Subtypes I and II of Ulva prolifera O.F. Müller: Dominant Green Tide Species in the Southern Yellow Sea and Their Responses to Natural Light and Temperature Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Identification and Thallus Culture
2.2. Gamete Collection, Observation, and Cultivation
2.3. Observation and Measurement of Gametophyte Morphology and Growth
2.4. Determination of Chlorophyll Fluorescence Parameters and Chloroplast Pigment Content
2.5. Statistical Analysis
3. Results
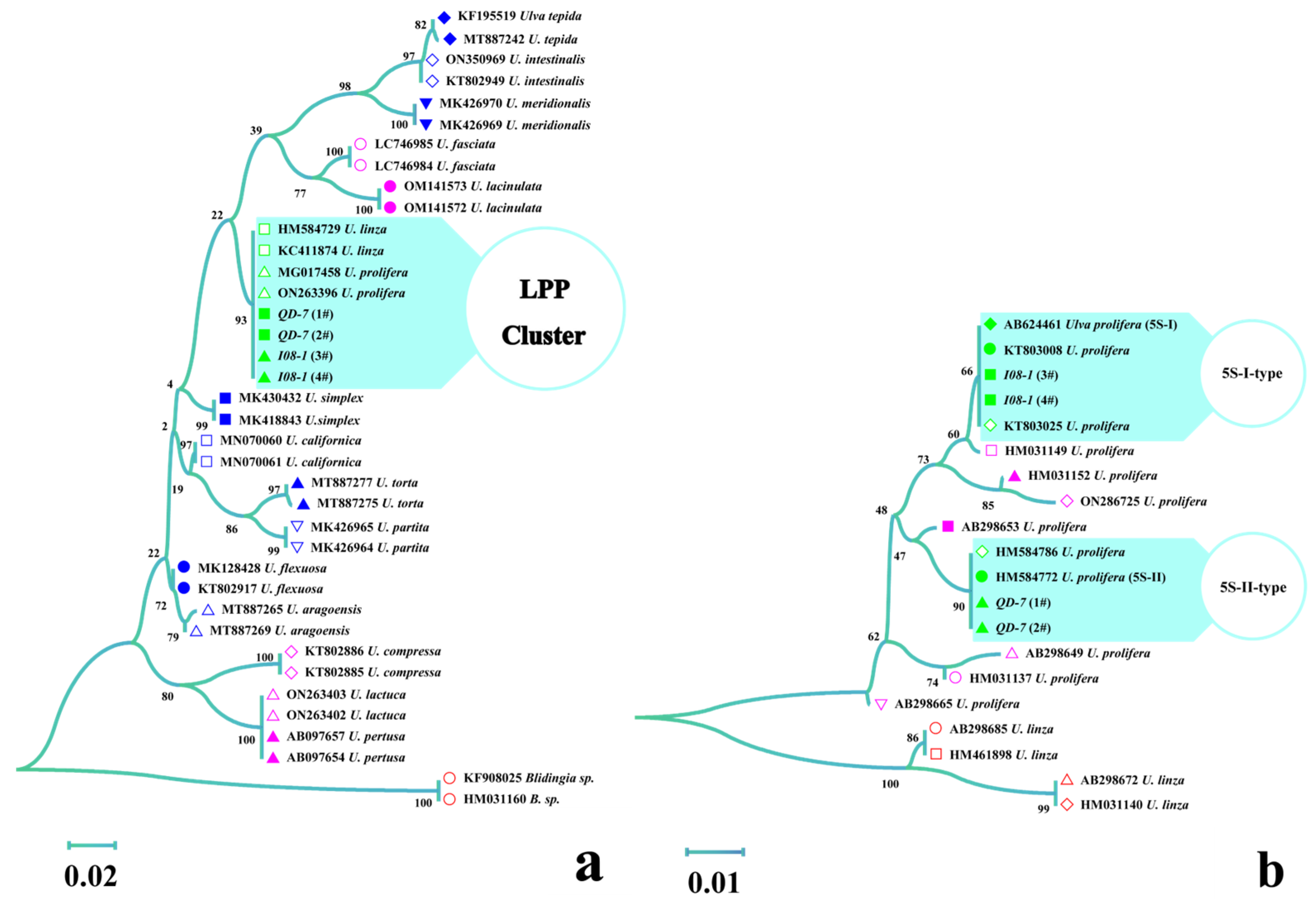
3.1. Identification of Ulva prolifera Strains
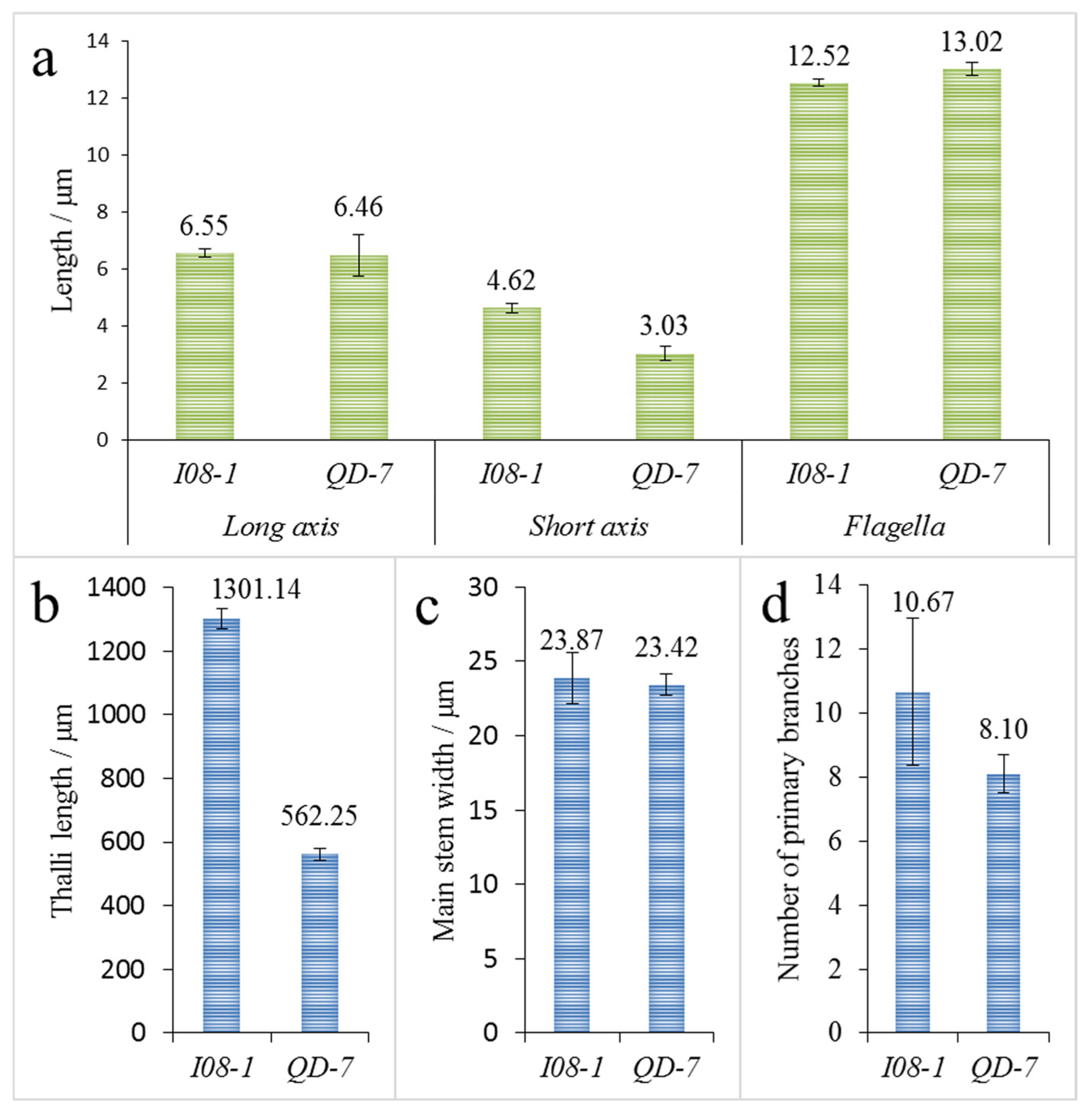
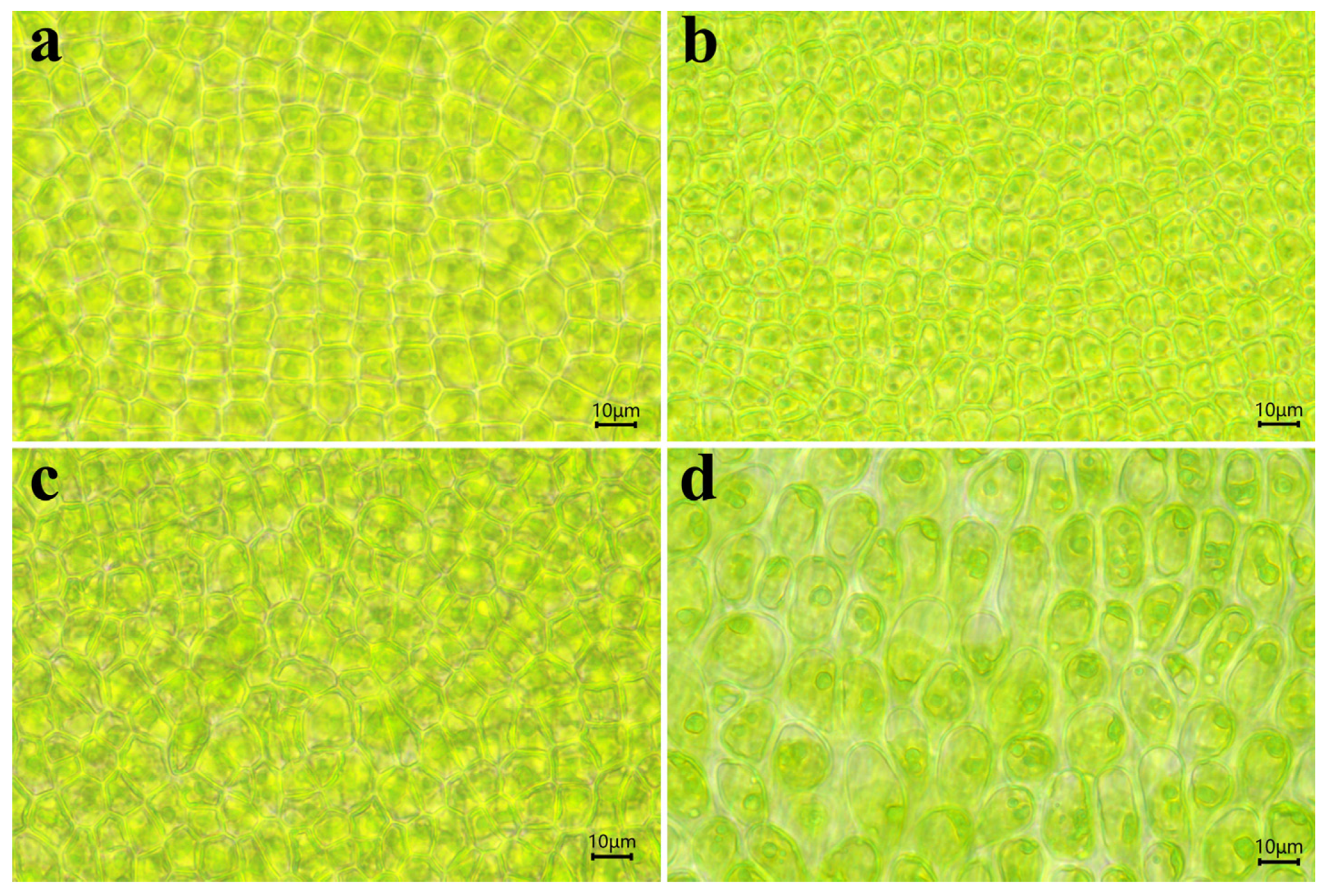
3.2. Gamete Germling Morphology
3.3. Adult Thalli Morphology
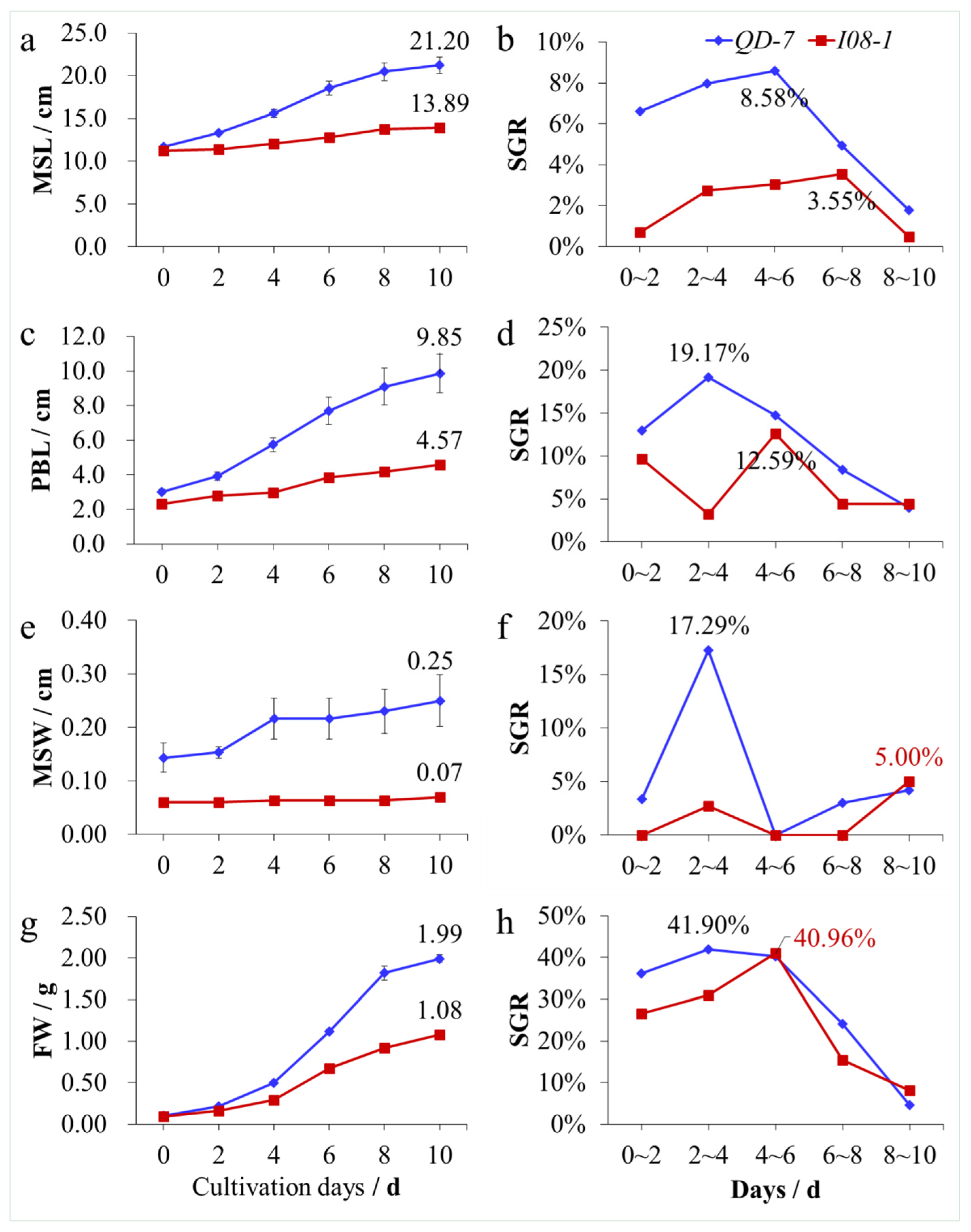
3.4. Adult Thalli Growth
3.5. Chlorophyll Fluorescence Parameters
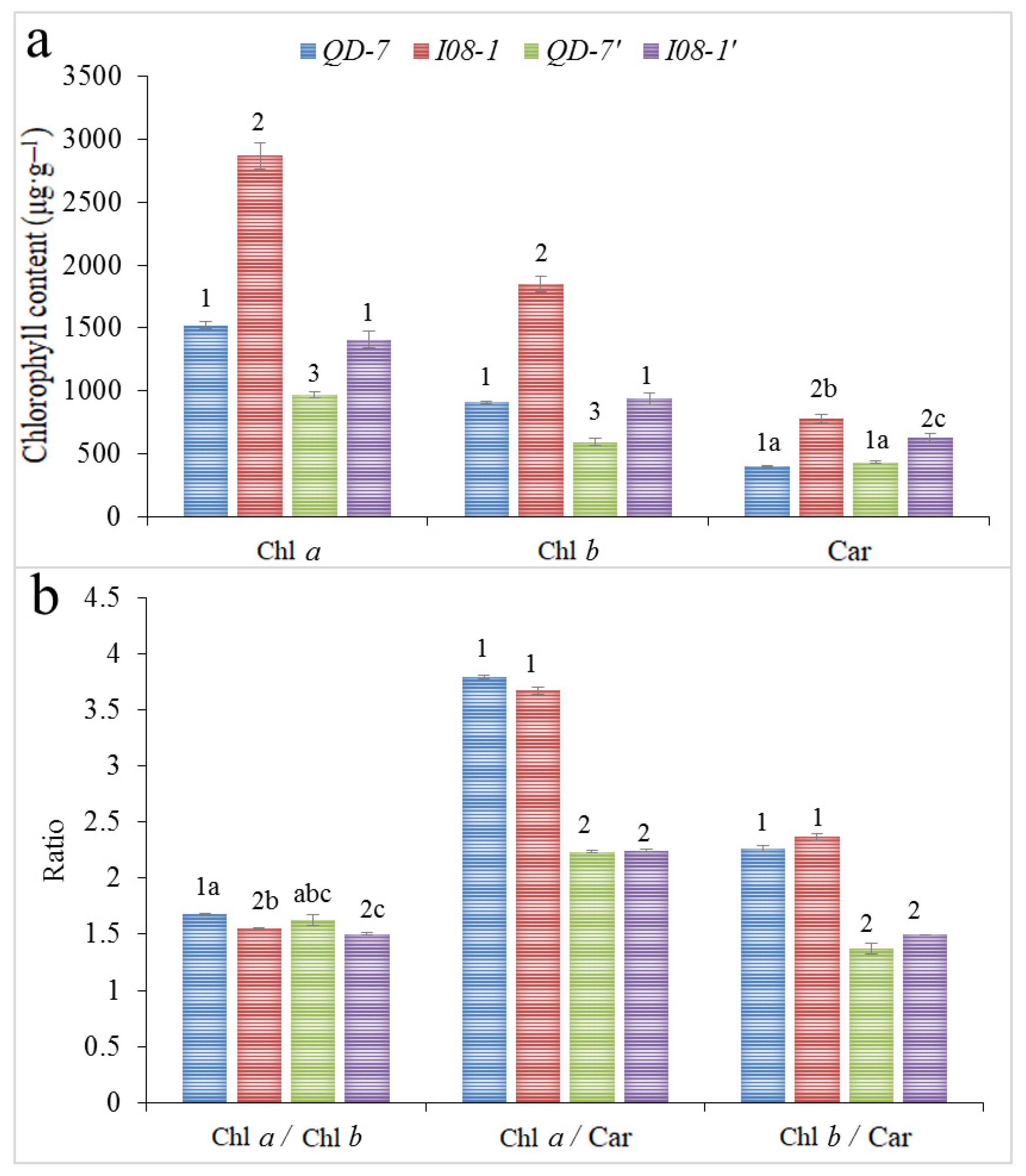
3.6. Chloroplast Pigment Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Liu, J.; Zhao, S.; Sun, Y.; Cui, Q.; Wu, L.; Gao, S.; Zhang, J.; He, P. Review of the development of the green tide and the process of control in the southern Yellow Sea in 2022. Estuar. Coast. Shelf Sci. 2024, 302, 108772. [Google Scholar] [CrossRef]
- Liu, N.; Yu, J.; Wang, Q.; Zhang, K.; Jiang, C.; Tian, S. Network and evolutionary analysis of green tide management policies in the Yellow Sea, China. Mar. Pollut. Bull. 2024, 206, 116755. [Google Scholar] [CrossRef] [PubMed]
- Huan, L.; Gu, W.; Wang, X.; Yan, Y.; Tang, Q.; Han, X.; Wang, Z.; Zhou, K.; Qiu, Q.; Xu, J.; et al. Reproductive traits of floating Ulva prolifera sporophytes and gametophytes and their contribution to the Yellow Sea green tide. Mar. Pollut. Bull. 2025, 214, 117752. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Liu, D.; Sun, Z.; Tang, R.; Ma, X.; Feng, Z. Loading of microplastics by two related macroalgae in a sea area where gold and green tides occur simultaneously. Sci. Total Environ. 2022, 814, 152809. [Google Scholar] [CrossRef]
- Sun, K.; Ren, J.; Bai, T.; Zhang, J.; Liu, Q.; Wu, W.; Zhao, Y.; Liu, Y. A dynamic growth model of Ulva prolifera: Application in quantifying the biomass of green tides in the Yellow Sea, China. Ecol. Model. 2020, 428, 109072. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Qu, T.; Zhong, Y.; Guan, C.; Hou, C.; Tang, L.; Tang, X.; Wang, Y. The causal link between nitrogen structure and physiological processes of Ulva prolifera as the causative species of green tides. Sci. Total Environ. 2024, 953, 176170. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, B.; Yao, Q.; Burnett, W.; Charette, M.; Su, R.; Lian, E.; Yu, Z. Nutrient-rich submarine groundwater discharge fuels the largest green tide in the world. Sci. Total Environ. 2021, 770, 144845. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zuo, P.; Yan, Y.; Qin, Y. Approaches, challenges and prospects for modeling macroalgal dynamics in the green tide: The case of Ulva prolifera. Mar. Pollut. Bull. 2025, 215, 117897. [Google Scholar] [CrossRef]
- Glibert, P. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Y.; Fang, Z.; Cui, X.; Liang, J.; Song, X. Spatiotemporal patterns and morphological characteristics of Ulva prolifera distribution in the Yellow Sea, China in 2016–2018. Remote Sens. 2019, 11, 445. [Google Scholar] [CrossRef]
- Li, D.; Gao, Z.; Song, D.; Shang, W.; Jiang, X. Characteristics and influence of green tide drift and dissipation in Shandong Rongcheng coastal water based on remote sensing. Estuar. Coast. Shelf Sci. 2019, 227, 106335. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Wang, J.; Zhou, H.; Guan, W.; Lou, X.; Cao, W.; Shi, A.; Chen, P.; Fan, K.; et al. Long time-series remote sensing analysis of the periodic cycle evolution of the inlets and ebb-tidal delta of Xincun Lagoon, Hainan Island, China. ISPRS J. Photogramm. Remote Sens. 2020, 165, 67–85. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, A.; Chen, R. China’s algal bloom suffocates marine life. Science 2021, 373, 751. [Google Scholar] [CrossRef]
- Wang, C.; Yu, R.; Zhou, M. Effects of the decomposing green macroalga Ulva (Enteromorpha) prolifera on the growth of four red-tide species. Harmful Algae 2012, 16, 12–19. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, Y.; Deng, P.; Chen, J.; Li, W.; Gao, Q.; Shen, H.; Peng, Q.; Chen, M.; Deng, X. Regulation of freshwater filamentous green algae (Cladophora) and its impact on malodorous volatile organic sulfur compound (DMS) by biomanipulation. Sci. Total Environ. 2024, 955, 176856. [Google Scholar] [CrossRef]
- Sun, X.; Wu, M.; Xing, Q.; Song, X.; Zhao, D.; Han, Q.; Zhang, G. Spatio-temporal patterns of Ulva prolifera blooms and the corresponding influence on chlorophyll-a concentration in the Southern Yellow Sea, China. Sci. Total Environ. 2018, 640, 807–820. [Google Scholar] [CrossRef]
- Norkko, A.; Bonsdorff, E. Altered benthic prey-availability due to episodic oxygen deficiency caused by drifting algal mats. Mar. Ecol. 1996, 17, 355–372. [Google Scholar] [CrossRef]
- Alvarez, S.; Brown, C.; Garcia Diaz, M.; O’Leary, H.; Solís, D. Non-linear impacts of harmful algae blooms on the coastal tourism economy. J. Environ. Manag. 2024, 351, 119811. [Google Scholar] [CrossRef]
- Alsaleh, M.; Wang, X. Aquaculture growth and coastal tourism development in the context sustainable blue economy. Sustain. Dev. 2024, 33, 2030–2046. [Google Scholar] [CrossRef]
- Raha, D.; Davies-Vollum, K.; Hemstock, S.; Boateng, I.; Islam, M.; Pierce, C. We need collaboration and co-creation to address challenges facing coastal communities. Nat. Hum. Behav. 2024, 8, 814–822. [Google Scholar] [CrossRef]
- Conover, J.; Green, L.; Thornber, C. Biomass decay rates and tissue nutrient loss in bloom and non-bloom-forming macroalgal species. Estuar. Coast. Shelf Sci. 2016, 178, 58–64. [Google Scholar] [CrossRef]
- Quillien, N.; Nordström, M.; Guyonnet, B.; Maguer, M.; Le Garrec, V.; Bonsdorff, E.; Grall, J. Large-scale effects of green tides on macrotidal sandy beaches: Habitat-specific responses of zoobenthos. Estuar. Coast. Shelf Sci. 2015, 164, 379–391. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Barnes, B.; Lapointe, B.; Chen, Y.; Xie, Y.; Wang, M. Climate and anthropogenic controls of seaweed expansions in the East China Sea and Yellow Sea. Geophys. Res. Lett. 2022, 49, e2022GL098185. [Google Scholar] [CrossRef]
- Son, Y.; Choi, B.; Kim, Y.; Park, Y. Tracing floating green algae blooms in the Yellow Sea and the East China Sea using GOCI satellite data and Lagrangian transport simulations. Remote Sens. Environ. 2015, 156, 21–33. [Google Scholar] [CrossRef]
- Li, D.; Gao, Z.; Xu, F. Research on the dissipation of green tide and its influencing factors in the Yellow Sea based on Google Earth Engine. Mar. Pollut. Bull. 2021, 172, 112801. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R.; et al. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.; Yu, Z.; Song, X. Investigation on the efficiency of a silicone antifouling coating in controlling the adhesion and germination of Ulva prolifera micro-propagules on rafts. Sci. China Earth Sci. 2017, 60, 391–396. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Zhuang, M.; Zhang, J.; Yu, K.; Zhao, S.; Sun, Y.; Tong, Y.; Xia, L.; Qin, Y.; et al. Controlling the source of green tides in the Yellow Sea: NaClO treatment of Ulva attached on Pyropia aquaculture rafts. Aquaculture 2021, 535, 736378. [Google Scholar] [CrossRef]
- Maggs, C.; Harries, D.; Priatna, D. Indonesian green tides: The problem is also the solution. Indones. J. Appl. Environ. Stud. 2024, 5, 55–57. [Google Scholar] [CrossRef]
- Fu, M.; Cao, S.; Li, J.; Zhao, S.; Liu, J.; Zhuang, M.; Qin, Y.; Gao, S.; Sun, Y.; Kim, J.; et al. Controlling the main source of green tides in the Yellow Sea through the method of biological competition. Mar. Pollut. Bull. 2022, 177, 113561. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Z.; Cao, X.; Tong, Y.; He, R.; Fu, M.; Sun, J.; Xu, H.; Xia, J.; Liu, J.; et al. A mixed acid treatment for the prevention of Ulva prolifera attachment to Neopyropia aquaculture rafts: Laboratory experimentation. Mar. Pollut. Bull. 2022, 184, 114134. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Yang, Y. Environmental and economic impacts of different disposal options for Ulva prolifera green tide in the Yellow Sea, China. ACS Sustain. Chem. Eng. 2022, 10, 11483–11492. [Google Scholar] [CrossRef]
- Tang, T.; Effiong, K.; Hu, J.; Li, C.; Xiao, X. Chemical prevention and control of the green tide and fouling organism Ulva: Key chemicals, mechanisms, and applications. Front. Mar. Sci. 2021, 8, 618950. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Zhang, J.; Cai, C.; He, P. Complete mitochondrial genome of Ulva prolifera, the dominant species of green macroalgal blooms in Yellow Sea, China. Mitochondrial DNA B-Resour. 2016, 1, 76–78. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Huo, Y.; Zhang, J.; Cui, J.; Wang, Y.; Yang, L.; Zhou, Q.; Lu, Y.; Yu, K.; He, P. Variations of dominant free-floating Ulva species in the source area for the world’s largest macroalgal blooms, China: Differences of ecological tolerance. Harmful Algae 2018, 74, 58–66. [Google Scholar] [CrossRef]
- Han, H.; Fan, S.; Song, W.; Li, Y.; Xiao, J.; Wang, Z.; Zhang, X.; Ding, D. The contribution of attached Ulva prolifera on Pyropia aquaculture rafts to green tides in the Yellow Sea. Acta Oceanol. Sin. 2020, 39, 101–106. [Google Scholar] [CrossRef]
- Cai, C.; Liu, X.; Zhao, H.; Jiang, T.; Jia, R.; He, P. Weakened growth, cell division, and energy metabolism, but enhanced resistance, signaling, and anabolism: Responses of Ulva prolifera to copper elucidated by omics. J. Appl. Phycol. 2021, 33, 3449–3465. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China?—An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Zheng, M.; Zhuo, P.; Xu, N. Photoperiod mediates the effects of elevated CO2 on the growth and physiological performance in the green tide alga Ulva prolifera. Mar. Environ. Res. 2018, 141, 24–29. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, Y.; Zheng, M.; Liu, N.; Li, Y.; Xu, N. High light might alleviate inhibitory effects of high temperature on growth and physiological parameters of Ulva prolifera. Aquac. Res. 2020, 51, 2062–2070. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Beardall, J.; Zhong, Z.; Gao, G.; Xu, J. Physiological acclimation of Ulva prolifera to seasonal environmental factors drives green tides in the Yellow Sea. Mar. Environ. Res. 2022, 179, 105695. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, M.; Cui, Y.; Tian, L.; Yang, P.; Zhao, L.; Xue, M.; Liu, J. What causes the great green tide disaster in the South Yellow Sea of China in 2021? Ecol. Indic. 2022, 140, 108988. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, Q.; Xu, Z.; Li, B.; Wu, H.; Zang, S.; Yan, F.; Xu, Z.; Liu, N. High-light pronounced the effects of stocking density on photosynthesis and nutrients uptake of the bloom-forming green alga, Ulva linza. Front. Mar. Sci. 2024, 11, 1440734. [Google Scholar] [CrossRef]
- Fan, X.; Xu, D.; Wang, Y.; Zhang, X.; Cao, S.; Mou, S.; Ye, N. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: Implications for the explosion in green tides. J. Appl. Phycol. 2014, 26, 537–544. [Google Scholar] [CrossRef]
- Gu, K.; Liu, Y.; Jiang, T.; Cai, C.; Zhao, H.; Liu, X.; He, P. Molecular response of Ulva prolifera to short-term high light stress revealed by a multi-omics approach. Biology 2022, 11, 1563. [Google Scholar] [CrossRef]
- Pan, Y.; Li, P.; Sun, J.; Liu, S.; Xing, L.; Yu, D.; Feng, Q. Potential Impact of Sea Surface Temperature Variability on the 2007 Sudden Bloom of Ulva prolifera in the Southern Yellow Sea. Remote Sens. 2024, 16, 4407. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Li, H.; Li, S.; Pan, C.; Yi, L.; Xu, J.; He, P. Ocean warming enhances the competitive advantage of Ulva prolifera over a golden tide alga, Sargassum horneri under eutrophication. Front. Mar. Sci. 2024, 11, 1440734. [Google Scholar] [CrossRef]
- Zhang, J.; Huo, Y.; Gao, S.; Yang, L.; Zhao, S.; Li, J.; He, P. Seasonal variation of dominant free-floating Ulva species in the Rudong coastal area, China: Differences of ecological adaptability to temperature and irradiance. Indian J. Geo-Mar. Sci. 2017, 46, 1758–1764. [Google Scholar]
- Keesing, J.; Liu, D.; Fearns, P.; Garcia, R. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar. Pollut. Bull. 2011, 62, 1169–1182. [Google Scholar] [CrossRef]
- Miao, X.; Xiao, J.; Pang, M.; Zhang, X.; Wang, Z.; Miao, J.; Li, Y. Effect of the large-scale green tide on the species succession of green macroalgal micro-propagules in the coastal waters of Qingdao, China. Mar. Pollut. Bull. 2018, 126, 549–556. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, L.; Liu, J.; Tong, Y.; Xia, J.; Zhao, X.; Zhao, S.; Fu, M.; Zhuang, M.; He, P.; et al. Prevention strategies for green tides at source in the Southern Yellow Sea. Mar. Pollut. Bull. 2022, 178, 113646. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.; Zhang, J.; Tian, X.; Hua, L.; He, Q.; Huo, Y.; Yu, K.; Shi, D.; Ma, J.; et al. Seasonal variation of dominant free-floating and attached Ulva species in Rudong coastal area, China. Harmful Algae 2013, 28, 46–54. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, R.; Chen, Z.; Qiu, L.; Wang, Y.; Kong, F.; Geng, H.; Zhao, Y.; Jiang, P.; Yan, T.; et al. Genetic evidence in tracking the origin of Ulva prolifera blooms in the Yellow Sea, China. Harmful Algae 2018, 78, 86–94. [Google Scholar] [CrossRef]
- Pang, S.; Liu, F.; Shan, T.; Xu, N.; Zhang, Z.; Gao, S.; Chopin, T.; Sun, S. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar. Environ. Res. 2010, 69, 207–215. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.; Zhao, X.; Hu, C. Quantitative, molecular and growth analyses of Ulva microscopic propagules in the coastal sediment of Jiangsu province where green tides initially occurred. Mar. Environ. Res. 2012, 74, 56–63. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Mao, Y.; Liang, C.; Zhuang, Z.; Wang, Q.; Ye, N. Somatic cells serve as a potential propagule bank of Enteromorpha prolifera forming a green tide in the Yellow Sea, China. J. Appl. Phycol. 2010, 22, 173–180. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Xu, Z.; Sun, Y. A study on the cultivation of the vegetative cells and protoplasts of Porphyra haitanensis. J. Shanghai Ocean Univ. 1986, 17, 1–7. (In Chinese) [Google Scholar]
- Zhao, S.; Xia, Z.; Liu, J.; Sun, J.; Zhang, J.; He, P. Morphology, growth, and photosynthesis of Ulva prolifera O.F. Müller (Chlorophyta, Ulvophyceae) gametophytes, the dominant green tide species in the Southern Yellow Sea. J. Sea Res. 2023, 193, 102375. [Google Scholar] [CrossRef]
- He, P.; Zhang, J.; Huo, Y.; Cai, C. Green Tides of China; Science Press: Beijing, China, 2019; pp. 1–438. (In Chinese) [Google Scholar]
- Liu, J.; Tong, Y.; Xia, J.; Sun, Y.; Zhao, X.; Sun, J.; Zhao, S.; Zhuang, M.; Zhang, J.; He, P.; et al. Ulva macroalgae within local aquaculture ponds along the estuary of Dagu River, Jiaozhou Bay, Qingdao. Mar. Pollut. Bull. 2022, 174, 113243. [Google Scholar] [CrossRef]
- Stein, J. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: London, UK, 1973; pp. 289–311. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J.; Huo, Y.; Zhou, L.; Wu, Q.; Chen, L.; Yu, K.; He, P. Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar. Pollut. Bull. 2015, 101, 660–666. [Google Scholar] [CrossRef]
- Li, H.; Sun, Q.; Zhao, S.; Zhang, W. Plant Physiological and Biochemical Experiment Principle and Technology; Higher Education Press: Beijing, China, 2000; pp. 134–137. (In Chinese) [Google Scholar]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Xia, Z.; Yuan, H.; Liu, J.; Zhao, S.; Tong, Y.; Sun, Y.; Li, S.; Li, A.; Cao, J.; Xia, J.; et al. Biomass and species composition of green macroalgae in the Binhai Harbor intertidal zone of the Southern Yellow Sea. Mar. Pollut. Bull. 2023, 186, 114407. [Google Scholar] [CrossRef]
- Nelson, T.; Nelson, A.; Tjoelker, M. Seasonal and spatial patterns of “green tides” (ulvoid algal blooms) and related water quality parameters in the coastal waters of Washington State, USA. Bot. Mar. 2003, 46, 263–275. [Google Scholar] [CrossRef]
- Charlier, R.; Morand, P.; Finkl, C. How Brittany and Florida coasts cope with green tides. Int. J. Environ. Stud. 2008, 65, 191–208. [Google Scholar] [CrossRef]
- Hughey, J.; Miller, K.; Gabrielson, P. Mitogenome analysis of a green tide forming Ulva from California, USA confirms its identity as Ulva expansa (Ulvaceae, Chlorophyta). Mitochondrial DNA B-Resour. 2018, 3, 1302–1303. [Google Scholar] [CrossRef]
- Yoshida, G.; Uchimura, M.; Hiraoka, M. Persistent occurrence of floating Ulva green tide in Hiroshima Bay, Japan: Seasonal succession and growth patterns of Ulva pertusa and Ulva spp. (Chlorophyta, Ulvales). Hydrobiologia 2015, 758, 223–233. [Google Scholar] [CrossRef]
- Kwon, H.; Kang, H.; Oh, Y.; Park, S.; Kim, G. Green tide development associated with submarine groundwater discharge in a coastal harbor, Jeju, Korea. Sci. Rep. 2017, 7, 6325. [Google Scholar] [CrossRef]
- Kirkendale, L.; Saunders, G.; Winberg, P. A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. J. Phycol. 2013, 49, 69–81. [Google Scholar] [CrossRef]
- Largo, D.; Sembrano, J.; Hiraoka, M.; Ohno, M. Taxonomic and ecological profile of “green tide” species of Ulva (Ulvales, Chlorophyta) in central Philippines. Hydrobiologia 2004, 512, 247–253. [Google Scholar] [CrossRef]
- Allanson, B.; Human, L.; Claassens, L. Observations on the distribution and abundance of a green tide along an intertidal shore, Knysna Estuary. S. Afr. J. Bot. 2016, 107, 49–54. [Google Scholar] [CrossRef]
- Yang, Y.; Hassan, S.; Awasthi, M.; Gajendran, B.; Sharma, M.; Ji, M.; Salama, E. The recent progress on the bioactive compounds from algal biomass for human health applications. Food Biosci. 2023, 51, 102267. [Google Scholar] [CrossRef]
- Bartold, M.; Kluczek, M. Estimating of chlorophyll fluorescence parameter Fv/Fm for plant stress detection at peatlands under Ramsar Convention with Sentinel-2 satellite imagery. Ecol. Inform. 2024, 81, 102603. [Google Scholar] [CrossRef]
- Tan, L.; Xu, W.; He, X.; Wang, J. The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar. Coast. Shelf Sci. 2019, 229, 106411. [Google Scholar] [CrossRef]
- Solomonova, E.; Shoman, N.; Akimov, A.; Rylkova, O. Impact of copper oxide nanoparticles on the physiology of different microalgal species. Reg. Stud. Mar. Sci. 2023, 66, 103128. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Hua, L.; He, P.; Qu, X.; Yu, K. Effects of sodium hypochlorite on chlorophyll fluorescence parameters and photosynthetic rate of Ulva compressa. J. Shanghai Ocean Univ. 2014, 23, 215–221. (In Chinese) [Google Scholar]
- Xu, L.; Lin, L.; Luo, L.; Zuo, X.; Cao, C.; Jin, X.; Jin, A.; Ma, Z.; Chen, B.; Wu, M. Organic acid treatment for removal of epiphytic Ulva L. attached to Sargassum fusiforme seedlings. Aquaculture 2022, 547, 737533. [Google Scholar] [CrossRef]
- Beer, S. Photosynthetic traits of the ubiquitous and prolific macroalga Ulva (Chlorophyta): A review. Eur. J. Phycol. 2022, 58, 390–398. [Google Scholar] [CrossRef]
- Terada, R.; Vo, T.; Nishihara, G.; Shioya, K.; Shimada, S.; Kawaguchi, S. The effect of irradiance and temperature on the photosynthesis and growth of a cultivated red alga Kappaphycus alvarezii (Solieriaceae) from Vietnam, based on in situ and in vitro measurements. J. Appl. Phycol. 2016, 28, 457–467. [Google Scholar] [CrossRef]
- Hupp, J.; McCoy, J.; Millgan, A.; Peers, G. Simultaneously measuring carbon uptake capacity and chlorophyll a fluorescence dynamics in algae. Algal Res. 2021, 58, 102399. [Google Scholar] [CrossRef]
- Ralph, P.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Dou, B.; Li, Y.; Wang, F.; Chen, L.; Zhang, W. Chassis engineering for high light tolerance in microalgae and cyanobacteria. Crit. Rev. Biotechnol. 2024, 45, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Kulikovsky, S.; Liveanu, V.; Eichenbaum, B.; Meir, A.; Isaacson, T.; Tadmor, Y.; Adir, N.; Schuster, G. The desert green algae Chlorella ohadii thrives at excessively high light intensities by exceptionally enhancing the mechanisms that protect photosynthesis from photoinhibition. Plant J. 2021, 108, 1851. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.; Ac, A.; Marek, M.; Kalin, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. Methods Enzymol. 2022, 674, 53–84. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Zhang, X.; Dong, M.; Fan, X.; Xu, J.; Cao, S.; Xu, D.; Wang, W.; Ye, N. Photoprotection in the green tidal alga Ulva prolifera: Role of LHCSR and PsbS proteins in response to high light stress. Plant Biol. 2013, 15, 1033–1039. [Google Scholar] [CrossRef]
- Sahay, S.; Grzybowski, M.; Schnable, J.; Głowacka, K. Genotype-specific nonphotochemical quenching responses to nitrogen deficit are linked to chlorophyll a to b ratios. J. Plant Physiol. 2024, 297, 154261. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Liu, J.; Xia, Z.; Sun, J.; Zhang, J.; He, P. Subtypes I and II of Ulva prolifera O.F. Müller: Dominant Green Tide Species in the Southern Yellow Sea and Their Responses to Natural Light and Temperature Conditions. Biology 2025, 14, 702. https://doi.org/10.3390/biology14060702
Zhao S, Liu J, Xia Z, Sun J, Zhang J, He P. Subtypes I and II of Ulva prolifera O.F. Müller: Dominant Green Tide Species in the Southern Yellow Sea and Their Responses to Natural Light and Temperature Conditions. Biology. 2025; 14(6):702. https://doi.org/10.3390/biology14060702
Chicago/Turabian StyleZhao, Shuang, Jinlin Liu, Zhangyi Xia, Jingyi Sun, Jianheng Zhang, and Peimin He. 2025. "Subtypes I and II of Ulva prolifera O.F. Müller: Dominant Green Tide Species in the Southern Yellow Sea and Their Responses to Natural Light and Temperature Conditions" Biology 14, no. 6: 702. https://doi.org/10.3390/biology14060702
APA StyleZhao, S., Liu, J., Xia, Z., Sun, J., Zhang, J., & He, P. (2025). Subtypes I and II of Ulva prolifera O.F. Müller: Dominant Green Tide Species in the Southern Yellow Sea and Their Responses to Natural Light and Temperature Conditions. Biology, 14(6), 702. https://doi.org/10.3390/biology14060702









