Light/Dark Cycle Lighting Influences Growth and Energy Use Efficiency of Hydroponic Lettuces in an LED Plant Factory
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Conditions and Plant Material
2.2. Light/Dark Cycle Treatments
2.3. Photosynthetic Parameters and Chlorophyll Fluorescence
2.4. Growth Parameters
2.5. Photon Yield and Energy Use Efficiency
2.6. Statistical Analysis
3. Results
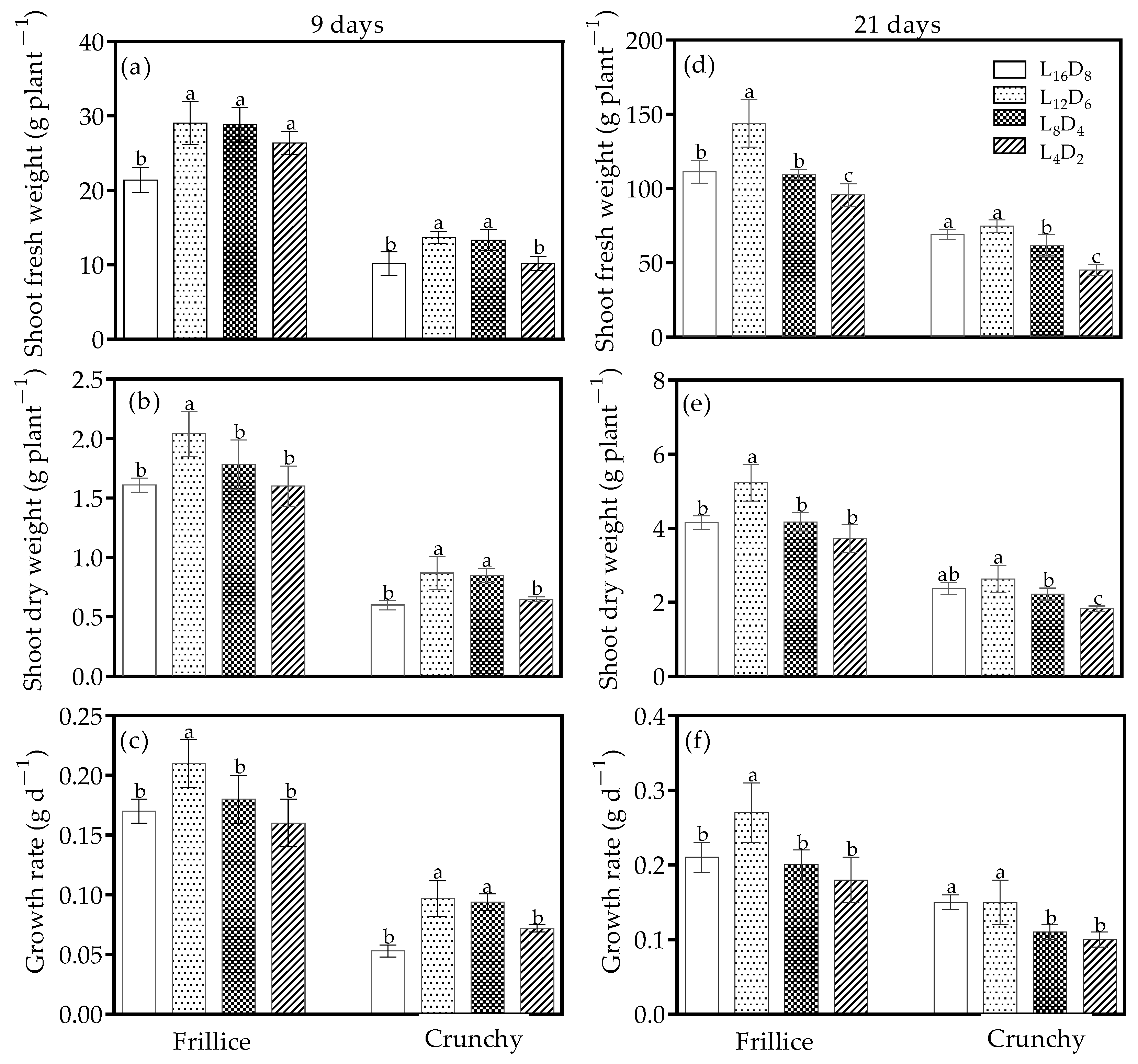
3.1. Leaf Number and Leaf Area
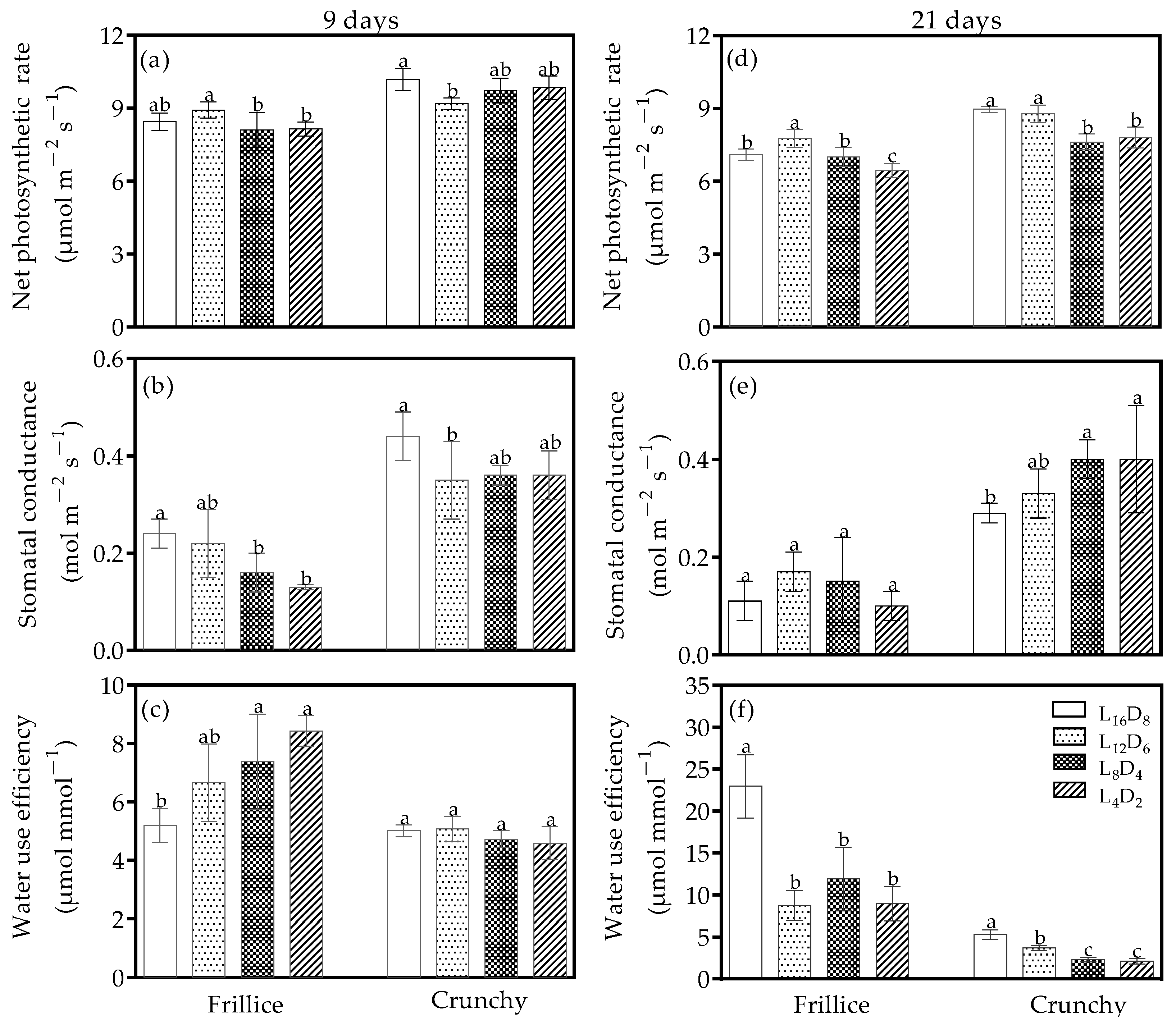
3.2. Photosynthetic Parameters
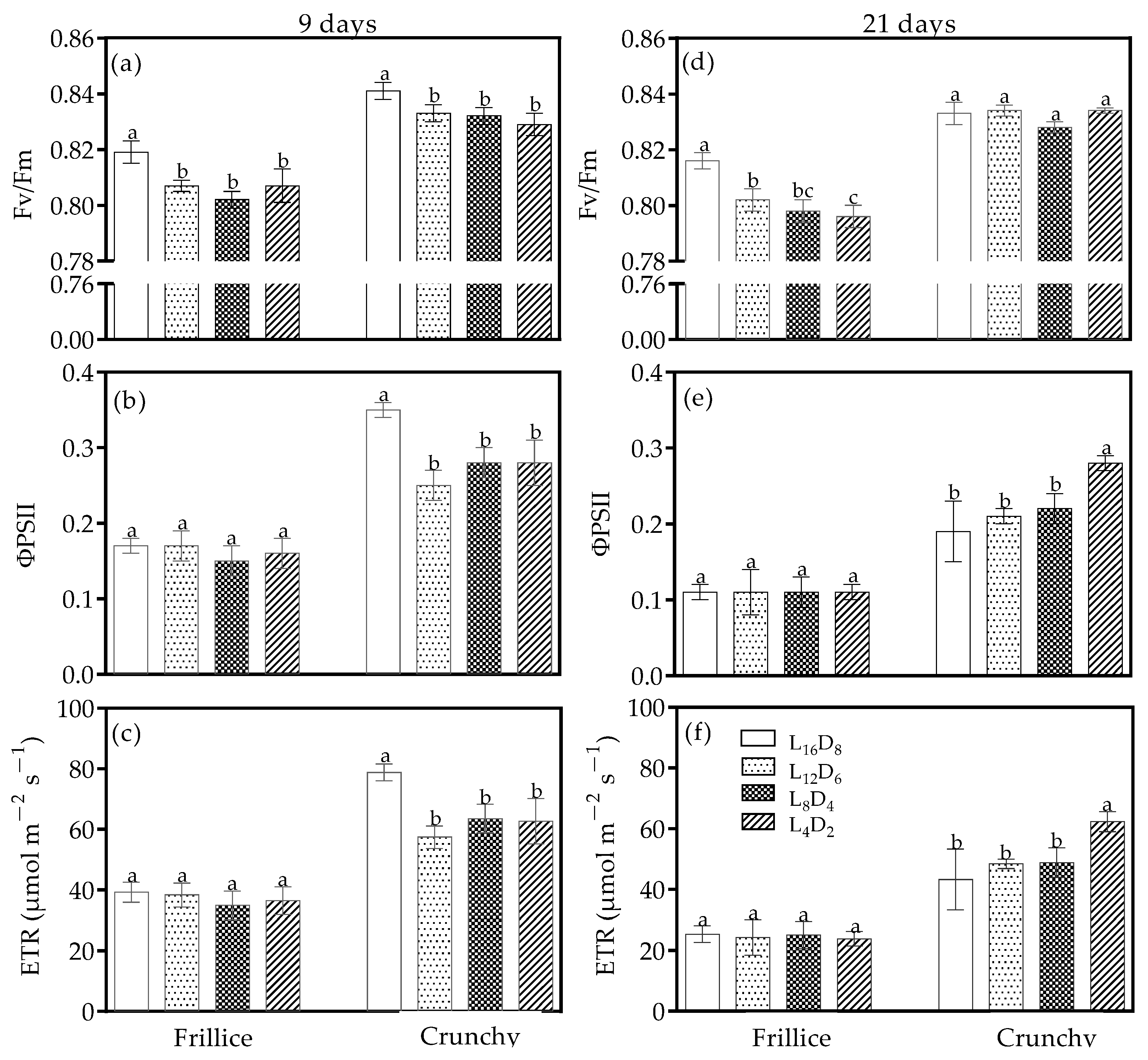
3.3. Chlorophyll Fluorescence
3.4. Shoot Weight and Growth Rate
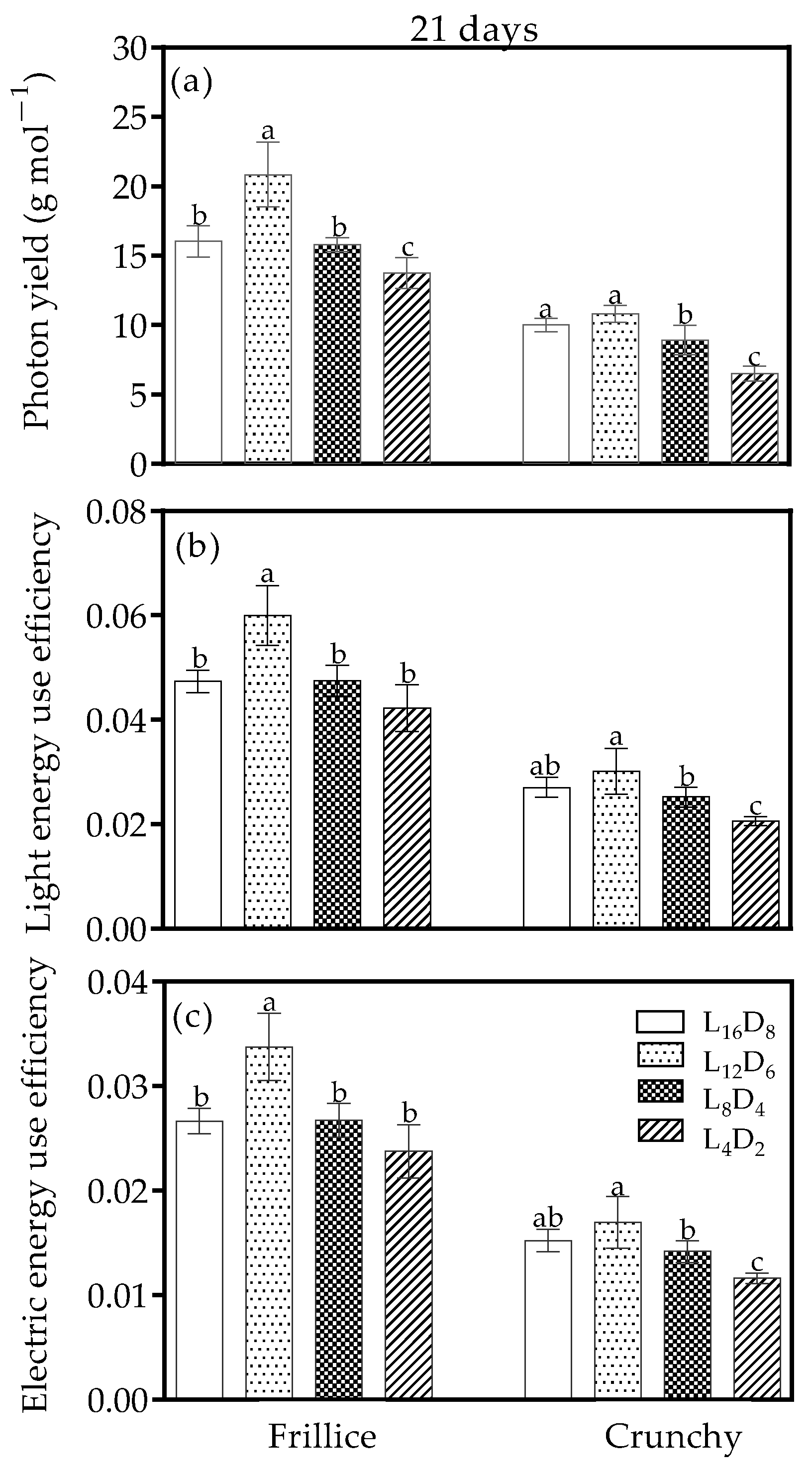
3.5. Photon Yield, Light Energy Use Efficiency, and Electric Energy Use Efficiency
4. Discussion
4.1. Lettuce Response to Light/Dark Cycle Was Cultivar-Dependent
4.2. Light/Dark Cycles Can Regulate Water Use Efficiency by Altering Stomatal Opening
4.3. Optimizing Light/Dark Cycle Could Enhance Productivity and Reduce Energy Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozai, T.; Kubota, C.; Chun, C.; Afreen, F.; Ohyama, K. Necessity and concept of the closed transplant production system. In Transplant Production in the 21st Century; Kubota, C., Chun, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 3–19. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables (ESA/P/WP/248); DESA Publications: New York, NY, USA, 2017. [Google Scholar]
- van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, L.; Klerkx, R.S.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Balázs, L.; Dombi, Z.; Csambalik, L.; Sipos, L. Characterizing the spatial uniformity of light intensity and spectrum for indoor crop production. Horticulturae 2022, 8, 644. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Custódio, F.B.; Knupp, E.A.N.; Palmieri, H.E.L.; Silva, J.B.B.; Glória, M.B.A. Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environ. Pollut. 2018, 242 Pt A, 383–389. [Google Scholar] [CrossRef]
- Roa, J. Informal Food Markets in Quezon City and Pasay City, Philippines: A Rapid Assessment. Resilient Cities Initiative Research Report; International Potato Center: Lima, Peru, 2023. [Google Scholar]
- Boros, I.F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED lighting environments on lettuce (Lactuca sativa L.) in PFAL systems—A review. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, L.; Yang, Q.; Guo, W. Responses of butter leaf lettuce to mixed red and blue light with extended light/dark cycle period. Sci. Rep. 2022, 12, 6924. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q. Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci. Hortic. 2018, 234, 220–226. [Google Scholar] [CrossRef]
- Dai, M.; Tan, X.; Ye, Z.; Chen, X.; Zhang, Y.; Ruan, Y.; Ma, B.; Kong, D. Analysis of lettuce transcriptome reveals the mechanism of different light/dark cycle in promoting the growth and quality. Front. Plant Sci. 2024, 15, 1394434. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Gessler, A. Circadian regulation of photosynthesis and transpiration from genes to ecosystems. Environ. Exp. Bot. 2018, 152, 37–48. [Google Scholar] [CrossRef]
- Kurata, H.; Mochizuki, A.; Okuda, N.; Seki, M.; Furusaki, S. Intermittent light irradiation with a second-scale interval enhances caffeine production by Coffea arabica cells. Biotechnol. Prog. 1998, 14, 797–799. [Google Scholar] [CrossRef]
- Kurata, H.; Mochizuki, A.; Okuda, N.; Seki, M.; Furusaki, S. Intermittent light irradiation with second- or hour-scale periods controls anthocyanin production by strawberry cells. Enzym. Microb. Technol. 2000, 26, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Ishii, M.; Ito, T.; Maruo, T.; Suzuki, K.; Matsuo, K. Plant growth and physiological characters of lettuce plants grown under artificial light of different irradiating cycles. Environ. Control Biol. 1995, 33, 143–149. [Google Scholar] [CrossRef]
- Higashi, T.; Nishikawa, S.; Okamura, N.; Fukuda, H. Evaluation of growth under non-24 h period lighting conditions in Lactuca sativa L. Environ. Control Biol. 2015, 53, 7–12. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; TÓth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.; Webb, A.A.R. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Yang, R.; Yang, H.; Ji, F.; He, D. Enhancing the photon yield of hydroponic lettuce through stage-wise optimization of the daily light integral in an LED plant factory. Agronomy 2024, 14, 2949. [Google Scholar] [CrossRef]
- Rosenqvist, E.; van Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Deell, J.R., Toivonen, P.M.A., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Fang, W. Quantification of performance in plant factory. Technology advances in protected horticulture. In Proceedings of the 2013 the 3rd High-Level International Forum on Protected Horticulture, Shouguang, China, 19–22 April 2013. [Google Scholar]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.; Yang, Q. Lettuce plant growth and tipburn occurrence as affected by airflow using a multi-fan system in a plant factory with artificial light. J. Therm. Biol. 2020, 88, 102496. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T. Current status of plant factories with artificial lighting (PFALs) and smart PFALs. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 3–13. [Google Scholar]
- Harmer, S.L. Circadian system in higher plants. Annu. Rev. Plant Biol. 2009, 60, 357–377. [Google Scholar] [CrossRef]
- Fukuda, H.; Nakamichi, N.; Hisatsune, M.; Murase, H.; Mizuno, T. Synchronization of plant circadian oscillators with a phase delay effect of the vein network. Phys. Rev. Lett. 2007, 99, 098102. [Google Scholar] [CrossRef]
- Fung-Uceda, J.; Lee, K.; Seo, P.J.; Polyn, S.; De Veylder, L.; Mas, P. The circadian clock sets the time of DNA replication licensing to regulate growth in Arabidopsis. Dev. Cell 2018, 45, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Morales, A.; Harbinson, J.; Heuvelink, E.; Prinzenberg, A.E.; Marcelis, L.F.M. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 2016, 6, 31252. [Google Scholar] [CrossRef]
- Chen, K.-M.; Piippo, M.; Holmström, M.; Nurmi, M.; Pakula, E.; Suorsa, M.; Aro, E.-M. A chloroplast-targeted DnaJ protein AtJ8 is negatively regulated by light and has rapid turnover in darkness. J. Plant Physiol. 2011, 168, 1780–1783. [Google Scholar] [CrossRef]
- Hayashi, F.; Ichino, T.; Osanai, M.; Wada, K. Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol. 2000, 41, 1096–1101. [Google Scholar] [CrossRef]
- Higashi, T.; Murase, H.; Fukuda, H. Entrainment of circadian rhythms to environmental cycles in Lactuca sativa L.—Characteristics of circadian rhythms in lettuce. Environ. Control Biol. 2014, 52, 21–27. [Google Scholar] [CrossRef]
- Simon, N.M.L.; Graham, C.A.; Comben, N.E.; Hetherington, A.M.; Dodd, A.N. The circadian clock influences the long-term water use efficiency of Arabidopsis. Plant Physiol. 2020, 183, 317–330. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Kong, D.; Guo, F.; Wei, H. Light-mediated signaling and metabolic changes coordinate stomatal opening and closure. Front. Plant Sci. 2020, 11, 601478. [Google Scholar] [CrossRef] [PubMed]
- Large-Scale Facility Horticulture and Plant Factories; Japan Greenhouse Horticulture Association: Tokyo, Japan, 2024; p. 81.


| Cultivar | Treatment | Light Time (h) | Dark Time (h) | Total Light/Dark Cycles |
|---|---|---|---|---|
| Frillice | L16D8 | 16 | 8 | 21 |
| L12D6 | 12 | 6 | 28 | |
| L8D4 | 8 | 4 | 42 | |
| L4D2 | 4 | 2 | 84 | |
| Crunchy | L16D8 | 16 | 8 | 21 |
| L12D6 | 12 | 6 | 28 | |
| L8D4 | 8 | 4 | 42 | |
| L4D2 | 4 | 2 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhong, L.; Ji, X.; Wang, J.; He, D. Light/Dark Cycle Lighting Influences Growth and Energy Use Efficiency of Hydroponic Lettuces in an LED Plant Factory. Biology 2025, 14, 571. https://doi.org/10.3390/biology14050571
Li W, Zhong L, Ji X, Wang J, He D. Light/Dark Cycle Lighting Influences Growth and Energy Use Efficiency of Hydroponic Lettuces in an LED Plant Factory. Biology. 2025; 14(5):571. https://doi.org/10.3390/biology14050571
Chicago/Turabian StyleLi, Wen, Luming Zhong, Xiang Ji, Jun Wang, and Dongxian He. 2025. "Light/Dark Cycle Lighting Influences Growth and Energy Use Efficiency of Hydroponic Lettuces in an LED Plant Factory" Biology 14, no. 5: 571. https://doi.org/10.3390/biology14050571
APA StyleLi, W., Zhong, L., Ji, X., Wang, J., & He, D. (2025). Light/Dark Cycle Lighting Influences Growth and Energy Use Efficiency of Hydroponic Lettuces in an LED Plant Factory. Biology, 14(5), 571. https://doi.org/10.3390/biology14050571








