1. Introduction
As a major contributor to global morbidity and mortality, stroke manifests in two primary forms: ischemic and hemorrhagic subtypes [
1], with ischemic stroke (IS) constituting approximately 70% of clinical presentations [
2]. The multifaceted pathophysiology of IS involves intricate interactions between neuroinflammatory cascades and oxidative damage mechanisms. These pathological processes ultimately compromise blood–brain barrier integrity and induce cerebral edema formation [
3].
Modern epidemiological studies have found that the incidence of IS is significantly higher in males than in females. Relevant research indicates that estrogens (e.g., estradiol) play a crucial role in the treatment of IS [
4,
5]. Modern medical research has found that a healthy lifestyle can effectively reduce the risk of stroke [
6], and that acute anger or emotional instability is strongly associated with the occurrence of IS and cerebral hemorrhage [
7]. Currently, endovascular thrombectomy (EVT) alone or in combination with intravenous thrombolysis remains the most effective treatment for IS, with no approved alternative therapy showing greater clinical effectiveness [
8]. IS treatment faces a narrow time window, and patients experience more severe reperfusion injury and some neurological function damage after the operation [
9]. Studies have shown that IS begins to occur when a thrombotic occlusion of the middle cerebral artery (MCA) is induced by cardiovascular or cerebrovascular diseases, or when atherosclerotic plaques lead to arterial stenosis. This pathological process triggers severe cerebral inflammation accompanied by intense oxidative stress and blood–brain barrier (BBB) damage [
10]. When IS induces a brain inflammatory response, NF-κB regulates the downstream NLRP3 inflammasome and triggers the Caspase-1 cascade, activating IL-1β and IL-18 and ultimately leading to the pyroptosis of brain cells [
11]. Thus, for populations at a high risk of the disease, developing drugs that can mitigate sudden disease-related injuries, alleviate brain inflammatory responses and oxidative stress damage caused by IS, and exert therapeutic effects on IS is of utmost importance [
12].
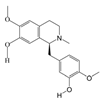
Periplaneta americana (L.) (PA) is a cockroach species in the family Blattidae. It has been used as a traditional Chinese medicinal insect since the Shennong Bencao Jing (written between 25–220 AD) [
13]. Historically employed in ethnopharmacological practices for addressing traumatic injuries, gastrointestinal disorders, and inflammatory pathologies [
14],
Periplaneta americana (L.) has been substantiated by contemporary pharmacological investigations to exhibit potent anti-pathogenic, redox-modulating, and regenerative properties in its extract (PAE) [
15,
16]. The preparation of medicine made from PA as raw material, “Kangfuxin Liquid”, has a history of more than 20 years and is still widely circulated and used in the market [
17]. It is rich in amino acids, peptides, nucleosides, polysaccharides, and fatty acids and has significant therapeutic effects on skin burns and scalds, fatty liver, colitis, and gastric bleeding [
18,
19,
20,
21,
22]. Recent studies have shown that PAE can activate the ERK/CREB/BDNF signaling pathway and exert neuroprotective and neurofunctional recovery effects on stroke [
23]. Meanwhile, the commercially available drug ‘Xinmailong’ injection, which is manufactured using PA as a raw material, has been shown to effectively reduce acute myocardial infarction injury in rats [
24] and act on various cardiovascular diseases [
24]. Thus, based on PA’s multiple excellent pharmacological activities, we have initiated the exploration of PA’s therapeutic effects on cerebral neural diseases.
Network pharmacology, a recently emerging method for drug prediction, can analyze and assess the relationship between multicomponent drugs and diseases from multiple perspectives and levels more systematically. The theoretical study of drug effects is consistent with the holistic concept of traditional Chinese medicine, and its advent has provided more scientific and reliable methods for exploring the mechanisms of complex Chinese herbal medicine systems in the treatment of diseases [
25].
Based on the results of the previous research conducted by our research group, this study reveals that PAS840 (an extract of PA, named according to the characteristics of the extraction process in
Section 2.1) can effectively alleviate the inflammatory response and oxidative stress damage induced by glutamate in PC12 nerve cells [
26]. It can also significantly reduce the area of cerebral infarction in rats and the neuronal apoptosis of brain tissue, and regulate the ecological balance of gut microbiota in rats [
27]. Therefore, we further analyzed the components of PAS840 using liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology and peptidomics, and predicted the disease targets of the identified components using network pharmacology approaches. Oxygen–glucose deprivation/reoxygenation (OGD/R) and transient middle cerebral artery occlusion (tMCAO) are widely-used contemporary models for IS [
28,
29]. Through in vitro and in vivo experiments, they can effectively mimic the process of oxygen and glucose deprivation in nerve cells and brain tissue following IS occurrence in animals or humans. As an internationally recognized in vitro experimental sample, microglial cells (BV-2) exhibit high consistency with primary cells in core phenotypes such as neuroinflammatory responses and phagocytic function [
30]. In this study, the OGD/R model was constructed using BV-2 cells, and the tMCAO model was established using rats to verify the effects of PAS840 on oxidative stress, the NLRP3/Caspase-1/IL-1β pyroptosis pathway, BBB integrity, and neural function repair. This study provides a scientific basis for the potential role of PAS840 in the prevention and treatment of IS.
2. Materials and Methods
2.1. Naming and Extraction Process of PAS840
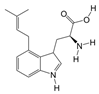
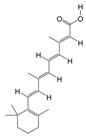
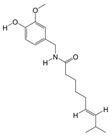
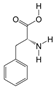
PAS840: The dried PA body powder of the PA was extracted with 90% ethanol at a ratio of five times the weight of the PA material, and the ethanol solution was concentrated under reduced pressure to obtain an ethanol extract. The ethanol extract was then dried to obtain a dehydrated extract. The defatted extract was adsorbed on an S-8 macroporous adsorption resin column, and the resin column was first washed with water and then washed with 40% ethanol to elute the resin column. The ethanol eluate was collected, concentrated at 60 °C under reduced pressure, and lyophilized to obtain the PAS840 sample.
2.2. Materials
Materials included Cell Counting Kit-8 (CCK-8) (Aladdin, Wuhan, China, Lot No.: L2-128-203), 96-well/24-well/6-well cell culture plates (NEST, Gretna, LA, USA, Lot No.: 052024BL01), MCC950 NLRP3 inhibitor (MedChemExpress, Shanghai, China, Lot No.: CP-456773), BV-2 mouse microglia specific culture medium/sugar-free DMEM culture medium (Wuhan PNase Life Science Technology Co., Ltd., Wuhan, China, Lot No.: WH4424E112/WHAA24P244), PBS buffer/4% paraformaldehyde/hematoxylin (Wuhan Saive Biotechnology Co., Ltd., Wuhan, China, Lot No.: GP21090061782/GP2-306304/G1004), crystal violet dye (Baiyuntian, Shaoguan, China, Lot No.: C0121-100), Transwell (Costar, Arlington, VA, USA, Lot No.: 02422025), Evans Blue Dye (MACKLIN, Shanghai, China, Lot No.: C14442662), triphenyltetrazolium chloride (TTC) stain (Aladdin, Shanghai, China, Lot No.: L2128203), qPCR detection kit (Aicrui Biotechnology Co., Ltd., Qingdao, China, Lot No.: a6a0298), primers (Shenggong Bioengineering Co., Ltd., Shanghai, China, Lot No.: 300371063), malondialdehyde (MDA) detection kit/superoxide dismutase (SOD) detection kit (Boxbio, Beijing, China, Lot No.: 10124071220/10124070650), total nitric oxide synthase (NOS) subtype detection kit/reactive oxygen species (ROS) detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China, Lot No.: 2024107/2023128), tMCAO monofilament/bupivacaine hydrochloride (Beijing Xichong Biotechnology Co., Ltd., Beijing, China, Lot No.: 20230506), neutral Gum/HE stain (Wuhan Bolf Biotechnology Co., Ltd., Wuhan, China, Lot No.: B0044/BHO001), NLRP3/NF-κB antibody/secondary antibody (Wuhan Punose Life Science Technology Co., Ltd., Wuhan, China, Lot No.: GB114320/GB11142/GB21303), and isoflurane (Shandong Ante Herding Technology Co., Ltd., Jinan, China, Lot No.: 2023090502).
2.3. Network Pharmacology Analysis of IS by PAS840
2.3.1. Identification of the Composition of PAS840 Substances
The determination of small and medium molecular compounds in PAS840 samples: Sample treatment: Mix an appropriate amount of sample with 600 µL of 2-chloro-L-phenylalanine (4 ppm) methanol solution, swirl for 30 s, grind at 50 Hz for 120 s using a tissue grinder (MB-96, wall), ultrasound at room temperature for 10 min, centrifuge at 412,000 rpm for 10 min at 4 °C, and filter supernatant through a 0.22 μm membrane. Analysis: Use a Thermo Vanquish (Thermo Fisher Scientific, Waltham, MA, USA) ultra-high performance liquid phase system with ACQUITY UPLC
® HSS T3 (2.1 × 100 mm, 1.8 µm) column (Waters, Milford, MA, USA) with a flow rate of 0.3 mL/min, column temperature of 40 °C, and sample size of 2 μL. Mobile phase: In positive ion mode, 0.1% acetonitrile formate (B2) and 0.1% water formate (A2); in negative ion mode, acetonitrile (B3) and 5 mM ammonium formate water (A3). Gradient elution is shown in
Table 1. Mass spectrometry: Use Thermo Q Exactive (Thermo Fisher Scientific, Waltham, MA, USA), ESI, and positive and negative ion modes with a positive ion spray voltage of 3.50 kV, negative ion spray voltage of -2.50 kV, sheath gas of 40 arb, auxiliary gas of 10 arb, and capillary temperature 325 °C. Conduct the first-level full scan at 70,000 resolution, m/z 100–1000 range. Then, perform second-level cracking by HCD, with a collision energy of 30 eV, and second-level resolution of 17,500, and break the first 10 ions before signal collection. Use dynamic exclusion to remove unnecessary MS/MS information.
PAS840 peptide component detection: Mobile phase: Liquids A (100% water and 0.1% formic acid) and B (80% acetonitrile and 0.1% formic acid). Sample prep: Dissolve lyophilized powder in 10 mL of A. For liquid–liquid detection, inject 1 µg supernatant after 20 min at 4 °C and 14,000 rmp centrifugation. Chromatography: Elution is shown in
Table 2. Mass spec: Use Nanospray FlexTM (NSI) ion source and Q Exactive HF-X (Thermo Fisher Scientific, Waltham, MA, USA). Use an ion spray voltage of 2.4 kV, and ion transfer tube temp of 275 °C. Acquire in data-dependent mode. 1st stage MS: full scan m/z 100–1500, resolution 120,000 (200 m/z), AGC 3 × 10
6, and max C-trap inj time 80 ms. Fragment the parent ion (highest intensity) by HCD. 2nd MS: resolution 15,000 (200 m/z), AGC 5 × 10
4, max inj time 45 ms, and peptide frag/collision energy 27%. Detection: Use raw data (.raw) for MS. Retrieve peptide sequences by de novo analysis with Peptides and De and Novo-only Peptide databases.
2.3.2. Acquisition of Disease Targets Corresponding to PAS840 Components
To obtain the related protein information, the peptide sequences that scored highest on the Peptides database analysis (−10 lgP value) and those that scored higher on de novo-only peptide analysis (>95 confidence level) were chosen and added to the Emboss database (
https://www.ebi.ac.uk/Tools/seqstats/emboss_pepstats/, accessed on 8 January 2024). Peptide sequences with a charge value of ≥0 and an isoelectric point value of ≤12 were screened according to the conditional characteristics of food-borne anti-inflammatory peptides and neuroprotective peptides [
31,
32]. The peptide sequence numbers were then entered into the NoverPor (
https://www.novoprolabs.com/, accessed on 12 January 2024) web tool to convert the sequence numbers to the smile format, and the similarity ensemble approach (SEA) (
https://sea.bkslab.org/, accessed on 12 January 2024) was used to obtain the target protein for each peptide prediction. After eliminating duplicates, the target protein information of the peptides and small molecules was combined to obtain the desired information.
2.3.3. IS Disease Target Acquisition
To identify the intersection of PAS840 targets with IS, the search term “cerebral ischemia” was chosen, and the OMIM (
https://omim.org/, accessed on 12 January 2024), GeneCards (
http://www.genecards.org/, accessed on 12 January 2024), and DisGeNET (
https://www.disgenet.org/search, accessed on 12 January 2024) databases’ search results were combined. Duplicate disease targets were removed, and the disease targets and PAS840 targets were imported into Venn 2.1.0.
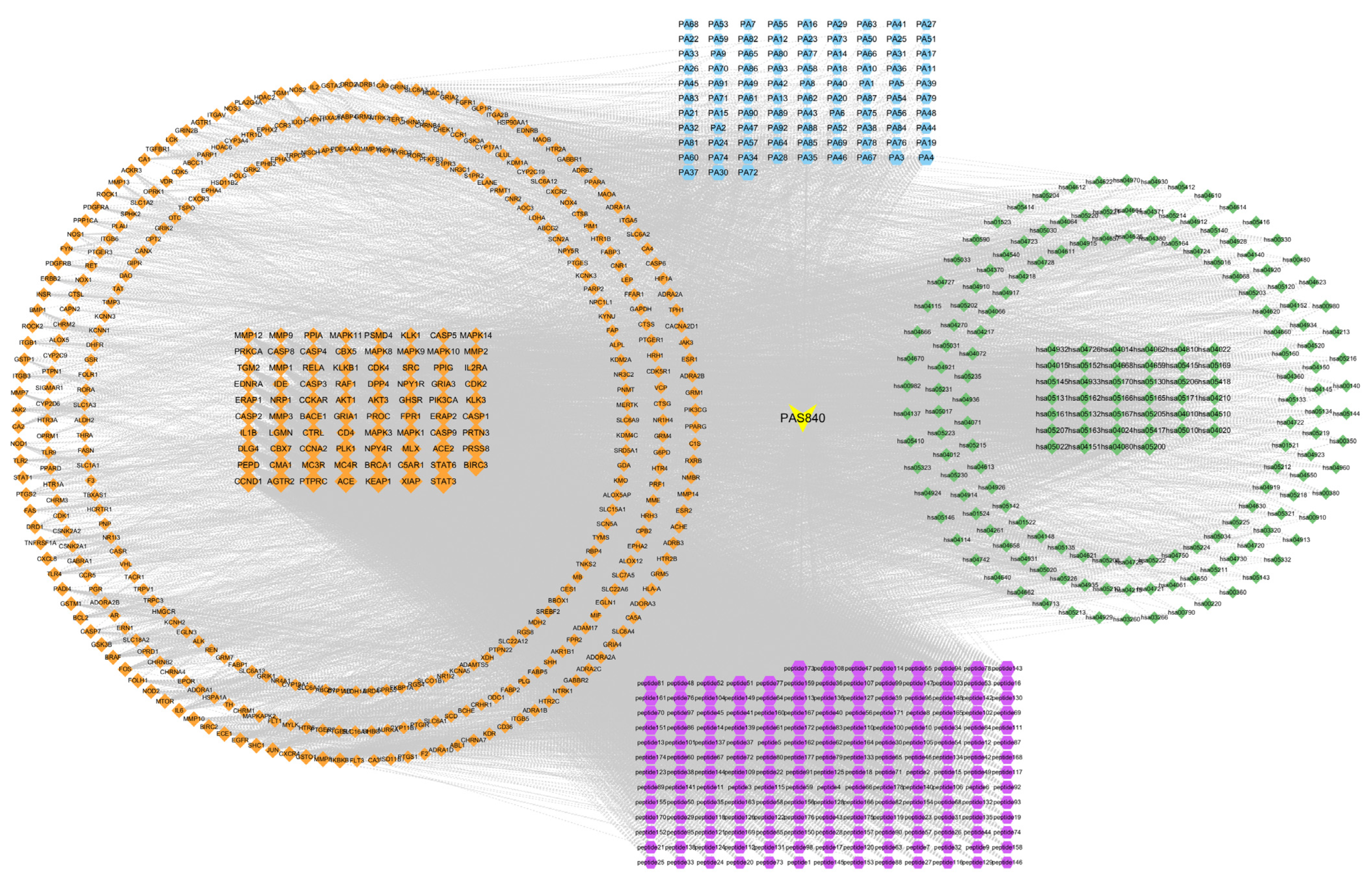
2.3.4. Disease Target Interaction Network Construction
The intersection illness target was entered into the STRING Protein Interaction Retrieval Prediction Database (
https://string-db.org/, accessed on 12 January 2024). The relevant data were produced by hiding the disconnected nodes in the network and setting the remaining parameters to their default values. The protein network was analyzed using Centiscape 2.2 of the Cytoscape 3.9.1 software. The core protein target was screened using default screening conditions of degree (>39.764434), closeness (>0.001091), and betweenness (>498.244804), and a protein–protein interaction (PPI) network map was created.
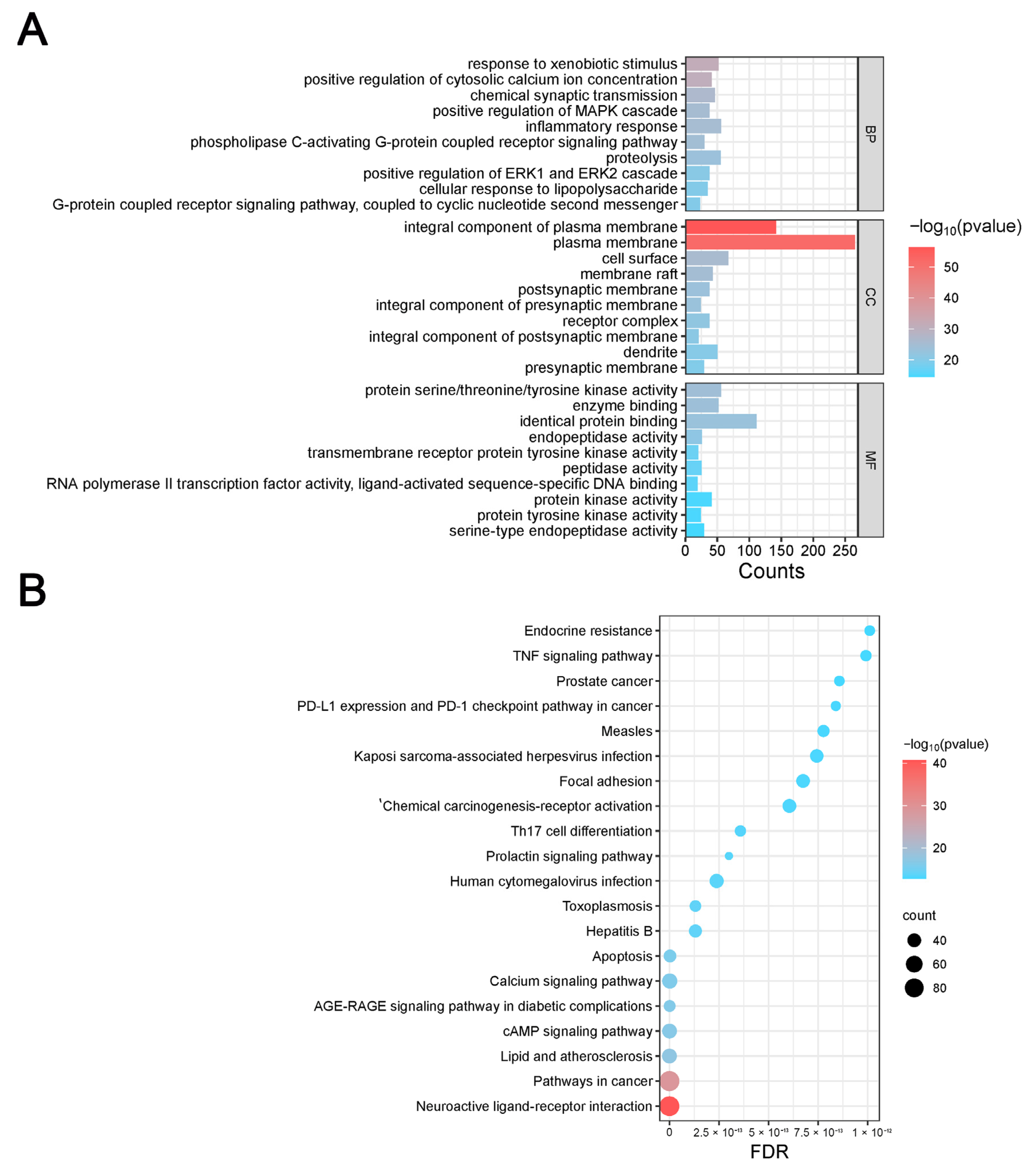
2.3.5. Gene Ontology and KEGG Enrichment Analysis
The DAVID database (
https://david.ncifcrf.gov/, accessed on 12 January 2024) was used to import disease target information, and the OFFICIAL_GENE_SYMBOL was chosen in DAVID for the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment and biological process gene ontology (GO) analyses. The online bioinformatics platform Microbiosense (
http://www.bioinformatics.com.cn/, accessed on 12 January 2024) was used to visualize the enrichment outcomes.
2.3.6. Component–Pharmacological Target–Disease Pathway Network Construction
The PAS840 target, intersection target, and KEGG pathway data were acquired and utilized to create a composition–pharmacodynamic target–disease pathway network worksheet. The data were then integrated into a tape file and imported into Cytoscape 3.9.1 software to create a network diagram of the composition–pharmacodynamic target–disease pathway. Simultaneously, node degree values were computed using Centiscape 2.2, a Cytoscape 3.9.1 plug-in, to assess the connections between the sample components, pharmacodynamic targets, and disease pathways.
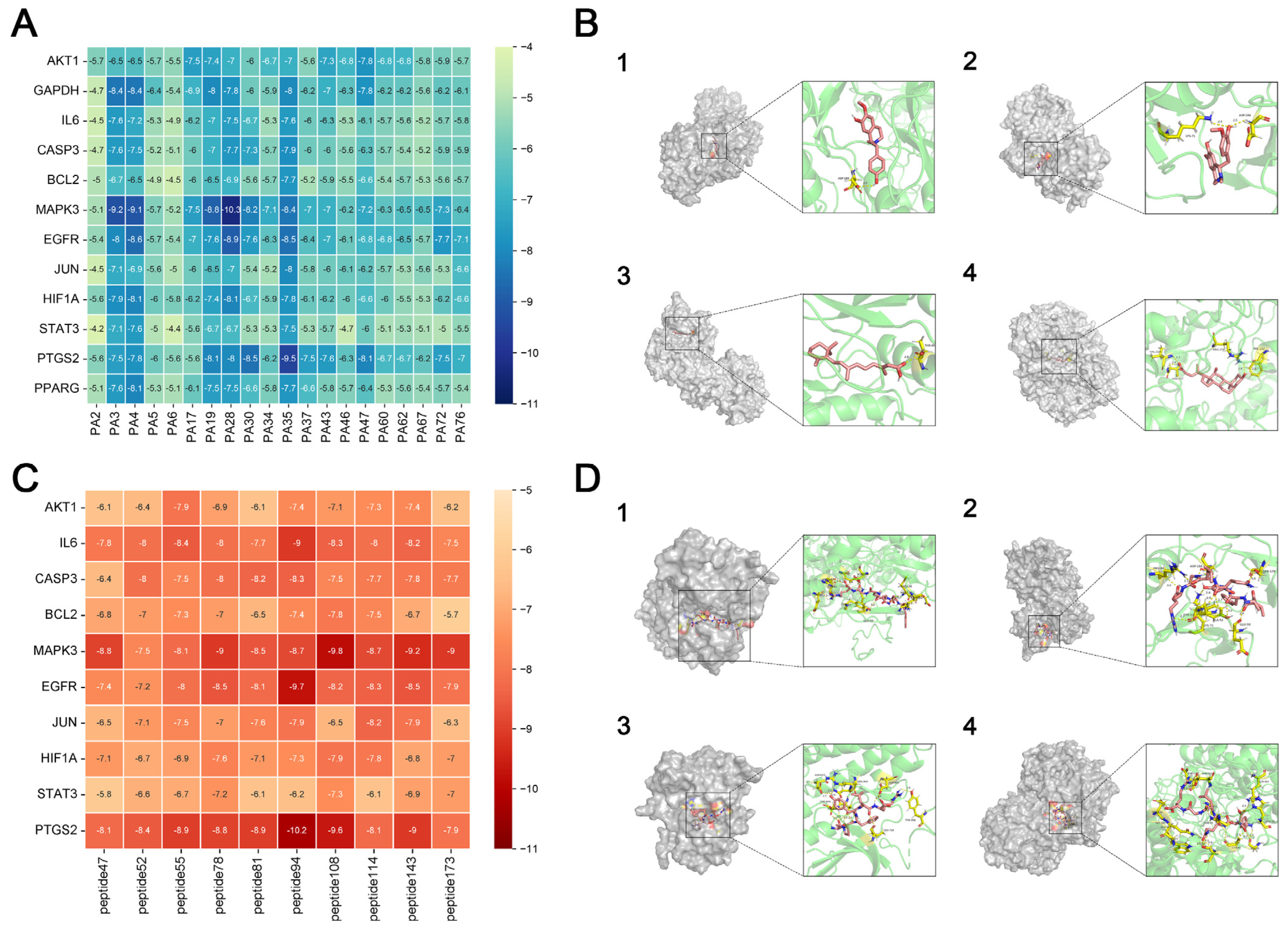
2.3.7. Molecular Docking
Target proteins with the highest degree of interaction and matching active components that were filtered out of the PPI network were subjected to molecular docking. Structural files for the target proteins and active ingredients were obtained from the PDB database (
https://www.rcsb.org/, accessed on 23 January 2024). PyMoL 4.6.0 software was used to dewater and deligand the proteins, and the active sites were chosen for molecular docking by focusing on the original ligands of the target proteins. Molecular docking was performed using the AutoDock Vina 1.5.7 software to confirm the binding activity between the key target proteins and matching active components, with the search space volume >27000 Angstrom3 (using random seed files and each protein box size, see raw data), record affinity value. PyMoL 4.6.0 software was used to visualize the outcomes.
2.4. In Vitro Experiments
2.4.1. The Effect of PAS840 on the Survival Rate of BV-2 Cells Was Detected by cck-8
Mouse microglial cells (BV-2) were provided by Wuhan Punose Life Science Technology Co. Ltd. (Lot number: 8R58KVUP17, Wuhan, China), the BV-2 cells used in the experiments were maintained within 10 passages. The logarithmic growth phase of BV-2 cells was adjusted to a cell suspension with a density of 1.5 × 105 cells/mL. A total of 100 μL of the cell suspension was seeded into 96-well plates and incubated at 37 °C and 5% CO2 for 24 h. Then, 100 μL of PAS840 complete culture medium solution at concentrations of 20, 40, 80, 160, 320, 640, and 1280 μg/mL were added to the corresponding wells (the drug is completely soluble in the culture medium without interference from phosphate-buffered saline or dimethyl sulfoxide), and the Control group was given 100 μL of the complete culture medium. After culturing for 24 h, 10 μL of CCK-8 was added to each well. The reaction was allowed to proceed for 1 h, and the optical density (OD) at 450 nm was measured using a multiwavelength microplate reader. Cell viability (%) and cell activity were calculated, and the safe dose of PAS840 was determined.
2.4.2. Experimental Grouping
Based on the CCK-8 assay results in
Section 2.4.1, select the dosage range that can effectively enhance cell viability without inhibiting cell survival rate as the administration dosage for subsequent experiments. To validate the effect of PAS840 on injured cells in the OGD/R model, we set up the following groups: the Control group without treatment, the OGD/R group, the NLRP3 inhibitor group (10 nm/L, MCC950 + OGD/R) [
33], and the PAS840-treated groups at low, medium, and high doses (40 μg/mL, PAS840 L + OGD/R; 80 μg/mL, PAS840 M + OGD/R; and 160 μg/mL, PAS840 H + OGD/R).
2.4.3. Establishment of OGD/R Model and Detection of Survival Rate of Injured Cells
Logarithmic proliferation period BV-2 cells were obtained, the cell density was adjusted to 1.5 × 10
5 cells/mL cell suspension, 100 μL of which was inoculated into 96-well plates, and the cells were cultured at 37 °C and 5% CO
2 for 24 h under normal conditions. The Control group continued to use a complete culture medium under normal conditions, and the OGD/R group was replaced with glucose-free DMEM. The sugar-free DMED containing PAS840 was replaced in the PAS840 treatment group and cultured separately in a 95% N
2, 5% CO
2 environment for 4, 6, and 8 h before being replaced with a complete medium and further cultured for 24 h. Refer to Methods
Section 2.4.1 for how the CCK-8 assay was used to detect cell viability and determine the subsequent modeling time. According to the grouping conditions described in Methods
Section 2.4.2, cells were seeded in 6-well plates and observed under an inverted microscope after 12 h of drug administration and modeling.
2.4.4. The Migration Ability of PAS840 to OGD/R-Injured Cells Was Detected
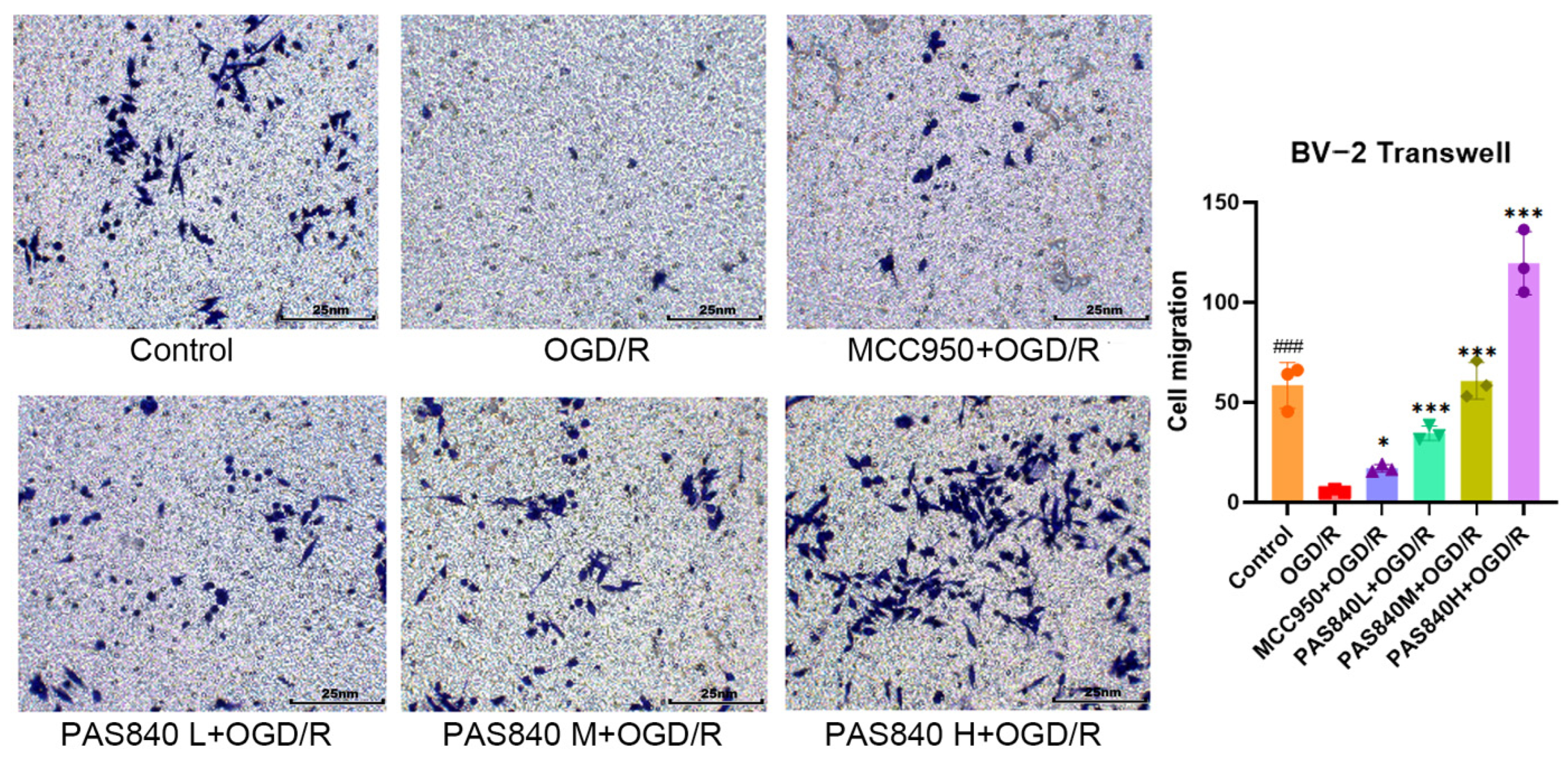
According to the grouping conditions specified in
Section 2.4.2, the cells were cultured in 6-well plates, treated, and modeled according to the method specified in paragraph 2.4.3. In a 24-well plate, 500 μL of medicated complete medium was added according to the grouping conditions specified in
Section 2.4.2, and the cells were suspended in DMEM medium without serum and adjusted to a cell density of 2.5 × 10
5 cells/mL. A total of 200 μL of the cell suspension was then placed on the upper chamber of the Transwell, and the Transwell was placed in the 24-well plate. The cells were cultured at 37 °C and 5% CO
2 for 48 h. After removing the Transwell plate, the cells were fixed with paraformaldehyde and stained with crystal violet for 20 min. The cells were then observed and counted under a microscope.
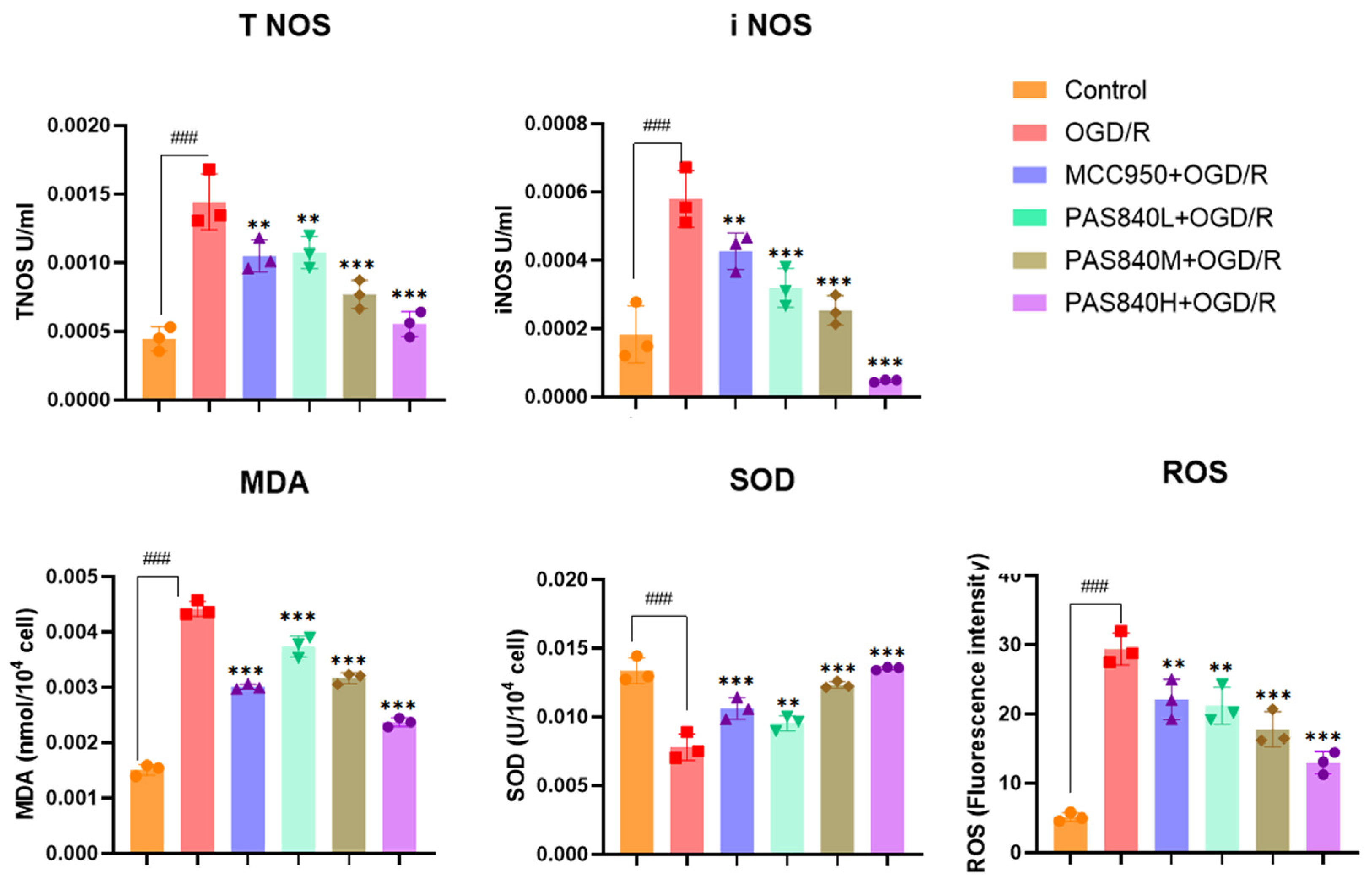
2.4.5. Biochemical Indicators Testing
According to the grouping conditions specified in
Section 2.4.2, the cells were cultured in 6-well plates, treated, and modeled according to the method specified in paragraph 2.4.3. According to the operation instructions of the MDA, SOD, NOS, and ROS kits, take 5 × 10
6 cells from each group of samples. Add the cell lysis buffer provided in the kits to fully lyse the cells. Then, add the working solution provided in the kits as described in the instructions. After a sufficient reaction, take 200 μL from each group of samples and add them to a 96-well plate. Measure the OD values and fluorescence values using a multi-wavelength microplate reader as required by the instructions, and calculate the expression levels of MDA, SOD, NOS, and ROS. All steps were performed in accordance with the manufacturer’s instructions in the test kit manuals.
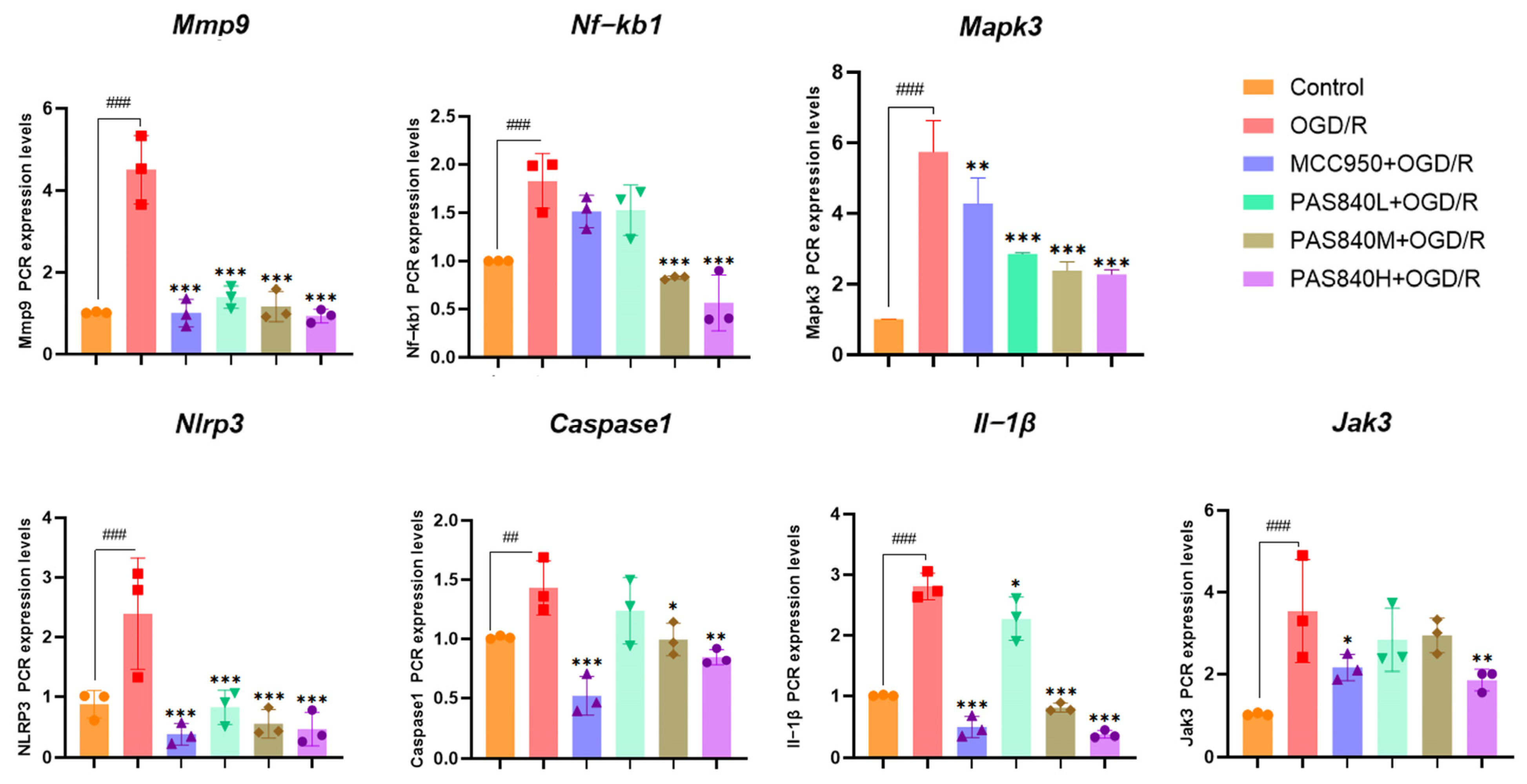
2.4.6. mRNA Expression Detection and Analysis by RT-qPCR in BV-2 Cells
According to the grouping conditions specified in
Section 2.4.2, the cells were seeded into 6-well plates, treated, and modeled according to the method specified in
Section 2.4.3. Add 1 milliliter of Working Solution 1 from the real-time quantitative polymerase chain reaction (RT-qPCR) kit to each group of cells. Extract the total RNA as instructed, and then measure the purity and concentration of the RNA using an ultraviolet spectrophotometer. The concentrations of the sample RNA were standardized, and cDNA was synthesized from total RNA through reverse transcription. The amplified products were analyzed by RT-qPCR using β-actin as an internal reference. The relative quantification of the target gene was performed using the 2
−ΔΔCT method. The primer sequences are listed in
Table 3.
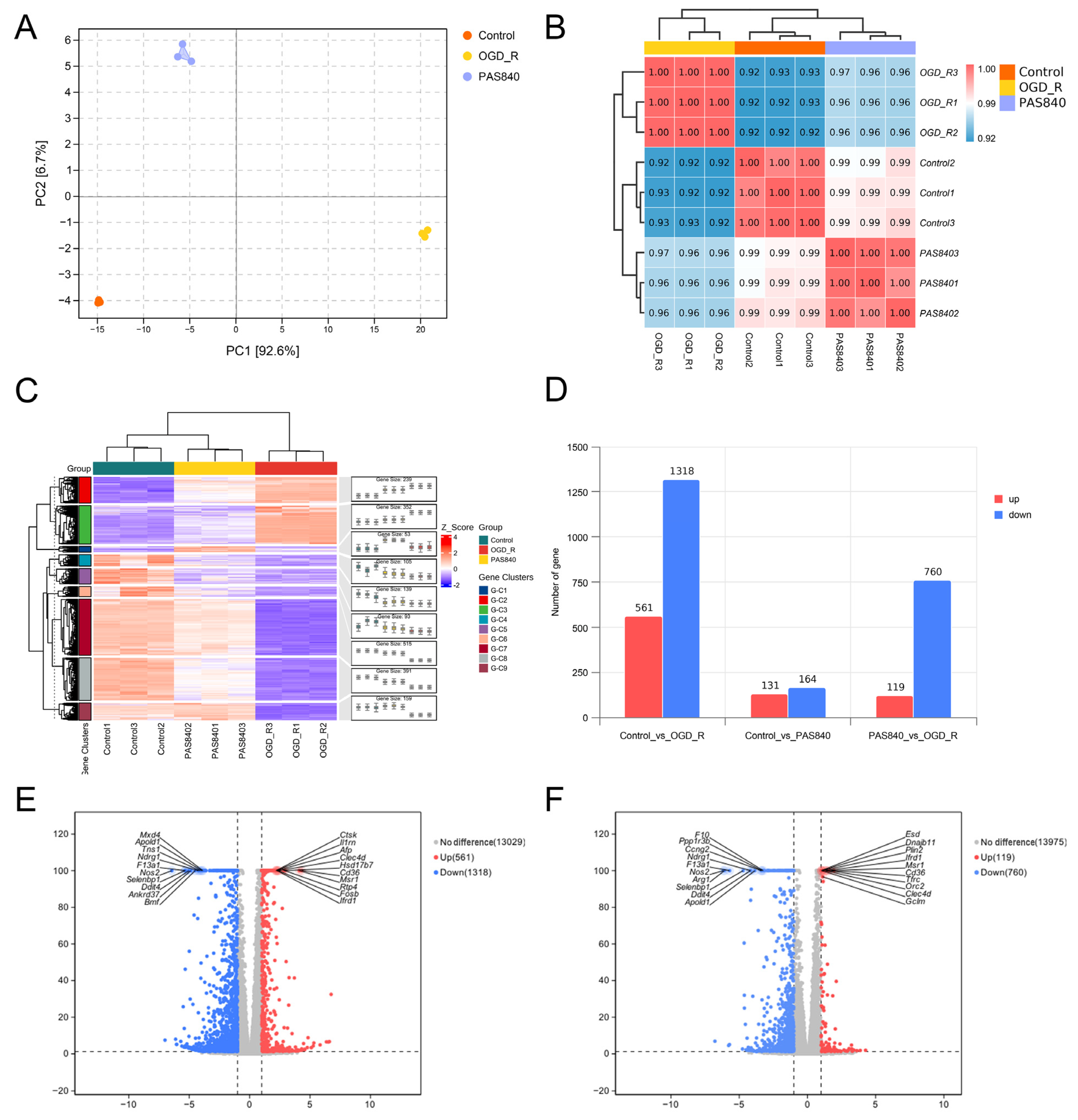
2.4.7. Transcriptomic Sequencing
Following cell inoculation into 6-well plates according to
Section 2.4.2 grouping protocols, modeling and drug treatments were performed as per Methods
Section 2.4.3. Total RNA from Control, OGD/R, and PAS840H + OGD/R groups was quantified and qualified (≥1 µg). RNA libraries were constructed using the NEBNext Ultra II RNA Library Prep Kit (Illumina) (New England Biolabs Inc; Ipswich, MA, USA): Fragmented mRNA templates were reverse-transcribed into double-stranded cDNA with random oligonucleotide primers. After purification and end repair, 3’-terminal “A” overhangs were added. AMPure XP beads were employed to select cDNA fragments (400–500 bp) for final library preparation. Equimolar pooled libraries were diluted to appropriate concentrations and sequenced on an Illumina platform (PE150 mode).
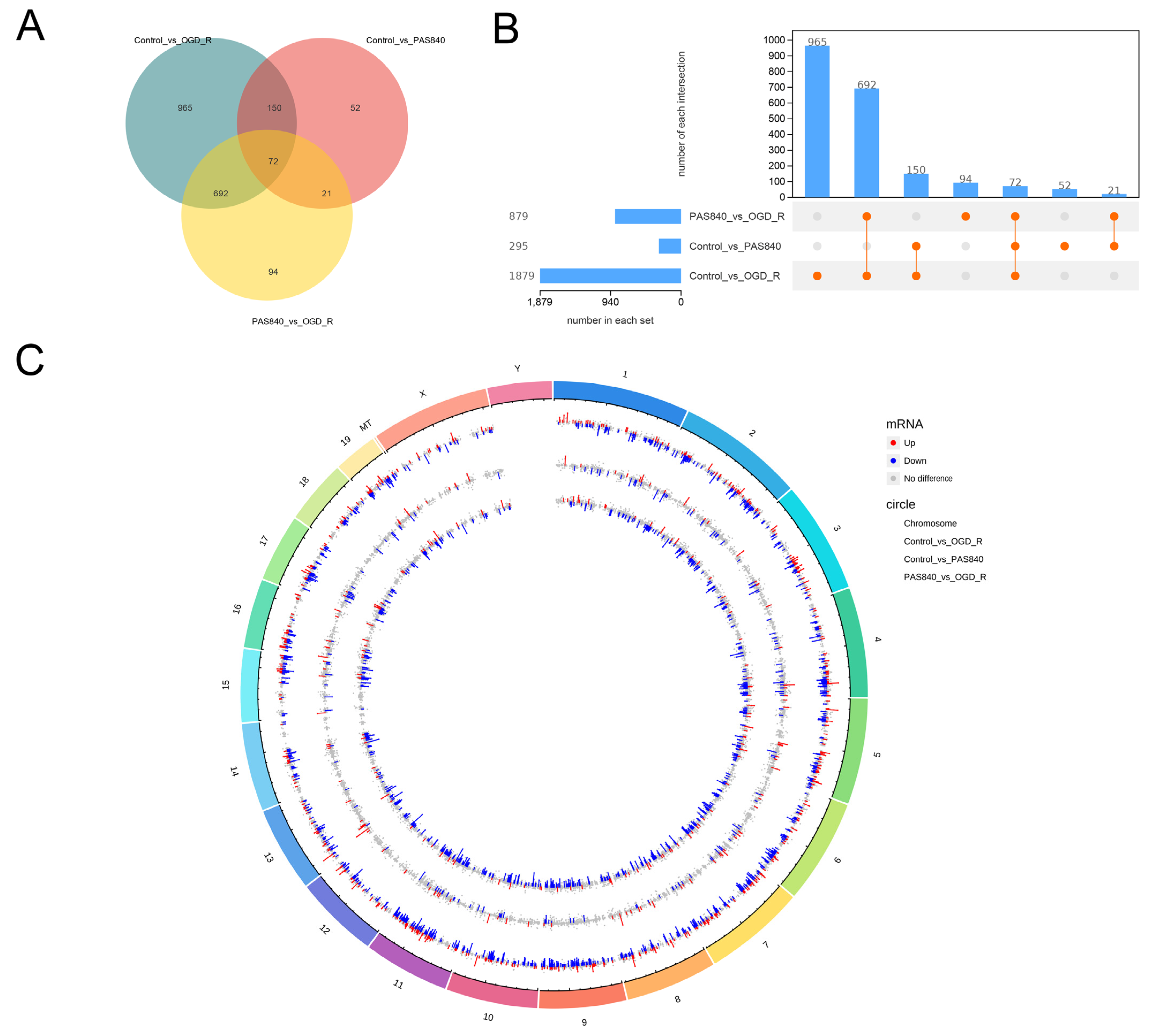
Raw FASTQ data were generated from image files via the sequencing system software. HISAT2 (v2.1.0) indexed the reference genome and aligned clean paired-end reads, while HTSeq (v0.9.1) quantified gene read counts. Differential expression analysis was conducted using DESeq (v1.38.3). GO enrichment analysis (via topGO) calculated p-values for annotated terms through the hypergeometric distribution method (significance threshold: p < 0.05). KEGG pathway analysis implemented with ClusterProfiler statistically evaluated pathway-level gene enrichment using the same significance testing framework.
2.5. In Vivo Experiments
2.5.1. Grouping and Dosing of Animals
In our preliminary studies, we found that female rats exhibited significantly better neurological function than male rats after model establishment. To minimize the interference of estrogen on the therapeutic effect of the disease and better compare drug efficacy, 36 male Sprague-Dawley (SD) rats were used in the experiment. These rats were purchased from Beijing Sipfer Biotechnology Co., Ltd. (License No.: SCXK 2019-0010). The housing conditions were SPF grade, selected rats were 15 weeks of age, and the weight of the rats at the time of modeling was 250 ± 20 g. All rats were placed in a strictly controlled environment (dark/light cycle: 12 h; temperature: 24 ± 2 °C; relative humidity: 65% ± 5%), and each rat was given 60Co irradiated sterilized rat food and purified water daily. The animals involved in this study were approved by the Experimental Animal Ethics Committee of the Dali University (approval number: 2024-PZ-002).
The rats were randomly divided into four groups, with 9 rats per group. They were named the Sham group, Model group, PAS840 L group (gavage: 30 mg/kg), and PAS840 H group (gavage: 120 mg/kg), The gavage dosage was selected based on previously published papers by our research team [
27]. The drugs were dissolved in normal saline for administration, with equivalent volumes of saline administered to both the Sham and Model groups. To investigate the effects of drugs on preventive and adjuvant treatment in high-risk populations for diseases, after 7 days of adaptive feeding, the tMCAO model was established in rats 12 h after the first drug administration. Following model establishment, the rats received the drug for an additional 7 days, with administrations conducted at fixed time points. The Sham group was administered an equivalent volume of normal saline. Based on previous studies by our team, PAS840 reached its absorption peak in rat cerebrospinal fluid 6 h after administration. Therefore, all rats were sacrificed 6 h after the last drug administration, and tissue samples were collected from the rats.
2.5.2. tMCAO Model Establishment
To ensure experimental uniformity, all model establishments for samples were independently completed by the first author within 24 h. A rat tMCAO model was established by a suture occlusion of the right middle cerebral artery. The right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed via a midline neck incision. Anesthesia was induced with 4% isoflurane and maintained by continuous inhalation at 1.75%. Post-anesthesia, the ECA was transected, and the tMCAO monofilament was inserted through the ECA to a depth of (18 ± 1) mm within the ICA. After wound suturing, the tMCAO monofilament was removed at 90 min. In the Sham group, only skin incision and vessel dissection were performed before suturing. Postoperatively, bupivacaine hydrochloride was administered to all rats for analgesia. The operation duration for a single rat was 20 to 25 min, and all sample sizes were selected to meet the animal welfare requirements specified by the Ethics Committee of Dali University and the minimum statistically significant sample size.
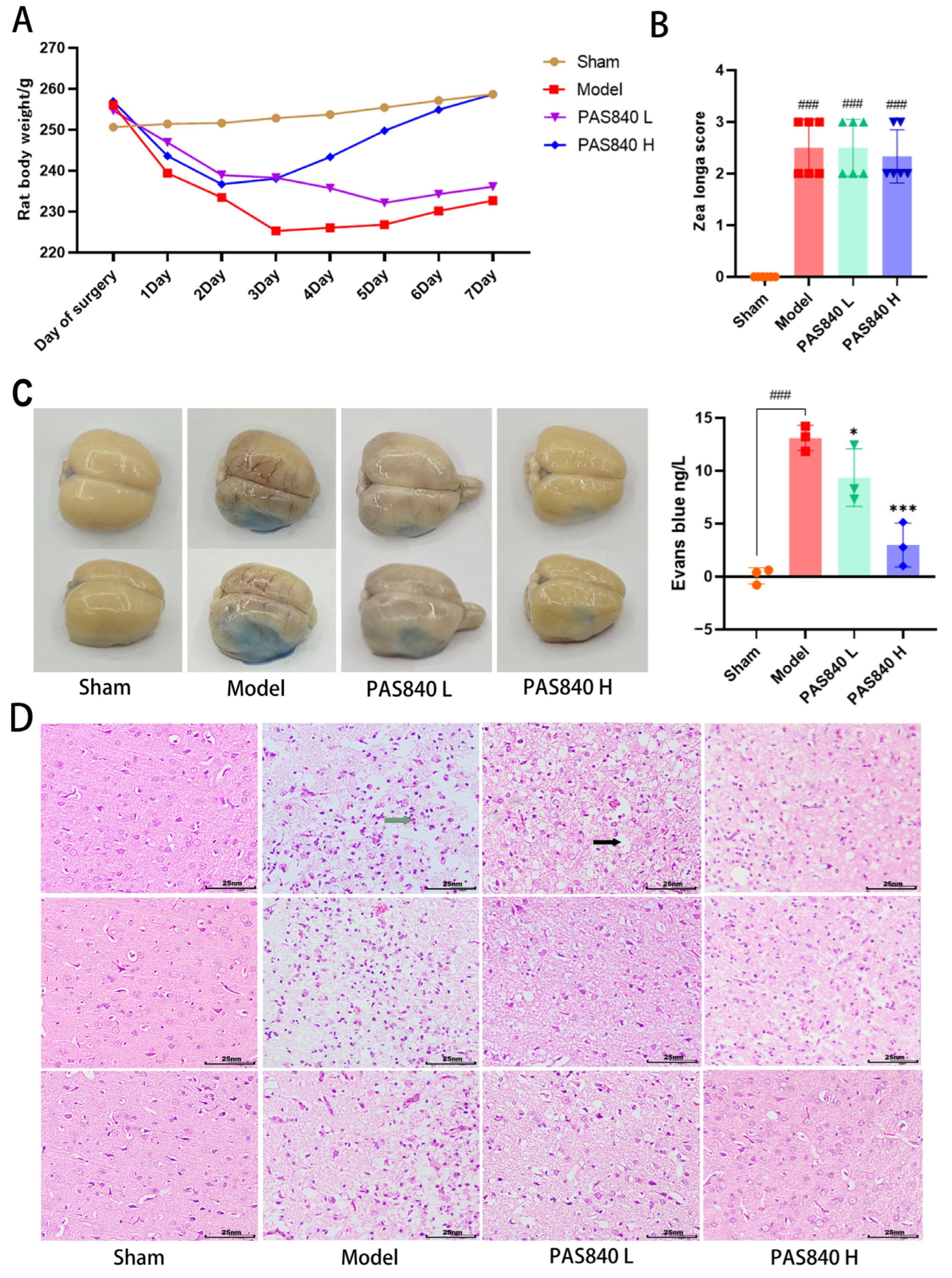
2.5.3. Weight Monitoring
On the day of modeling, as well as after the model was completed, the fasting body weight of each group of rats was monitored daily, and a dynamic weight monitoring curve graph was drawn.
2.5.4. Neurological Function Measurement
Twenty-four hours post-modeling, neurological function in each rat group (
n = 6) was assessed via the Zea Longa scale [
34]. The scoring criteria was as follows: 4 (complete paralysis), 3 (unilateral paralysis), 2 (paralyzed-side dragging during walking), 1 (torso twisting with impaired forelimb extension upon tail lift), and 0 (no deficits). Scores ≥1 confirmed successful nerve injury modeling.
2.5.5. Evans Blue Dyeing
Each group of rats (n = 3) was injected with 2% Evans blue (EB) solution via the tail vein. One hour later, they were anesthetized with isoflurane and perfused with saline. After perfusion was completed, the entire rat brain was removed and photographed. Then, 100 mg of brain tissue was ground using 1 mL of formamide solution, incubated at 37 °C for 24 h, and the OD value at 620 nm was measured using a multi-wavelength microplate reader. A standard curve was plotted using the EB standard solution, and the EB content of all samples was calculated.
2.5.6. TTC Staining
The brain tissue samples from rats (n = 3) were isolated and subjected to TTC histochemical processing. Following cryopreservation at −20 °C for twenty minutes, intact brains were sectioned coronally into five sequential 2 mm thick slices. These tissue sections were subsequently incubated with 4% TTC staining solution under controlled conditions (37 °C, avoiding light) for 20 min.
2.5.7. HE Staining of Brain Tissue
For each group, the brains were collected (n = 3). After wax-embedding, sectioning, dewaxing, and hydration, the brain sections were placed on glass slides. Then, they were stained with hematoxylin and eosin (HE) staining solution, dehydrated, cleared, and sealed. Subsequently, the pathological morphology of the cerebral cortex was observed under a microscope.
2.5.8. mRNA Expression Detection and Analysis by RT-qPCR in Brain Tissue
Taking the cortical part of the infarcted brain tissue of rats in
Section 2.5.7 (
n = 3), and following the experimental method described in
Section 2.4.5, β-actin was used as an internal reference to perform a relative quantitative analysis of the target gene using the 2
−ΔΔCT method. The primer sequences are listed in
Table 4.
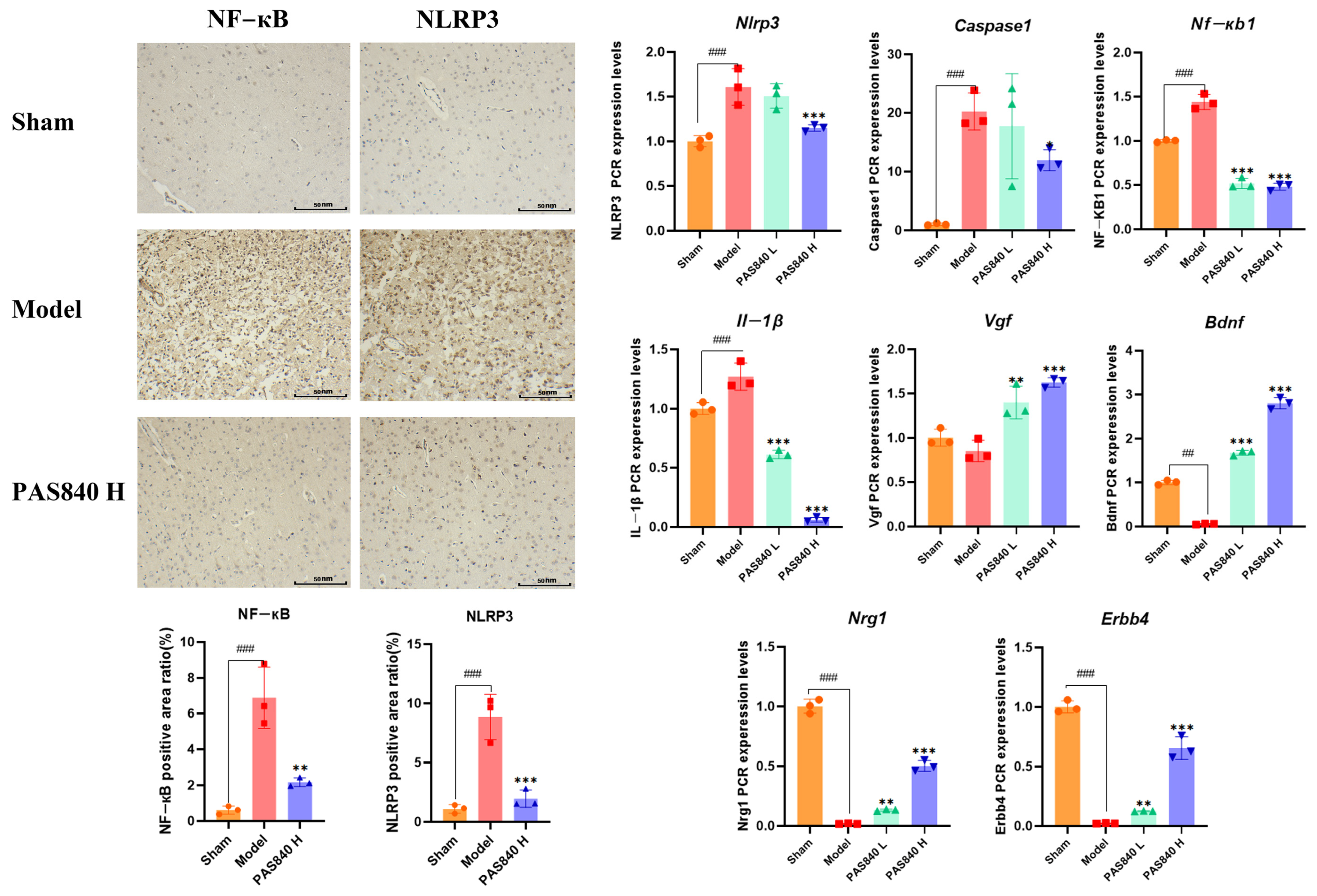
2.5.9. Immunohistochemical Analysis of NLRP3 and NF-κB Expression
Take
Section 2.5.7 rat brain tissue samples (
n = 3), section them with paraffin, dewax them to water, perform antigen repair, block endogenous peroxidases, incubate with 3% rabbit serum at room temperature for 30 min, incubate with the primary antibody at 4 °C overnight, incubate with the secondary antibody at room temperature for 50 min, add a freshly prepared DAB chromogen solution, counterstain with hematoxylin for 3 min, dehydrate, mount the sections, and observe and photograph them under a microscope. Use ImageJ 1.53t for statistical analysis.
2.6. Statistical Analysis
All data were analyzed using the ParsonalBio GenesCloud platform (
https://www.genescloud.cn/cloudClassroom, accessed on 18 November 2024) and SPSS 27. For statistical analysis in SPSS 27, both “Tukey” and “Dunnett-t” were selected as post-hoc multiple comparison methods in the ANOVA results window. Analysis results assumed homogeneity of variance with a significance level set at 0.05, where
p < 0.05 was considered statistically significant. When homogeneity of variance was confirmed, the Tukey method was used for analysis; when homogeneity of variance was not confirmed, the Dunnett-t method was applied instead. The analysis results were visualized and plotted using GraphPad Prism 8.0.2 and the Parsonalbio Genescloud platform. Cytoscape 3.9.1 was used to construct relevant network diagrams.
4. Discussion
IS represents a human condition with limited treatment options, an extremely narrow therapeutic window, and extremely high morbidity and mortality [
35]. The pathogenesis of IS is very complex, and currently, it includes various theories such as brain nerve inflammation, calcium overload, and ferroptosis; however, they all point to the death of neuronal cells caused by intense stimulation injury, which leads to local inflammation in the injured brain region [
36]. This localized brain inflammation exacerbates secondary brain injury by aggravating BBB injury, oxidative stress, and brain edema and directly induces neuronal cell death [
37]. In addition to local inflammation in the brain regions, increasing evidence shows that the inflammatory response following stroke extends throughout the brain, leading to complex secondary conditions [
38]. Thus, for high-risk populations of the disease, reducing inflammation and oxidative stress damage to thereby mitigate sudden disease-related injuries, and minimizing disease-associated complications and sequelae, represent key focuses for drug development targeting this condition.
Microglia are intrinsic immune effector cells of the central nervous system (CNS) that play a crucial role in neuroinflammation. In acute neurodegenerative diseases (stroke, cerebral hypoxia, and brain trauma), the phenotype of microglia changes, inflammatory mediators are released, and a transformation from the early anti-inflammatory phenotype (M2) to the pro-inflammatory phenotype (M1) occurs, which causes neuroinflammation and ultimately leads to varying degrees of inflammatory responses [
39]. M1 phenotype cells secrete various pro-inflammatory factors, such as nitric oxide (NO), reactive oxygen species (ROS), tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and chemokines, etc. [
40]. Intense oxidative stress injury mediates the activation of NF-κB and NLRP3 inflammasomes and stimulates the activation of caspase-1 and IL-1β. Therefore, controlling the oxidative stress response and the cascade reaction of NLRP3 inflammasomes has become key to the treatment of IS.
Related studies have shown that PAE exhibits favorable pharmacological effects, such as anti-inflammation, anti-oxidation, the promotion of tissue repair, the reduction of edema, and the promotion of angiogenesis in various injury-related diseases [
41], which are highly consistent with IS treatment goals. This study predicted the effects of IS using network pharmacology and combined in vitro experiments to construct a BV-2 cell OGD/R injury model and a rat brain tMCAO injury model to detect the activity of oxidative stress injury and inflammasome pathways induced by IS. We investigated the effects of PAS840 on IS-mediated cerebral cell inflammatory responses and pyroptosis to elucidate the possible mechanisms by which PAS840 treats IS.
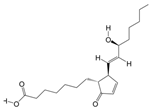
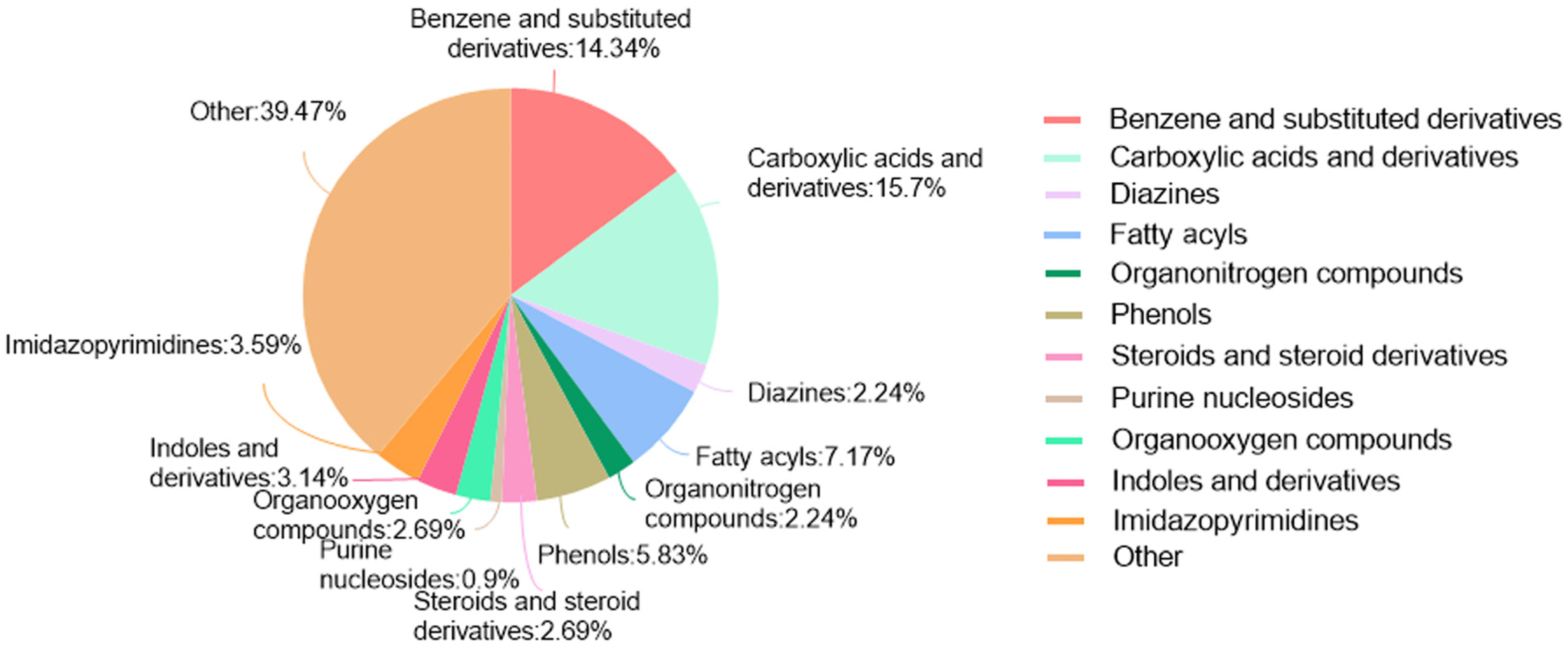
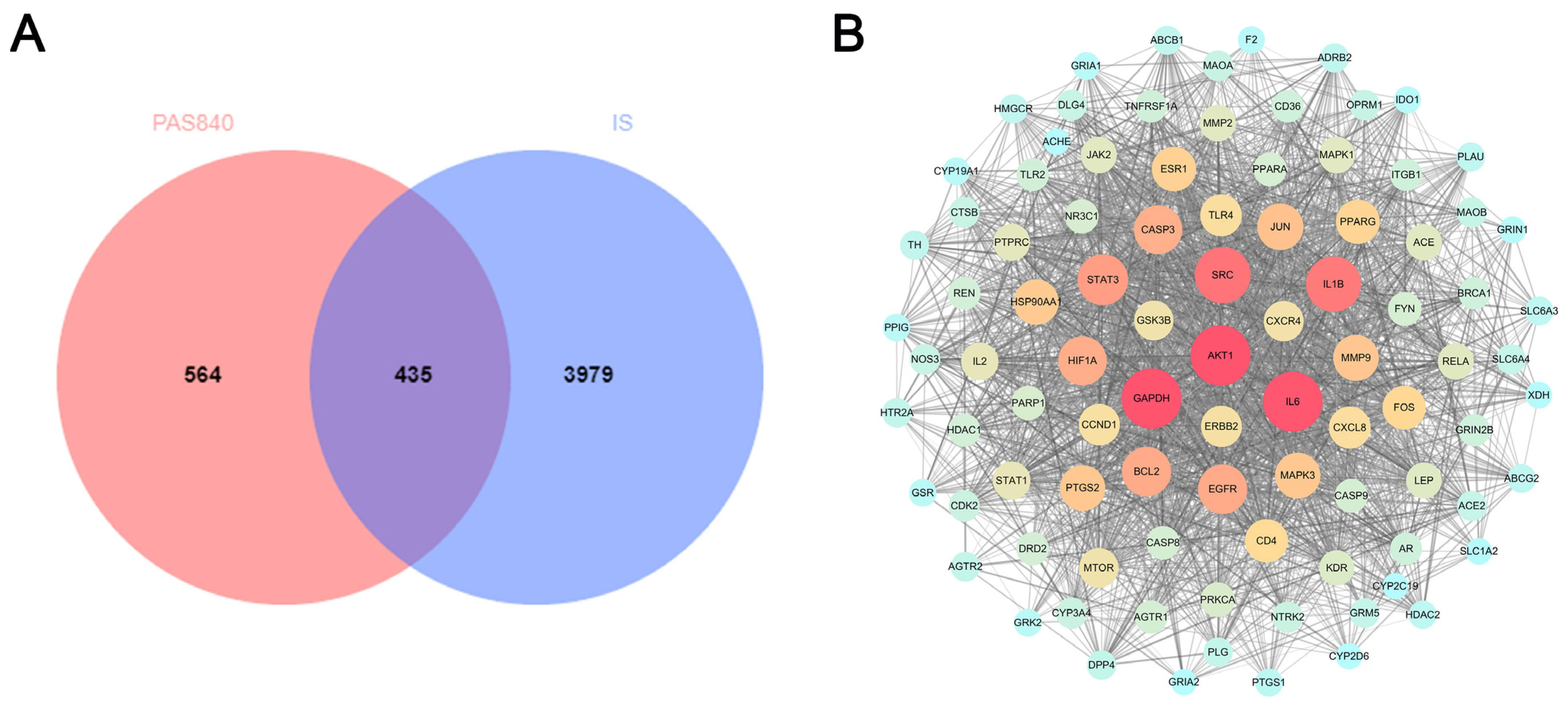
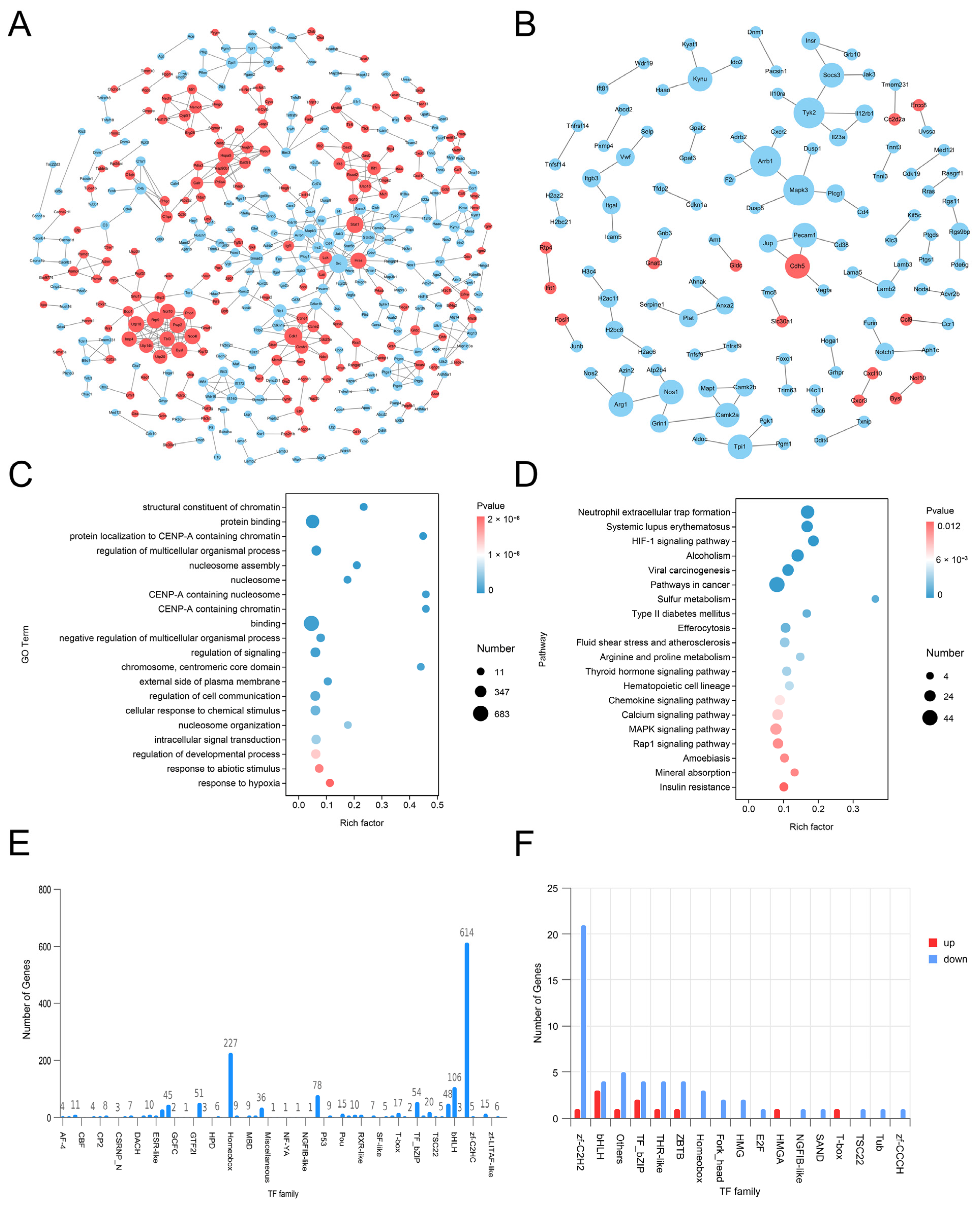
LC-MS/MS and peptidomics analyses revealed that PAS840 contained abundant material components. Through network pharmacology prediction analysis and molecular docking, we screened out 93 key therapeutic targets, among which classical inflammatory signal targets such as NOS, MAPK, Caspase, IL-1β, and IL-6 had relatively high correlation scores. These target proteins suggest that inhibiting the body’s oxidative stress response and inflammation and enhancing the body’s resistance to stimulation-induced apoptosis are the keys to the treatment of IS. In subsequent in vitro cell experiments, PAS840 effectively inhibited the levels of ROS, MDA, and NOS and increased SOD activity, thereby alleviating the oxidative stress caused by OGD/R. It could effectively inhibit the activation of the NF-κB/NLRP3/Caspase-1/IL-1β inflammasome pathway, and reduce the inflammatory response and pyroptosis of BV-2 cells caused by OGD/R injury.
By comparing the core therapeutic targets predicted by network pharmacology with the differential genes between the PAS840 and OGD/R groups in transcriptomics, a high degree of overlap was observed, indicating that the predictions based on network pharmacology in this study possessed a high degree of credibility. A further analysis of the core therapeutic targets and differentially expressed genes revealed that both network pharmacology and transcriptome enrichment analysis results demonstrated PAS840’s positive regulatory role in enhancing cellular resistance to stimulation-induced injury and inflammation, regulating neural signal conduction, and promoting anti-apoptotic processes. It also participates in the treatment of various diseases, including immune dysregulation and toxoplasmosis. Notably, signaling pathways, such as neural receptor–ligand engagement, calcium channels, and neural signal propagation, which are intimately associated with a myriad of neurological pathologies, demonstrate pronounced involvement in these conditions and show therapeutic efficacy.
Microvascular endothelial cells form the structural basis of the BBB. The BBB strictly controls the exchange of nutrients and metabolites between the blood and brain tissue and plays a vital role in maintaining the homeostasis of the cerebral microenvironment and normal neural functions [
42]. BBB disruption is a major pathological manifestation of IS and continuously aggravates the disease course at different stages of cerebral ischemia. These processes include matrix metalloproteinase (MMP) activation, inflammatory expression, vesicular transport, cellular oxidative stress, and cytoskeleton disruption, which further undermine the maintenance of cell stability and nutrient and metabolic transport in the CNS, thereby exacerbating ischemic brain injury and tissue-type plasminogen activator injury [
42]. Therefore, BBB injury and leakage caused by IS and the resulting series of complications are important causes of aggravated IS injury and impeded recovery from the disease course [
43]. Thus, in BBB repair, inhibiting oxidative stress and brain inflammation, reconstructing the microvascular system, and activating neurotrophic factors are important strategies for the modern medical treatment of IS and for reducing the occurrence of complications [
44].
In in vivo animal experiments, the results of HE staining showed that the administration of PAS840 could significantly reduce brain tissue damage. We further explored the in vivo expression of the EB dye solution and its impact on IS through EB staining. We found that after the administration of PAS840, the physiological state of the rats in the treatment group was significantly better than that in the Model group, and the EB staining area and EB content in the brain tissue decreased significantly. Through qPCR experiments and immunohistochemistry experiments, it was found that PAS840 could effectively reduce the expression of the NF-κB/NLRP3/Caspase-1/IL-1β inflammasome pathway and simultaneously activate the BDNF/VGF/NGR1/Erbb4 neurotrophic factors as well as vascular endothelial growth factor. The results of both in vivo and in vitro experiments indicated that PAS840 could effectively inhibit the expression of the inflammasome pathway. This is highly consistent with the results of in vitro cell experiments, and the inhibition of NLRP3 expression, alleviation of oxidative stress, and protection of neuronal cells and the BBB are key factors in modern IS treatment.
Research findings demonstrate that PAS840 mitigates the inflammatory response and oxidative stress levels in BV-2 cells after OGD/R injury, enhances cell viability and migratory capacity, and decreases inflammation and oxidative stress levels in rat brain tissue after tMCAO. PAS840 also alleviates BBB injury and reduces neuronal cell pyroptosis by suppressing the NF-κB/NLRP3/Caspase-1/IL-1β inflammasome pathway and activating the expression of BDNF/VGF/NGR1/Erbb4 neurotrophic factors and vascular endothelial growth factor. This indicates that the pharmacological effects in question likely represent the pivotal mechanism by which PAS840 exerts its therapeutic benefits in the treatment of IS.
Although this study indicated that PAS840 exerts neuroprotective and therapeutic effects against IS-induced brain injury in cellular and animal models, and also indicated its protective role against IS occurrence, it is limited by its reliance on BV-2 microglial cell analysis and exclusive focus on neuroinflammation, thus lacking an exploration of other pathological sites of IS. While immortalized cells enhance experimental reproducibility, model expansion is required, including neuronal cells, human cerebral vascular endothelial cells, and organoid co-culture systems, to validate therapeutic feasibility.
In future work, we will investigate and evaluate the effects of preoperative and postoperative administration regimens of this drug on IS, conduct in vivo experiments in female rats to explore its efficacy, and complete acute toxicity tests and long-term safety assessments. Finally, the integration of multi-omics approaches will be used to decipher mechanisms and optimize synergies with existing therapies, thereby advancing the drug’s development as a preventive and surgical adjuvant for further research and clinical translation.
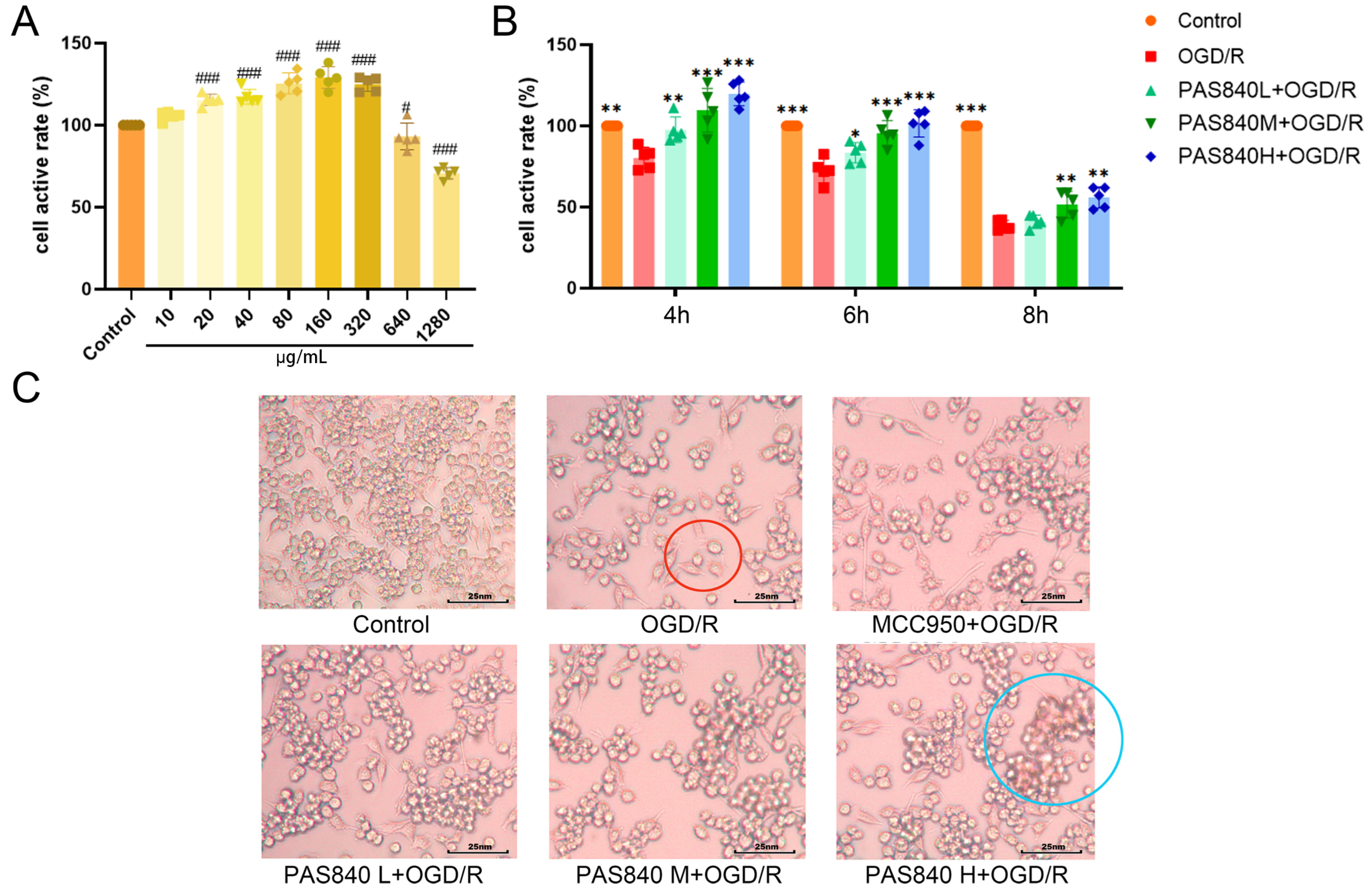
 indicates cellular vacuolation and
indicates cellular vacuolation and  indicates inflammatory cell invasion.
indicates inflammatory cell invasion.
 indicates cellular vacuolation and
indicates cellular vacuolation and  indicates inflammatory cell invasion.
indicates inflammatory cell invasion.