 287–292. [Google Scholar] [CrossRef]
- Macusi, E.D.; Liguez, A.K.O.; Digal, L.N. Factors influencing catch and support for the implementation of the closed fishing season in Davao Gulf, Philippines. Mar. Policy 2021, 217, 105997. [Google Scholar] [CrossRef]
- Zorrozua, N.; Granado, I.; Fernandes-Salvador, J.A.A.; Louzao, M.; Basterretxea, M.; Arizaga, J. Evaluating the dependence of opportunistic Yellow-legged Gulls (Larus michahellis) on marine habitat and fishing discards. Int. J. Avian Sci. 2023, 166, 112–128. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Cottalorda, J.M.; Dominici, J.M.; Harmelin, J.G.; Diréach, L.L.; Ruitton, S. Effects of reserve protection level on the vulnerable fish species Sciaena umbra and implications for fishing management and policy. Glob. Ecol. Conserv. 2015, 3, 279–287. [Google Scholar] [CrossRef]
- Muawanah, U.; Yusuf, G.; Adrianto, L.; Kalther, J.; Pomeroy, R.; Abdullah, H.; Ruchimat, T. Review of national laws and regulation in Indonesia in relation to an ecosystem approach to fisheries management. Mar. Policy 2018, 91, 150–160. [Google Scholar] [CrossRef]
- Ormerod, S.J. Current issues with fish and fisheries: Editor’s overview and introduction. J. Appl. Ecol. 2003, 40, 204–213. [Google Scholar] [CrossRef]






| River | Number | Number | Body Length/mm | Body Weight/g | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Range | Mean | Range | Mean | ||
| Duoxiong Zangbo | 499 | 164 | 209 | 30~389 | 205.89 | 0.3~695.2 | 190.63 |
| Nianchu River | 483 | 235 | 175 | 35~355 | 142.07 | 0.6~563.6 | 73.23 |
| Lhasa River | 814 | 364 | 264 | 20~412 | 179.24 | 0.3~859.7 | 151.47 |
| Niyang River | 262 | 142 | 95 | 40~385 | 168.24 | 1.0~791.0 | 109.89 |
| Index | River | Equation |
|---|---|---|
| Body length growth rate | Duoxiong Zangbo | dL/dt = 63.838 e−0.154(t+0.556) |
| Lhasa River | dL/dt = 67.491 e−0.174(t+0.695) | |
| Niyang River | dL/dt = 67.763 e−0.172(t+0.668) | |
| Nianchu River | dL/dt = 64.900 e−0.162(t+0.407) | |
| Body length growth acceleration | Duoxiong Zangbo | d2L/dt2 = −9.831 e−0.154(t+0.556) |
| Lhasa River | d2L/dt2 = −11.743 e−0.174(t+0.695) | |
| Niyang River | d2L/dt2 = −11.655 e−0.172(t+0.668) | |
| Nianchu River | d2L/dt2 = −10.514 e−0.162(t+0.407) | |
| Body weight growth rate | Duoxiong Zangbo | dW/dt = 540.037 e−0.154(t+0.556) [1 − e−0.154(t+0.556)]1.9689 |
| Lhasa River | dW/dt = 565.268 e−0.174(t+0.695) [1 − e−0.174(t+0.695)]1.9881 | |
| Niyang River | dW/dt = 488.768 e−0.172(t+0.668) [1 − e−0.172(t+0.668)]2.0694 | |
| Nianchu River | dW/dt = 515.932 e−0.162(t+0.407) [1 − e−0.162(t+0.407)]1.9697 | |
| Body weight growth acceleration | Duoxiong Zangbo | d2W/dt2 = 83.166 e−0.154(t+0.556) [1 − e−0.145(t+0.556)]0.9689 [2.9689 e−0.154(t+0.556) − 1] |
| Lhasa River | d2W/dt2 = 98.357 e−0.174(t+0.695) [1 − e−0.174(t+0.695)]0.9881 [2.9881 e−0.174(t+0.695) − 1] | |
| Niyang River | d2W/dt2 = 85.134 e−0.172(t+0.668) [1 − e−0.172(t+0.668)]1.0714 [3.0714 e−0.172(t+0.668) − 1] | |
| Nianchu River | d2W/dt2 = 83.581 e−0.162(t+0.407) [1 − e−0.162(t+0.407)]0.9697 [2.9697 e−0.162(t+0.407) − 1] |
| River | Inflection Point Age | Critical Age | Growth Performance Index |
|---|---|---|---|
| Duoxiong Zangbo | 6.51 | 5.32 | 4.42 |
| Nianchu River | 6.31 | 5.13 | 4.41 |
| Lhasa River | 5.60 | 4.40 | 4.42 |
| Niyang River | 5.85 | 4.46 | 4.43 |
| River | Total Mortality (Z) | Natural Mortality (M) | Exploitation Rate (E) | E-Max |
|---|---|---|---|---|
| Duoxiong Zangbo | 0.453 | 0.311 | 0.313 | 0.557 |
| Lhasa River | 1.066 | 0.364 | 0.659 | 0.617 |
| Niyang River | 1.67 | 0.373 | 0.777 | 0.616 |
| Nianchu River | 0.693 | 0.331 | 0.522 | 0.448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Wang, L.; Zhang, C.; Li, H.; Ma, B. Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet. Biology 2025, 14, 707. https://doi.org/10.3390/biology14060707
Han H, Wang L, Zhang C, Li H, Ma B. Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet. Biology. 2025; 14(6):707. https://doi.org/10.3390/biology14060707
Chicago/Turabian StyleHan, Haoxiang, Lin Wang, Chi Zhang, Hongchi Li, and Bo Ma. 2025. "Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet" Biology 14, no. 6: 707. https://doi.org/10.3390/biology14060707
APA StyleHan, H., Wang, L., Zhang, C., Li, H., & Ma, B. (2025). Population Structure, Growth Characteristics, Resource Dynamics, and Management Strategies of Schizopygopsis younghusbandi in Four Tributaries of the Yarlung Zangbo River, Tibet. Biology, 14(6), 707. https://doi.org/10.3390/biology14060707






