Simple Summary
Drought and high-severity wildfires significantly affect bird communities, including migratory stop-over populations. These disturbances are interconnected, influencing ecosystems over different timescales. Using fall bird-banding data, this study examined how wildfire and drought shape avian relative abundance, taxonomic, functional, and phylogenetic diversity. After the fire, an increase in migratory insectivores contributed to a less phylogenetically diverse community. Some community changes appeared temporary, suggesting a return to pre-fire conditions over time. However, varying monsoonal conditions showed a lasting association with functional and phylogenetic diversity, emphasizing the importance of disentangling the combined effects of short-term disturbances (fire) and long-term environmental pressures (drought). While bird populations can recover from fire-driven habitat changes, persistent drought may continue to impact community composition.
Abstract
Drought and high-severity, stand-replacing wildfires can have substantial impacts on the composition of avian communities, including stop-over communities during migration. An inextricable link exists between drought and wildfire, each operating and impacting across different timescales. Many studies have found nonlinear avian abundance trends in breeding community time series data that include pre- and post-fire observations, describing an initial decrease in abundance followed by rapid increases that can attenuate over time. Here, we use a fall bird-banding dataset to evaluate shifts in a drought-impacted avian community following wildfire from taxonomic, functional, and phylogenetic perspectives. We looked at the community as a whole and also categorized birds as residents, migrants, and breeders to assess potential varying responses at the study site. We observed post-fire shifts in functional and phylogenetic diversity that corresponded to changes in vegetation. An influx of migratory insectivores post-fire drove much of the variation between pre- and post-fire avian communities and toward a more related, less phylogenetically dispersed community. A concurrent monsoon season drought was also associated with functional and phylogenetic diversity, highlighting the intertwined pulse press effects on avian communities. Overall, our results suggest that, although bird communities are immediately impacted by fire-driven resource changes, they can rebound over time, it is unclear how long-term drought may continue to shape the composition of these avian communities.
1. Introduction
Drought and wildfire can drive substantial shifts in species distributions and patterns of habitat use worldwide [1]. Species’ life history traits, such as migratory strategy, can buffer species from disturbances or increase their vulnerability [2]. In the southwest of the Unites States of America, intense and persistent drought—coupled with historical fire suppression—has increased wildfire frequency, extent, and severity [3,4,5]. Both drought and high-severity, stand-replacing wildfires can have notable impacts on ecosystems and their avian communities [6,7,8,9,10].
Depending on its intensity and/or frequency, wildfire can have immediate and substantial impacts on the composition and demographics of avian communities [9,11,12]. Steel et al. [12] found prominent reductions in avian community richness within severely burned habitat; however, many studies have found nonlinear abundance trends in time series data that include pre- and post-fire observations that describe an initial decrease in density and abundance followed by rapid increases that can attenuate over time [9,11,13,14]. Unlike the relatively short-term effects of fire, drought can have long-term and disproportionate impacts among species and in different habitats [7,10]. Although the response to drought can vary among avian species that have differing life histories and behavioral traits, the impacts of drought are capable of imposing sizable shifts in abundance, richness, and composition on avian communities [7]. For example, decreases in avian insectivore abundance have been repeatedly recorded during periods of drought [6,15,16,17].
An inextricable link exists between drought and wildfire, each operating across different timescales. Researchers are increasingly recognizing impacts on the dynamic interaction between drought and wildfire, describing variable community responses to fire during times of drought [9,17]. The ecosystem-resetting effects of fire can alter a landscape’s response to drought. Depending on their taxonomic makeup, post-fire successional vegetative communities can create novel microclimates that buffer against the effects of drought [18]. The constituent members of avian communities are affected differently by drought and fire, revealing patterns that suggest restructuring along multiple axes of biological organization [15,19].
Migration is a critical time in a migratory bird’s life cycle, when they are pushed to their physiological limits [20] and experience the highest annual mortality rates [21,22], especially during droughts [8,16]. Species’ migratory strategies can dramatically impact their vulnerability to disturbance-induced mortality [2]. Avian communities typically comprise resident, breeding, and transient species, whose relative proportions vary throughout the year. In this context, wildfire can be expected to more severely alter the composition of migrating transient species if they have the ability to select unburned habitat. Conversely, resident communities can be more constrained by local adaptation, low dispersal tendencies, and site fidelity and, therefore, resist disturbance-induced shifts. Most research on the compound impacts of drought and wildfire on avian communities has focused on the breeding season—when bird communities are at their most stable and transient individuals are rare [7,8,9,11,14]. Far less research is available on how fire and drought in the monsoon season impact communities during migration. Compared with breeding communities, migratory communities are composed of many transient individuals that shift with phenological sequences of species arrival and departure [23,24]. Migration has been theorized to be an evolved response to escape low food availability or harsh climatic conditions during the nonbreeding season [25,26,27,28]. Changes in resource availability or other habitat conditions along migratory routes can, therefore, influence the selection of migration stopover sites. One recent fall migration study found increases in avian abundance and decreases in body fat associated with the number of acres burned [29], suggesting that a bird’s migration pathway and health can be severely affected by wildfires. Evaluating avian community responses between the migratory and resident components of a bird community across periods of drought interrupted by wildfire can elucidate unobserved ecological processes and improve our understanding of the relative risk wildfire and drought pose to birds that have variable migratory strategies.
Environmental disturbances can drive shifts in functional diversity through range expansion or contraction, phenological changes, local extinctions, or colonizations [30,31]. Local extinction and emigration from recently burned habitat can lower functional diversity [32,33]; however, as documented in plant communities, some measures of functional diversity can rapidly increase following the initial impacts of wildfire [34]. The dynamism in community function following wildfire is understudied and can enhance descriptions of community change and the ecosystem impacts from wildfire.
In the summer of 2011, a record-setting wildfire punctuated a fall bird-banding effort of more than 5 years at Bandelier National Monument in Sandoval County, New Mexico. The wildfire was the largest in New Mexico history at the time and was exacerbated by a persistent and ongoing drought in the region. Here, we use this fall bird-banding dataset to compare the relative impact of wildfire and drought during the monsoon season on community composition between migratory and resident species in an avian community from taxonomic, functional, and phylogenetic perspectives. This study highlights species-specific responses that underlie functional measures of community diversity and ties them to processes of ecological reorganization. Examining avian community change through multiple ecologically relevant axes can offer insight into the relative ability of migratory and resident communities to recover, persist, or reassemble in the face of persisting drought and ever-increasing, large-scale wildfires in the western United States.
2. Materials and Methods
2.1. Study Area
The fall migration monitoring site, Upper Alamo in Bandelier National Monument, is located at the Alamo Boundary trailhead (35.834 N, 106.608 W) in the northwestern area of Bandelier in the state of New Mexico in the United States of America. The site is a mixed-conifer forest with dispersed meadows. In addition, the bird-banding station is located at high elevation (2718 m) in a national monument that starts below 1676 m near the Rio Grande and rises in elevation westward from there. The habitat changed substantially when the Las Conchas wildfire started on 26 June 2011, and burned a total area of 64,246 ha, much of which occurred in the park. The fire was a severe, stand-replacing fire that burned 0.40 ha per second for the first 8 h. The pre-fire forest was a mature, mixed-conifer forest composed primarily of ponderosa pine, Douglas fir, white fir, limber pine, and aspen of varying size classes. Before the fire, Rocky Mountain maple, spruce, and piñon were also present. The successionally diverse mixed-conifer forest was replaced with early successional species, primarily quaking aspens and young fir trees. Remnant, dead snags and a few remaining mature ponderosa pine and white fir trees survived the fire.
2.2. Bird Captures
The fall migratory bird-banding station was started with the goal of learning about the species diversity and quantity of birds that use this high-elevation location during their migration period—a critical part of a bird’s annual life cycle. These data generate useful information about the status and trends [35] of birds that migrate through Bandelier National Monument. All work performed with live wild birds followed published guidelines for the use of wild birds in research [36]. The bird-banding station consisted of 20 standard, 30 mm mesh nylon mist nets that measure 12 m long by 2.5 m high. During the study, net locations were not changed. All nets were operated from August through October. Banding operations at the station were performed twice per week from the beginning of August to mid-October in 2005, 2006, and 2008 through 2021. The number of banding sessions per year varied due to weather or lack of personnel and ranged from 16 to 32 sessions with an average of 22 sessions per year. Each banding day, nets were open at sunrise to 12 pm at the latest. Net hours were recorded within 10 min of open and close times and averaged 2081 net hours per year. The birds per 100 net hour value was used to standardize the uneven effort over the course of the study.
We identified, aged, sexed—based on Pyle [37], weighed, measured, fat-scored, and checked all birds for signs of molt.
2.3. Vegetation Data
We used data from a separate vegetation monitoring project and, therefore, had limited vegetation data available for the nearby area for pre- and post-fire years. We selected data from 16 plots located within 1.6 km of the fall bird-banding site. The pre-fire data (from 2008 and 2009) and the post-fire data (from 2019 and 2020) included live tree density by diameter at breast height (DBH) category and shrub density. Data were also collected on tree and shrub species. The DBH categories were sapling = 2.5–15.0 cm, small trees = 15.1–30.0 cm, medium trees = 30.1–45.0, and large trees > 45.1 cm.
Additionally, we used normalized difference vegetation index (NDVI) which is a measure of the density of green vegetation, to assess changes in vegetation to characterize habitat change. We downloaded September NDVI values for the centroid coordinates of the banding site during the study period at 250 m resolution (Landsat Spectral Indices products courtesy of the United States Geological Survey Earth Resources Observation and Science Center). We chose September NDVI values because we felt it best reflected the greenness during much of the banding session each year.
2.4. Statistical Analyses
To standardize variable levels of effort for each year, we calculated capture rates for each species as birds per 100 net hours [38]. A net hour is a unit of measure used to calculate the amount of time that nets are open. For example, one net that is open for 1 h is equal to one net hour. For the full 16-year analysis, we excluded same-year recaptures because we wanted to compare avian species among the years to assess community changes pre- and post-fire. Additionally, we grouped subspecies into species categories to standardize the data for further analyses. For example, gray-headed juncos and Oregon juncos were considered dark-eyed juncos for this dataset. We assigned species to three categories based on Birds of the World species accounts [39], local breeding occurrence data, and expert knowledge: (1) resident birds that use the habitat in the surrounding area year-round, (2) migratory birds that represent transient individuals that use the habitat as a stopover site, and (3) breeders that represent long-distance migrants suspected or known to breed at and around the study site (Table S2). To evaluate how resident, breeding, or migratory species may differ in their response, we performed subset analysis with the three assigned categories.
We evaluated taxonomic change in the migratory and resident components of the avian community from 2005 to 2021 (omitting 2007 due to a mismatch in effort) over our study period, using species richness and relative abundance based on birds per net hour. The year of the fire (2011) was not considered in pre- and post-fire comparison tests. Species richness is calculated as the number of species in a sample (year, in our case). We included Palmer Drought Severity Index (PDSI) values as a measure of the annual monsoon season (July–September) [40]. Although the average annual value of PDSI was positively correlated to the annual monsoon season values (r = 0.585, p = 0.017, n = 16), we decided that the annual monsoon values more directly dictate resources pulses that would affect habitat usage during fall migration (mid-August–mid-October) and eliminates noise from combining PDSI variation during the previous spring and winter months. We compared vegetation variables, species richness, PDSI, and NDVI pre- and post-fire medians and tested for differences between the groups using Wilcoxon signed-rank tests due to nonparametric data distributions. We used nonparametric median-based, linear models (α = 0.05) as a robust way to evaluate relationships between diversity metrics and climatic or habitat variables with our small dataset where we could not confidently assume normally distributed response variables.
We also used nonmetric multidimensional scaling (NMDS) with a Jaccard dissimilarity matrix to assess changes in the presence and absence of different avian species within the community pre- (2005–2010) and post-fire (2012–2021); we did not use relative abundance for this analysis. We tested for differences in the pre- and post-fire avian community groups using analysis of similarities (ANOSIM) [41,42] and investigated the species driving the distribution patterns pre- and post-fire in the NMDS using the function envfit in the package vegan v.2.6.8 [43]. Additionally, we used an Indicator Species Analysis using the function multipatt in the package indicspecies [44] to assess the differential occurrence of species in post-fire years. Before this multivariate analysis, we removed rare species from the avian community dataset; we defined rare species as those that occurred two times or fewer over the course of the 16-year sampling period. Due to the nature of migration and the chance for vagrants in our dataset we decided that two times over the course of the 16-year sampling period would remove most vagrants. We determined the appropriate number of dimensions by plotting final stress versus the number of dimensions and chose the number of axes beyond which reductions in stress were small [45].
To evaluate functional change in the avian community over our study period, we compiled trait data related to foraging and dispersal from AVONET [46]. We used bill length, bill width, bill depth, tarsus length, Kipp’s distance, hand–wing index, trophic niche, and lifestyle mode (aerial, perching, and generalist) as functional traits. We additionally included migration category (migrant, breeder, and resident) to calculate functional richness and identity for the full community. As with our NMDS, we removed rare species from the avian community dataset. For each study year and migration category, we generated a presence–absence matrix from which we constructed a matrix of functional Gower distances, which can handle both continuous and categorical data. We weighted all traits equally. Then, using principal coordinate analysis (PCoA), we constructed multidimensional functional spaces and assessed their quality using the quality.fspaces function from the R package mFD v.1.0.7 [47]. We selected the three PCoA dimensions with the mean absolute lowest deviation (MAD) and used those dimensions for calculation of functional richness, which measures the volume of the convex hulls of species trait space not weighted by abundance using the R package mFD v.1.0.7 [47]. Additionally, because culmen length correlated with the PCoA axis showing the smallest MAD, we plotted the average community culmen length over time (Figures S1 and S2).
To remove the effect of species richness on year-to-year community comparisons, we calculated standard effect sizes (SES) for functional richness [48]. We first constructed null community models (n = 1000) by randomizing traits among species but preserving trait covariance as in Ortega-Martínez et al. [49] and used these expected community values to calculate a standard effect size. We considered SES values statistically significant (α threshold < 0.05) if they fell outside the range of −1.96 to 1.96 standard deviations [50]. SES values that fell below −1.96 indicate under-dispersion and are thought to represent ecological filtering whereas values over 1.96 indicate over-dispersion and are thought to be connected to limiting similarity; values that fell within the range of −1.96 to 1.96 are assumed to represent stochastic processes [49].
We calculated the phylogenetic distance (PD) using the R package picante v.1.8.2 [51]. We downloaded 100 trees from birdtree.org using the Hackett backbone [52]. We pruned the phylogenetic trees to match our community data. We accounted for branch length and topological uncertainty by calculating PD across the entire posterior sample of 100 bird trees. For each topology and randomized presence–absence community matrix, we calculated the phylogenetic distance standard effect size (PD SES) by shuffling taxa tip labels (n = 1000) to generate null models. We visualized PD SES in ggplot2 v.3.5.1. We used the R statistical software v. 4.2.3 for all data analyses [53].
We tested for differences between pre- (2005–2010) and post- (2012–2021) fire years’ taxonomic, functional, and phylogenetic richness using a non-parametric Wilcoxon signed-rank test in R. To identify changes in species richness, SESs of functional richness, and SES PD associated with changes in PDSI and over time, we ran median-based linear models using the R package mblm v.0.12.1 [54]. The models are a robust form of regression and are less sensitive to data irregularities and outliers [54]. They implement a nonparametric Wilcoxon signed-rank test to evaluate significant relationships [54]. We used Spearman’s rank correlation to test for correlations between SES functional richness values.
3. Results
We banded 13,731 birds representing 77 species over the course of 16 years. Over the 16 sampling years, PDSI during the monsoon months significantly decreased, indicating increased drought over time during monsoon months (MAD = 0.41, V = 2666, p = 0.016), but showed no difference pre- and post-fire when tested as binary time bins (W = 32, p = 0.426; Figure S3a). NDVI was significantly different pre- and post-fire when tested as binary time bins (W = 50, p = 0.001; Figure S3b) and significantly dropped in the years post-wildfire (W = 40, p = 0.002; Figure S4). The results showed decreases in most categories of live vegetation post-fire except for young live trees (2.5–15 cm DBH; p = 0.021; Figure S5). We saw more young live trees in the 2.5–15 cm DBH category post-fire, and the majority were quaking aspen (Figure S6), although their distribution was variable on the landscape.
Although our dataset contained only 5 years pre-fire, our results showed some compelling patterns associated with wildfire and drought. Species richness showed a significant increase post-fire when tested as categorical time bins (W = 6, p = 0.023; Figure S3c). Species richness significantly increased over the time period for all species (MAD = 0.42, V = 134, p = 0.0007; Figure 1A), even when broken into the subset categories of migrants (MAD = 0.20, V = 136, p = 0.0005), residents (MAD = 0.11, V = 119, p = 0.0009), and breeders (MAD = 0.23, V = 104, p = 0.002). However, species richness showed a significant negative relationship with PDSI but only for breeding birds (MAD = 0.74, V = 14, p = 0.030). Birds per 100 net hours increased immediately after the fire (Figure 1B) and significantly increased over time for breeders (MAD = 0.054, V = 119, p = 0.009) and migrants (MAD = 0.053, V = 111, p = 0.028). We did not detect a significant relationship (p > 0.05) between PDSI and changes in birds per 100 net hours over the length of the study period (Figure 1B).
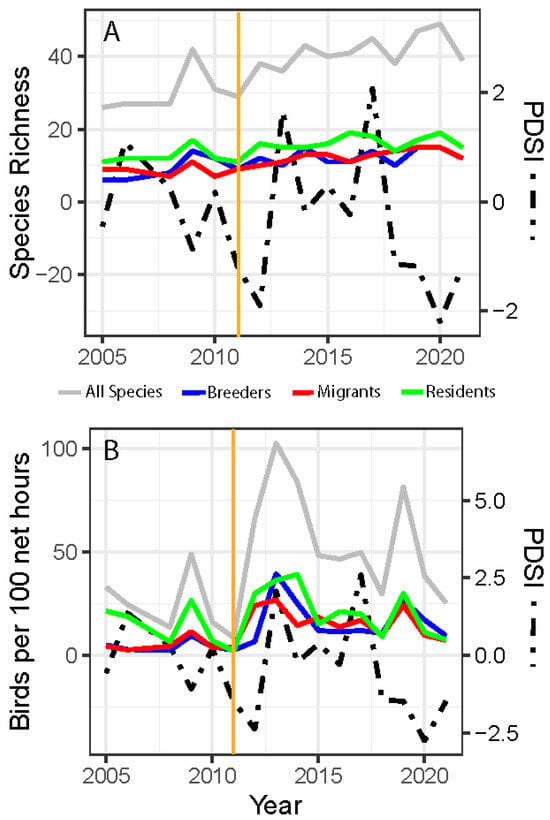
Figure 1.
Species richness (A) and birds per 100 net hours (B) displayed on the left y-axis and average monsoon (June–September) PDSI values on the right y-axis from 2005, 2006, and 2008 to 2021; all species are shown with a gray line, residents are shown in green, migrants are shown in red, and breeders are shown in blue; orange vertical lines indicate the fire event.
NMDS results were generated from two convergent solutions and two dimensions, with stress = 0.129. We assessed differences in community composition and structure for pre- and post-fire years using the NMDS results (R = 0.67, p = 0.001). To investigate species driving the distribution patterns, we displayed vectors of species (p < 0.05) that showed strong influences on the community structure, all of which were associated with post-fire years (Figure 2a); these included Brewer’s sparrow, Cassin’s vireo, Cordilleran flycatcher, dark-eyed junco, downy woodpecker, Grace’s warbler, green-tailed towhee, lazuli bunting, mountain bluebird, olive-sided flycatcher, plumbeous vireo, red-breasted nuthatch, violet-green swallow, Virginia’s warbler, warbling vireo, white-breasted nuthatch, white-crowned sparrow, and western wood-pewee. The length of the vectors corresponds to the strength of the influence, with longer vectors having stronger influences and shorter vectors being less significant. To further assess specific species associated with post-fire conditions, we identified the differential occurrence of species in post-fire years using a species indicator analysis. The post-fire indicator species for our site are displayed with boxes around the four-letter species code in Figure 2a and included Virginia’s warbler, warbling vireo, plumbeous vireo, lazuli bunting, and Cassin’s vireo. Among bird species driving the difference between pre- and post-fire communities, the relative proportions of migrants and breeders showed a general increase in the years following the fire (Figure 2b) as did the relative proportions of insectivores and omnivores within the community (Figure 2c).
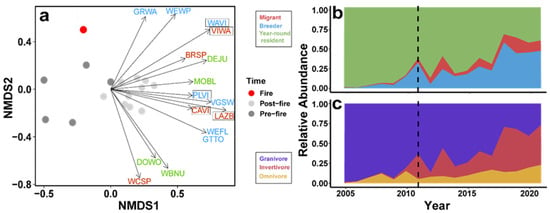
Figure 2.
Avian community composition over time. (a) Nonmetric multidimensional scaling (NMDS) of the avian community pre- (2005, 2006, and 2008–2010) and post-fire (2012–2021; R = 0.69, p = 0.003). Dark gray points represent pre-fire years, the red point indicates the year of fire, and light gray points indicate post-fire years. Vector arrows show significant species colored by migratory category and the direction of influence driving the distribution patterns pre- and post-fire in the NMDS (p < 0.05); species with boxes around them are indicator species for post-fire conditions (p < 0.05). BRSP = Brewer’s sparrow, CAVI = Cassin’s vireo, WEFL = western flycatcher, DEJU = dark-eyed junco, DOWO = downy woodpecker, GRWA = Grace’s warbler, GTTO = green-tailed towhee, LAZB = lazuli bunting, MOBL = mountain bluebird, PLVI = plumbeous vireo, VGSW = violet-green swallow, VIWA = Virginia’s warbler, WAVI = warbling vireo, WBNU = white-breasted nuthatch, WCSP = white-crowned sparrow, WEWP = western wood-pewee. (b) Area plot showing relative abundance of migratory groups of significant NMDS species over time. (c) Area plot showing relative abundance of trophic groups of significant NMDS species over time. Relative widths of color bands indicate shifts in proportions of migratory and foraging modes.
Functional diversity as measured by functional richness showed inconsistent trends over time and with climate. Three traits were significantly correlated with the PCoA axes showing the lowest MAD: posterior and anterior bill length and feeding guild (Figure S2). Only a single SES functional richness value significantly differed from random expectations and all values showed no temporal trends for the overall community or any subset (Figure S7a,c,e,f). Only resident species in 2006 showed significantly lower than expected values of SES functional richness. All other annual standard effect size estimates fell within null expectations. The SES functional richness values showed significant associations with time. The overall community SES functional richness declined over time (MAD = 0.207, V = 2167, p < 0.001; Figure S7a), running counter to the trend in species richness and SES functional richness of community components. SES functional richness significantly increased over time for the migratory, breeding, and resident subsets of the community (migrant: MAD = 0.125, V = 4924, p < 0.001; breeder: MAD = 0.156, V = 4789, p = 0.002; resident: MAD = 0.185, V = 4436, p = 0.035; Figure S7b–d). SES functional richness of the overall community and breeding component subset showed no differences between pre- and post-fire years (overall community: W = 29, p = 0.665; breeders: W = 39, p = 0.093). SES of functional richness of the migratory and resident components showed significant increases post-fire (migratory: W = 48, p = 0.006; resident: W = 43, p = 0.031). The SES functional richness of the overall community was not associated with mean monsoon PDSI (MAD = 0.845, V = 3183, p = 0.4305; Figure S7e). However, the functional richness of resident and migratory species showed significant negative relationships with decreasing drought (migrant: MAD = 0.501, V = 2658, p = 0.016, resident: MAD = 0.742, V = 2594, p = 0.010; Figure S7f,h). The average culmen length of the overall bird community decreased in the years following the fire, shifting toward a community with shorter bills (Figure 3a), but the difference between pre- and post-fire years was only marginally significant (W = 4, p = 0.052). Only 3 years showed significant SES PD values. The SES PD in 2009 was higher than null expectations, whereas 2008 and 2019 were significantly lower than null expectations (Figure 3b). The trend in SES PD fluctuated over time but showed a significant decreasing trend (MAD = 0.3546, V = 2169, p < 0.001). SES PD also showed a weaker significant negative association with mean monsoon PDSI (MAD = 1.07, V = 2093, p = 0.0276). The SES PD showed a strong positive correlation with average culmen length in the community (MAD = 2.43, V = 5540, p < 0.001; Figure 3).
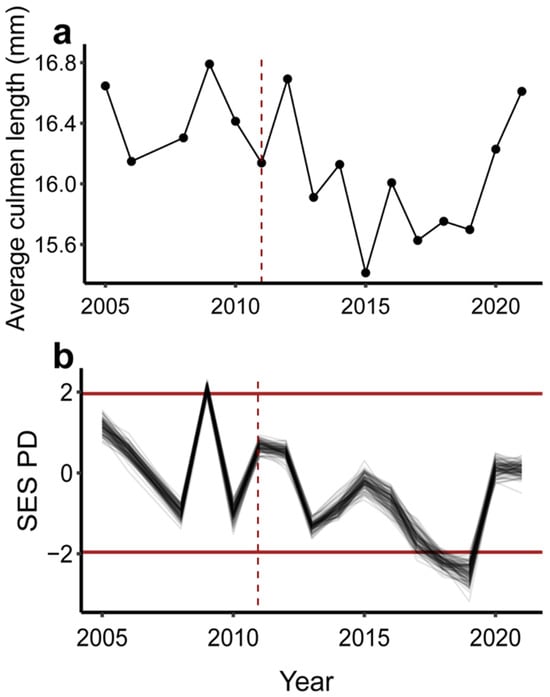
Figure 3.
Functional change, as measured by average community culmen length, and phylogenetic distance over time. (a) Average community culmen length over time. Points represent the mean culmen length of the overall community each year. The black line connects points to emphasize chronology. The red dashed vertical line indicates year of fire. (b) Standard effect sizes for phylogenetic distance (SES PD) over time. Black lines connect yearly SES PD values. Each line represents yearly SES PD values for 1 of 100 different phylogenetic tree topologies. Red horizontal dark lines indicate significance thresholds for SES PD values. Red dashed vertical line indicates year of fire.
4. Discussion
The duration of extreme droughts and instances and intensity of wildfire are expected to increase. Birds will need to adapt to keep up with changing resource bases within their breeding habitats and along their migratory pathways [55]. Our study illustrates the associations along multiple axes of biological variation of both wildfire and drought on an avian community during fall migration.
Wildfires can act to reset communities to varying degrees, depending on their severity and/or extent. Initially, wildfires can deplete long-standing resources for higher-order consumers. But over time, competition and an influx of carbon and nitrogen can drive rapid succession in the community of primary producers [56,57,58]. Unlike the pulse effects wildfire can have, drought operates over longer timescales. The dichotomy between short- and long-duration ecosystem impacts has been framed as pulse–press dynamics [59,60].
Pulse-like, nonlinear changes in avian abundance and species diversity indices following wildfire have been documented for breeding communities [61]. Our results show similar short-duration shifts in a migratory stopover community post-fire. We saw the overall species richness (for all categories) increased over time (Figure 1A), and an increase in birds caught per net hour for migrants and breeders at the site following the 2011 wildfire (Figure 1B), presumably due to the changes in habitat created after the fire. This reorganization is reflected in species-level shifts in relative abundance and the overall community structure pre- and post-fire (Figure 1 and Figure 2) and could be driven in part by mismatches among the ecological requirements of species, post-fire resource availability, and habitat conditions, forcing emigration or discouraging use of the area as a stopover site. The post-fire vegetation shift to dense but patchy areas of quaking aspen cover was reflected by the drop in September NDVI values at the banding site after 2011. Unlike mature conifers, young deciduous trees like aspens have lower canopy structure, chlorophyll density, and overall leaf area, each of which can lead to lower NDVI values [62,63] (Figure S4). These same changes in the habitat structure and net primary productivity of the post-fire successional plant community (Figures S4 and S5) could have led to functional changes in the avian community by attracting species that would normally have bypassed the habitat [64]. For example, the increased cover provided by patchy areas of young, dense, early successional quaking aspens (Figure S6) could attract migratory avian insectivores by providing increased high-density cover from predators and the elements and by promoting insect abundance [65,66]. We saw this pattern emerge in our dataset where insectivorous migratory species were documented more often in post-fire years (Figure 2b,c). The species identified in our indicator analysis for post-fire conditions also suggested this trend because all were migratory insectivores (Figure 2a).
Recent studies have highlighted how the foraging behavior of birds influences their post-fire occurrence. Birds that forage on open ground were more abundant in the years soon after fire [67], whereas birds that depend on well-developed, midstory structure were more abundant in later successional vegetation [68]. At our study site, the chipping sparrow (Spizella passerina) —a ground forager—showed one of the highest relative abundances immediately after the wildfire during the same year, and Wilson’s warbler showed one of the highest relative abundances in later years post-fire (Table S1). Irruptive patterns have been recorded in Spizella sparrows [69], but we are skeptical that irruptive migration drove the dominance of chipping sparrows in 2011 because their numbers were still relatively low compared with other years pre- and post-fire.
Multiple community properties showed associations with both fire and drought. Among species driving pre- and post-fire community separation, the relative abundance of migratory insectivores increased in the years immediately following the fire (Figure 2). This increase coincided with a shift toward a more related, less phylogenetically dispersed community (Figure 3b), which coincided with a shift in apparent community function toward shorter-billed birds (Figure 3a). The initial post-fire shift could be the result of high temporal turnover while species less likely to forage in mixed-conifer forests investigate newly structured and homogenized habitat during migration. Functional richness showed no clear association with wildfire. While functional richness increased significantly over time in the migratory, breeding, and resident components of the community, only the migratory and resident components had significantly higher diversity during post-fire years; this could reflect post-fire vegetation changes attracting migratory insectivores. However, we urge caution interpreting the functional richness metrics since nearly all values fell within significance thresholds, indicating that most years were in line with expectations given stochastic community assembly. Furthermore, overall functional richness showed an opposing pattern relative to the community components, illustrating how Simpson’s paradox and our small samples sizes may hinder the interpretation of component trends of functional richness [70]. The breeding community subset may also be absorbing statistical power from our migrant group, because all birds from this category are long-distance migrants that may be utilizing the local habitat for longer periods of time. More compelling, however, was the overall community relationship between mean culmen length and phylogenetic diversity suggesting a decrease in functional diversity—with respect to this foraging trait—of the community post-wildfire (Figure S8). It is possible that other measures of functional diversity might capture signatures of wildfire, but we restricted our analyses to functional richness and average culmen length because neither are abundance weighted, and mist-netting data are thought to bias abundance estimates within bird communities [38,71].
The more pervasive and subtle effects of drought have downstream effects on all ecosystem services and could be responsible for slow habitat degradation along migratory routes. Many migratory birds track environmental conditions on their breeding and non-breeding grounds [72,73], but much less is known about how migratory birds select stopover habitat [74,75,76,77]. Drought is known to negatively impact birds on their breeding grounds [7,78]. It stands to reason that drought-degraded habitat could cost migratory birds time and energy, as well as influence migratory bird abundance [8]. Running counter to our expectations, we found that in years when the monsoon produced wetter conditions, functional richness of resident species and migrant species, overall community phylogenetic diversity, and breeding bird species richness decreased. The precipitation-driven habitat shifts in relation to a wetter monsoon could favor certain groups of birds, leading to declines in species and functional richness, as well as phylogenetic distance at a local scale. For example, resource pulses following the monsoon season could alter local and long-distance migration phenology for all species by directly influencing the timing and availability of food sources. Alternatively, because wetter monsoon years were mostly pre-fire (PDSI monsoon season median = 0.050), the relationship may be driven by post-fire condition effects on increased species diversity which also happened to be the drier years (PDSI monsoon season median = −0.715) in our relatively small dataset (Figure S3a). Ultimately parsing the effects of long-term drought and wildfire was not possible with our data from a single site and small sample sizes.
Overall, we found some evidence that the wildfire-changed habitat at our study site attracted and supported new species combinations during migration and shifted community composition. However, the taxonomic, functional, and phylogenetic shifts that follow fire could be variable and short-lived. Birds’ capability to select habitats during migration will hinge on the availability of adequate stopover sites, highlighting the growing importance of preserving productive microhabitats in a drought-stricken region. Moving forward, researchers should expand on our localized analyses and leverage open-access banding data and occurrence records to model the associations between taxonomic, functional, and phylogenetic diversity and pulse–press disturbances such as wildfire and drought at a continental scale. Larger studies at regional or continental scales could leverage the wealth of open-access data to tease apart the effects of wildfire and drought with more complex and powerful modeling approaches.
5. Conclusions
While instances of extreme drought and wildfire occurrences and severity increase, migratory bird communities could shift to alternate areas and migratory routes that have the resources available to support their needs. Our results suggest that migratory birds can rapidly respond to changing resources along their migratory pathways to find suitable environmental conditions as they move across the landscape. However, facilitating conservation through site identification and protection, such as using wildland fire forest management and the conservation and creation of areas that provide stopover refuge, is increasingly essential in the protection of migratory birds during this crucial time in their annual life cycle—when refuge could mean the difference between a successful migration and death.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14060597/s1, Figure S1: Quality of first six functional spaces. Plot (created in mFD R package) was used to determine the highest quality PCoA axis with the lowest raw and mean absolute deviance for the full bird community; Figure S2: Relationships among traits and PCoA for two highest quality functional spaces (PCoA 4-5). Blue-colored panels indicate significant relationships from linear models for continuous traits and Kruskal–Wallis tests for categorical traits; Figure S3: Boxplot of pre- and post-fire values for climate, habitat, and species richness over study period (a) drought as measured by Palmer Drought Severity Index (PDSI), (b) habitat greenness as measured by Normalized Difference Vegetation Index, (c) species richness for the site. Boxes and horizontal black lines indicate interquartile range and median, respectively. Vertical black lines indicate 95% quantiles. Points show raw values horizontally jittered for clarity. Statistics in plot indicate results of Wilcoxon signed-rank tests between pre-and post-fire values. Figure S4: Changes in NDVI at banding site over study period. Points show median September values at 250 m resolution of NDVI for the centroid of the banding site. Black line connects points to emphasize temporal trend. Dashed red line indicates 2011 fire. Figure S5: Box and whisker plots of medians and range for shrub and live-tree densities pre- and post-fire of varying DBH sizes; differences among the groups were tested using Mann–Whitney tests due to nonparametric data distributions (α = 0.05); Figure S6: Pre- and post-fire medians and ranges for small live-tree species (2.5–15 cm DBH) per acre; Figure S7: Standard effect size (SES) of functional richness over time (a–d) and as a function of drought (e–h) for the entire community (a,e), migrants (b,f), breeding migrants (c,g), and resident species (d,h). Red dashed lines indicate significance thresholds for SES values. The circles indicate year of fire. Grey dashed lines in panels a, b, and h show a significant relationship between resident functional richness and year or drought from median-based linear models; Figure S8: The relationship between standard effect size (SES) of phylogenetic distance (PD) and mean culmen length (mm) for the entire community. Red point indicates values from year of fire. Grey line shows significant relationship from median-based linear models; Table S1: Bird Banding Data; Table S2: All species migration status.
Author Contributions
Conceptualization, J.E.S. and C.R.G.; formal analysis, J.E.S. and C.R.G.; data curation, S.E.M., K.A.T., S.M.F., C.D.H., B.E.T., and L.L.T.; writing—original draft preparation, J.E.S., C.R.G., K.A.T., S.E.M., C.D.H., and B.E.T.; writing—review and editing, J.E.S., C.R.G., C.D.H., S.E.M., K.A.T., B.E.T., L.L.T., and S.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This publication and contribution to fieldwork was funded by Triad National Security, LLC, manager of the LANL for the United States Department of Energy’s National Nuclear Security Administration under Contract No. 89233218CNA000001. The fieldwork at BNM and compilation of these data was funded by the United States National Park Service.
Institutional Review Board Statement
This study was conducted in accordance with the Institutional Animal Care and Use Committee at Los Alamos National Laboratory (protocol number 20-60 approved 7 July 2021 and prior protocols).
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available climate datasets were analyzed in this study. These data can be found here: https://www.ncdc.noaa.gov/cag/ (accessed on 11 April 2025). Publicly available avian trait data related to foraging and dispersal from the AVONET database were analyzed in this study and can be found here: https://opentraits.org/datasets/avonet.html (accessed on 10 April 2025). The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank all of the interns, technicians, volunteers, and staff, past and present, who have helped in this long-term study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOSIM | Analysis of Similarities |
| DBH | diameter at breast height |
| MAD | mean absolute deviation |
| NMDS | nonmetric multidimensional scaling |
| PCoA | Principal Coordinate Analysis |
| PD | phylogenetic diversity |
| PDSI | Palmer Drought Severity Index |
| SES | standard effect sizes |
References
- Chen, I.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Crick, H.; Learmonth, J.; Maclean, I.; Thomas, C.; Bairlein, F.; Forchhammer, M.; Francis, C.; Gill, J.; Godley, B.; et al. Travelling through a warming world: Climate change and migratory species. Endanger. Species Res. 2009, 7, 87–99. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Farella, J.; Roos, C.I.; Liebmann, M.J.; Falk, D.A.; Allen, C.D. Multiscale perspectives of fire, climate and humans in western North America and the Jemez Mountains, USA. Phil. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150168. [Google Scholar] [CrossRef]
- Crockett, J.L.; Westerling, A.L. Greater temperature and precipitation extremes intensify western U.S. droughts, wildfire severity, and Sierra Nevada tree mortality. J. Clim. 2018, 31, 341–354. [Google Scholar] [CrossRef]
- Wasserman, T.N.; Mueller, S.E. Climate influences on future fire severity: A synthesis of climate-fire interactions and impacts on fire regimes, high-severity fire, and forests in the western United States. Fire Ecol. 2023, 19, 1–22. [Google Scholar] [CrossRef]
- Jones, G.M.; Shirk, A.J.; Yang, Z.; Davis, R.J.; Ganey, J.L.; Gutiérrez, R.J.; Healey, S.; Hedwall, S.; Hoagland, S.; Maes, R.; et al. Spatial and temporal dynamics of Mexican spotted owl habitat in the southwestern US. Landsc. Ecol. 2023, 38, 7–22. [Google Scholar]
- Albright, T.P.; Pidgeon, A.M.; Rittenhouse, C.D.; Clayton, M.K.; Flather, C.H.; Culbert, P.D.; Wardlow, B.D.; Radeloff, V.C. Effects of drought on avian community structure. Glob. Chang. Biol. 2010, 16, 2158–2170. [Google Scholar] [CrossRef]
- Albright, T.P.; Pidgeon, A.M.; Rittenhouse, C.D.; Clayton, M.K.; Wardlow, B.D.; Flather, C.H.; Culbert, P.D.; Radeloff, V.C. Combined effects of heat waves and droughts on avian communities across the conterminous United States. Ecosphere 2010, 1, 1–22. [Google Scholar] [CrossRef]
- Saracco, J.F.; Fettig, S.M.; San Miguel, G.L.; Mehlman, D.W.; Thompson, B.E.; Albert, S.K. Avian demographic responses to drought and fire: A community-level perspective. Ecol. Appl. 2018, 28, 1773–1781. [Google Scholar] [CrossRef]
- Selwood, K.E.; McGeoch, M.A.; Clarke, R.H.; Mac Nally, R. High-productivity vegetation is important for lessening bird declines during prolonged drought. J. Appl. Ecol. 2018, 55, 641–650. [Google Scholar] [CrossRef]
- Smucker, K.M.; Hutto, R.L.; Steele, B.M. Changes in bird abundance after wildfire: Importance of fire severity and time since fire. Ecol. Appl. 2005, 15, 1535–1549. [Google Scholar] [CrossRef]
- Steel, Z.L.; Fogg, A.M.; Burnett, R.; Roberts, L.J.; Safford, H.D. When bigger isn’t better—Implications of large high-severity wildfire patches for avian diversity and community composition. Divers. Distrib. 2021, 28, 439–453. [Google Scholar] [CrossRef]
- Earnst, S.L.; Newsome, H.L.; LaFramboise, W.L.; LaFramboise, N. Avian response to wildfire in interior Columbian basin shrubsteppe. Condor Ornithol. Appl. 2009, 111, 370–376. [Google Scholar]
- Taillie, P.J.; Burnett, R.D.; Roberts, L.J.; Campos, B.R.; Peterson, M.N.; Moorman, C.E. Interacting and non-linear avian responses to mixed-severity wildfire and time since fire. Ecosphere 2018, 9, e02291. [Google Scholar] [CrossRef]
- Burbidge, A.A.; Fuller, P.J. Gibson Desert birds: Responses to drought and plenty. Emu Austral Ornithol. 2007, 107, 126–134. [Google Scholar] [CrossRef]
- Stanek, J.E.; Thompson, B.E.; Milligan, S.E.; Tranquillo, K.A.; Fettig, S.M.; Hathcock, C.D. Does age, residency, or feeding guild coupled with a drought index predict avian health during fall migration? Animals 2022, 12, 454. [Google Scholar] [CrossRef]
- Stevens, H.C.; Watson, D.M. Interacting impacts of drought and fire on bird populations—Insights from a long-term study in the Warrumbungles. Aust. Zool. 2022, 42, 608–630. [Google Scholar] [CrossRef]
- Crockett, J.L.; Hurteau, M.D. Post-fire early successional vegetation buffers surface microclimate and increases survival of planted conifer seedlings in the southwestern United States. Can. J. For. Res. 2021, 52, 416–425. [Google Scholar] [CrossRef]
- Zlonis, E.J.; Walton, N.G.; Sturtevant, B.R.; Wolter, P.T.; Niemi, G.J. Burn severity and heterogeneity mediate avian response to wildfire in a hemiboreal forest. For. Ecol. Manage. 2019, 439, 70–80. [Google Scholar] [CrossRef]
- McWilliams, S.R.; Guglielmo, C.; Pierce, B.; Klaassen, M. Flying, fasting, and feeding in birds during migration: A nutritional and physiological ecology perspective. J. Avian Biol. 2004, 35, 377–393. [Google Scholar] [CrossRef]
- Klaassen, R.H.G.; Hake, M.; Strandberg, R.; Koks, B.J.; Trierweiler, C.; Exo, K.; Bairlein, F.; Alerstam, T. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 2014, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sergio, F.; Tavecchia, G.; Tanferna, A.; Blas, J.; Blanco, G.; Hiraldo, F. When and where mortality occurs throughout the annual cycle changes with age in a migratory bird: Individual vs. population implications. Sci. Rep. 2019, 9, 17352. [Google Scholar] [CrossRef] [PubMed]
- Gordo, O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 2007, 35, 37–58. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; van de Pol, M.; Bairlein, F. Autumn bird migration phenology: A potpourri of wind, precipitation and temperature effects. Glob. Change Biol. 2019, 25, 4064–4080. [Google Scholar] [CrossRef] [PubMed]
- Kendeigh, S.C.; Dol’Nik, V.R.; Gavrilov, V.M.; Pinowski, J. Avian energetics. In Granivorous Birds in Ecosystems: Their Evolution, Populations, Energetics, Adaptations, Impacts and Control (International Biological Programme 12), 1st ed.; Pinowski, J., Kendeigh, S.C., Eds.; Cambridge University Press: New York, NY, USA, 1977; pp. 127–203. [Google Scholar]
- Fretwell, S.D. Evolution of migration in relation to factors regulating bird numbers. In Migrant Birds in the Neotropics: Ecology, Behavior, Distribution, and Conservation, 1st ed.; Keast, A., Morton, E.S., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1980; pp. 517–527. [Google Scholar]
- Levey, D.J.; Stiles, F.G. Evolutionary precursors of long-distance migration: Resource availability and movement patterns in Neotropical landbirds. Am. Nat. 1992, 140, 447–476. [Google Scholar] [CrossRef]
- Wiener, P.; Tuljapurkar, S. Migration in variable environments: Exploring life-history evolution using structured population models. J. Theor. Biol. 1994, 166, 75–90. [Google Scholar] [CrossRef]
- Kittelberger, K.D.; Miller, M.K.; Şekercioğlu, C.H. Fall bird migration in western North America during a period of heightened wildfire activity. Avian Conserv. Ecol. 2022, 17, 43. [Google Scholar] [CrossRef]
- Boucek, R.E.; Rehage, J.S. Climate extremes drive changes in functional community structure. Glob. Change Biol. 2014, 20, 1821–1831. [Google Scholar] [CrossRef]
- Barnagaud, J.Y.; Gaüzère, P.; Zuckerberg, B.; Princé, K.; Svenning, J.C. Temporal changes in bird functional diversity across the United States. Oecologia 2017, 185, 737–748. [Google Scholar] [CrossRef]
- Docherty, T.D.S.; Hethcoat, M.G.; MacTavish, L.M.; MacTavish, D.; Dell, S.; Stephens, P.A.; Willis, S.G. Burning savanna for avian species richness and functional diversity. Ecol. Appl. 2020, 30, e02091. [Google Scholar] [CrossRef]
- Knaggs, M.; Haché, S.; Nielsen, S.E.; Pankratz, R.F.; Bayne, E. Avian response to wildfire severity in a northern boreal region. Forests 2020, 11, 1330. [Google Scholar] [CrossRef]
- Ocampo-Zuleta, K.; Parrado-Rosselli, Á. Functional diversity in an Andean subpáramo affected by wildfire in Colombia. Plant Divers. 2023, 45, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hussell, D.J.; Ralph, C.J. Recommended methods for monitoring change in landbird populations by counting and capturing migrants. North Am. Bird Bander 2005, 30, 2. [Google Scholar]
- Fair, J.M.; Paul, E.; Jones, J.; Bies, L. (Eds.) Guidelines to the Use of Wild Birds in Research, 4th ed.; The Ornithological Council: Washington, DC, USA, 2023; pp. 42–54. [Google Scholar]
- Pyle, P. Identification Guide to North American Passerines; Slate Creek Press: Bolinas, CA, USA, 1987; p. 273. Volume 1. [Google Scholar]
- Karr, J.R. Surveying birds with mist nets. Stud. Avian Biol. 1981, 6, 62–67. [Google Scholar]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. (Eds.) Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar]
- NOAA National Centers for Environmental Information, Climate at a Glance: National Time Series. National Oceanic and Atmospheric Administration, Published March 2025. Available online: https://www.ncei.noaa.gov/access/monitoring/climate-at-a-glance/national/time-series (accessed on 20 March 2025).
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 41, 213–226. [Google Scholar] [CrossRef]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. vegan: Community Ecology Package. R Package Version 2.6-8. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 March 2025).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- McCune, B.P.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Tobias, J.A.; Sheard, C.; Pigot, A.L.; Devenish, A.J.M.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, ecological and geographical data for all birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef]
- Magneville, C.; Loiseau, N.; Albouy, C.; Casajus, N.; Claverie, T.; Escalas, A.; Leprieur, F.; Maire, E.; Mouillot, D.; Villéger, S. mFD: An R package to compute and illustrate the multiple facets of functional diversity. Ecography 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Swenson, N.G. Functional and Phylogenetic Ecology in R; Springer: New York, NY, USA, 2014; Volume 639. [Google Scholar]
- Ortega-Martínez, I.J.; Moreno, C.E.; Rios-Díaz, C.L.; Arellano, L.; Rosas, F.; Castellanos, I. Assembly mechanisms of dung beetles in temperate forests and grazing pastures. Sci. Rep. 2020, 10, 391. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 March 2025).
- Komsta, L. Package ‘mblm’ Median-Based Linear Models. R Package Version 0.12.1. 2019. Available online: https://cran.r-project.org/web/packages/mblm/mblm.pdf (accessed on 20 March 2025).
- Robertson, E.P.; La Sorte, F.A.; Mays, J.D.; Taillie, P.J.; Robinson, O.J.; Ansley, R.J.; O’connell, T.J.; Davis, C.A.; Loss, S.R. Decoupling of bird migration from the changing phenology of spring green-up. Proc. Natl. Acad. Sci. USA 2024, 121, e2308433121. [Google Scholar] [CrossRef]
- Wan, S.; Hui, D.; Luo, Y. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Smith, E.A.; O’Loughlin, D.; Buck, J.R.; St Clair, S.B. The influences of conifer succession, physiographic conditions and herbivory on quaking aspen regeneration after fire. For. Ecol. Manage. 2011, 262, 325–330. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Reich, P.B. Shocks to the system: Community assembly of the oak savanna in a 40-year fire frequency experiment. Ecology 2012, 93, S52–S69. [Google Scholar] [CrossRef]
- Bender, E.A.; Case, T.J.; Gilpin, M.E. Perturbation experiments in community ecology: Theory and practice. Ecology 1984, 65, 1–13. [Google Scholar] [CrossRef]
- Ives, A.R. Predicting the response of populations to environmental change. Ecology 1995, 76, 926–941. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Brotons, L.; Pons, P. Aridity influences the recovery of vegetation and shrubland birds after wildfire. PLoS ONE 2017, 12, e0173599. [Google Scholar] [CrossRef]
- Song, C.; Woodcock, C.E. Monitoring forest succession with multitemporal Landsat images: Factors of uncertainty. IEEE Trans. Geosci. Remote Sens. 2003, 41, 2557–2567. [Google Scholar] [CrossRef]
- Lentile, L.B.; Holden, Z.A.; Smith, A.M.S.; Falkowski, M.J.; Hudak, A.T.; Morgan, P.; Lewis, S.A.; Gessler, P.E.; Benson, N.C. Remote sensing techniques to assess active fire characteristics and post-fire effects. Int. J. Wildland Fire 2006, 15, 319–345. [Google Scholar] [CrossRef]
- Rainsford, F.W.; Giljohann, K.M.; Bennett, A.F.; Clarke, M.F.; MacHunter, J.; Senior, K.; Sitters, H.; Watson, S.; Kelly, L.T. Ecosystem type and species’ traits help explain bird responses to spatial patterns of fire. Fire Ecol. 2023, 19, 59. [Google Scholar] [CrossRef]
- Bailey, J.K.; Whitman, T.G. Interactions among fire, aspen, and elk affect insect diversity: Reversal of a community response. Ecology 2002, 83, 1701–1712. [Google Scholar] [CrossRef]
- Wenninger, A.; Hollingsworth, T.; Wagner, D. Predatory hymenopteran assemblages in boreal Alaska: Associations with forest composition and post-fire succession. Ecoscience 2019, 26, 205–220. [Google Scholar] [CrossRef]
- Rainsford, F.W.; Kelly, L.T.; Leonard, S.W.J.; Bennett, A.F. Fire and functional traits: Using functional groups of birds and plants to guide management in a fire-prone, heathy woodland ecosystem. Divers. Distrib. 2021, 28, 372–385. [Google Scholar] [CrossRef]
- Rainsford, F.W.; Kelly, L.T.; Leonard, S.W.J.; Bennett, A.F. Post-fire habitat relationships for birds differ among ecosystems. Biol. Conserv. 2021, 260, 109218. [Google Scholar] [CrossRef]
- Veit, R.R. Do vagrant birds in Massachusetts reflect population growth and dispersal rather than weather patterns? Bird Obs. 1990, 18, 5. [Google Scholar]
- Simpson, E.H. The interpretation of interaction in contingency tables. J. R. Stat. Soc. Ser. B 1951, 13, 238–241. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr.; Good, D.A. Misuse of data from mist-net captures to assess relative abundance in bird populations. Auk 1996, 113, 381–398. [Google Scholar] [CrossRef]
- Gómez, C.; Tenorio, E.A.; Montoya, P.; Cadena, C.D. Niche-tracking migrants and niche-switching residents: Evolution of climatic niches in New World warblers (Parulidae). Proc. R. Soc. B Biol. Sci. 2016, 283, 2015–2458. [Google Scholar] [CrossRef]
- Zurell, D.; Gallien, L.; Graham, C.H.; Zimmermann, N.E. Do long-distance migratory birds track their niche through seasons? J. Biogeogr. 2018, 45, 1459–1468. [Google Scholar] [CrossRef]
- Fandos, G.; Tellería, J.L. Seasonal niche-tracking behaviour of two partially migratory passerines. Ibis 2020, 162, 307–317. [Google Scholar] [CrossRef]
- Lindstrom, Å. The role of predation risk in stopover habitat selection in migrating bramblings, Fringilla montifringilla. Behav. Ecol. 1990, 1, 102–106. [Google Scholar] [CrossRef]
- Moore, F.R.; Aborn, D.A. Mechanisms of en route habitat selection: How do migrants make habitat decisions during stopover? Stud. Avian Biol. 2000, 20, 34–42. [Google Scholar]
- Cohen, E.B.; Horton, K.G.; Marra, P.P.; Clipp, H.L.; Farnsworth, A.; Smolinsky, J.A.; Sheldon, D.; Buler, J.J. A place to land: Spatiotemporal drivers of stopover habitat use by migrating birds. Ecol. Lett. 2021, 24, 38–49. [Google Scholar] [CrossRef]
- Cady, S.M.; O’Connell, T.J.; Loss, S.R.; Jaffe, N.E.; Davis, C.A. Species-specific and temporal scale-dependent responses of birds to drought. Glob. Change Biol. 2019, 25, 2691–2702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).