Spatio-Temporal Projections of the Distribution of the Canopy-Forming Algae Sargassum in the Western North Pacific Under Climate Change Scenarios Using the MAXENT Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
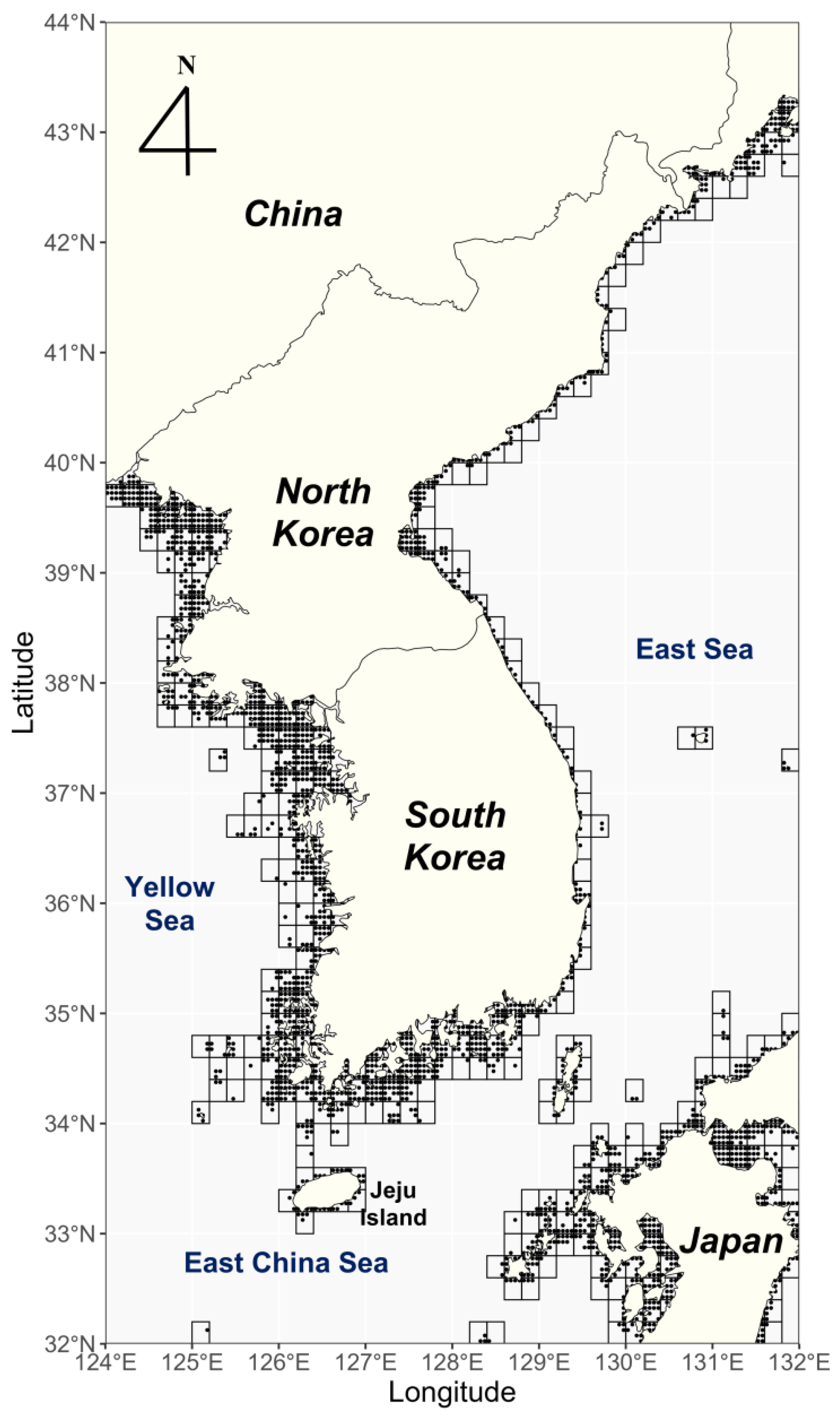
2.1. Study Area
2.2. Collection of Data on Sargassum Occurrence
2.3. Environmental Variables
2.4. Species Distribution Modeling and Evaluation of Sargassum
3. Results
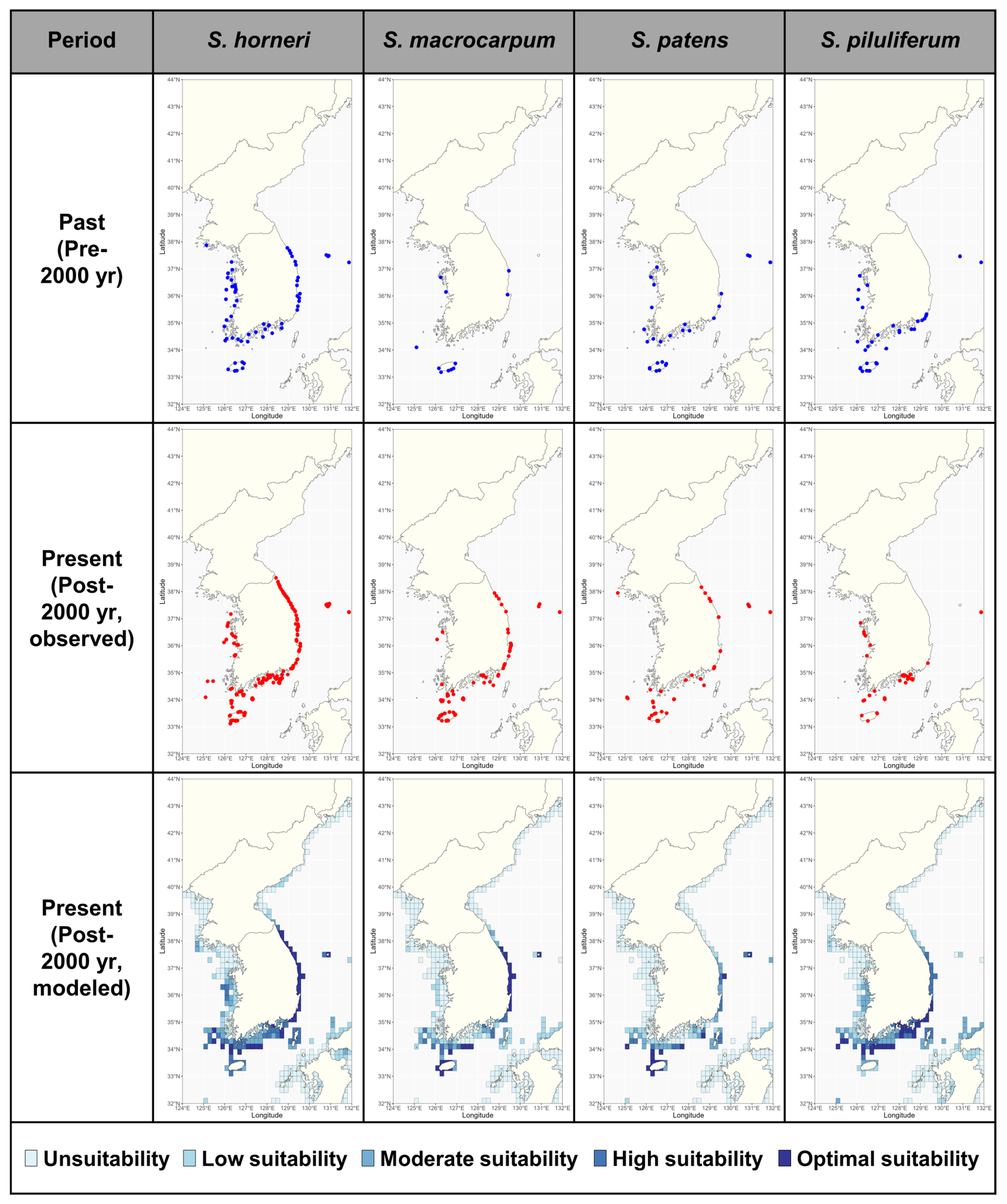
3.1. Past and Present Distribution of Sargassum in South Korea
3.2. Optimal Conditions and Performance of the MAXENT Model
3.3. Analysis of Habitat Suitability for Sargassum
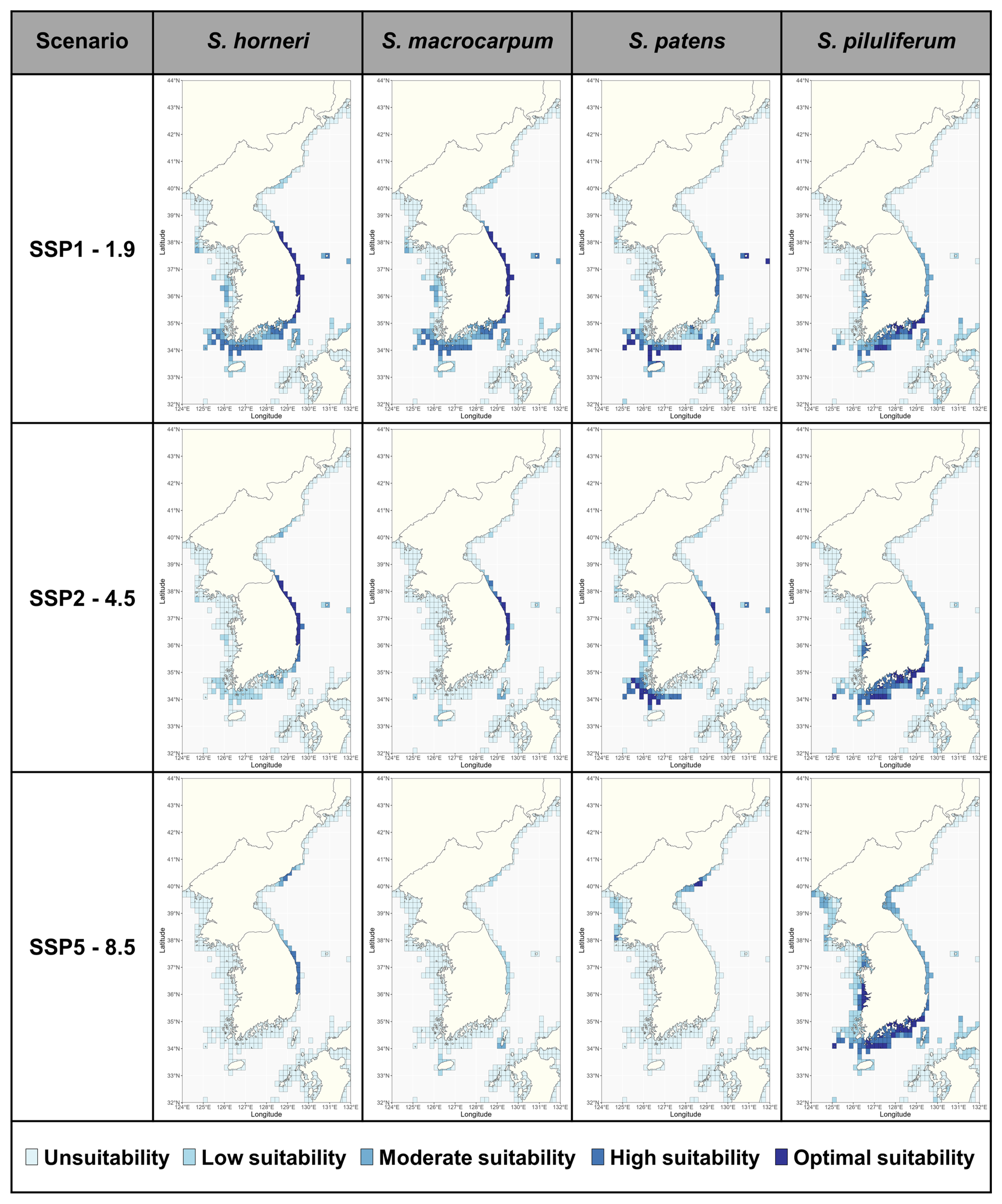
3.4. Potential Suitable Habitats for Sargassum
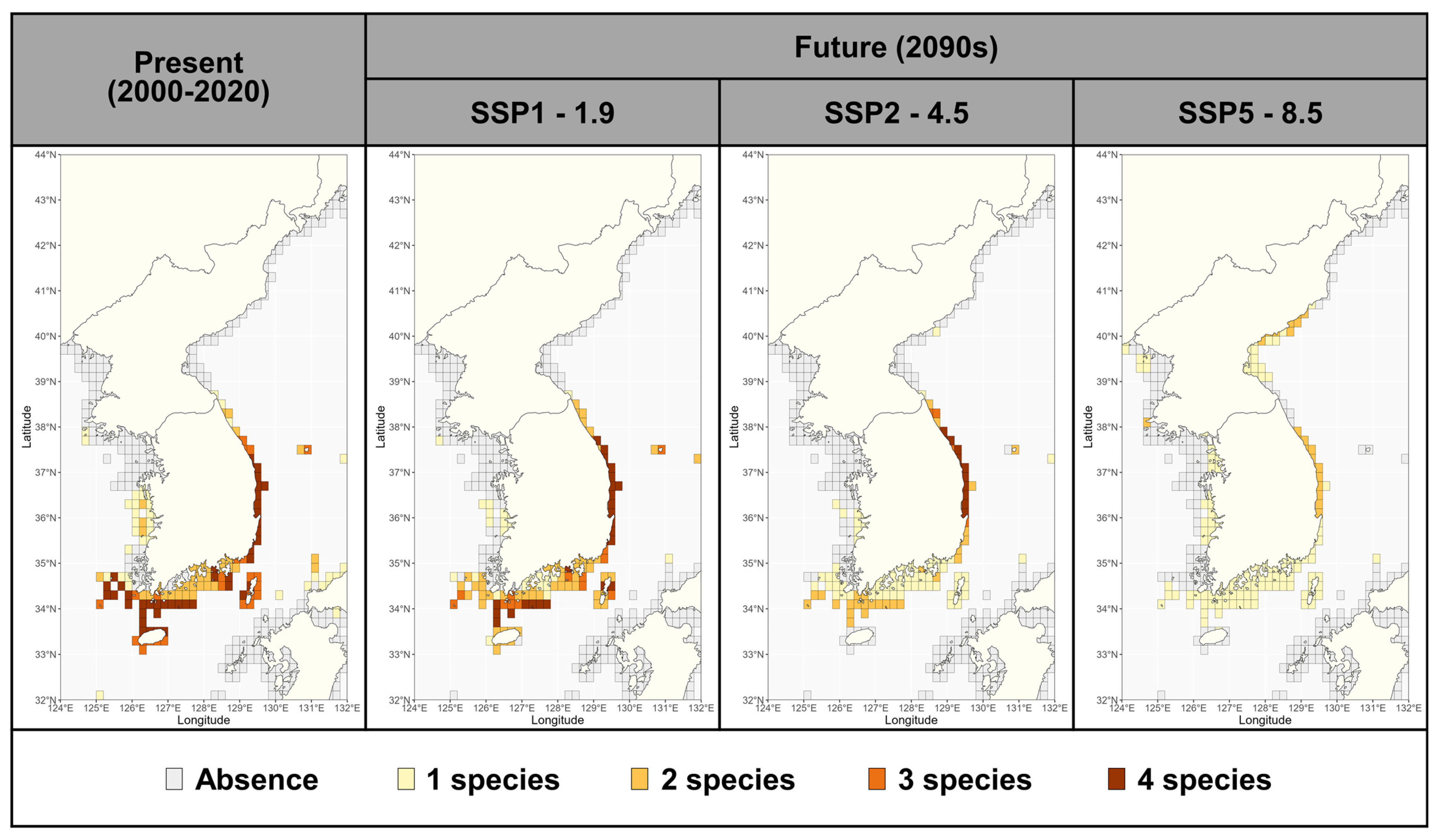
3.5. Latitudinal Centroid Shift and Community Changes Under Climate Change
4. Discussion
4.1. Environmental Influence on Sargassum Distribution
4.2. Habitat Shifts and Changes in the Past, Present, and Future
4.3. Ecological and Conservation Implications for Sargassum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSI | Habitat suitability index |
| MAXENT | Maximum Entropy |
| SDM | Species distribution model |
| SSP | Shared Socioeconomic Pathway |
| MPA | Marine protected area |
References
- Garcia-Soto, C.; Cheng, L.; Caesar, L.; Schmidtko, S.; Jewett, E.B.; Cheripka, A.; Rigor, I.; Caballero, A.; Chiba, S.; Báez, J.C. An overview of ocean climate change indicators: Sea surface temperature, ocean heat content, ocean pH, dissolved oxygen concentration, arctic sea ice extent, thickness and volume, sea level and strength of the AMOC (Atlantic Meridional Overturning Circulation). Front. Mar. Sci. 2021, 8, 642372. [Google Scholar]
- Reid, P.C.; Fischer, A.C.; Lewis-Brown, E.; Meredith, M.P.; Sparrow, M.; Andersson, A.J.; Antia, A.; Bates, N.R.; Bathmann, U.; Beaugrand, G. Impacts of the oceans on climate change. Adv. Mar. Biol. 2009, 56, 1–150. [Google Scholar] [PubMed]
- Alexander, M.A.; Scott, J.D.; Friedland, K.D.; Mills, K.E.; Nye, J.A.; Pershing, A.J.; Thomas, A.C. Projected sea surface temperatures over the 21st century: Changes in the mean, variability and extremes for large marine ecosystem regions of Northern Oceans. Elem. Sci. Anth. 2018, 6, 9. [Google Scholar] [CrossRef]
- Lima, F.P.; Wethey, D.S. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 2012, 3, 704. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q. Analysis on long-term change of sea surface temperature in the China Seas. J. Ocean Univ. China 2013, 12, 295–300. [Google Scholar] [CrossRef]
- Bao, B.; Ren, G. Climatological characteristics and long-term change of SST over the marginal seas of China. Cont. Shelf Res. 2014, 77, 96–106. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, T.; Son, Y.B.; Park, S.R. Threats to a temperate kelp forest species, Ecklonia cava, through tropical fish herbivory associated with sea surface warming in the East China Sea. Diversity 2024, 16, 253. [Google Scholar] [CrossRef]
- Mei, W.; Xie, S.-P.; Primeau, F.; McWilliams, J.C.; Pasquero, C. Northwestern Pacific typhoon intensity controlled by changes in ocean temperatures. Sci. Adv. 2015, 1, e1500014. [Google Scholar] [CrossRef]
- Rodgers, E.B.; Adler, R.F.; Pierce, H.F. Contribution of tropical cyclones to the North Pacific climatological rainfall as observed from satellites. J. Appl. Meteorol. 2000, 39, 1658–1678. [Google Scholar] [CrossRef]
- Bi, Y.; Feng, M.; Zhang, Y.; Liang, J.; Wang, W. Spatial distribution patterns of Sargassum horneri in the coastal waters of the Ma’an Archipelago. Acta Ecol. Sin. 2018, 38, 309–315. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Z.; Song, X.; Cao, X. The nitrogen isotopic composition of dissolved nitrate in the Yangtze River (Changjiang) estuary, China. Estuar. Coast. Shelf Sci. 2009, 85, 641–650. [Google Scholar] [CrossRef]
- Son, Y.B.; Choi, J.-K. Mapping the Changjiang diluted water in the East China Sea during summer over a 10-year period using GOCI satellite sensor data. Front. Mar. Sci. 2022, 9, 1024306. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.H.; Kim, T.-H.; Park, S.R. Recovery pattern and seasonal dynamics of kelp species, Ecklonia cava population formed following the large-scale disturbance. J. Korean Soc. Oceanogr. 2016, 21, 103–111. [Google Scholar]
- Lin, C.-l.; Ning, X.-r.; Su, J.-l.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Marine Syst. 2005, 55, 223–234. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Bishop, M.J.; Booth, D.J. Implications of climate change for macrophytic rafts and their hitchhikers. Mar. Ecol. Prog. Ser. 2011, 443, 285–292. [Google Scholar] [CrossRef]
- Peck, L.S. Prospects for survival in the Southern Ocean: Vulnerability of benthic species to temperature change. Antarct. Sci. 2005, 17, 497–507. [Google Scholar] [CrossRef]
- Hanley, M.E.; Firth, L.B.; Foggo, A. Victim of changes? Marine macroalgae in a changing world. Ann. Bot. 2024, 133, 1–16. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R. Climate change, human impacts, and coastal ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, T.H.; Kang, Y.H.; Kim, S.; Kim, T.-H.; Kim, J.K.; Lee, T.; Son, Y.B.; Lee, H.J.; Park, S.R. Changes in the dynamics and nutrient budget of a macroalgal community exposed to land-based fish farm discharge off Jeju Island, Korea. Sustainability 2021, 13, 11793. [Google Scholar] [CrossRef]
- Choi, S.K.; Oh, H.-J.; Yun, S.-H.; Lee, H.J.; Lee, K.; Han, Y.S.; Kim, S.; Park, S.R. Population dynamics of the ‘golden tides’ seaweed, Sargassum horneri, on the southwestern coast of Korea: The extent and formation of golden tides. Sustainability 2020, 12, 2903. [Google Scholar] [CrossRef]
- Tsukidate, J. Ecology of Sargassum spp. and Sargassum forest formation. NOAA Tech. Rep. NMFS 1992, 106, 63–72. [Google Scholar]
- Oak, J.-H.; Lee, I.-K. Taxonomy of the genus Sargassum (Fucales, Phaeophyceae) from Korea 1. Subgenus Bactrophycus Section Teretia. Algae 2005, 20, 77–90. [Google Scholar] [CrossRef]
- Oak, J.-H.; Lee, I.-K. Taxonomy of the genus Sargassum (Fucales, Phaeophyceae) from Korea 2. Subgenus Bactrophycus section Halochloa and Repentia. Algae 2006, 21, 393–405. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.K.; Van, S.; Kim, S.T.; Kang, Y.H.; Park, S.R. Geographic differentiation of morphological characteristics in the brown seaweed Sargassum thunbergii along the Korean coast: A response to local environmental conditions. J. Mar. Sci. Eng. 2022, 10, 549. [Google Scholar] [CrossRef]
- Gorman, D.; Horta, P.; Flores, A.A.; Turra, A.; Berchez, F.A.d.S.; Batista, M.B.; Lopes Filho, E.S.; Melo, M.S.; Ignacio, B.L.; Carneiro, I.M. Decadal losses of canopy-forming algae along the warm temperate coastline of Brazil. Glob. Change Biol. 2020, 26, 1446–1457. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G. Alpha and beta diversity in Mediterranean macroalgal assemblages: Relevancy and type of effect of anthropogenic stressors vs natural variability. Mar. Biol. 2020, 167, 32. [Google Scholar] [CrossRef]
- Terawaki, T.; Yoshikawa, K.; Yoshida, G.; Uchimura, M.; Iseki, K. Ecology and restoration techniques for Sargassum beds in the Seto Inland Sea, Japan. Mar. Pollut. Bull. 2003, 47, 198–201. [Google Scholar] [CrossRef]
- Maestro, M.; Pérez-Cayeiro, M.L.; Chica-Ruiz, J.A.; Reyes, H. Marine protected areas in the 21st century: Current situation and trends. Ocean Coast. Manag. 2019, 171, 28–36. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.; Berkhout, J. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef]
- Grorud-Colvert, K.; Sullivan-Stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J. The MPA Guide: A framework to achieve global goals for the ocean. Science 2021, 373, eabf0861. [Google Scholar] [CrossRef]
- Phillips, J.A.; Blackshaw, J.K. Extirpation of macroalgae (Sargassum spp.) on the subtropical east Australian coast. Conserv. Biol. 2011, 25, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [PubMed]
- Guerra-García, J.M.; Maestre, M.J.; González, A.R.; García-Gómez, J.C. Assessing a quick monitoring method using rocky intertidal communities as a bioindicator: A multivariate approach in Algeciras Bay. Environ. Monit. Assess. 2006, 116, 345–361. [Google Scholar] [CrossRef][Green Version]
- Juanes, J.A.; Guinda, X.; Puente, A.; Revilla, J.A. Macroalgae, a suitable indicator of the ecological status of coastal rocky communities in the NE Atlantic. Ecol. Indic. 2008, 8, 351–359. [Google Scholar] [CrossRef]
- Li, J.-J.; Huang, S.-H.; Liu, Z.-Y.; Bi, Y.-X. Climate-driven range shifts of brown seaweed Sargassum horneri in the Northwest Pacific. Front. Mar. Sci. 2020, 7, 730. [Google Scholar] [CrossRef]
- Martínez, B.; Viejo, R.M.; Carreño, F.; Aranda, S.C. Habitat distribution models for intertidal seaweeds: Responses to climatic and non-climatic drivers. J. Biogeogr. 2012, 39, 1877–1890. [Google Scholar] [CrossRef]
- Hwang, E.K.; Choi, H.G.; Kim, J.K. Seaweed resources of Korea. Bot. Mar. 2020, 63, 395–405. [Google Scholar] [CrossRef]
- Jung, S.W.; Rho, H.S.; Choi, C.G. Seaweed Beds and Community Structure in the East and South Coast of Korea. J. Mar. Sci. Eng. 2022, 10, 689. [Google Scholar] [CrossRef]
- Kang, R.-S. A review of destruction of seaweed habitats along the coast of the Korean Peninsula and its consequences. Bull. Fish. Res. Agen. 2010, 32, 25–31. [Google Scholar]
- Jueterbock, A.; Smolina, I.; Coyer, J.A.; Hoarau, G. The fate of the Arctic seaweed Fucus distichus under climate change: An ecological niche modeling approach. Ecol. Evol. 2016, 6, 1712–1724. [Google Scholar] [CrossRef]
- Sainz-Villegas, S.; de la Hoz, C.F.; Juanes, J.A.; Puente, A. Predicting non-native seaweeds global distributions: The importance of tuning individual algorithms in ensembles to obtain biologically meaningful results. Front. Mar. Sci. 2022, 9, 1009808. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Fan, X.; Duan, Q.; Shen, C.; Wu, Y.; Xing, C. Global surface air temperatures in CMIP6: Historical performance and future changes. Environ. Res. Lett. 2020, 15, 104056. [Google Scholar] [CrossRef]
- Chang, K.-I.; Teague, W.; Lyu, S.; Perkins, H.; Lee, D.-K.; Watts, D.; Kim, Y.-B.; Mitchell, D.; Lee, C.; Kim, K. Circulation and currents in the southwestern East/Japan Sea: Overview and review. Prog. Oceanogr. 2004, 61, 105–156. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Park, K.-A. Change in the recent warming trend of sea surface temperature in the East Sea (Sea of Japan) over decades (1982–2018). Remote Sens. 2019, 11, 2613. [Google Scholar] [CrossRef]
- Mi, B.; Zhang, Y.; Mei, X. The sediment distribution characteristics and transport pattern in the eastern China seas. Quat. Int. 2022, 629, 44–52. [Google Scholar] [CrossRef]
- Son, Y.B.; Kim, E.; Cho, J.H.; Choi, S.K.; Kang, D. Analysis of regional variation of water transparency in the Yellow Sea and East China Sea based on MODIS data. Reg. Stud. Mar. Sci. 2025, 84, 104093. [Google Scholar] [CrossRef]
- Lee, Y.P.; Kang, S.Y. A Catalogue of the Seaweeds in Korea; Jeju National University: Jeju, Republic of Korea, 2002; p. 662. [Google Scholar]
- Assis, J.; Fernández Bejarano, S.J.; Salazar, V.W.; Schepers, L.; Gouvêa, L.; Fragkopoulou, E.; Leclercq, F.; Vanhoorne, B.; Tyberghein, L.; Serrão, E.A. Bio-ORACLE v3. 0. Pushing marine data layers to the CMIP6 Earth System Models of climate change research. Glob. Ecol. Biogeogr. 2024, 33, e13813. [Google Scholar] [CrossRef]
- Jueterbock, A. R Package MaxentVariableSelection: Selecting the Best Set of Relevant Environmental Variables Along with the Optimal Regularization Multiplier for Maxent Niche Modeling. R Foundation for Statistical Computing. 2015. Available online: https://cran.r-project.org/web/packages/MaxentVariableSelection/index.html (accessed on 4 June 2018).
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Z.; Peng, D.; Chu, J. Potential impacts of climate change on cephalopods in a highly productive region (Northwest Pacific): Habitat suitability and management. Sci. Total Environ. 2024, 953, 175794. [Google Scholar] [CrossRef]
- Bekkby, T.; Smit, C.; Gundersen, H.; Rinde, E.; Steen, H.; Tveiten, L.; Gitmark, J.K.; Fredriksen, S.; Albretsen, J.; Christie, H. The abundance of kelp is modified by the combined impact of depth, waves and currents. Front. Mar. Sci. 2019, 6, 475. [Google Scholar] [CrossRef]
- Hurd, C.L. Water motion, marine macroalgal physiology, and production. J. Phycol. 2000, 36, 453–472. [Google Scholar] [CrossRef]
- Komatsu, T.; Murakami, S.-I. Influence of a Sargassum forest on the spatial distribution of water flow. Fish. Oceanogr. 1994, 3, 256–266. [Google Scholar] [CrossRef]
- Wheeler, W.N. Pigment content and photosynthetic rate of the fronds of Macrocystis pyrifera. Mar. Biol. 1980, 56, 97–102. [Google Scholar] [CrossRef]
- Cheang, C.C.; Chu, K.H.; Ang, J.; Put, O. Morphological and genetic variation in the populations of Sargassum hemiphyllum (phaeophyceae) in the Northwestern Pacific 1. J. Phycol. 2008, 44, 855–865. [Google Scholar] [CrossRef]
- Báez, J.C.; Olivero, J.; Peteiro, C.; Ferri-Yáñez, F.; Garcia-Soto, C.; Real, R. Macro-environmental modelling of the current distribution of Undaria pinnatifida (Laminariales, Ochrophyta) in northern Iberia. Biol. Invasions 2010, 12, 2131–2139. [Google Scholar] [CrossRef]
- Lapointe, B.; Brewton, R.; Herren, L.; Wang, M.; Hu, C.; McGillicuddy, D., Jr.; Lindell, S.; Hernandez, F.; Morton, P. Nutrient content and stoichiometry of pelagic Sargassum reflects increasing nitrogen availability in the Atlantic Basin. Nat. Commun. 2021, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Jiang, H.; Ma, Y.; Cui, C.; Qin, H.; Liu, L.; Zang, S.; Xing, H.; Xu, Z.; Wu, H. Combined influences of light and nitrogen enrichment on the physiological performance of a golden tide alga (Sargassum horneri). J. Mar. Sci. Eng. 2022, 10, 1195. [Google Scholar] [CrossRef]
- Sasaki, Y.N.; Umeda, C. Rapid warming of sea surface temperature along the Kuroshio and the China coast in the East China Sea during the twentieth century. J. Clim. 2021, 34, 4803–4815. [Google Scholar] [CrossRef]
- Endo, H.; Nishigaki, T.; Yamamoto, K.; Takeno, K. Subtidal macroalgal succession and competition between the annual, Sargassum horneri, and the perennials, Sargassum patens and Sargassum piluliferum, on an artificial reef in Wakasa Bay, Japan. Fish. Sci. 2019, 85, 61–69. [Google Scholar] [CrossRef]
- Endo, H.; Nishigaki, T.; Yamamoto, K.; Takeno, K. Age-and size-based morphological comparison between the brown alga Sargassum macrocarpum (Heterokonta; Fucales) from different depths at an exposed coast in northern Kyoto, Japan. J. Appl. Phycol. 2013, 25, 1815–1822. [Google Scholar] [CrossRef]



| Variable | Evaluation | S. horneri | S. macrocarpum | S. patens | S. piluliferum |
|---|---|---|---|---|---|
| Beta multiplier | 2.5 | 1.0 | 1.0 | 1.5 | |
| Ocean temperature (Ltmax) | Contribution | 15.18 | 10.79 | - | - |
| VIF | 3.22 | 2.19 | - | - | |
| Ocean temperature (Ltmin) | Contribution | 39.45 | 26.24 | - | 27.04 |
| VIF | 4.38 | 2.61 | - | 1.52 | |
| Ocean temperature (Mean) | Contribution | - | - | 36.61 | - |
| VIF | - | - | 1.09 | - | |
| Salinity (Ltmin) | Contribution | - | - | - | 9.30 |
| VIF | - | - | - | 1.23 | |
| Salinity (Ltmax) | Contribution | 9.43 | - | - | - |
| VIF | 2.46 | - | - | - | |
| Sea water velocity (Mean) | Contribution | 35.94 | 55.28 | 39.17 | 48.75 |
| VIF | 1.15 | 1.10 | 1.04 | 1.05 | |
| Primary productivity (Mean) | Contribution | - | 7.69 | 36.61 | - |
| VIF | - | 1.48 | 1.09 | - | |
| Nitrate (Mean) | Contribution | - | - | - | 14.91 |
| VIF | - | - | - | 1.29 |
| Species | AUC | TSS |
|---|---|---|
| S. horneri | 0.8894 ± 0.0212 | 0.6330 ± 0.0663 |
| S. macrocarpum | 0.9110 ± 0.0175 | 0.6990 ± 0.0597 |
| S. patens | 0.9024 ± 0.0468 | 0.7256 ± 0.0860 |
| S. piluliferum | 0.8498 ± 0.0465 | 0.5594 ± 0.0957 |
| Scenarios | S. horneri | S. macrocarpum | S. patens | S. piluliferum | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (×103 km2) | Trend (%) | Area (×103 km2) | Trend (%) | Area (×103 km2) | Trend (%) | Area (×103 km2) | Trend (%) | Area (×103 km2) | Trend (%) | ||
| Present | 14.40 | - | 6.61 | - | 5.61 | - | 13.85 | - | 16.92 | - | |
| SSP1-1.9 | 2030s | 10.33 | −28.25 | 4.34 | −34.42 | 6.17 | +9.91 | 10.74 | −22.49 | 14.12 | −16.5 |
| 2060s | 9.81 | −31.87 | 3.69 | −44.12 | 5.93 | +5.78 | 12.04 | −13.09 | 15.08 | −10.8 | |
| 2090s | 10.21 | −29.12 | 4.44 | −32.89 | 6.26 | +11.66 | 9.73 | −29.80 | 13.69 | −19.0 | |
| SSP2-4.5 | 2030s | 12.40 | −13.91 | 5.46 | −17.49 | 6.29 | +12.14 | 13.04 | −5.78 | 15.83 | −6.4 |
| 2060s | 6.80 | −52.81 | 2.14 | −67.61 | 4.61 | −17.87 | 12.15 | −12.27 | 14.71 | −13.0 | |
| 2090s | 2.77 | −80.78 | 1.45 | −78.00 | 4.69 | −16.32 | 11.37 | −17.94 | 14.11 | −16.5 | |
| SSP5-8.5 | 2030s | 9.62 | −33.18 | 3.62 | −45.20 | 5.25 | −6.51 | 14.12 | +1.93 | 15.62 | −7.6 |
| 2060s | 2.37 | −83.57 | 1.48 | −77.63 | 4.28 | −23.70 | 16.98 | +22.58 | 18.06 | +6.7 | |
| 2090s | 1.52 | −89.42 | 0.10 | −98.45 | 0.90 | −83.96 | 18.19 | +31.29 | 19.07 | +12.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.K.; Son, Y.B.; Jeong, H.W.; Go, S.; Park, S.R. Spatio-Temporal Projections of the Distribution of the Canopy-Forming Algae Sargassum in the Western North Pacific Under Climate Change Scenarios Using the MAXENT Model. Biology 2025, 14, 590. https://doi.org/10.3390/biology14060590
Choi SK, Son YB, Jeong HW, Go S, Park SR. Spatio-Temporal Projections of the Distribution of the Canopy-Forming Algae Sargassum in the Western North Pacific Under Climate Change Scenarios Using the MAXENT Model. Biology. 2025; 14(6):590. https://doi.org/10.3390/biology14060590
Chicago/Turabian StyleChoi, Sun Kyeong, Young Baek Son, Hyun Woo Jeong, Seonggil Go, and Sang Rul Park. 2025. "Spatio-Temporal Projections of the Distribution of the Canopy-Forming Algae Sargassum in the Western North Pacific Under Climate Change Scenarios Using the MAXENT Model" Biology 14, no. 6: 590. https://doi.org/10.3390/biology14060590
APA StyleChoi, S. K., Son, Y. B., Jeong, H. W., Go, S., & Park, S. R. (2025). Spatio-Temporal Projections of the Distribution of the Canopy-Forming Algae Sargassum in the Western North Pacific Under Climate Change Scenarios Using the MAXENT Model. Biology, 14(6), 590. https://doi.org/10.3390/biology14060590






