Neurocognitive Outcomes After Extracranial Surgery and General Anesthesia in Patients with a History of Mild-to-Moderate Traumatic Brain Injury: Systemic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Study Design
2.2.2. Population/Participants
2.2.3. Intervention/Exposure
2.2.4. Control
2.2.5. Outcomes
2.3. Study Selection
2.4. Data Extraction
2.5. Risk-of-Bias Assessment
2.6. Data Synthesis and Statistical Analysis
3. Results
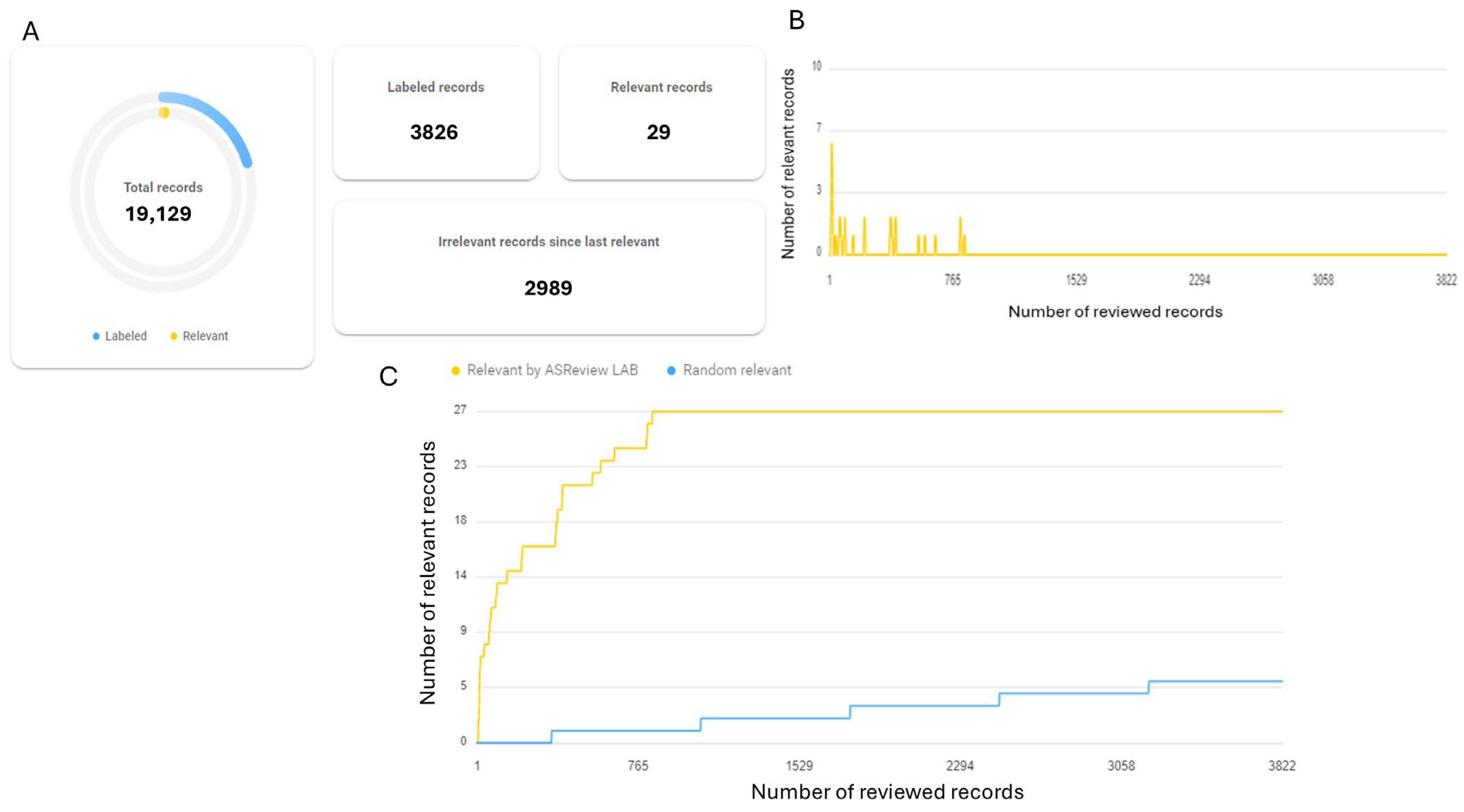
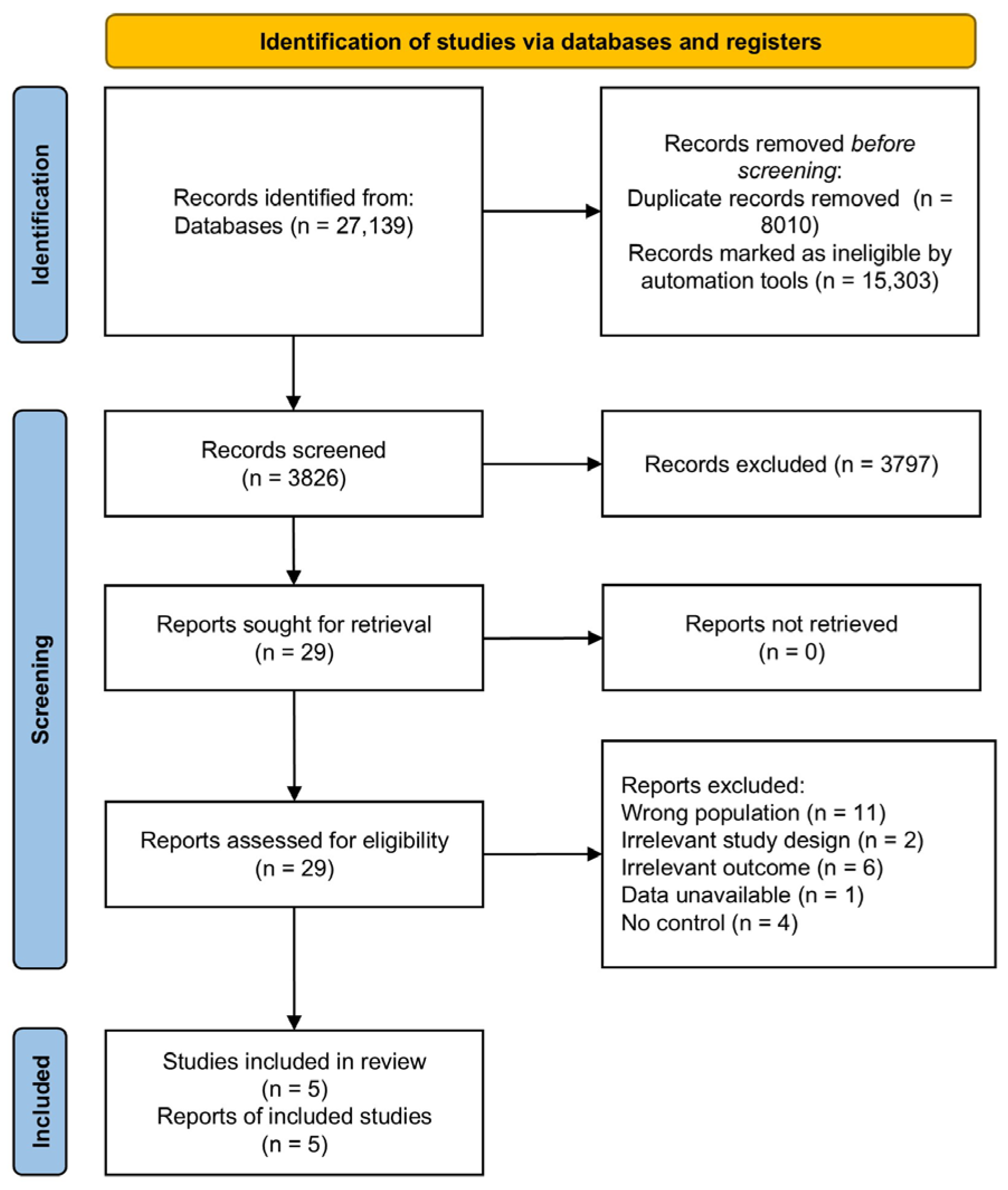
3.1. Study Search and Selection
3.2. Study Characteristics
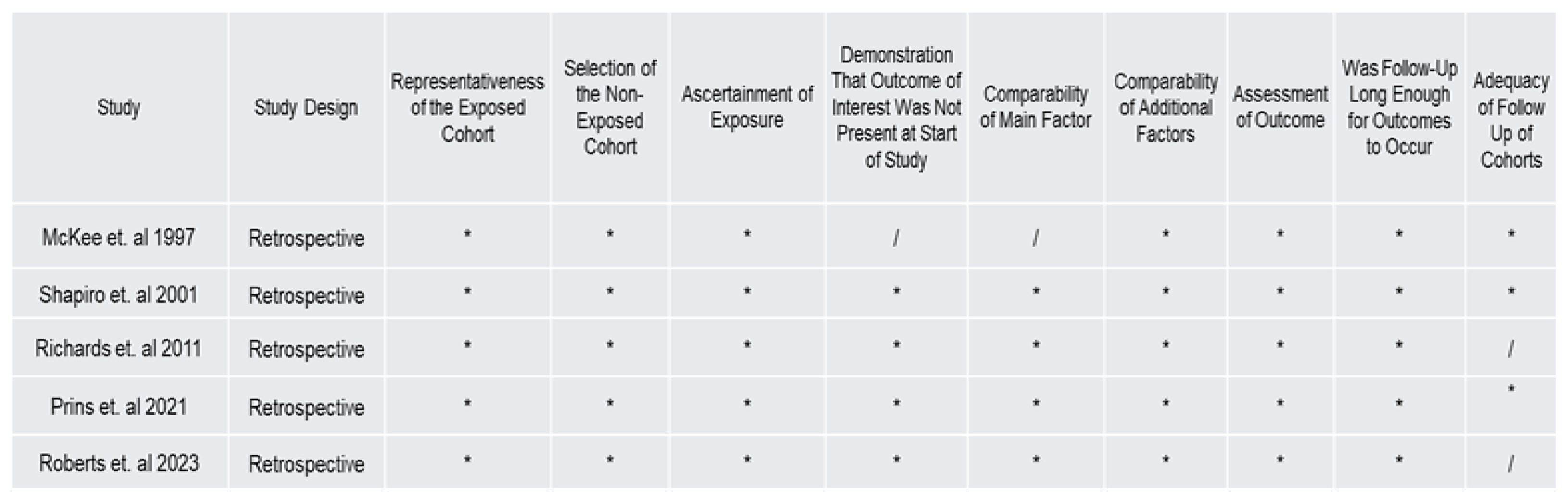
3.3. Risk of Bias and Quality Reporting
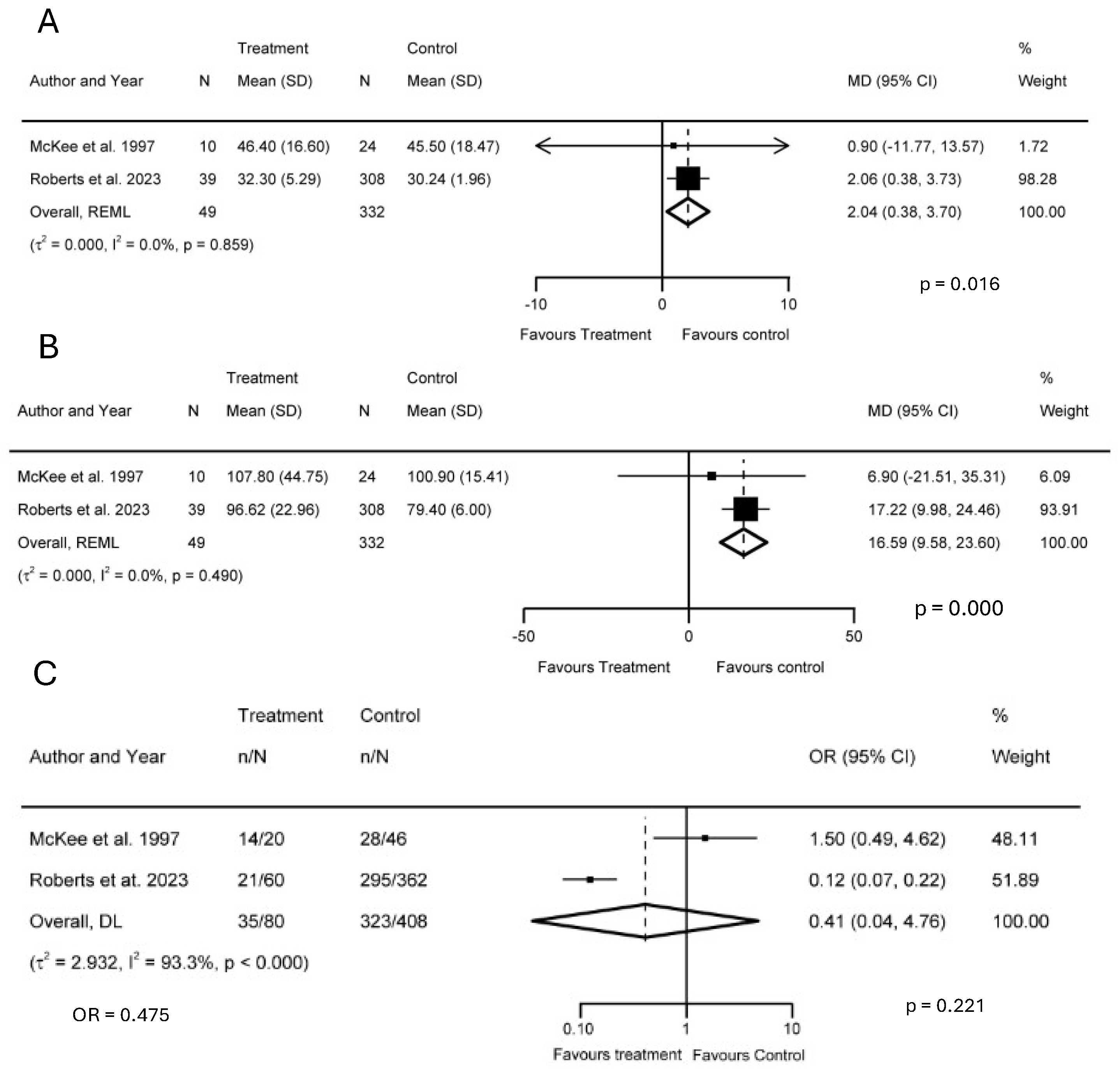
3.4. Meta-Analysis
3.4.1. Trail-Making Tests A and B
3.4.2. Glasgow Outcome Scale
3.4.3. Length of ICU and Hospital Stays
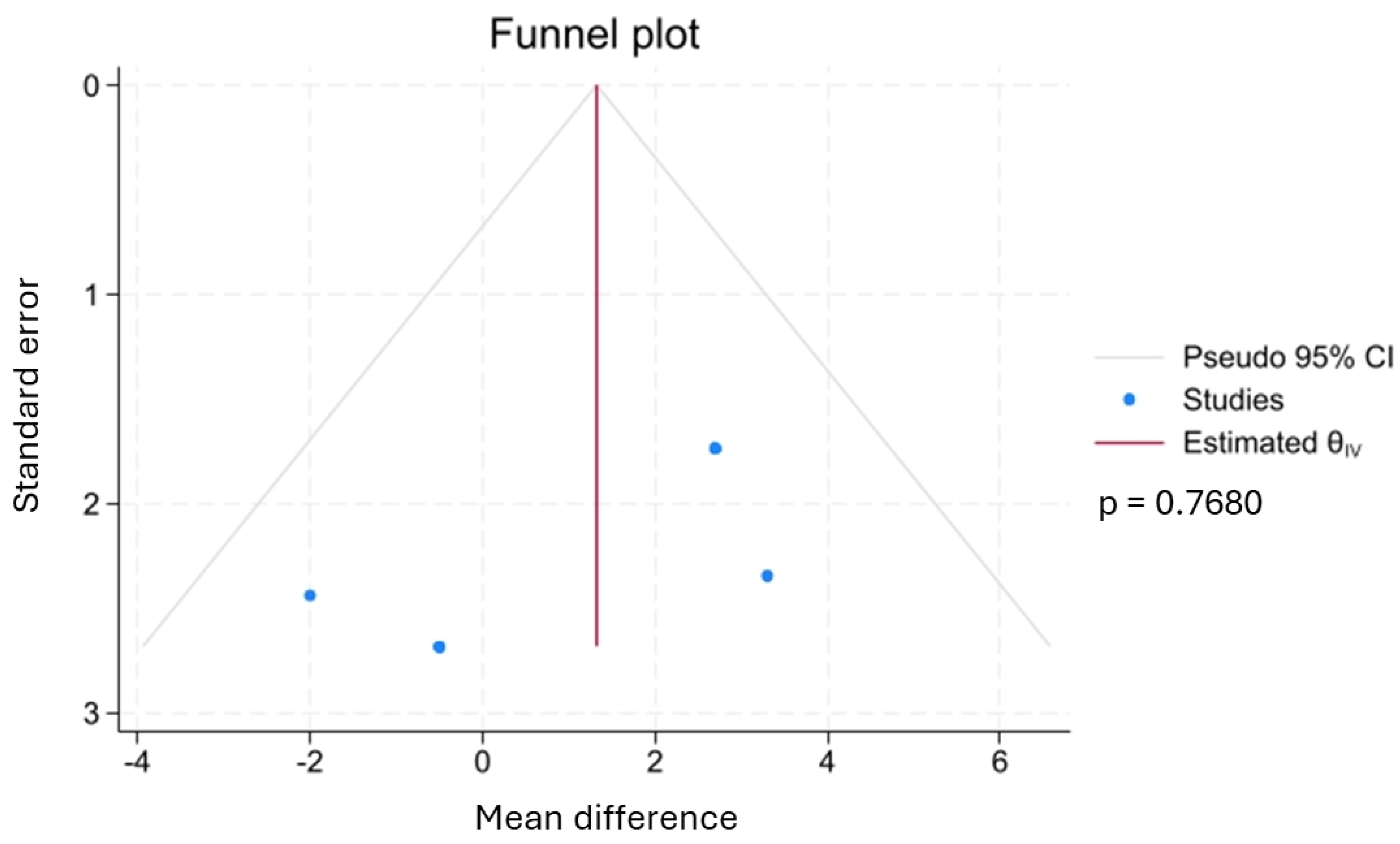
3.4.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, H.; Xu, L.-M.; Wang, D.-X. Perioperative neurocognitive disorders: A narrative review focusing on diagnosis, prevention, and treatment. CNS Neurosci. Ther. 2022, 28, 1147–1167. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.; Kim, L.D. Post-operative delirium: A review of diagnosis and treatment strategies. J. Xiangya Med. 2018, 3, 8. [Google Scholar] [CrossRef][Green Version]
- Dilmen, O.K.; Meco, B.C.; Evered, L.A.; Radtke, F.M. Postoperative neurocognitive disorders: A clinical guide. J. Clin. Anesth. 2024, 92, 111320. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Foti, L.; Piazzini, T.; Russo, G.; Verrengia, M.; Sangermano, C.; Bilotta, F.; Romagnoli, S. Clinical literature on postoperative delirium and neurocognitive disorders: A historical systematic review. J. Anesth. Analg. Crit. Care 2022, 2, 11. [Google Scholar] [CrossRef]
- Vacas, S.; Cole, D.J.; Cannesson, M. Cognitive decline associated with anesthesia and surgery in older patients. JAMA 2021, 326, 863–864. [Google Scholar] [CrossRef]
- Needham, M.J.; Webb, C.E.; Bryden, D.C. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br. J. Anaesth. 2017, 119, i115–i125. [Google Scholar] [CrossRef]
- Terrando, N.; Brzezinski, M.; Degos, V.; Eriksson, L.I.; Kramer, J.H.; Leung, J.M.; Miller, B.L.; Seeley, W.W.; Vacas, S.; Weiner, M.W.; et al. Perioperative cognitive decline in the aging population. Mayo Clin. Proc. 2011, 86, 885–893. [Google Scholar] [CrossRef]
- Coronado, V.G.; Haileyesus, T.; Cheng, T.A.; Bell, J.M.; Haarbauer-Krupa, J.; Lionbarger, M.R.; Flores-Herrera, J.; McGuire, L.C.; Gilchrist, J. Trends in Sports- and Recreation-Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J. Head Trauma Rehabil. 2015, 30, 185–197. [Google Scholar] [CrossRef]
- VanItallie, T.B. Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metab. Clin. Exp. 2019, 100S, 153943. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- van der Horn, H.J.; Out, M.L.; de Koning, M.E.; Mayer, A.R.; Spikman, J.M.; Sommer, I.E.; van der Naalt, J. An integrated perspective linking physiological and psychological consequences of mild traumatic brain injury. J. Neurol. 2020, 267, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic brain injury and alzheimer’s disease: The cerebrovascular link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic brain injury and risk of alzheimer’s disease and related dementias in the population. J. Alzheimers Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic brain injury and risk of neurodegenerative disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawai, Y.; Iwamura, A.; Maegawa, N.; Fukushima, H.; Okuchi, K. Outcomes after Traumatic Brain Injury with Concomitant Severe Extracranial Injuries. Neurol. Med. Chir. 2018, 58, 393–399. [Google Scholar] [CrossRef]
- Robba, C.; Bonatti, G.; Pelosi, P.; Citerio, G. Extracranial complications after traumatic brain injury: Targeting the brain and the body. Curr. Opin. Crit. Care 2020, 26, 137–146. [Google Scholar] [CrossRef]
- Greil, M.E.; Pan, J.; Barber, J.K.; Temkin, N.R.; Bonow, R.H.; Videtta, W.; Vega, M.J.; Lujan, S.; Petroni, G.; Chesnut, R.M. Global Neurotrauma Research Group Collaborators Extracranial Complications in Monitored and Nonmonitored Patients with Traumatic Brain Injury in the BEST TRIP Trial and a Companion Observational Cohort. World Neurosurg. 2024, 190, e424–e434. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Held, K.; Wagner, P. Cognitive impairment following traumatic brain injury. Curr. Treat. Options Neurol. 2002, 4, 43–57. [Google Scholar] [CrossRef]
- Wu, H.; Ahammed, Y.; Tian, S.; Liu, Y.; Sanders, R.D.; Ma, D. Brain Structural and Functional Changes Associated with Postoperative Neurocognitive Disorders: Research Update. Anesth. Analg. 2025. [Google Scholar] [CrossRef]
- Sharma, D. Anesthesia for Patients with Acute Traumatic Brain Injury. Wolters Kluwer 2019. Available online: https://www.uptodate.com/contents/anesthesia-for-patients-with-acute-traumatic-brain-injury (accessed on 7 April 2025).
- Kim, H. Anesthetic management of the traumatic brain injury patients undergoing non-neurosurgery. Anesth. Pain Med. 2023, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Sumsuzzman, D.M.; Duran, T.A.; Ju, L.-S.; Seubert, C.N.; Martynyuk, A.E. Perioperative Neurocognitive Disorder in Individuals with a History of Traumatic Brain Injury: Protocol for a Systematic Review and Meta-Analysis. Biology 2025, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Iverson, L.M. Glasgow Coma Scale. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Gilbey, T.; Milne, B.; de Somer, F.; Kunst, G. Neurologic complications after cardiopulmonary bypass—A narrative review. Perfusion 2023, 38, 1545–1559. [Google Scholar] [CrossRef]
- Cheung, A.T.; Messé, S.R. Preventing brain injury after cardiopulmonary bypass will require more than just dialing up the pressure. Circulation 2018, 137, 1781–1783. [Google Scholar] [CrossRef]
- Moitra, V.K.; Guerra, C.; Linde-Zwirble, W.T.; Wunsch, H. Relationship Between ICU Length of Stay and Long-Term Mortality for Elderly ICU Survivors. Crit. Care Med. 2016, 44, 655–662. [Google Scholar] [CrossRef]
- Rydingsward, J.E.; Horkan, C.M.; Mogensen, K.M.; Quraishi, S.A.; Amrein, K.; Christopher, K.B. Functional status in ICU survivors and out of hospital outcomes: A cohort study. Crit. Care Med. 2016, 44, 869–879. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Nakamura, K.; Inoue, S.; Liu, K.; Yamakawa, K.; Nishida, T.; Ohshimo, S.; Hashimoto, S.; Kanda, N.; Aso, S.; et al. Two-year trajectory of functional recovery and quality of life in post-intensive care syndrome: A multicenter prospective observational study on mechanically ventilated patients with coronavirus disease-19. J. Intensive Care 2025, 13, 7. [Google Scholar] [CrossRef]
- Quan, Y.; Tytko, T.; Hui, B. Utilizing ASReview in screening primary studies for meta-research in SLA: A step-by-step tutorial. Res. Methods Appl. Linguist. 2024, 3, 100101. [Google Scholar] [CrossRef]
- Hindriks, S.J. A Study on the User Experience of the ASReview Software Tool for Experienced and Unexperienced Users. Bachelor’s Thesis, Utrecht University, Utrecht, The Netherlands, 2020. [Google Scholar]
- Jelicic Kadic, A.; Vucic, K.; Dosenovic, S.; Sapunar, D.; Puljak, L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J. Clin. Epidemiol. 2016, 74, 119–123. [Google Scholar] [CrossRef]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Dryden, D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. WJG 2017, 5, 80. [Google Scholar] [CrossRef]
- Jing, Y.; Lin, L. Comparisons of the mean differences and standardized mean differences for continuous outcome measures on the same scale. JBI Evid. Synth. 2024, 22, 394–405. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New Jersey, NJ, USA, 1988; pp. 79–81. ISBN 9780203771587. [Google Scholar]
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Dirnagl, U.; Lauritzen, M. Fighting publication bias: Introducing the negative results section. J. Cereb. Blood Flow. Metab. 2010, 30, 1263–1264. [Google Scholar] [CrossRef]
- McKee, M.D.; Schemitsch, E.H.; Vincent, L.O.; Sullivan, I.; Yoo, D. The effect of a femoral fracture on concomitant closed head injury in patients with multiple injuries. J. Trauma 1997, 42, 1041–1045. [Google Scholar] [CrossRef]
- Shapiro, M.B.; Nance, M.L.; Schiller, H.J.; Hoff, W.S.; Kauder, D.R.; Schwab, C.W. Nonoperative management of solid abdominal organ injuries from blunt trauma: Impact of neurologic impairment. Am. Surg. 2001, 67, 793–796. [Google Scholar] [CrossRef]
- Richards, J.E.; Guillamondegui, O.D.; Archer, K.R.; Jackson, J.C.; Ely, E.W.; Obremskey, W.T. The association of reamed intramedullary nailing and long-term cognitive impairment. J. Orthop. Trauma 2011, 25, 707–713. [Google Scholar] [CrossRef]
- Prins, J.T.H.; Van Lieshout, E.M.M.; Ali-Osman, F.; Bauman, Z.M.; Caragounis, E.-C.; Choi, J.; Christie, D.B.; Cole, P.A.; DeVoe, W.B.; Doben, A.R.; et al. Outcome after surgical stabilization of rib fractures versus nonoperative treatment in patients with multiple rib fractures and moderate to severe traumatic brain injury (CWIS-TBI). J. Trauma Acute Care Surg. 2021, 90, 492–500. [Google Scholar] [CrossRef]
- Roberts, C.J.; Barber, J.; Temkin, N.R.; Dong, A.; Robertson, C.S.; Valadka, A.B.; Yue, J.K.; Markowitz, A.J.; Manley, G.T.; Nelson, L.D.; et al. Clinical Outcomes After Traumatic Brain Injury and Exposure to Extracranial Surgery: A TRACK-TBI Study. JAMA Surg. 2024, 159, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.T.H.; Van Lieshout, E.M.M.; Ali-Osman, F.; Bauman, Z.M.; Caragounis, E.-C.; Choi, J.; Christie, D.B.; Cole, P.A.; DeVoe, W.B.; Doben, A.R.; et al. Surgical stabilization versus nonoperative treatment for flail and non-flail rib fracture patterns in patients with traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2022, 48, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Fazaluddin, A.; Andrew, K.N. Recognition of categorised words: Repetition effects in rote study. Memory 2013, 21, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. What cognitive abilities are involved in trail-making performance? Intelligence 2011, 39, 222–232. [Google Scholar] [CrossRef]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The trail making test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- O’Rourke, J.J.F.; Beglinger, L.J.; Smith, M.M.; Mills, J.; Moser, D.J.; Rowe, K.C.; Langbehn, D.R.; Duff, K.; Stout, J.C.; Harrington, D.L.; et al. The Trail Making Test in prodromal Huntington disease: Contributions of disease progression to test performance. J. Clin. Exp. Neuropsychol. 2011, 33, 567–579. [Google Scholar] [CrossRef]
- Lange, R.T.; Iverson, G.L.; Zakrzewski, M.J.; Ethel-King, P.E.; Franzen, M.D. Interpreting the trail making test following traumatic brain injury: Comparison of traditional time scores and derived indices. J. Clin. Exp. Neuropsychol. 2005, 27, 897–906. [Google Scholar] [CrossRef]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef]
- Hafiz, N.J.; Lohse, A.; Haas, R.; Reiche, S.; Sedlaczek, L.; Brandl, E.J.; Riemer, T.G. Trail making test error analysis in subjective cognitive decline, mild cognitive impairment, and alzheimer’s dementia with and without depression. Arch. Clin. Neuropsychol. 2023, 38, 25–36. [Google Scholar] [CrossRef]
- Arbuthnott, K.; Frank, J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Linari, I.; Juantorena, G.E.; Ibáñez, A.; Petroni, A.; Kamienkowski, J.E. Unveiling Trail Making Test: Visual and manual trajectories indexing multiple executive processes. Sci. Rep. 2022, 12, 14265. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Parra, J.E.; Restrepo-de-Mejía, F. The Trail Making Test (part B) is associated with working memory: A concurrent validity study. Appl. Neuropsychol. Adult 2025, 32, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Miskin, N.; Thesen, T.; Barr, W.B.; Butler, T.; Wang, X.; Dugan, P.; Kuzniecky, R.; Doyle, W.; Devinsky, O.; Blackmon, K. Prefrontal lobe structural integrity and trail making test, part B: Converging findings from surface-based cortical thickness and voxel-based lesion symptom analyses. Brain Imaging Behav. 2016, 10, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Frisvold, S.; Coppola, S.; Ehrmann, S.; Chiumello, D.; Guérin, C. Respiratory challenges and ventilatory management in different types of acute brain-injured patients. Crit. Care 2023, 27, 247. [Google Scholar] [CrossRef]
- Taylor, S.V.; Loo, G.T.; Richardson, L.D.; Legome, E. Patient factors associated with prolonged length of stay after traumatic brain injury. Cureus 2024, 16, e59989. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Wilson, L.; Boase, K.; Nelson, L.D.; Temkin, N.R.; Giacino, J.T.; Markowitz, A.J.; Maas, A.; Menon, D.K.; Teasdale, G.; Manley, G.T. A Manual for the Glasgow Outcome Scale-Extended Interview. J. Neurotrauma 2021, 38, 2435–2446. [Google Scholar] [CrossRef]
- Georges, A.; Das, J.M. Traumatic brain injury (archive). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 2012, 43, 299–307. [Google Scholar] [CrossRef]
- Mitka, M. Reports of concussions from youth sports rise along with awareness of the problem. JAMA 2010, 304, 1775–1776. [Google Scholar] [CrossRef]
- Mahanna-Gabrielli, E.; Schenning, K.J.; Eriksson, L.I.; Browndyke, J.N.; Wright, C.B.; Culley, D.J.; Evered, L.; Scott, D.A.; Wang, N.Y.; Brown, C.H.; et al. State of the clinical science of perioperative brain health: Report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br. J. Anaesth. 2019, 123, 464–478. [Google Scholar] [CrossRef]
- Barreto Chang, O.L.; Possin, K.L.; Maze, M. Age-Related Perioperative Neurocognitive Disorders: Experimental Models and Druggable Targets. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.R.; Campbell, A. Surgery, Anesthesia, and TBI Outcomes-Unraveling the Complex Interplay. JAMA Surg. 2024, 159, 259. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.; Althoff, R.R.; Boomsma, D.I. Anesthesia and cognitive performance in children: No evidence for a causal relationship. Twin Res. Hum. Genet. 2009, 12, 246–253. [Google Scholar] [CrossRef] [PubMed]
- DiMaggio, C.; Sun, L.S.; Li, G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth. Analg. 2011, 113, 1143–1151. [Google Scholar] [CrossRef]
- Yang, H.W.; Bae, J.B.; Oh, D.J.; Moon, D.G.; Lim, E.; Shin, J.; Kim, B.J.; Lee, D.W.; Kim, J.L.; Jhoo, J.H.; et al. Exploration of cognitive outcomes and risk factors for cognitive decline shared by couples. JAMA Netw. Open 2021, 4, e2139765. [Google Scholar] [CrossRef]
- Vitaliano, P.P.; Echeverria, D.; Yi, J.; Phillips, P.E.M.; Young, H.; Siegler, I.C. Psychophysiological mediators of caregiver stress and differential cognitive decline. Psychol. Aging 2005, 20, 402–411. [Google Scholar] [CrossRef]
- Vitaliano, P.P.; Zhang, J.; Young, H.M.; Caswell, L.W.; Scanlan, J.M.; Echeverria, D. Depressed mood mediates decline in cognitive processing speed in caregivers. Gerontologist 2009, 49, 12–22. [Google Scholar] [CrossRef]
- Caswell, L.W.; Vitaliano, P.P.; Croyle, K.L.; Scanlan, J.M.; Zhang, J.; Daruwala, A. Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Exp. Aging Res. 2003, 29, 303–318. [Google Scholar] [CrossRef]

| Author, Year and Country | Age (Years) | BMI/Body Weight (Kg) | Gender | Type of Surgery | Route of Administration | Anesthetics Used | Outcome | Duration After Which Neurocognitive Tests Were Assessed | Technique/Scale | Journal |
|---|---|---|---|---|---|---|---|---|---|---|
| McKee et al., 1997, Canada [41] | 16–63 (Mean = 37.5) | n/a | Male (Study Group): 35 Female (Study Group): 11 Male (Control): 75 Female (Control): 24 | EC (orthopedic) -femoral fracture | n/a | n/a | Executive functioning, reasoning, problem-solving, visual motor attention, cognitive impairment | avg 32 months (range 12–59 months) | Return to work status, Glasgow Outcome Scale (GOS), the Category test, and the Trails A and B tests | The Journal of Trauma: Injury, Infection, and Critical Care |
| Shapiro et al., 2001, USA [42] | 35.6 | n/a | n/a | laparaotomy (intra-abdominal) | n/a | n/a | Quality of life (postoperative recovery), Injury recovery | n/a | ISS, AIS, Revised Trauma Score (RTS), GCS, hospital LOS, ICU LOS, presence of hollow viscus injusry involving urinary bladder or gastrointestinal tract, mortality | The American Surgeon |
| Richards et al., 2011, USA [43] | 42.7 (SD = 16.8) | n/a | Number (%) Male (Study Group): 9 (50) Female (Study Group): 9 (50) Male (Control): 54 (60) Female (Control): 36 (40) | EC (orthopedic)—reamed intramedullary nailing | n/a | n/a | Global cognition, verbal and visual memory, visuospatial construction, processing speed, visual attention/verbal attention, executive functioning (verbal fluency and set shifting), and depressive and posttraumatic stress disorder symptoms | 12 months after injury | Mini Mental State Exam, Rey Auditory Verbal Learning Test, Rey Osterreith Complex Figure Test–Delay, Rey Osterreith Complex Figure–Copy, Digit Symbol Coding, Trailmaking Test A, Digit Span, FAS, Trailmaking Test B Additionally, and Beck Depression Inventory-II and the Davidson Trauma Scale ISS, AIS | Journal of Orthopaedic Trauma |
| Prins et al., 2021, Muti-national [44] | Overall = 50 (37–63) SSRF = 50 (37–61) Nonoperative = 50 (37–63) | Overall = 26 (24–30) kg/m2 SSRF = 28 (25–31) kg/m2 Nonoperative = 26 (23–29) kg/m2 | (male) Overall = 76.7% SSRF = 72.7% Nonoperative = 78% | Stabilization of rib fractures (SSRF) | n/a | n/a | Quality of life (postoperative recovery) | n/a | Ventilator free days, ICU-LOS, Hospital LOS, rate of/time to tracheostomy, occurrence of complications, GCS, mortality | Journal of Trauma Acute Care Surgery |
| Roberts et al., 2024, USA [45] | 42.2 +/− 17.8 (SD) | n/a | Male: 1279 Female: 556 | EC | n/a | n/a | Executive functioning, processing speed, memory, neurocognitive impairment | 2 weeks and 6 months | GOSE-ALL, GOSE-TBI, Trail Making Test Parts A and B, WAIS-PSI, RAVLT Immediate Recall, and RAVLT-L, AIS, ISS | JAMA Surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Z.A.; Duran, T.A.; Sumsuzzman, D.M.; Ju, L.-S.; Seubert, C.N.; Martynyuk, A.E. Neurocognitive Outcomes After Extracranial Surgery and General Anesthesia in Patients with a History of Mild-to-Moderate Traumatic Brain Injury: Systemic Review and Meta-Analysis. Biology 2025, 14, 640. https://doi.org/10.3390/biology14060640
Khan ZA, Duran TA, Sumsuzzman DM, Ju L-S, Seubert CN, Martynyuk AE. Neurocognitive Outcomes After Extracranial Surgery and General Anesthesia in Patients with a History of Mild-to-Moderate Traumatic Brain Injury: Systemic Review and Meta-Analysis. Biology. 2025; 14(6):640. https://doi.org/10.3390/biology14060640
Chicago/Turabian StyleKhan, Zeeshan A., Tahiris A. Duran, Dewan Md. Sumsuzzman, Ling-Sha Ju, Christoph N. Seubert, and Anatoly E. Martynyuk. 2025. "Neurocognitive Outcomes After Extracranial Surgery and General Anesthesia in Patients with a History of Mild-to-Moderate Traumatic Brain Injury: Systemic Review and Meta-Analysis" Biology 14, no. 6: 640. https://doi.org/10.3390/biology14060640
APA StyleKhan, Z. A., Duran, T. A., Sumsuzzman, D. M., Ju, L.-S., Seubert, C. N., & Martynyuk, A. E. (2025). Neurocognitive Outcomes After Extracranial Surgery and General Anesthesia in Patients with a History of Mild-to-Moderate Traumatic Brain Injury: Systemic Review and Meta-Analysis. Biology, 14(6), 640. https://doi.org/10.3390/biology14060640









