Evaluation of the Bioactivity of Phenolic Compounds from the Sargassum pallidum and Development of Their Stable Emulsion and Cream
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Equipment and Reagents
2.2. Determination of Phenolic Compounds
2.3. Culture RAW264.7 Cell
2.4. Cytotoxicity Assay
2.5. Intracellular NO Levels
2.6. Detection of Inflammation-Related Mediators
2.7. Measurement of Reactive Oxygen Species (ROS) Levels
2.8. RT-PCR Analysis
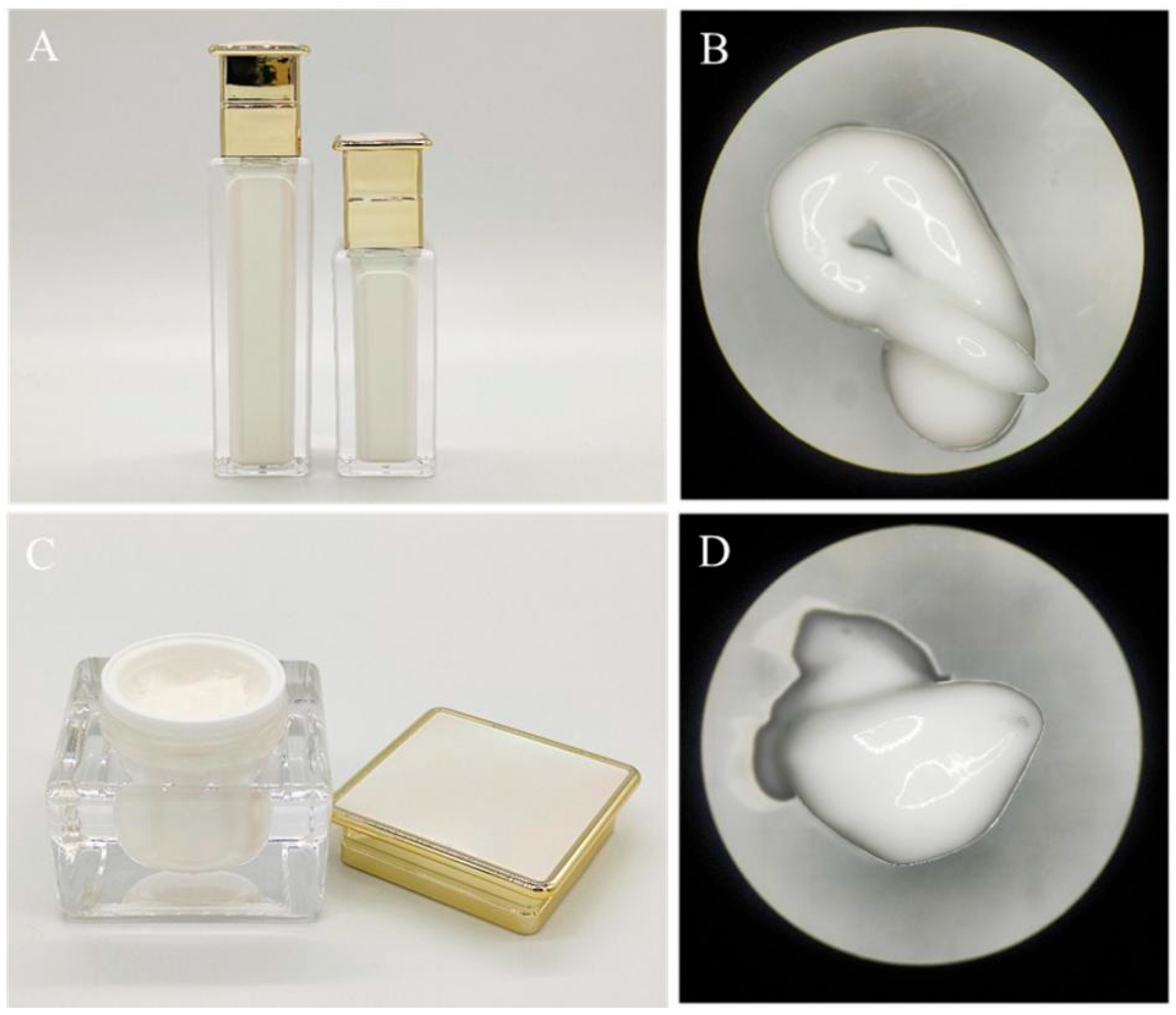
2.9. Preparation of Emulsions and Creams
2.10. Evaluating the Effectiveness of Emulsions and Creams
2.11. Statistical Analysis
3. Results
3.1. TLC Polyphenol Characterization
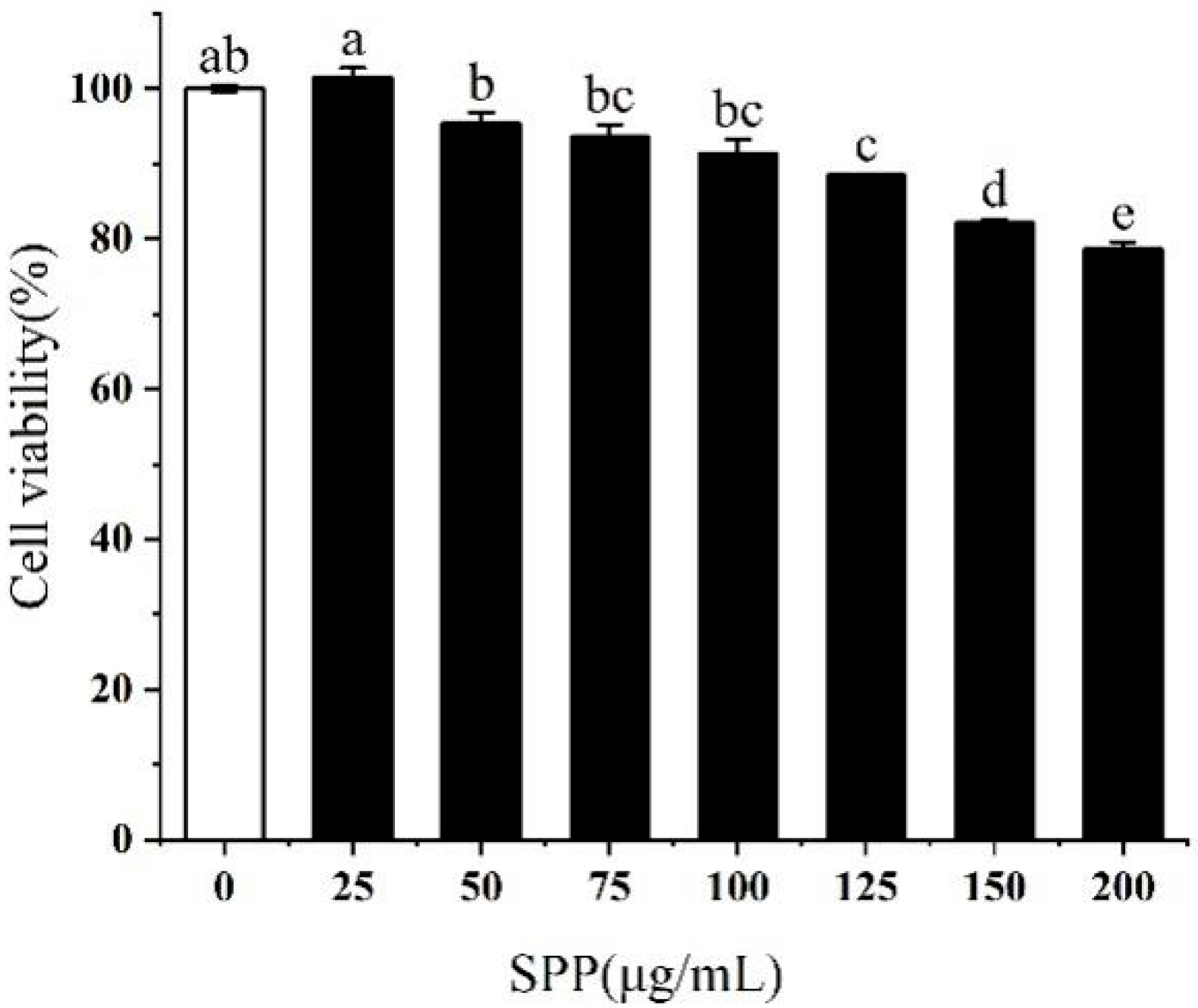
3.2. Effect of SPP on the Viability of RAW264.7 Cells
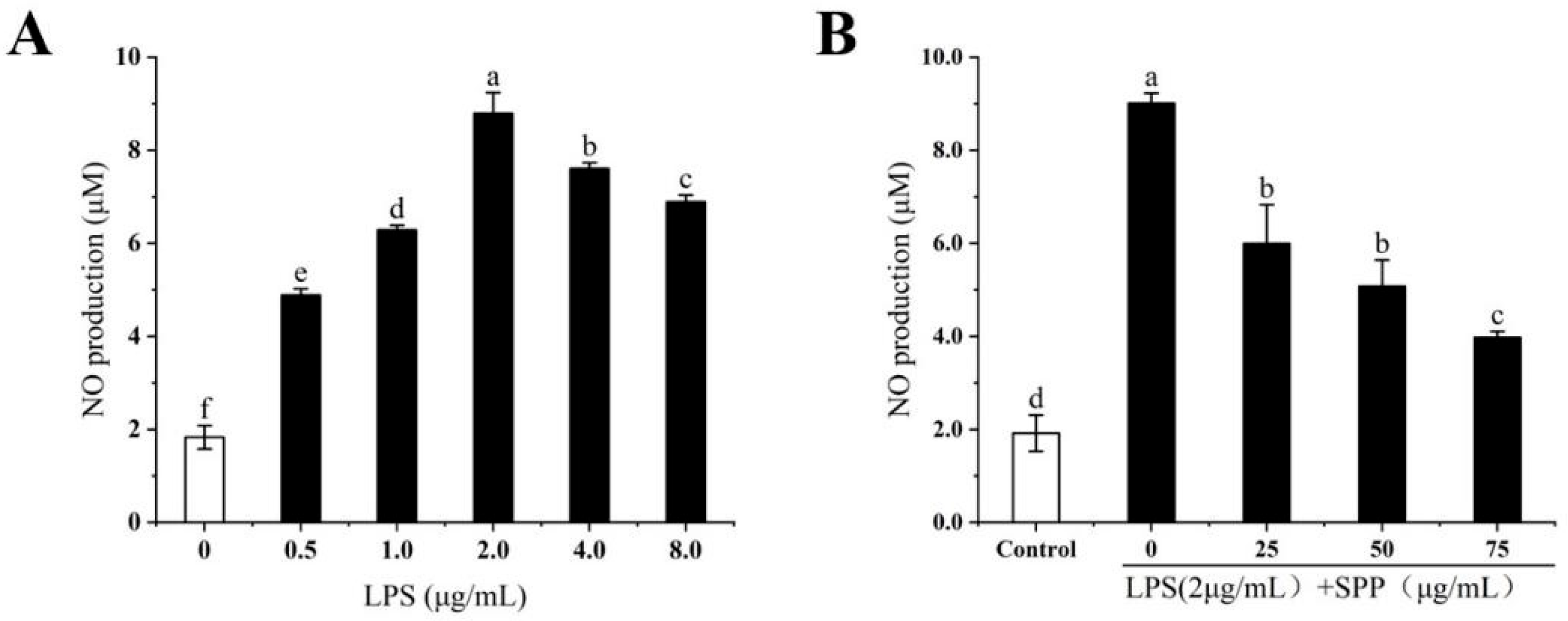
3.3. The Anti-Inflammatory Effects of SPP
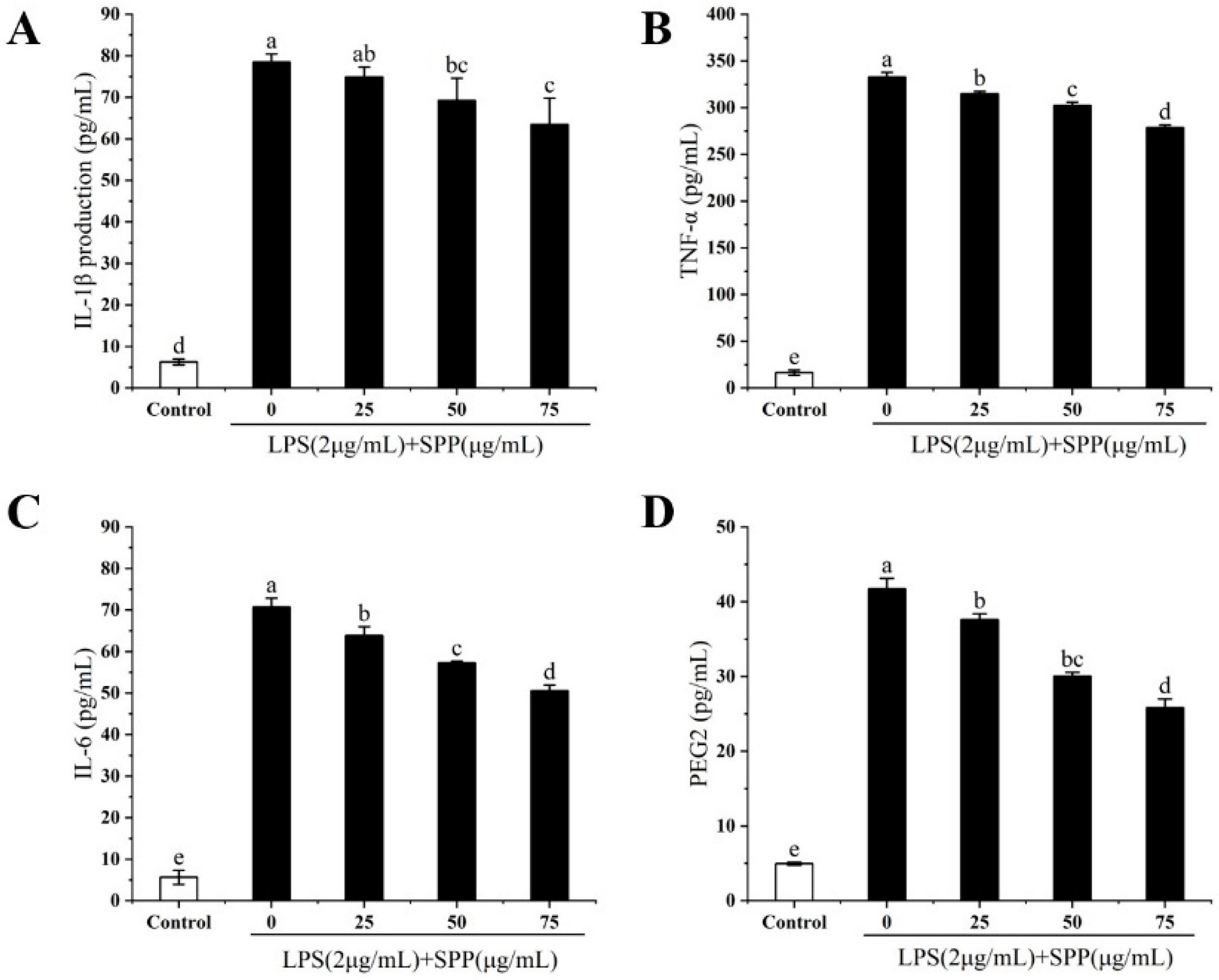
3.4. Effect of SPP on the Expression of Relevant Inflammatory Elements (TNF-α, IL-1β, IL-6, PGE2)
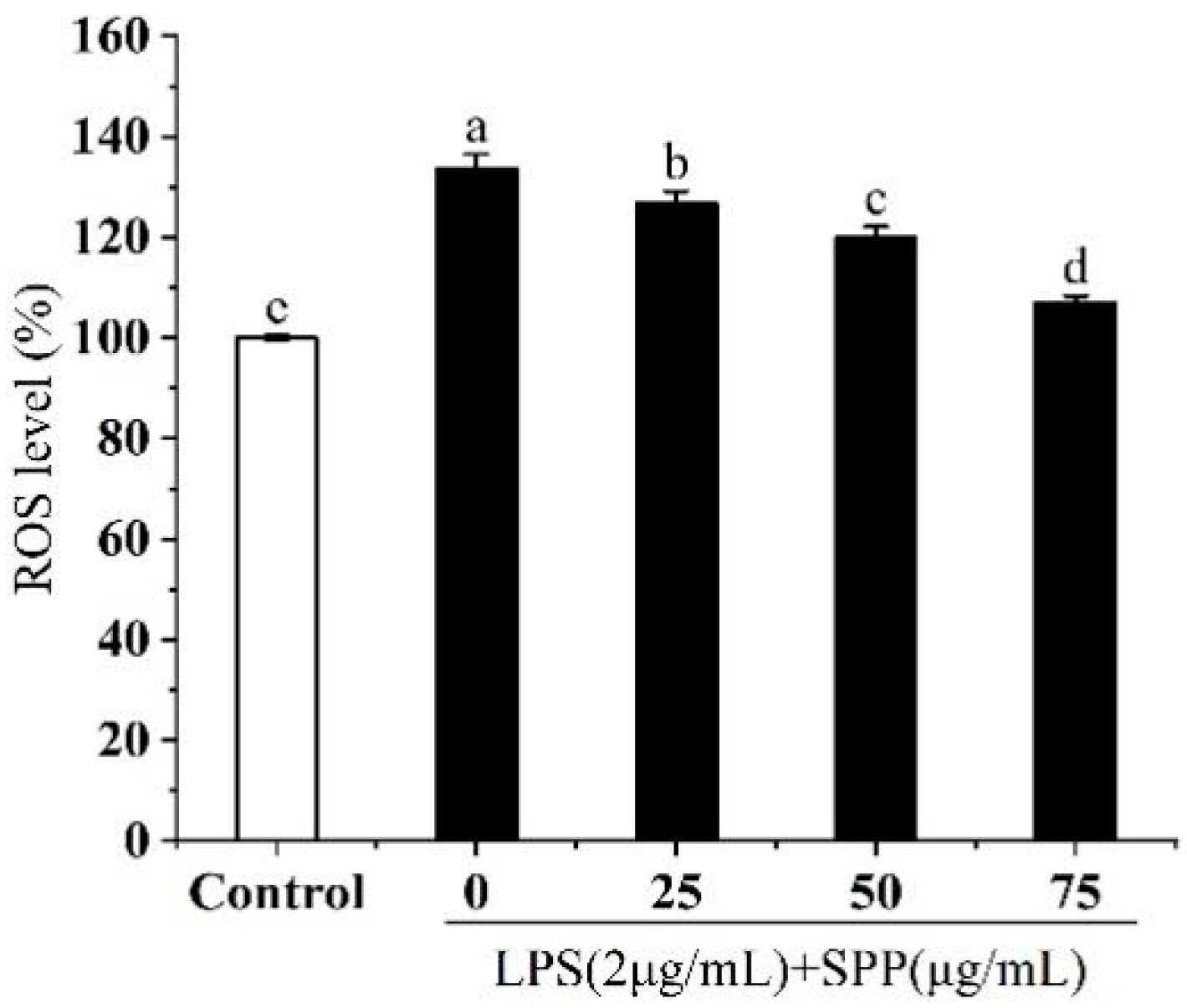
3.5. Effect of SPP on Intracellular ROS Generation
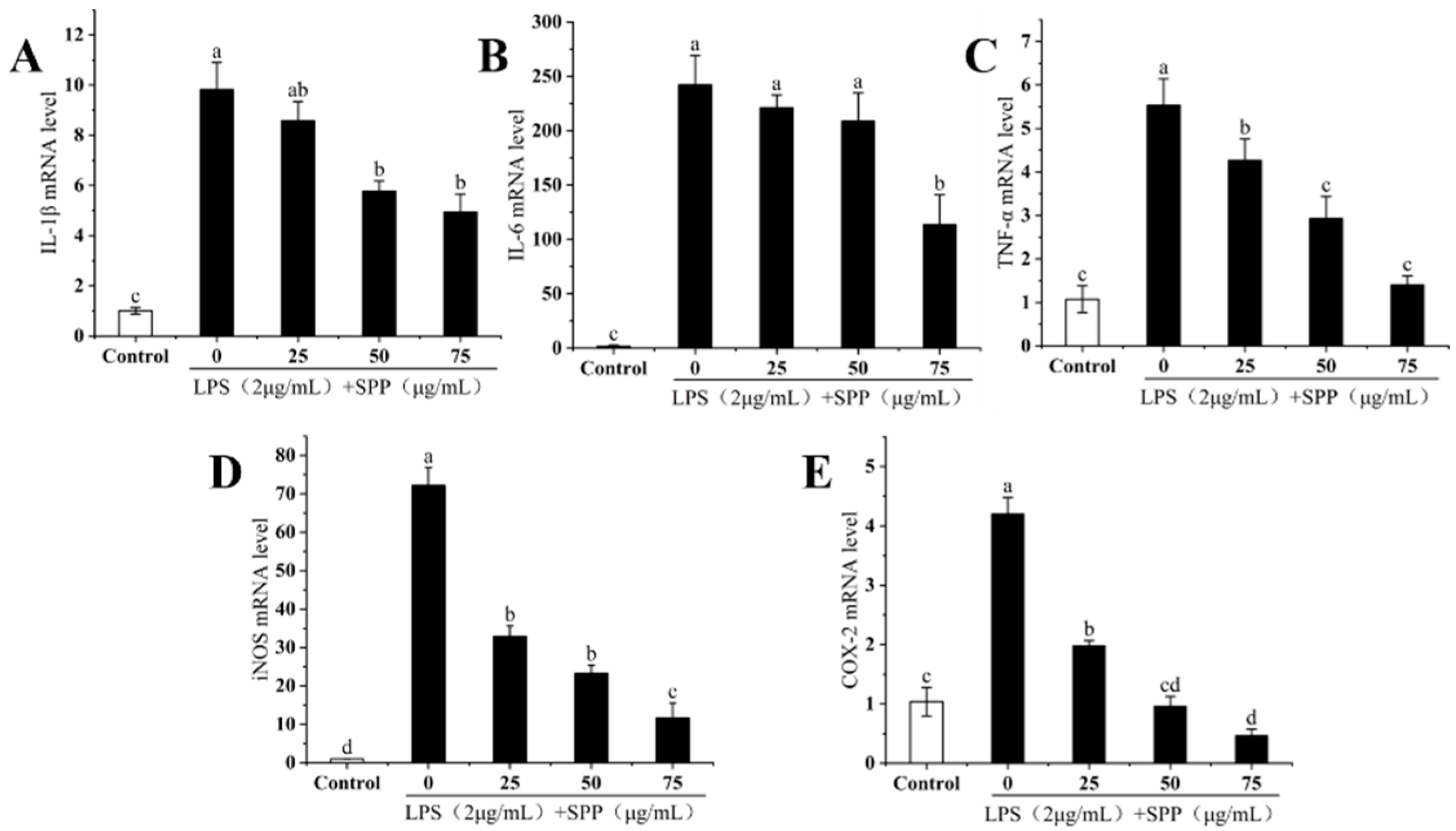
3.6. The IL-1β, IL-6, TNF-α, iNOS, and COX-2 mRNA Expressions
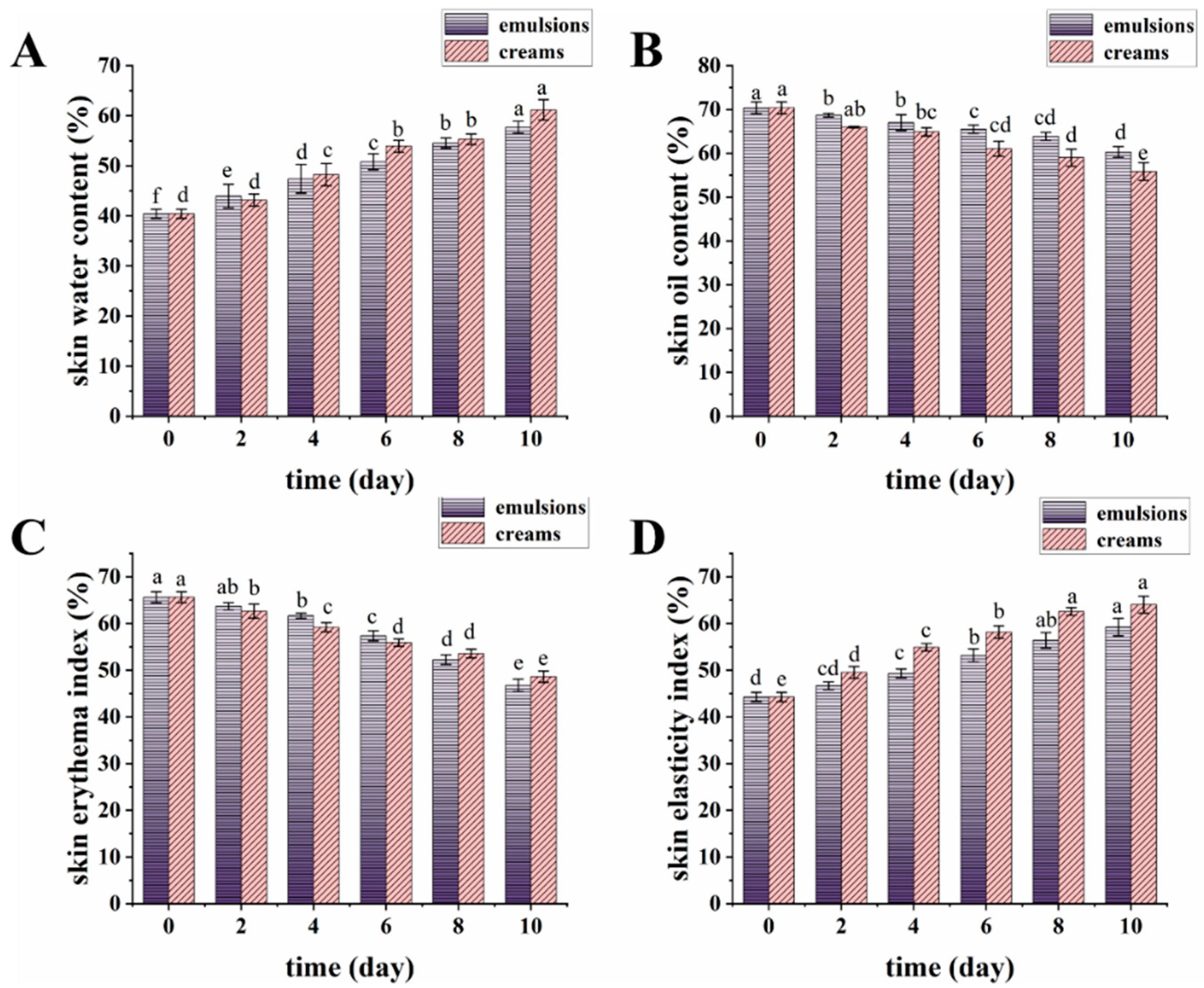
3.7. Testing the Physicochemical Properties of Emulsions and Creams
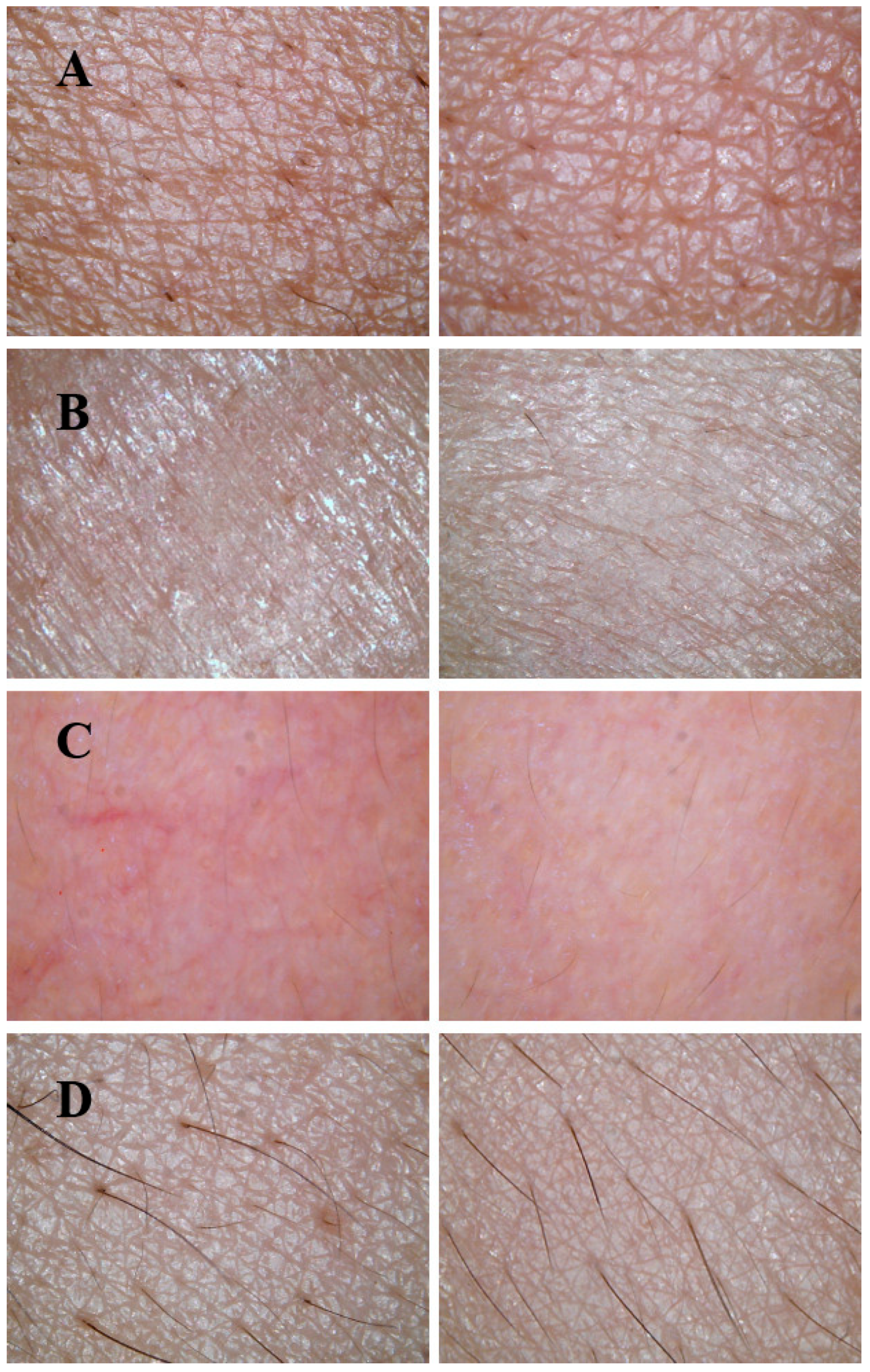
3.8. Skin Benefits of Emulsions and Creams
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, C.; Sa, Y. The rapeutic potential of autophagy in immunity and inflammation: Current and future perspectives. Pharmacol. Rep. 2023, 75, 499–510. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chi, Z.; Lai, D.; Liu, K.; Liu, Z.; Zheng, Y. Advances in the microbial synthesis of active ingredients in cosmetics. Sheng Wu Gong Cheng Xue Bao 2024, 40, 2489–2512. [Google Scholar]
- Selvaraj, B.; Ganapathy, D. Exploration of Sargassum wightii: Extraction, Phytochemical Analysis, and Antioxidant Potential of Polyphenol. Cureus 2024, 16, e63706. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Lee, J.H.; Je, J.G.; Yu, H.-S.; Jeon, Y.-J.; Kim, H.J.; Jee, Y. Sargassum horneri (Turner) C. Agardh containing polyphenols attenuates particulate matter-induced inflammatory response by blocking TLR-mediated MYD88-dependent MAPK signaling pathway in MLE-12 cells. J. Ethnopharmacol. 2021, 265, 113340. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.; Koszarska, M.; Atanasov, A.G.; Król-Szmajda, K.; Jóźwik, A.; Stelmasiak, A.; Hejna, M. Bioactive Potential of Algae and Algae-Derived Compounds: Focus on Anti-Inflammatory, Antimicrobial, and Antioxidant Effects. Molecules 2024, 29, 4695. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Kim, A.-R.; Lee, B.; Joung, E.-J.; Gwon, W.-G.; Utsuki, T.; Kim, N.-G.; Kim, H.-R. 6,6’-Bieckol suppresses inflammatory responses by down-regulating nuclear factor-κB activation via Akt, JNK, and p38 MAPK in LPS-stimulated microglial cells. Immunopharmacol. Immunotoxicol. 2016, 38, 244–252. [Google Scholar] [CrossRef]
- Han, E.-J.; Jayawardena, T.U.; Jang, J.-H.; Fernando, I.P.S.; Jee, Y.; Jeon, Y.-J.; Lee, D.-S.; Lee, J.-M.; Yim, M.-J.; Wang, L.; et al. Sargachromenol Purified from Sargassum horneri Inhibits Inflammatory Responses via Activation of Nrf2/HO-1 Signaling in LPS-Stimulated Macrophages. Mar. Drugs 2021, 19, 497. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Han, E.J.; Jee, Y.; Ahn, G.; Rho, J.-R.; Jeon, Y.-J. Loliolide, isolated from Sargassum horneri; abate LPS-induced inflammation via TLR mediated NF-KB, MAPK pathways in macrophages. Algal Res.-Biomass Biofuels Bioprod. 2021, 56, 102297. [Google Scholar]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, Y.X.; Zhou, B.X.; Liu, Z.; Xue, W.Q.; Shi, J.F.; Zheng, J.Q.; Li, J.J.; Ji, J.; Qin, K.M.; et al. Extraction, purification and pharmacological activity of Polysaccharides from sea weed Sargassum pallidum. J. Dalian Ocean. Univ. 2022, 37, 894–902. [Google Scholar]
- Su, D.; Li, Q.; Lai, X.; Song, Y.; Li, H.; Ai, Z.; Zhang, Q.; Shao, W.; Yang, M.; Zhu, G. Sargassum pallidum reduces inflammation to exert antidepressant effect by regulating intestinal microbiome and ERK1/2/P38 signaling pathway. Front. Pharmacol. 2024, 15, 1424834. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Wu, Y.R.; Li, L.; Sun, X.C.; Wang, J.; Ma, C.Y.; Zhang, Y.; Qu, H.L.; Xu, R.X.; Li, J.J. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 322–332. [Google Scholar] [CrossRef]
- Ghosh, P.; Mukherjee, S.; Ghosh, S.; Gangopadhyay, A.; Keswani, T.; Sengupta, A.; Sarkar, S.; Bhattacharyya, A. Estimating nitric oxide (NO) from MDSCs by Griess method. Methods Cell Biol. 2024, 184, 149–158. [Google Scholar] [PubMed]
- Kim, S.Y.; Ahn, G.; Kim, H.S.; Je, J.G.; Kim, K.N.; Jeon, Y.J. Diphlorethohydroxycarmalol (DPHC) Isolated from the Brown Alga Ishige okamurae Acts on Inflammatory Myopathy as an Inhibitory Agent of TNF-α. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J. Appl. Phycol. 2018, 30, 1955–1970. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxidative Med. Cell. Longev. 2021, 1, 9974890. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Wang, X.Y.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Huang, X.; Li, F.; Wang, F. Neural Regulation of Innate Immunity in Inflammatory Skin Diseases. Pharmaceuticals 2023, 16, 246. [Google Scholar] [CrossRef]
- Shanmugam, G. Polyphenols: Potent protectors against chronic diseases. Nat. Prod. Res. 2024, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Yao, H.Z.; Li, Y.Q.; Li, Y.; Wang, P.Y. Progress of Research on the Anti-inflammatory Activity of Dietary Polyphenols and the Mechanisms whereby They Affect Inflammatory Signaling Pathways. Mod. Food Sci. Technol. 2024, 40, 398–408. [Google Scholar]
- Liu, M.; Chu, W.; Guo, T.; Zeng, X.; Shangguan, Y.; He, F.; Liang, X. Challenges of Cell Counting in Cell Therapy Products. Cell Transplant. 2024, 33, 09636897241293628. [Google Scholar] [CrossRef]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zaborova, V.; Budanova, E.; Kryuchkova, K.; Rybakov, V.; Shestakov, D.; Isaikin, A.; Romanov, D.; Churyukanov, M.; Vakhnina, N.; Zakharov, V.; et al. Nitric oxide: A gas transmitter in healthy and diseased skin. Med. Gas Res. 2025, 15, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Lei, M.; Bai, Y.X. Chronic Stress Mediates Inflammatory Cytokines Alterations and Its Role in Tumorigenesis. J. Inflamm. Res. 2025, 18, 1067–1090. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging-Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Arezki, N.R.; Williams, A.C.; Cobb, A.J.A.; Brown, M.B. Design, synthesis and characterization of linear unnatural amino acids for skin moisturization. Int. J. Cosmet. Sci. 2017, 39, 72–82. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.E.; Censi, R.; Zara, S.; Di Martino, P.; Gigliobianco, M.R. Activation of polyphenolic compounds as new ingredients for sustainable cosmetic formulation. J. Drug Deliv. Sci. Technol. 2025, 108, 106918. [Google Scholar] [CrossRef]
- Di Nicolantonio, L.; Ferrati, M.; Cristino, M.; Peregrina, D.V.; Zannotti, M.; Vitali, L.A.; Ciancia, S.I.; Giovannetti, R.; Ferraro, S.; Zara, S.; et al. Evaluation of Physicochemical and Microbial Properties of Extracts from Wine Lees Waste of Matelica’s Verdicchio and Their Applications in Novel Cosmetic Products. Antioxidants 2023, 12, 816. [Google Scholar] [CrossRef]
- du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.-C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]

| Gene | Primer Sequences |
|---|---|
| IL-1β | F: 5′-TTCAGGCAGGCAGTATCACTC -3′ |
| R: 5′-GAAGGTCCACGGGAAAGACAC-3′ | |
| IL-6 | F: 5′-TCCATCCAGTTGCCTTCTTG-3′ |
| R: 5′-AAGCCTCCGACTTGTGAAGTG-3′ | |
| TNF-α | F: 5′-ACTGGCAGAAGAGGCACTCC-3′ |
| R: 5′-GCCACAAGCAGGAATGAGAA-3′ | |
| iNOS | F: 5′-CCTCCTCGTTCAGCTCACCT-3′ |
| R: 5′-CAATCCACAACTCGCTCCAA-3′ | |
| COX-2 | F: 5′-CCTGGTGAACTACGACTGCTA-3′ |
| R: 5′-AGTGGAGAACGTCTTCAGATGAG-3′ | |
| GAPDH | F: 5′-GTGAAGGTGACAGCAGTCGGTT-3′ |
| R: 5′-GAAGTGGGGTGGCTTTTAGGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, L.; Sun, Y.; Yang, J.; Liu, W.; Wu, T.; Jia, R. Evaluation of the Bioactivity of Phenolic Compounds from the Sargassum pallidum and Development of Their Stable Emulsion and Cream. Biology 2025, 14, 625. https://doi.org/10.3390/biology14060625
Wang S, Wang L, Sun Y, Yang J, Liu W, Wu T, Jia R. Evaluation of the Bioactivity of Phenolic Compounds from the Sargassum pallidum and Development of Their Stable Emulsion and Cream. Biology. 2025; 14(6):625. https://doi.org/10.3390/biology14060625
Chicago/Turabian StyleWang, Shuoqi, Li Wang, Yiqing Sun, Jia Yang, Wei Liu, Tingting Wu, and Rui Jia. 2025. "Evaluation of the Bioactivity of Phenolic Compounds from the Sargassum pallidum and Development of Their Stable Emulsion and Cream" Biology 14, no. 6: 625. https://doi.org/10.3390/biology14060625
APA StyleWang, S., Wang, L., Sun, Y., Yang, J., Liu, W., Wu, T., & Jia, R. (2025). Evaluation of the Bioactivity of Phenolic Compounds from the Sargassum pallidum and Development of Their Stable Emulsion and Cream. Biology, 14(6), 625. https://doi.org/10.3390/biology14060625







