Optimizing LED Light Intensity and Photoperiod to Promote Growth and Rooting of Medicinal Cannabis in Photoautotrophic Micropropagation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Experimental Design
2.3. Measurements
2.3.1. Growth Indicators
2.3.2. Photosynthetic Pigment Content
2.3.3. Antioxidant Enzyme Activity and Root Activity
2.3.4. Net CO2 Exchange Amount
- k: Conversion factor from volume to molecular weight of CO2 (41.9 mol m−3 at 18 °C, 41.3 mol m−3 at 22 °C);
- N: The air exchange rate per min of the GA-7 (min−1).
- V: The ventilation volume of the GA-7 (m3);
- Cout: The CO2 concentration in the culture room (μmol mol−1);
- Ci1: The CO2 concentration inside the GA-7 at time t1 (μmol mol−1);
- Ci2: The CO2 concentration inside the GA-7 at time t2 (μmol mol−1).
2.3.5. Heatmap Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Growth Parameters
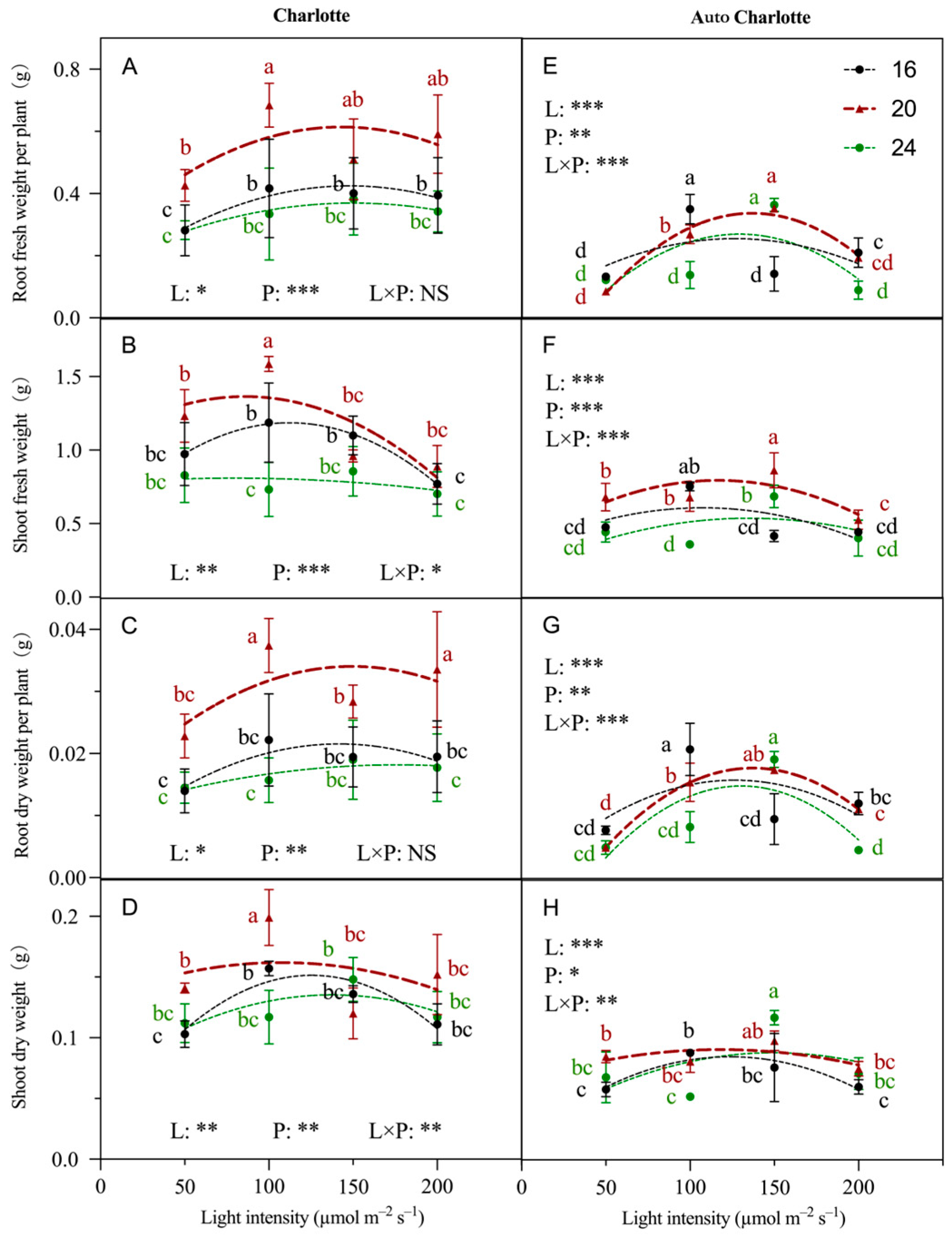
3.3. Biomass
3.4. Photosynthetic Pigment Content
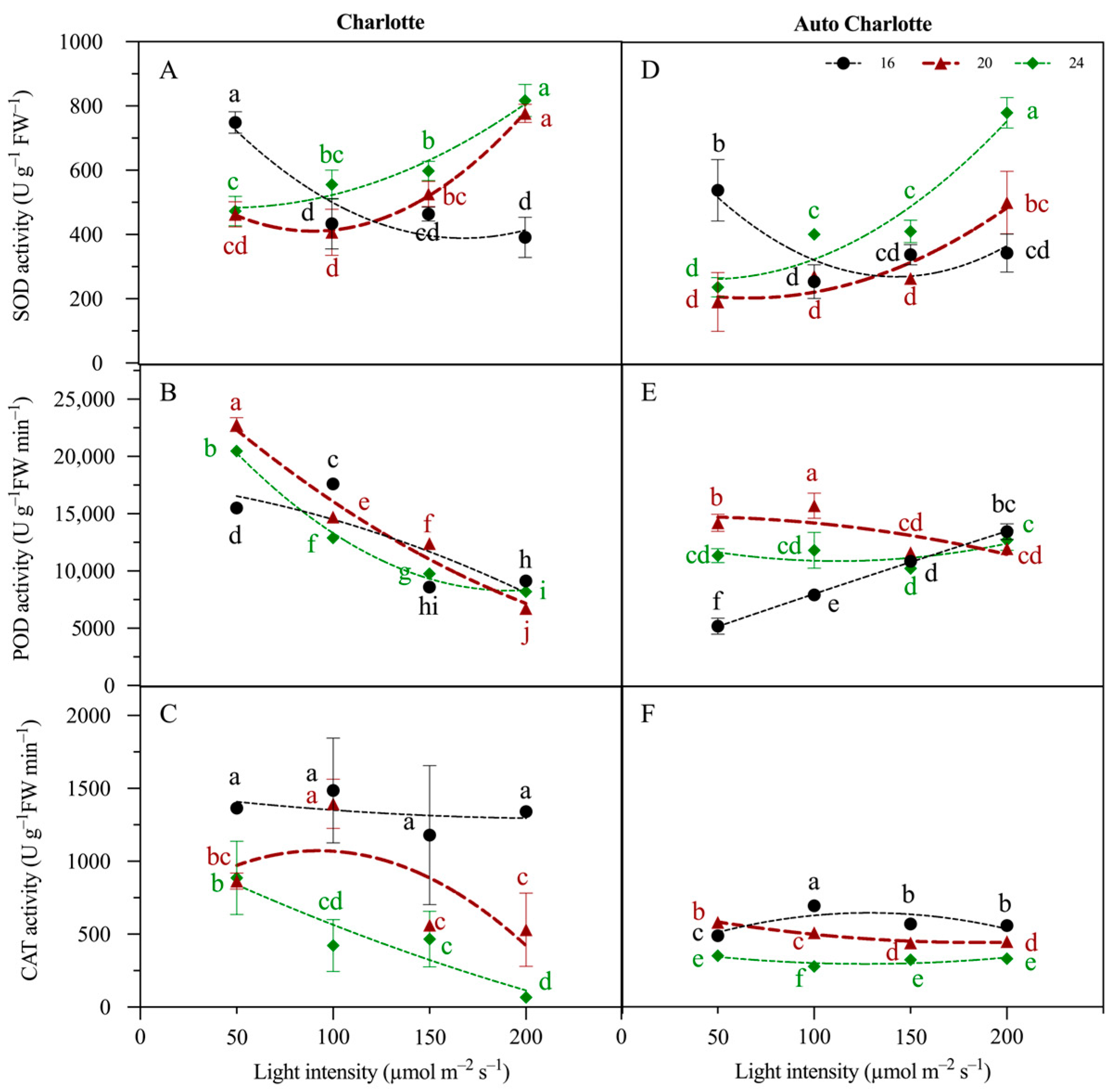
3.5. Antioxidant Enzyme Activity
3.6. Root Activity
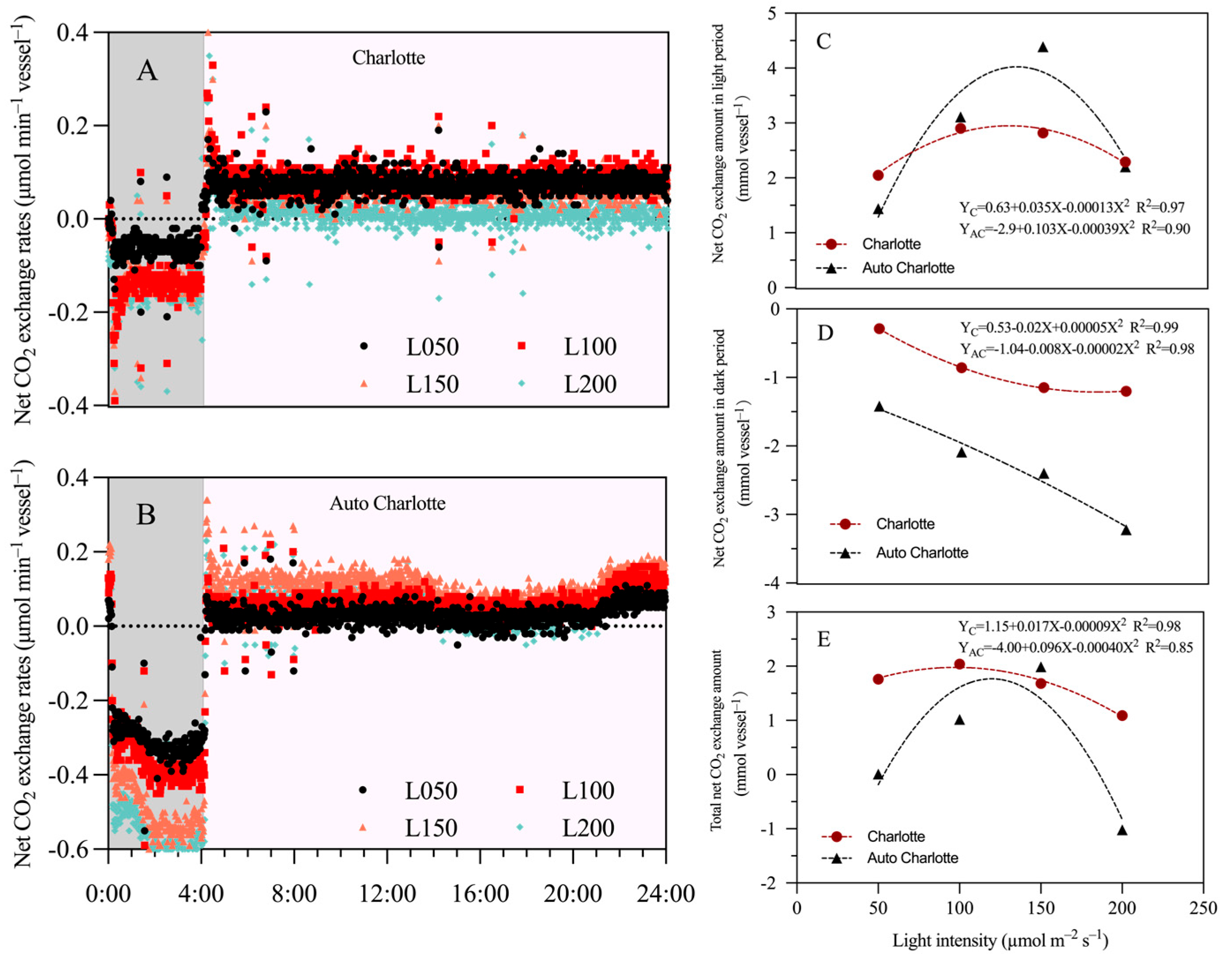
3.7. Net CO2 Exchange Amount
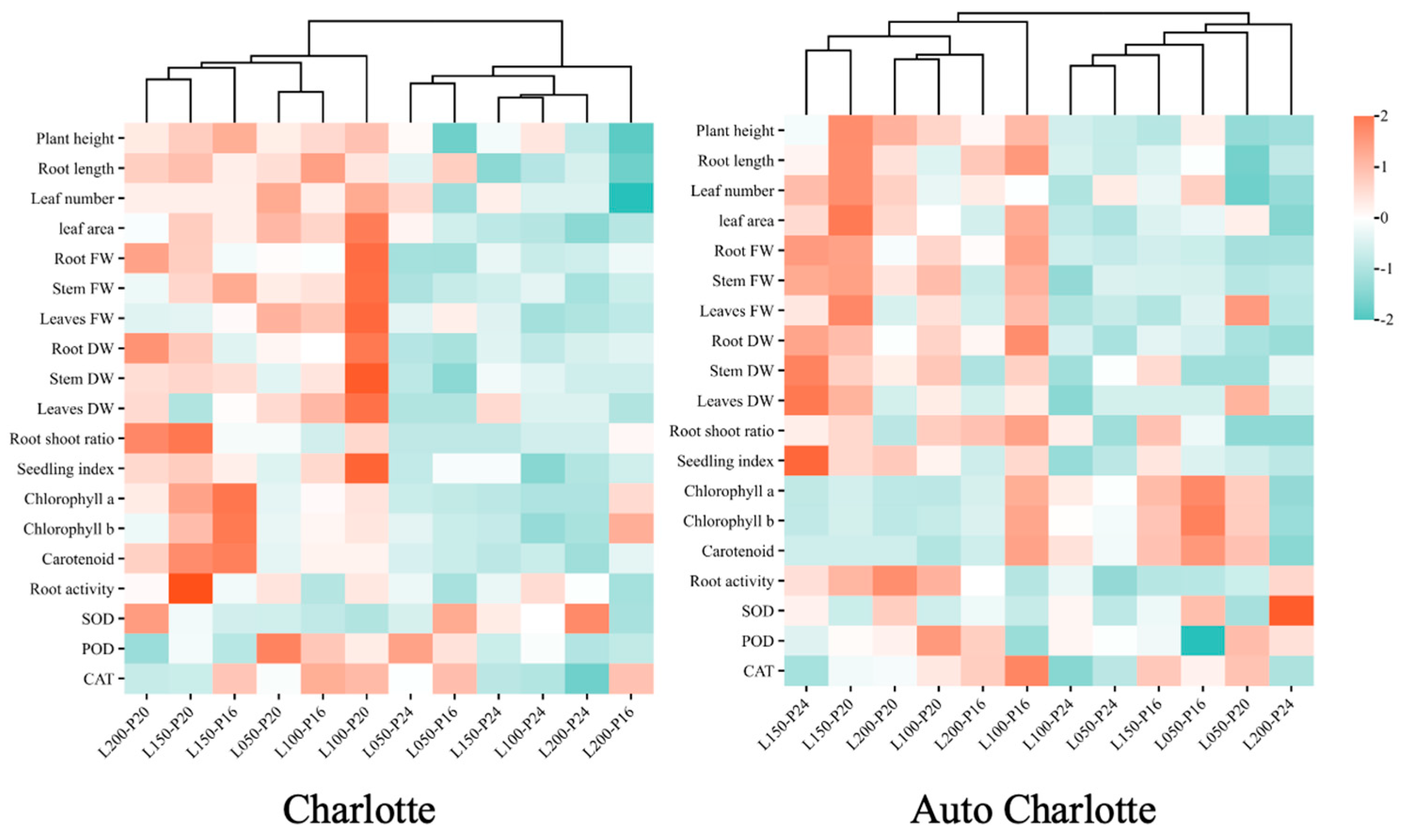
3.8. Cluster Heatmap Analysis
4. Discussion
4.1. Moderate Increases in Light Intensity and Photoperiod Promote Growth and Rooting
4.2. Continuous Lighting Is Not Conducive to Cannabis Plantlets in PAM
4.3. The Influence of Light Intensity on Net CO2 Exchange Amount
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarei, A.; Feissa, B.A.; Davis, B.; Tavakouli Dinani, E. Cannabis synthetic seeds: An alternative approach for commercial scale of clonal propagation and germplasm conservation. Plants 2022, 11, 3186. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Behdarvandi, B.; Tavakouli Dinani, E.; Maccarone, J. Cannabis sativa L. photoautotrophic micropropagation: A powerful tool for industrial scale in vitro propagation. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 932–941. [Google Scholar] [CrossRef]
- McLeod, A.; Vining, K.; Hoskins, T.; Contreras, R. Impact of indole-3-butyric acid concentration and formulation and propagation environment on rooting success of ‘I3’ hemp by stem cuttings. HortTechnology 2022, 32, 321–324. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Page, S.R.G.; Monthony, A.S.; Jones, A.M.P. DKW basal salts improve micropropagation and callogenesis compared with MS basal salts in multiple commercial cultivars of Cannabis sativa. Botany 2020, 99, 269–279. [Google Scholar] [CrossRef]
- Monthony, A.S.; Bagheri, S.; Zheng, Y.; Jones, A.M.P. Flower power: Floral reversion as a viable alternative to nodal micropropagation in Cannabis sativa. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 1018–1030. [Google Scholar] [CrossRef]
- Holmes, J.E.; Lun, S.; Collyer, D.; Punja, Z.K. Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front. Plant Sci. 2021, 12, 1885. [Google Scholar]
- Adamek, K.; Jones, A.M.P.; Torkamaneh, D. Somatic mutation accumulations in micropropagated Cannabis are proportional to the number of subcultures. Plants 2024, 13, 1910. [Google Scholar] [CrossRef]
- Punja, Z.K. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 514–527. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar]
- Cranshaw, W.S.; Halbert, S.E.; Favret, C.; Britt, K.E.; Gary, L. Phorodon cannabis Passerini (Hemiptera: Aphididae), a newly recognized pest in North America found on industrial hemp. Insecta Mundi 2018, 662, 1–12. [Google Scholar]
- Cranshaw, W.; Schreiner, M.; Britt, K.; Kuhar, T.P.; McPartland, J.; Grant, J. Developing insect pest management systems for hemp in the United States: A work in progress. J. Integr. Pest Manag. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Buirs, L.; Punja, Z.K. Integrated management of pathogens and microbes in Cannabis sativa L. (Cannabis) under greenhouse conditions. Plants 2024, 13, 786. [Google Scholar] [CrossRef]
- Lubell-Brand, J.D.; Kurtz, L.E.; Brand, M.H. An in vitro–ex vitro micropropagation system for hemp. HortTechnology 2021, 31, 199–207. [Google Scholar] [CrossRef]
- Basile, G.N.; Tedone, L.; Pulvento, C.; Mastro, G.D.; Ruta, C. Establishment of an efficient in vitro propagation protocol for Cannabis sativa L. subsp. ruderalis Janish. Horticulturae 2023, 9, 1241. [Google Scholar] [CrossRef]
- Shujat, S.; Robinson, G.I.; Norouzkhani, F.; Kovalchuk, I. Using advanced biotechnological techniques to improve cannabis cultivars. Biocatal. Agric. Biotechnol. 2024, 60, 103250. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.; ElSohly, M.A. Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L. In Vitro Cell. Dev. Biol.-Plant 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical, and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Wang, R.; He, L.S.; Xia, B.; Tong, J.F.; Li, N.; Peng, F. A micropropagation system for cloning of hemp (Cannabis sativa L.) by shoot tip culture. Pak. J. Bot. 2009, 41, 603–608. [Google Scholar]
- Chaohua, C.; Gonggua, Z.; Lining, Z.; Chunsheng, G.; Qing, T.; Jianhua, C.; Xinbo, G.; Dingxiang, P.; Jianguang, S. A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.). Ind. Crops Prod. 2016, 83, 61–65. [Google Scholar] [CrossRef]
- Piunno, K.F.; Golenia, G.; Boudko, E.A.; Downey, C.; Jones, A.M.P. Regeneration of shoots from immature and mature inflorescences of Cannabis sativa. Can. J. Plant Sci. 2019, 99, 556–559. [Google Scholar] [CrossRef]
- Kozai, T. Autotrophic (sugar-free) tissue culture for promoting the growth of plantlets in vitro and for reducing biological contamination. In Proceedings of the International Symposium on Application of Biotechnology for Small Industries in Developing Countries, Bangkok, Thailand, 21–24 September 1988. [Google Scholar]
- Ashrafzadeh, S.; Leung, D.W.M. Photoautotrophic micropropagation system (PAM): A novel approach deserving increased uptake for commercial plant production. Vegetos 2021, 34, 13–18. [Google Scholar] [CrossRef]
- Kodym, A.; Leeb, C.J. Back to the roots: Protocol for the photoautotrophic micropropagation of medicinal Cannabis. Plant Cell Tissue Organ Cult. 2019, 138, 399–402. [Google Scholar] [CrossRef]
- Zarei, A.; Davis, B.; Feyissa, B.A.; Dinani, E.T.; Simons, B. Improvement of mineral nutrition and rooting efficiency of Cannabis sativa L. for in vitro large-scale propagation. In Vitro Cell. Dev. Biol.-Plant 2023, 59, 95–105. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Propagation of Cannabis for clinical research: An approach towards modern herbal medicinal products development. Front. Plant Sci. 2020, 11, 958. [Google Scholar] [CrossRef]
- Massa, M.; Llewellyn, D.; Jones, M.; Zheng, Y. Light intensity can be used to modify the growth and morphological characteristics of Cannabis during the vegetative stage of indoor production. Ind. Crops Prod. 2022, 183, 114942. [Google Scholar]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Mohamed, E.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.; et al. Medical Cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 627204. [Google Scholar] [CrossRef]
- Murphy, R.; Adelberg, J. Physical factors increased quantity and quality of micropropagated shoots of Cannabis sativa L. in a repeated harvest system with ex vitro rooting. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 923–931. [Google Scholar] [CrossRef]
- Moher, M.; Llewellyn, D.; Golem, S.; Foley, E.; Dinka, S.; Jones, M.; Zheng, Y. Light spectra have minimal effects on rooting and vegetative growth responses of clonal Cannabis cuttings. HortScience 2023, 58, 215–221. [Google Scholar] [CrossRef]
- Wannida, S.; Heuvelink, E.; Kohlen, W.; Argyri, E.; Nicole, C.C.S.; Marcelis, L.F.M. Effect of far-red and blue light on rooting in medicinal Cannabis cuttings and related changes in endogenous auxin and carbohydrates. Sci. Hortic. 2024, 325, 112992. [Google Scholar]
- McKay, M.; Faust, J.E.; Taylor, M.; Adelberg, J. The effects of blue light and supplemental far-red on an in vitro multiple harvest system for the production of Cannabis sativa. Plants 2025, 14, 966. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Baiton, A.; Alizadeh, M.; Pepe, M.; Torkamaneh, D.; Jones, A.M.P. Advances and perspectives in tissue culture and genetic engineering of Cannabis. Int. J. Mol. Sci. 2021, 22, 5671. [Google Scholar] [CrossRef]
- Simiyu, D.C.; Jang, J.H.; Lee, O.R. Understanding Cannabis sativa L.: Current status of propagation, use, legalization, and haploid-inducer-mediated genetic engineering. Plants 2022, 11, 1201. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Dynamics of mineral uptake and plant function during development of drug-type medical cannabis plants. Agronomy 2023, 13, 2865. [Google Scholar] [CrossRef]
- Li, H. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar]
- Zheng, B.S. Modern Plant Physiological and Biochemical Research Techniques; Meteorological Press: Beijing, China, 2006; pp. 91–92. [Google Scholar]
- Sun, Q.; Hu, J.J. Plant Physiological Research Techniques; Northwest Agriculture and Forestry University Press: Yangling, China, 2005; pp. 167–170. [Google Scholar]
- Gao, J.F. Experimental Guidance of Plant Physiology; Higher Education Press: Beijing, China, 2006; pp. 57–59. [Google Scholar]
- Kozai, T.; Fujiwara, K.; Watanabe, I. Fundamental studies on environments in plant tissue culture vessels. (2) Effects of stoppers and vessels on gas exchange rates between inside and outside of vessels closed with stoppers. J. Agric. Meteorol. 1986, 42, 119–127. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kozai, T.; Watanabe, I. Measurements of carbon dioxide gas concentration in closed vessels containing tissue cultured plantlets and estimates of net photosynthetic rates of the plantlets. J. Agric. Meteorol. 1987, 43, 21–30. [Google Scholar] [CrossRef]
- Niu, G.; Kozai, T.; Kubota, C. A system for measuring the in situ CO2 exchange rates of in vitro plantlets. HortScience 1998, 33, 1076–1078. [Google Scholar] [CrossRef]
- Niu, G. Modelling and Simulation in Photoautotrophic Micropropagation. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Kozai, T., Afreen, F., Zobayed, S., Eds.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Kimura, K.; Yasutake, D.; Koikawa, K.K.M. Spatiotemporally variable incident light, leaf photosynthesis, and yield across a greenhouse: Fine-scale hemispherical photography and a photosynthesis model. Precis. Agric. 2023, 24, 114–138. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.; Ieperen, W.V.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Eva, R.; Carl-Otto, O. Continuous light increases growth, daily carbon gain, antioxidants, and alters carbohydrate metabolism in a cultivated and a wild tomato species. Front. Plant Sci. 2015, 6, 522. [Google Scholar] [CrossRef] [PubMed]
- Srajer Gajdosik, M.; Vicic, A.; Gvozdic, V.; Galic, V.; Begovic, L.; Mlinaric, S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. Int. J. Mol. Sci. 2022, 23, 9702. [Google Scholar] [CrossRef] [PubMed]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Injamum-Ul- Hoque, M.; Shaffique, S.; Ayoobi, A.; Rahman, M.A.; Rahman, M.M.; Choi, H.W. Illuminating Cannabis sativa L.: The Power of Light in Enhancing, C. sativa Growth and Secondary Metabolite Production. Plants 2024, 13, 2774. [Google Scholar] [CrossRef]
- Ma, X.; Song, L.; Yu, W.; Hu, Y.; Yang, L.; Wu, J.; Ying, Y. Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Front. Plant Sci. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Leonardos, E.; Grodzinski, B. Quantifying growth non-destructively using whole-plant CO2 exchange is a powerful tool for phenotyping. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 571–589. [Google Scholar]
- Xu, Z.H.; Zhao, Y.D. Study on monitoring methods for net CO2 exchange rate of individual standing tree. Russ. J. Plant Physiol. 2024, 71, 76. [Google Scholar] [CrossRef]
- Pepe, M.; Leonardos, E.D.; Marie, T.R.J.G.; Kyne, S.T.; Hesami, M.; Jones, A.M.P.; Grodzinski, B. A noninvasive gas exchange method to test and model photosynthetic proficiency and growth rates of in vitro plant cultures: Preliminary implication for Cannabis sativa L. Biology 2022, 11, 729. [Google Scholar] [CrossRef]
- Shi, X.; Collado, C.E.; Hernandez, R. Improve Cannabis sativa micropropagation through increasing air change rate in photoautotrophic and traditional tissue culture. Sci. Hortic. 2024, 333, 113238. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Development of an in vitro hydroponic culture system for wasabi nursery plant production—Effects of nutrient concentration and supporting material on plantlet growth. Sci. Hortic. 2019, 245, 237–243. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Growth and physiological characteristics of wasabi plantlets cultured by photoautotrophic micropropagation at different temperatures. Plant Cell Tissue Organ Cult. 2020, 143, 87–96. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Xiao, Y.; Kozai, T. Photoautotrophic micropropagation. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Burlington, NJ, USA, 2016; pp. 271–283. [Google Scholar]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Lucchesini, M.; Monteforti, G.; Mensuali-Sodi, A.; Serra, G. Leaf ultrastructure, photosynthetic rate, and growth of myrtle plantlets under different in vitro culture conditions. Plant Biol. 2006, 50, 161–168. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Substituting far-red for traditionally defined photosynthetic photons results in equal canopy quantum yield for CO2 fixation and increased photon capture during long-term studies: Implications for re-defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Iwanami, Y. Effect of CO2 enrichment and sucrose concentration under light photon fluxes on plantlet growth of carnation (Dianthus caryophyllus L.) in tissue culture during the preparation stage. J. Jpn. Soc. Hortic. Sci. 1988, 57, 279–288. [Google Scholar] [CrossRef]



| Treatment | Light Intensity (μmol m−2 s−1) | Actual Light Intensity (μmol m−2 s−1) | Photoperiod (h d−1) | Daily Light Integral (mol m−2 d−1) | ||
|---|---|---|---|---|---|---|
| L050-P16 | 50 | 53.5 | ± | 0.8 | 16 | 3.1 |
| L100-P16 | 100 | 105.0 | ± | 0.3 | 16 | 6.0 |
| L150-P16 | 150 | 152.8 | ± | 1.2 | 16 | 8.8 |
| L200-P16 | 200 | 190.5 | ± | 5.9 | 16 | 11.0 |
| L050-P20 | 50 | 52.1 | ± | 1.9 | 20 | 3.8 |
| L100-P20 | 100 | 102.4 | ± | 1.9 | 20 | 7.4 |
| L150-P20 | 150 | 151.8 | ± | 3.3 | 20 | 10.9 |
| L200-P20 | 200 | 196.4 | ± | 7.5 | 20 | 14.1 |
| L050-P24 | 50 | 53.7 | ± | 1.4 | 24 | 4.6 |
| L100-P24 | 100 | 103.2 | ± | 2.3 | 24 | 8.9 |
| L150-P24 | 150 | 152.8 | ± | 1.2 | 24 | 13.2 |
| L200-P24 | 200 | 193.2 | ± | 2.8 | 24 | 16.7 |
| Cultivar | Treatment | Plant Height (cm) | Root Length (cm) | Stem Diameter (mm) | Number of Leaves | Leaf Area (cm2) | Water Content (%) | Root Shoot Ratio | Seedling Index (DQI) |
|---|---|---|---|---|---|---|---|---|---|
| Charlotte | L050-P16 | 6.9 ± 1.7 c | 12.7 ± 2.3 ab | 2.2 ± 0.3 ab | 8.3 ± 0.6 bc | 30.0 ± 5.7 c | 90.5 ± 1.3 a | 0.13 ± 0.02 c | 0.0032 ± 0.0009 b |
| L100-P16 | 10.7 ± 1.2 ab | 14.8 ± 0.7 a | 2.3 ± 0.1 ab | 9.7 ± 0.6 ab | 43.6 ± 13.2 b | 88.4 ± 2.3 b | 0.14 ± 0.04 c | 0.0036 ± 0.0004 ab | |
| L150-P16 | 11.8 ± 1.3 a | 11.3 ± 0.6 bc | 2.2 ± 0.2 ab | 9.7 ± 1.5 ab | 39.1 ± 2.6 bc | 89.6 ± 0.6 ab | 0.16 ± 0.01 bc | 0.0034 ± 0.0011 b | |
| L200-P16 | 10.2 ± 1.1 ab | 5.4 ± 1.0 e | 2.3 ± 0.1 ab | 7.3 ± 0.6 c | 26.7 ± 3.6 c | 88.7 ± 0.8 b | 0.17 ± 0.04 bc | 0.0029 ± 0.0003 b | |
| L050-P20 | 6.6 ± 1.3 c | 12.0 ± 2.7 b | 2.3 ± 0.2 ab | 10.7 ± 1.5 a | 48.2 ± 7.7 ab | 90.1 ± 0.9 ab | 0.16 ± 0.02 bc | 0.0030 ± 0.0004 b | |
| L100-P20 | 11.3 ± 1.5 ab | 11.7 ± 1.0 b | 2.4 ± 0.3 a | 10.7 ± 1.5 a | 57.5 ± 2.1 a | 89.6 ± 1.0 ab | 0.19 ± 0.01 b | 0.0046 ± 0.0006 a | |
| L150-P20 | 11.0 ± 0.8 ab | 13.4 ± 0.8 ab | 2.3 ± 0.1 ab | 9.7 ± 0.6 ab | 44.7 ± 10.8 b | 90.0 ± 0.8 ab | 0.25 ± 0.00 a | 0.0037 ± 0.0011 ab | |
| L200-P20 | 10.3 ± 0.4 ab | 12.7 ± 0.1 ab | 2.2 ± 0.2 ab | 9.7 ± 1.2 ab | 35.8 ± 6.1 bc | 87.5 ± 0.7 bc | 0.24 ± 0.02 a | 0.0036 ± 0.0010 ab | |
| L050-P24 | 9.9 ± 0.5 ab | 9.3 ± 1.2 c | 2.1 ± 0.2 b | 10.0 ± 1.0 ab | 38.3 ± 5.8 bc | 88.5 ± 1.2 b | 0.13 ± 0.02 c | 0.0028 ± 0.0002 b | |
| L100-P24 | 10.4 ± 1.7 ab | 7.7 ± 1.3 de | 1.8 ± 0.3 c | 9.0 ± 1.0 b | 26.4 ± 3.8 c | 87.2 ± 2.5 bc | 0.14 ± 0.03 c | 0.0024 ± 0.0001 b | |
| L150-P24 | 9.5 ± 1.4 ab | 6.3 ± 0.3 e | 2.2 ± 0.1 ab | 9.7 ± 1.2 ab | 27.4 ± 2.0 c | 86.3 ± 1.3 c | 0.13 ± 0.03 c | 0.0032 ± 0.0003 b | |
| L200-P24 | 8.4 ± 2.5 b | 8.9 ± 1.1 d | 2.0 ± 0.2 bc | 9.0 ± 1.0 b | 21.4 ± 5.1 d | 87.1 ± 0.5 c | 0.14 ± 0.01 c | 0.0027 ± 0.0003 b | |
| L | ** | ** | NS | NS | ** | * | ** | NS | |
| P | * | *** | ** | * | *** | ** | *** | *** | |
| L × P | * | *** | NS | NS | * | NS | NS | *** | |
| Auto Charlotte | L050-P16 | 6.6 ± 0.3 d | 8.2 ± 1.7 c | 2.0 ± 0.1 a | 9.7 ± 0.6 ab | 17.5 ± 1.2 c | 89.2 ± 0.9 ab | 0.13 ± 0.02 bc | 0.0016 ± 0.0002 c |
| L100-P16 | 9.1 ± 0.6 ab | 12.6 ± 1.9 b | 2.0 ± 0.2 ab | 9.0 ± 1.0 ab | 24.6 ± 3.1 ab | 90.2 ± 0.4 ab | 0.23 ± 0.05 a | 0.0021 ± 0.0001 b | |
| L150-P16 | 6.1 ± 0.3 de | 5.6 ± 0.5 de | 1.9 ± 0.3 b | 8.7 ± 1.2 ab | 14.3 ± 0.9 cd | 86.8 ± 2.3 b | 0.20 ± 0.08 ab | 0.0020 ± 0.0005 bc | |
| L200-P16 | 7.7 ± 0.1 bc | 8.0 ± 1.7 c | 1.8 ± 0.1 b | 9.3 ± 0.6 ab | 14.8 ± 1.0 c | 89.1 ± 0.8 ab | 0.20 ± 0.01 ab | 0.0015 ± 0.0002 c | |
| L050-P20 | 5.5 ± 0.9 e | 4.3 ± 0.6 e | 1.7 ± 0.2 bc | 7.3 ± 0.6 c | 20.3 ± 1.9 b | 88.2 ± 0.9 b | 0.06 ± 0.01 d | 0.0015 ± 0.0001 c | |
| L100-P20 | 8.5 ± 0.6 b | 8.0 ± 0.5 c | 1.8 ± 0.2 b | 8.7 ± 1.5 ab | 17.5 ± 0.9 c | 89.8 ± 0.7 ab | 0.19 ± 0.03 ab | 0.0019 ± 0.0002 bc | |
| L150-P20 | 9.5 ± 0.3 ab | 15.6 ± 1.4 a | 1.9 ± 0.1 b | 10.7 ± 0.6 a | 25.1 ± 5.8 a | 90.5 ± 0.3 a | 0.18 ± 0.01 ab | 0.0021 ± 0.0002 bc | |
| L200-P20 | 9.8 ± 0.4 a | 10.7 ± 2.3 b | 1.9 ± 0.2 b | 9.7 ± 2.1 ab | 19.5 ± 3.8 bc | 88.1 ± 0.1 b | 0.09 ± 0.00 c | 0.0022 ± 0.0003 b | |
| L050-P24 | 6.4 ± 0.7 de | 8.5 ± 0.4 c | 1.8 ± 0.2 b | 9.3 ± 1.2 ab | 16.0 ± 2.8 c | 87.7 ± 2.1 b | 0.07 ± 0.01 d | 0.0014 ± 0.0003 c | |
| L100-P24 | 7.2 ± 1.4 cd | 6.3 ± 1.1 d | 1.6 ± 0.1 c | 8.0 ± 1.0 b | 16.4 ± 4.3 c | 87.9 ± 0.5 b | 0.16 ± 0.04 b | 0.0012 ± 0.0002 c | |
| L150-P24 | 7.3 ± 0.4 c | 11.5 ± 0.2 b | 1.8 ± 0.2 b | 10.0 ± 1.0 ab | 15.8 ± 3.7 c | 87.1 ± 0.3 b | 0.16 ± 0.02 b | 0.0029 ± 0.0000 a | |
| L200-P24 | 6.3 ± 1.2 de | 6.8 ± 0.6 cd | 1.6 ± 0.2 c | 7.7 ± 0.6 bc | 13.0 ± 1.0 d | 84.7 ± 0.3 c | 0.06 ± 0.01 d | 0.0014 ± 0.0001 c | |
| L | *** | *** | NS | NS | NS | * | *** | *** | |
| P | *** | * | * | NS | ** | ** | ** | NS | |
| L × P | *** | *** | NS | * | ** | * | NS | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Ji, F.; Zhou, Q.; He, D. Optimizing LED Light Intensity and Photoperiod to Promote Growth and Rooting of Medicinal Cannabis in Photoautotrophic Micropropagation. Biology 2025, 14, 706. https://doi.org/10.3390/biology14060706
Liang J, Ji F, Zhou Q, He D. Optimizing LED Light Intensity and Photoperiod to Promote Growth and Rooting of Medicinal Cannabis in Photoautotrophic Micropropagation. Biology. 2025; 14(6):706. https://doi.org/10.3390/biology14060706
Chicago/Turabian StyleLiang, Juwen, Fang Ji, Qing Zhou, and Dongxian He. 2025. "Optimizing LED Light Intensity and Photoperiod to Promote Growth and Rooting of Medicinal Cannabis in Photoautotrophic Micropropagation" Biology 14, no. 6: 706. https://doi.org/10.3390/biology14060706
APA StyleLiang, J., Ji, F., Zhou, Q., & He, D. (2025). Optimizing LED Light Intensity and Photoperiod to Promote Growth and Rooting of Medicinal Cannabis in Photoautotrophic Micropropagation. Biology, 14(6), 706. https://doi.org/10.3390/biology14060706








