Epidemiological and Histopathological Characteristics of Fetuses with Congenital Disorders: A Study in Greece
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Sources
2.2. Outcomes
2.3. Exposures
2.4. Statistical Analysis
2.5. Ethics Approval
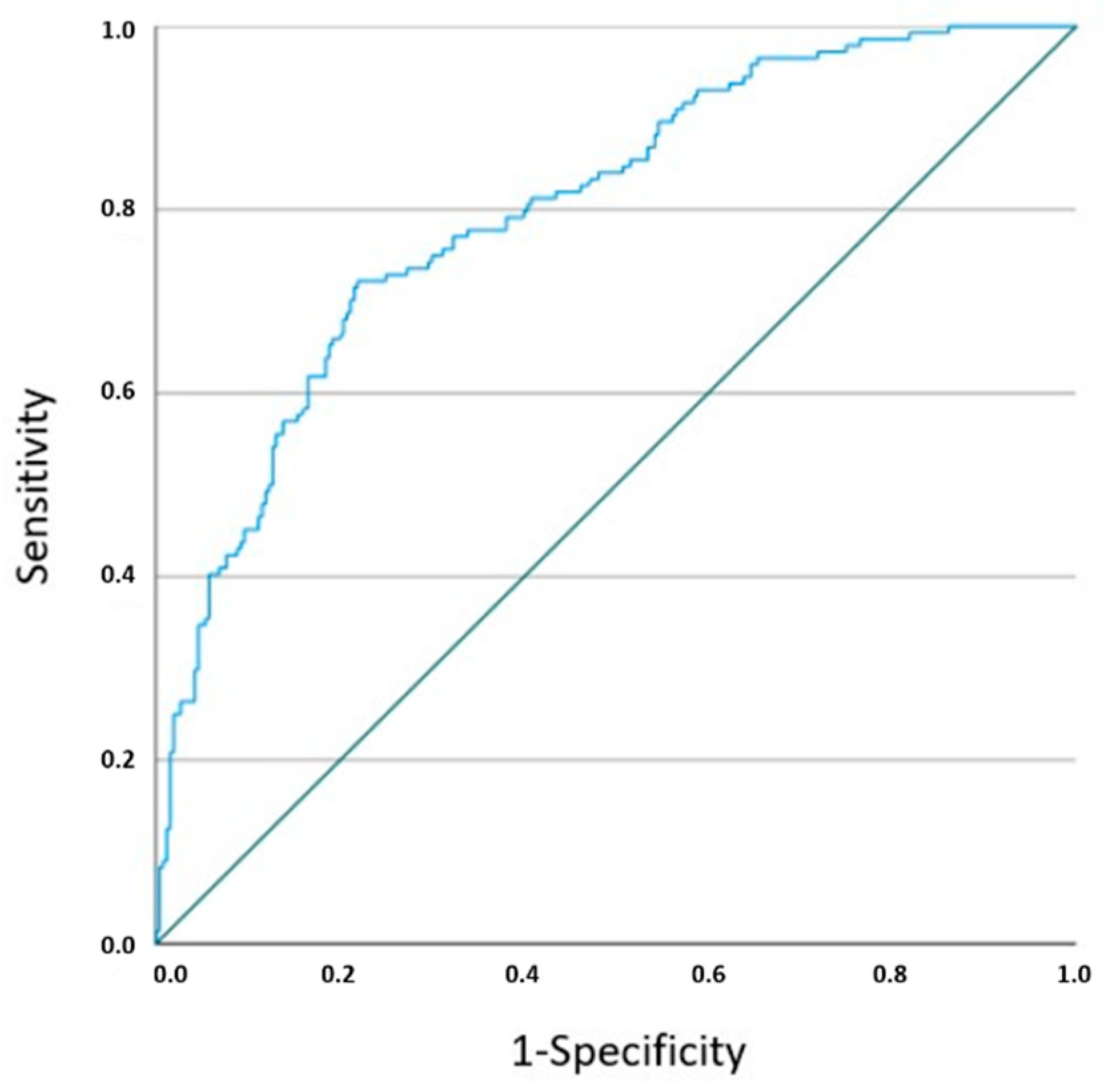
3. Results
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geda, Y.F.; Lamiso, Y.Y.; Berhe, T.M.; Mohammed, S.J.; Chibsa, S.E.; Adeba, T.S.; Mossa, K.A.; Abeje, S.; Gesese, M.M. Structural Congenital Anomalies in Resource-Limited Settings: A Systematic Review and Meta-Analysis. Sci. Rep. 2024, 14, 74015. [Google Scholar] [CrossRef]
- Wojcik, M.H.; Agrawal, P.B. Deciphering Congenital Anomalies for the Next Generation. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005504. [Google Scholar] [CrossRef]
- Dolk, H.; Loane, M.; Garne, E. The Prevalence of Congenital Anomalies in Europe. Adv. Exp. Med. Biol. 2010, 686, 349–364. [Google Scholar] [CrossRef]
- Swanson, J.; Ailes, E.C.; Cragan, J.D.; Grosse, S.D.; Tanner, J.P.; Kirby, R.S.; Waitzman, N.J.; Reefhuis, J.; Salemi, J.L. Inpatient Hospitalization Costs Associated with Birth Defects Among Persons Aged <65 Years—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kinsner-Ovaskainen, A.; Perraud, A.; Lanzoni, M.; Morris, J.; Garne, E. JRC-EUROCAT Report on Statistical Monitoring of Congenital Anomalies (2009–2018); European Monitoring of Congenital Anomalies: Ispra, Italy, 2021. [Google Scholar]
- Mamasoula, C.; Addor, M.C.; Carbonell, C.C.; Dias, C.M.; Echevarría-González-de-Garibay, L.J.; Gatt, M.; Khoshnood, B.; Klungsoyr, K.; Randall, K.; Stoianova, S.; et al. Prevalence of congenital heart defects in Europe, 2008–2015: A registry-based study. Birth Defects Res. 2022, 114, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Türkbay, T.; Öztürk, Z.A.; Akın, M.A.; Akın, L. The birth prevalence of selected major congenital anomalies: Six-year’s experience in a tertiary care maternity hospital. Turk. Pediatr. Ars. 2020, 55, 393–400. [Google Scholar] [CrossRef]
- Adane, F.; Afework, M.; Seyoum, G.; Gebrie, A. Prevalence and associated factors of birth defects among newborns in sub-Saharan African countries: A systematic review and meta-analysis. Pan Afr. Med. J. 2020, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Warmerdam, B.; Wyatt, K.; Ahmad, S.; Shaw, G.M. Prevalence of Birth Defects among American-Indian Births in California, 1983–2010. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 105–110. [Google Scholar] [CrossRef]
- Gunn-Charlton, J.K. Impact of Comorbid Prematurity and Congenital Anomalies: A Review. Front. Physiol. 2022, 13, 880891. [Google Scholar] [CrossRef]
- Honein, M.A.; Kirby, R.S.; Meyer, R.E.; Xing, J.; Skerrette, N.I.; Yuskiv, N.; Marengo, L.; Petrini, J.R.; Davidoff, M.J.; Mai, C.T.; et al. The Association between Major Birth Defects and Preterm Birth. Matern. Child. Health J. 2009, 13, 164–175. [Google Scholar] [CrossRef]
- Al-Dewik, N.; Al-Mulla, M.; El-Ayoubi, R.; Al-Mansoori, L.; Al-Thani, M.; Al-Khater, H.; Al-Mulla, F. Prevalence, Predictors, and Outcomes of Major Congenital Anomalies: A Population-Based Retrospective Study in the State of Qatar. Sci. Rep. 2023, 13, 27935. [Google Scholar] [CrossRef]
- Ghanchi, A.; Rahshenas, M.; Bonnet, D.; Derridj, N.; LeLong, N.; Salomon, L.J.; Goffinet, F.; Khoshnood, B. Prevalence of Growth Restriction at Birth for Newborns With Congenital Heart Defects: A Population-Based Prospective Cohort Study EPICARD. Front. Pediatr. 2021, 9, 676994. [Google Scholar] [CrossRef] [PubMed]
- Bomback, M.; Everett, S.; Lyford, A.; Sahni, R.; Kim, F.; Baptiste, C.; Motelow, J.E.; Tolia, V.; Clark, R.; Dugoff, L.; et al. Genetic Disorders and Their Association with Morbidity and Mortality in Early Preterm Small for Gestational Age Infants. Am. J. Obstet. Gynecol. 2025, 232, 487.e1–487.e14. [Google Scholar] [CrossRef]
- Puccio, G.; Giuffré, M.; Piccione, M.; Piro, E.; Rinaudo, G.; Corsello, G. Intrauterine Growth Restriction and Congenital Malformations: A Retrospective Epidemiological Study. Ital. J. Pediatr. 2013, 39, 23. [Google Scholar] [CrossRef] [PubMed]
- Nikkilä, A.; Källén, B.; Maršál, K. Fetal Growth and Congenital Malformations. Ultrasound Obstet. Gynecol. 2007, 29, 289–295. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, W.; Zhao, Q.; Zhang, Y.; Wang, X.; Wang, J.; Wang, R.; Hao, B.; Liao, S. Pregnancy outcomes of 4200 fetuses with increased nuchal translucency in Henan, China. Front. Med. 2025, 12, 1514504. [Google Scholar] [CrossRef] [PubMed]
- Hematian, M.N.; Hessami, K.; Torabi, S.; Saleh, M.; Nouri, B.; Saleh, M. A prospective cohort study on association of first-trimester serum biomarkers and risk of isolated foetal congenital heart defects. Biomarkers 2021, 26, 747–751. [Google Scholar] [CrossRef]
- Bellai-Dussault, K.; Dougan, S.D.; Fell, D.B.; Little, J.; Meng, L.; Okun, N.; Walker, M.C.; Armour, C.M.; Potter, B.K. Ultrasonographic Fetal Nuchal Translucency Measurements and Cytogenetic Outcomes. JAMA Netw. Open 2024, 7, e243689. [Google Scholar] [CrossRef]
- Niroomanesh, S.; Nadimzadeh, N.; Rahimi-Sharbaf, F.; Shirazi, M.; Golshahi, F.; Sahebdel, B.; Feizabad, E. Pregnancy outcomes of normal karyotype fetuses with increased nuchal translucency. Caspian J. Intern. Med. 2023, 14, 732–736. [Google Scholar] [CrossRef]
- Ebbing, C.; Kessler, J.; Moster, D.; Rasmussen, S. Single Umbilical Artery and Risk of Congenital Malformation: Population-Based Study in Norway. Ultrasound Obstet. Gynecol. 2020, 55, 510–515. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Q.; Kong, F.; Ning, Y. Diagnosis of single umbilical artery and risk of foetal congenital malformations by prenatal ultrasound: A retrospective study. BMC Pregnancy Childbirth 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, H.; Rafeei, K.; Dalili, M.; Asadi, N.; Seirfar, N.; Akbarzadeh-Jahromi, M. Prevalence of Single Umbilical Artery, Clinical Outcomes and Its Risk Factors: A Cross-Sectional Study. Int. J. Reprod. Biomed. 2021, 19, 441. [Google Scholar] [CrossRef]
- Saxena, M.; Hungund, B. Single umbilical artery and associated birth defects in perinatal autopsies: Prenatal diagnosis and management. J. Pathol. Transl. Med. 2024, 58, 214–218. [Google Scholar] [CrossRef]
- Dawson, A.L.; Tinker, S.C.; Jamieson, D.J.; Hobbs, C.A.; Berry, R.J.; Rasmussen, S.A.; Anderka, M.; Keppler-Noreuil, K.M.; Lin, A.E.; Reefhuis, J. Twinning and Major Birth Defects, National Birth Defects Prevention Study, 1997–2007. J. Epidemiol. Community Health (1978) 2016, 70, 1114–1121. [Google Scholar] [CrossRef]
- Ting, Y.; Ting, Z.; Fei, Z.; Chun-Fang, L.; Zhen, H. Prenatal diagnosis of structural anomaly among singletons and twins: Eight-year experience in a Chinese tertiary center. J. Ultrasound Med. 2023, 42, 185–192. [Google Scholar] [CrossRef]
- Gijtenbeek, M.; Shirzada, M.R.; Ten Harkel, A.D.J.; Oepkes, D.; Haak, M.C. Congenital heart defects in monochorionic twins: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Best, K.E.; Rankin, J. Increased Risk of Congenital Heart Disease in Twins in the North of England between 1998 and 2010. Heart 2015, 101, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Racusin, D.A.; Chen, H.Y.; Bhalwal, A.; Wiley, R.; Chauhan, S.P. Chorioamnionitis and adverse outcomes in low-risk pregnancies: A population-based study. J. Matern. Fetal Neonatal Med. 2022, 35, 5555–5563. [Google Scholar] [CrossRef]
- Jain, V.G.; Kline, J.E.; He, L.; Kline-Fath, B.M.; Altaye, M.; Muglia, L.J.; DeFranco, E.A.; Ambalavanan, N.; Parikh, N.A.; Cincinnati Infant Neurodevelopment Early Prediction Study Investigators. Acute Histologic Chorioamnionitis Independently and Directly Increases the Risk for Brain Abnormalities Seen on Magnetic Resonance Imaging in Very Preterm Infants. Am. J. Obstet. Gynecol. 2022, 227, 623.e1–623.e13. [Google Scholar] [CrossRef]
- Tsamantioti, E.; Lisonkova, S.; Muraca, G.M.; Örtqvist, A.K.; Razaz, N. Chorioamnionitis and Risk of Long-Term Neurodevelopmental Disorders in Offspring: A Population-Based Cohort Study. Am. J. Obstet. Gynecol. 2022, 227, 287.e1–287.e17. [Google Scholar] [CrossRef]
- Xiao, D.; Zhu, T.; Qu, Y.; Gou, X.; Huang, Q.; Li, X.; Mu, D. Maternal Chorioamnionitis and Neurodevelopmental Outcomes in Preterm and Very Preterm Neonates: A Meta-Analysis. PLoS ONE 2018, 13, e0208302. [Google Scholar] [CrossRef]
- Silva, G.V.; Gontijo, C.T.; Lunguinho, A.P.C.; Caetano, M.S.G.; Callado, G.Y.; Araujo Júnior, E.; Peixoto, A.B. Perinatal Outcomes Related to the Presence of a Nuchal Cord During Delivery: A Retrospective Cohort Study. Diagnostics 2025, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Tonni, G.; Lituania, M.; Cecchi, A.; Carboni, E.; Resta, S.; Bonasoni, M.P.; Ruano, R. Umbilical Cord Diseases Affecting Obstetric and Perinatal Outcomes. Healthcare 2023, 11, 2634. [Google Scholar] [CrossRef]
- Hayes, D.J.L.; Warland, J.; Parast, M.M.; Bendon, R.W.; Hasegawa, J.; Banks, J.; Clapham, L.; Heazell, A.E.P. Umbilical Cord Characteristics and Their Association with Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0239630. [Google Scholar] [CrossRef]
- Lichtman, Y.; Wainstock, T.; Walfisch, A.; Sheiner, E. The Significance of True Knot of the Umbilical Cord in Long-Term Offspring Neurological Health. J. Clin. Med. 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.L.; Bitar, L.; Rajagopalan, V.; Spong, C.Y. Interdependence of placenta and fetal cardiac development. Prenat. Diagn. 2024, 44, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Andescavage, N.N.; Limperopoulos, C. Placental Abnormalities in Congenital Heart Disease. Transl. Pediatr. 2021, 10, 2148–2156. [Google Scholar] [CrossRef]
- Wardinger, J.E.; Ambati, S. Placental Insufficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Günay, T.; Yardımcı, O.D. How Does Subchorionic Hematoma in the First Trimester Affect Pregnancy Outcomes? Arch. Med. Sci. 2021, 18, 639–646. [Google Scholar] [CrossRef]
- Pethő, B.; Mátrai, Á.; Agócs, G.; Veres, D.S.; Harnos, A.; Váncsa, S.; Bánhidy, F.; Hegyi, P.; Ács, N. Maternal age is highly associated with non-chromosomal congenital anomalies: Analysis of a population-based case-control database. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1217–1225. [Google Scholar] [CrossRef]
- Zhou, X.; He, J.; Wang, A.; Hua, X.; Li, T.; Shu, C.; Fang, J. Multivariate logistic regression analysis of risk factors for birth defects: A study from population-based surveillance data. BMC Public Health 2024, 24, 1037. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, S.; Sheng, Q.; Yang, J.; Liu, J.; Li, H.; Yuan, P.; Zhao, Y. Association of maternal age with adverse pregnancy outcomes: A prospective multicenter cohort study in China. J. Glob. Health 2023, 13, 04161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Kim, J.; Kang, J.; Kim, Y.H.; Kim, K. Congenital Anomalies and Maternal Age: A Systematic Review and Meta-Analysis of Observational Studies. Acta Obstet. Gynecol. Scand. 2022, 101, 484–498. [Google Scholar] [CrossRef]
- Black, A.J.; Lu, D.Y.; Yefet, L.S.; Baird, R. Sex differences in surgically correctable congenital anomalies: A systematic review. J. Pediatr. Surg. 2020, 55, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Williford, E.M.; Yang, W.; Howley, M.M.; Ma, C.; Collins, R.T.; Weber, K.A.; Heinke, D.; Petersen, J.M.; Agopian, A.J.; Archer, N.P.; et al. Factors associated with infant sex and preterm birth status for selected birth defects from the National Birth Defects Prevention Study, 1997–2011. Birth Defects Res. 2024, 116, e2294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, D.; Jiang, Y.; Fang, J. Prevalence and death rate of birth defects from population-based surveillance in Hunan Province, China, 2010–2020. Sci. Rep. 2024, 14, 14609. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Li, J.; Sun, L.; Qin, W.; Ding, G.; Wang, Q.; Zhu, J.; Yu, Z.; Xie, S.; et al. Gender Differences in the Association between Body Mass Index and Health-Related Quality of Life among Adults: A Cross-Sectional Study in Shandong, China. BMC Public Health 2019, 19, 1021. [Google Scholar] [CrossRef]
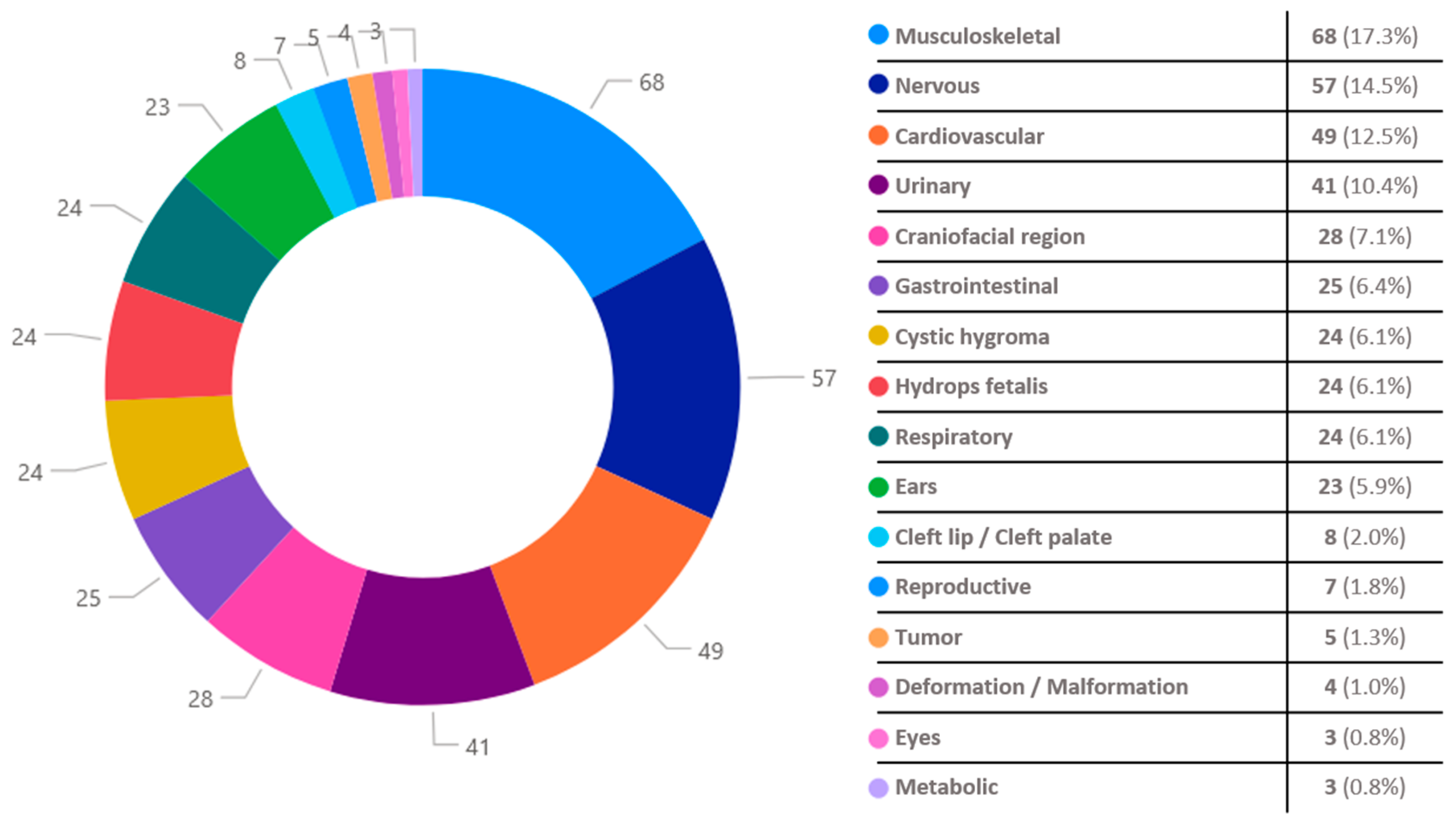
| Total Fetuses (N = 649) | Fetuses with Congenital Disorders (N = 256) | Fetuses Without Congenital Disorders (N = 393) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Mother’s age (years) * | 29.86 (5.653) | 30.10 (5.756) | 29.70 (5.590) | 0.205 |
| Gestational age (weeks) * | 23.00 (12.00) | 22.00 (7.00) | 23.00 (14.00) | 0.020 |
| Sex | ||||
| Male | 335 (51.6) | 128 (50.0) | 207 (52.7) | 0.506 |
| Female | 265 (40.8) | 101 (39.5) | 164 (41.7) | 0.564 |
| Cannot be identified + | 49 (7.6) | 27 (10.5) | 22 (5.6) | 0.020 |
| Fetal weight (grams) * | 540.00 (1227) | 530.00 (934) | 543.50 (1407) | 0.665 |
| Twin pregnancy | 30 (4.6) | 3 (1.2) | 27 (6.9) | <0.001 |
| Type of abortion/delivery | ||||
| Stillbirth | 518 (80.9) | 160 (64.5) | 358 (91.3) | <0.001 |
| Iatrogenic | 79 (12.3) | 62 (25.0) | 17 (4.3) | <0.001 |
| Vaginal delivery/C-section | 43 (6.7) | 26 (10.5) | 17 (4.3) | 0.002 |
| Autopsy and histopathological findings of fetuses and extraembryonic tissues | ||||
| Nuchal oedema | 32 (4.9) | 27 (10.5) | 5 (1.3) | <0.001 |
| Intrauterine growth restriction | 174 (36.2) | 78 (43.1) | 96 (32.0) | 0.014 |
| Chorioamnionitis (acute/chronic) | 59 (9.1) | 8 (3.1) | 51 (13.0) | <0.001 |
| Placenta | ||||
| Infarction | 122 (18.8) | 28 (10.9) | 94 (23.9) | <0.001 |
| Intervillous thrombus | 49 (7.6) | 12 (4.7) | 37 (9.4) | 0.026 |
| Villitis | 28 (4.3) | 7 (2.7) | 21 (5.3) | 0.110 |
| Abruption | 12 (1.8) | 1 (0.4) | 11 (2.8) | 0.034 |
| Umbilical cord | ||||
| Single umbilical artery | 29 (4.5) | 18 (7.0) | 11 (2.8) | 0.011 |
| Nuchal cord | 21 (3.2) | 3 (1.2) | 18 (4.6) | 0.016 |
| Funisitis | 13 (2.0) | 4 (1.6) | 9 (2.3) | 0.518 |
| Thrombosis of umbilical artery | 8 (1.2) | 2 (0.8) | 6 (1.5) | 0.490 |
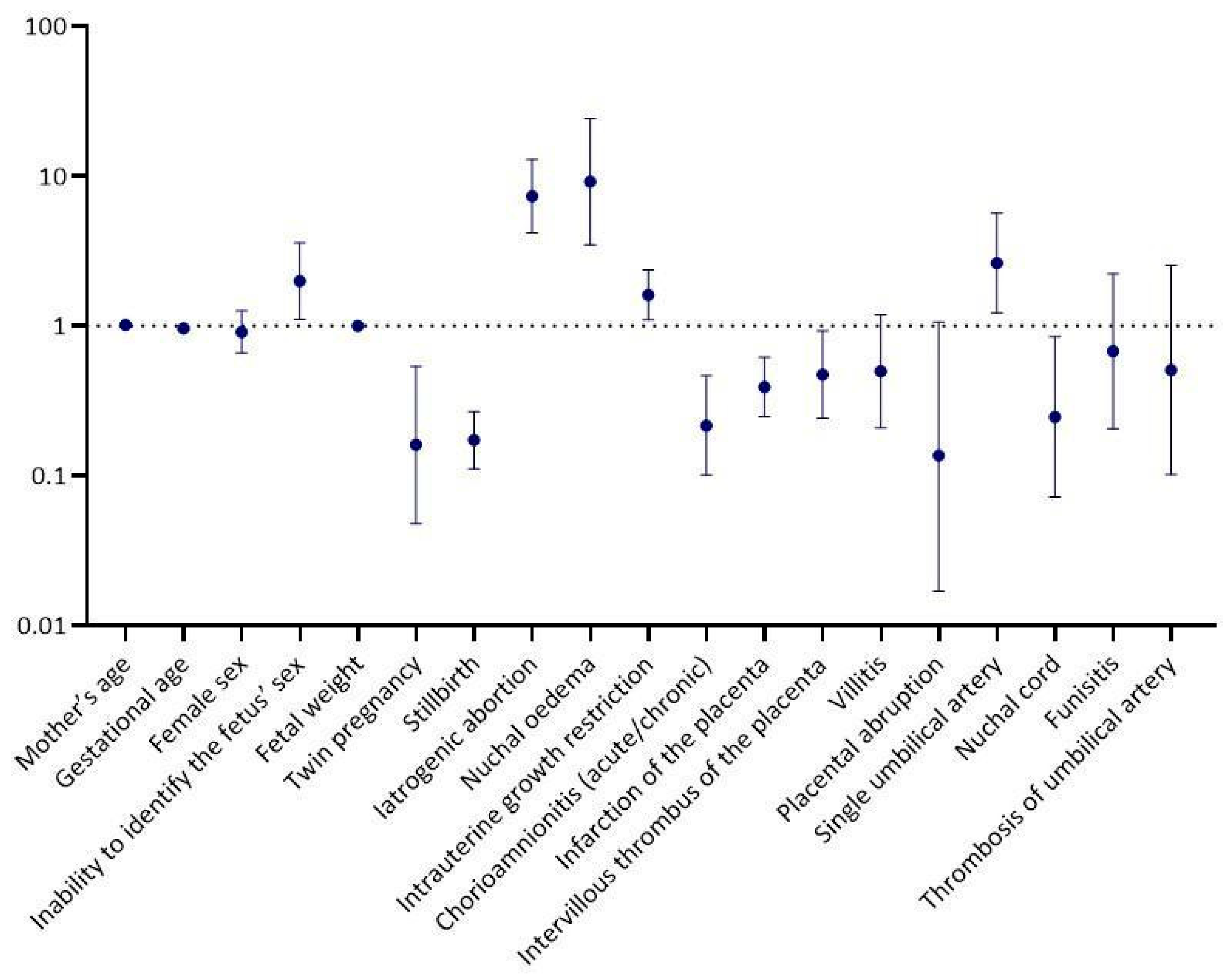
| Univariable Logistic Regression Analysis | ||
|---|---|---|
| p-Value | OR a (95% CI b) | |
| Mother’s age | 0.409 | 1.01 (0.98, 1.04) |
| Gestational age | 0.002 | 0.97 (0.94, 0.99) |
| Female sex | 0.564 | 0.91 (0.66, 1.25) |
| Inability to identify the fetus’ sex | 0.022 | 1.99 (1.11, 3.57) |
| Fetal weight | 0.187 | 1.00 (1.00, 1.00) |
| Twin pregnancy | 0.003 | 0.16 (0.05, 0.54) |
| Stillbirth | <0.001 | 0.17 (0.11, 0.27) |
| Iatrogenic abortion | <0.001 | 7.35 (4.18, 12.93) |
| Nuchal οedema | <0.001 | 9.15 (3.48, 24.09) |
| Intrauterine growth restriction | 0.014 | 1.61 (1.10, 2.36) |
| Chorioamnionitis (acute/chronic) | <0.001 | 0.22 (0.10, 0.46) |
| Infarction of the placenta | <0.001 | 0.39 (0.25, 0.62) |
| Intervillous thrombus of the placenta | 0.029 | 0.47 (0.24, 0.93) |
| Villitis | 0.116 | 0.50 (0.21, 1.19) |
| Placental abruption | 0.057 | 0.14 (0.02, 1.06) |
| Single umbilical artery | 0.014 | 2.63 (1.22, 5.66) |
| Nuchal cord | 0.026 | 0.25 (0.07, 0.85) |
| Funisitis | 0.520 | 0.68 (0.21, 2.22) |
| Thrombosis of umbilical artery | 0.409 | 0.51 (0.10, 2.54) |
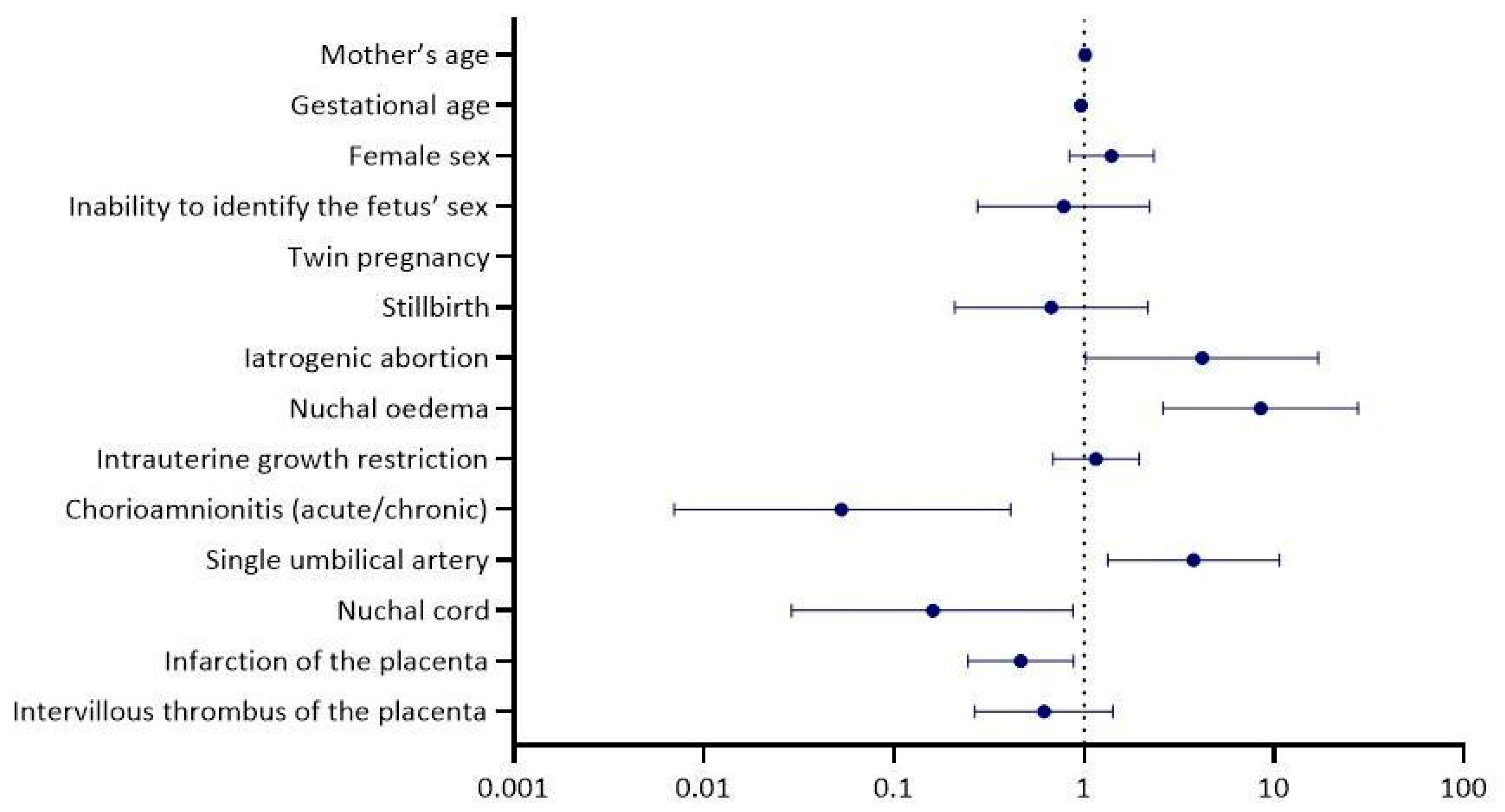
| Multivariable logistic regression analysis | ||
| p-value | OR a (95% CI b) | |
| Mother’s age | 0.548 | 1.01 (0.97, 1.06) |
| Gestational age | 0.051 | 0.97 (0.94, 1.00) |
| Female sex | 0.199 | 1.40 (0.84, 2.33) |
| Inability to identify the fetus’ sex | 0.642 | 0.78 (0.28, 2.22) |
| Twin pregnancy | 0.998 | 0.00 (0.00, 0.00) |
| Stillbirth | 0.506 | 0.67 (0.21, 2.17) |
| Iatrogenic abortion | 0.046 | 4.18 (1.02, 17.10) |
| Nuchal oedema | <0.001 | 8.49 (2.61, 27.66) |
| Intrauterine growth restriction | 0.583 | 1.16 (0.69, 1.95) |
| Chorioamnionitis (acute/chronic) | 0.005 | 0.05 (0.01, 0.41) |
| Single umbilical artery | 0.012 | 3.77 (1.33, 10.66) |
| Nuchal cord | 0.035 | 0.16 (0.03, 0.88) |
| Infarction of the placenta | 0.019 | 0.47 (0.25, 0.88) |
| Intervillous thrombus of the placenta | 0.254 | 0.62 (0.27, 1.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nteli, D.; Nteli, M.; Konstantinidis, K.; Ouzounidou, M.; Theotokis, P.; Manthou, M.-E.; Dermitzakis, I.; Miliara, X.; Gouta, C.; Angelidou, S.; et al. Epidemiological and Histopathological Characteristics of Fetuses with Congenital Disorders: A Study in Greece. Biology 2025, 14, 626. https://doi.org/10.3390/biology14060626
Nteli D, Nteli M, Konstantinidis K, Ouzounidou M, Theotokis P, Manthou M-E, Dermitzakis I, Miliara X, Gouta C, Angelidou S, et al. Epidemiological and Histopathological Characteristics of Fetuses with Congenital Disorders: A Study in Greece. Biology. 2025; 14(6):626. https://doi.org/10.3390/biology14060626
Chicago/Turabian StyleNteli, Despoina, Maria Nteli, Konstantinos Konstantinidis, Maria Ouzounidou, Paschalis Theotokis, Maria-Eleni Manthou, Iasonas Dermitzakis, Xeni Miliara, Chrysoula Gouta, Stamatia Angelidou, and et al. 2025. "Epidemiological and Histopathological Characteristics of Fetuses with Congenital Disorders: A Study in Greece" Biology 14, no. 6: 626. https://doi.org/10.3390/biology14060626
APA StyleNteli, D., Nteli, M., Konstantinidis, K., Ouzounidou, M., Theotokis, P., Manthou, M.-E., Dermitzakis, I., Miliara, X., Gouta, C., Angelidou, S., Miliaras, D., & Meditskou, S. (2025). Epidemiological and Histopathological Characteristics of Fetuses with Congenital Disorders: A Study in Greece. Biology, 14(6), 626. https://doi.org/10.3390/biology14060626











