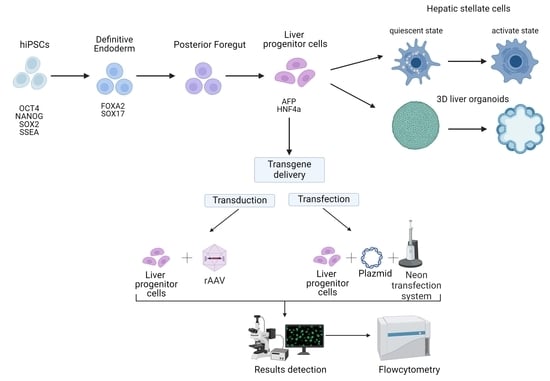
The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtained hiPSC’s Cell Line
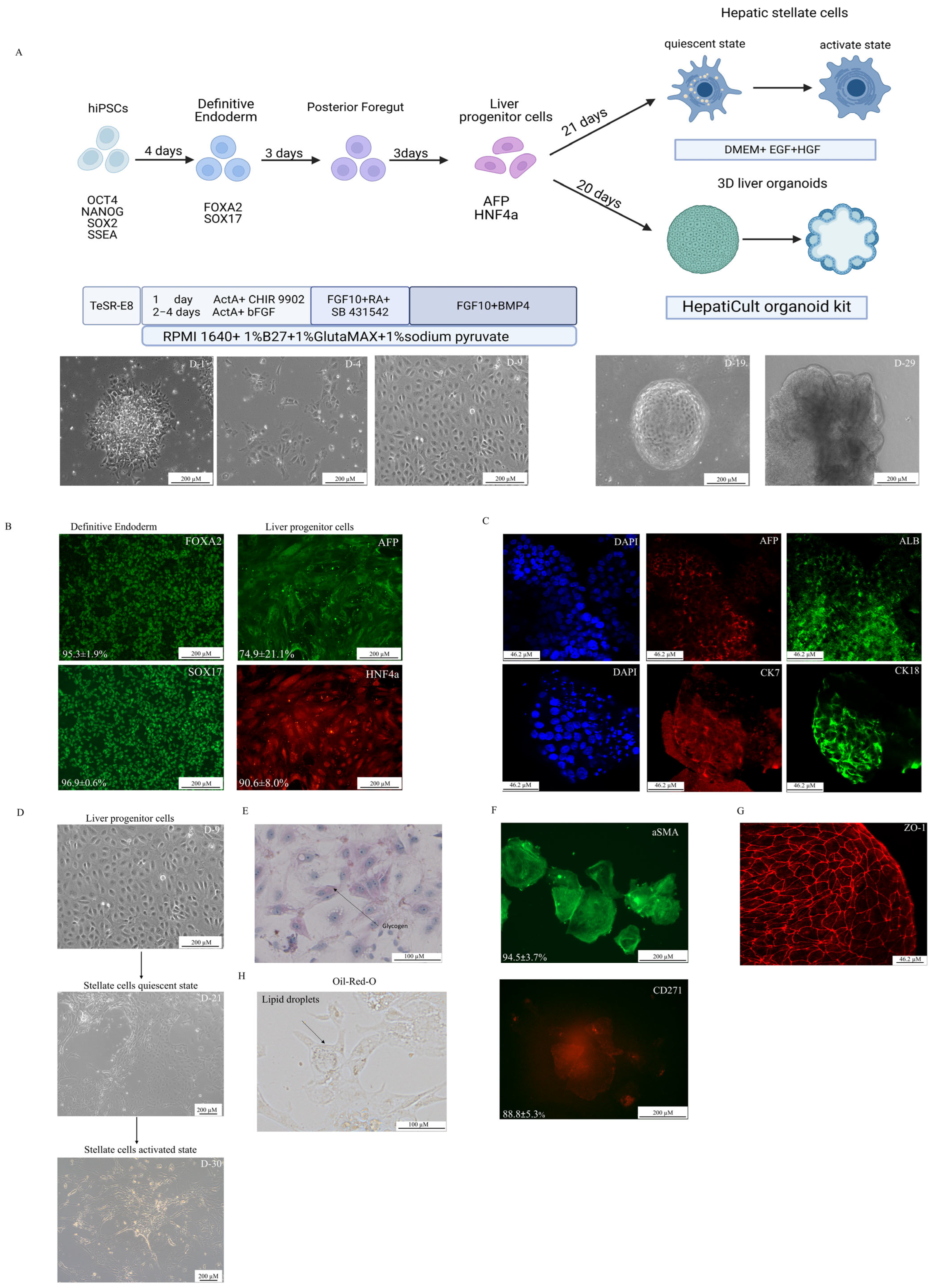
2.2. Directed Differentiation
2.3. Immunocytochemistry
2.4. Oil-Red-O Staining
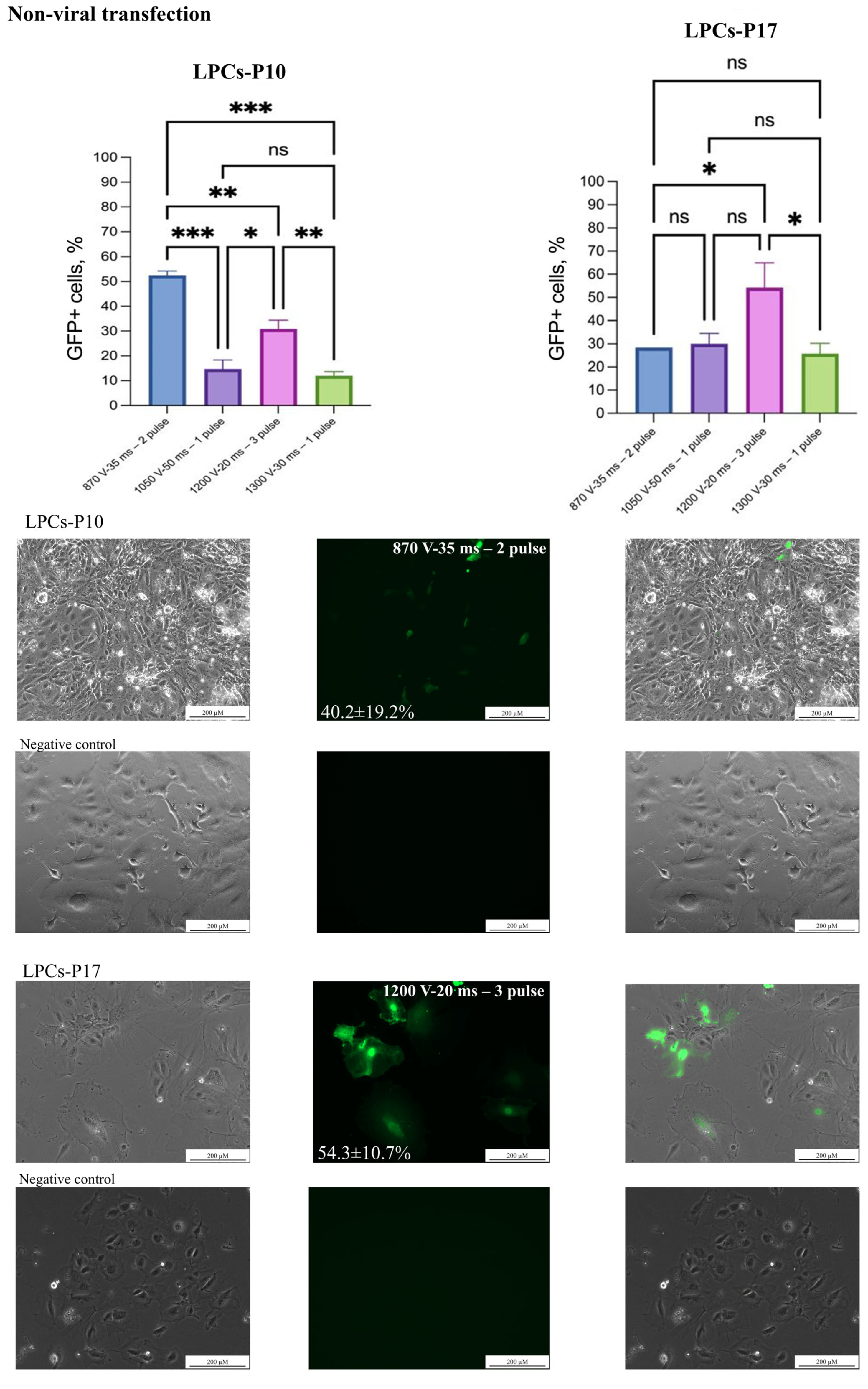
2.5. Non-Viral and Viral Transgene Delivery
2.6. Statistical Analysis
2.7. Gene Expression Analysis
3. Results
3.1. Directed Differentiation
3.2. Non-Viral Transfection
3.3. Viral Transduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altmaier, S.; Meiser, I.; Lemesre, E.; Chanrion, B.; Steeg, R.; Leonte, L.E.; Holst, B.; Nielsen, B.S.; Clausen, C.; Schmidt, K.; et al. Human iPSC-derived hepatocytes in 2D and 3D suspension culture for cryopreservation and in vitro toxicity studies. Reprod. Toxicol. 2022, 111, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Mich-Basso, J.D.; Lin, B.; Yang, L. High Efficient Differentiation of Functional Hepatocytes from Porcine Induced Pluripotent Stem Cells. PLoS ONE 2014, 9, e100417. [Google Scholar] [CrossRef] [PubMed]
- Chikada, H.; Kamiya, A. Molecular Mechanisms Regulating the Proliferation and Maturation of Hepatic Progenitor Cells During Liver Development. In Stem Cells and Cancer in Hepatology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–34. [Google Scholar]
- Coll, M.; Perea, L.; Boon, R.; Leite, S.B.; Vallverdú, J.; Mannaerts, I.; Smout, A.; El Taghdouini, A.; Blaya, D.; Rodrigo-Torres, D.; et al. Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell 2018, 23, 101–113.e7. [Google Scholar] [CrossRef]
- Bronsard, J.; Savary, C.; Massart, J.; Viel, R.; Moutaux, L.; Catheline, D.; Rioux, V.; Clement, B.; Corlu, A.; Fromenty, B.; et al. 3D multi-cell-type liver organoids: A new model of non-alcoholic fatty liver disease for drug safety assessments. Toxicol. Vitr. 2024, 94, 105728. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, D.; Garfin, P.M.; Ehmer, U.; Hurwitz, M.; Enns, G.; Michie, S.; Wu, M.; Zheng, M.; Nishimura, T.; et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017, 2, e94954. [Google Scholar] [CrossRef]
- Wu, F.; Wu, D.; Ren, Y.; Huang, Y.; Feng, B.; Zhao, N.; Zhang, T.; Chen, X.; Chen, S.; Xu, A. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J. Hepatol. 2019, 70, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.J.; Hong, Y.-H.; Shin, Y.; Lee, J.; Cho, H.-S.; Kim, D.-S.; Chung, K.-S.; Son, M.J. Efficient and reproducible generation of human induced pluripotent stem cell-derived expandable liver organoids for disease modeling. Sci. Rep. 2023, 13, 22935. [Google Scholar] [CrossRef]
- Blaszkiewicz, J.; Duncan, S.A. Use of stem cell-derived hepatocytes to model liver disease. J. Hepatol. 2024, 80, 826–828. [Google Scholar] [CrossRef]
- Hu, Y.; Geng, Q.; Wang, L.; Wang, Y.; Huang, C.; Fan, Z.; Kong, D. Research progress and application of liver organoids for disease modeling and regenerative therapy. J. Mol. Med. 2024, 102, 859–874. [Google Scholar] [CrossRef]
- Kostadinova, R.; Boess, F.; Applegate, D.; Suter, L.; Weiser, T.; Singer, T.; Naughton, B.; Roth, A. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2013, 268, 1–16. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Wang, Y.; Liu, J.; Lavrijsen, M.; Li, Y.; Zhang, R.; Verstegen, M.M.A.; Wang, Y.; Li, T.-C.; et al. Recapitulating hepatitis E virus–host interactions and facilitating antiviral drug discovery in human liver–derived organoids. Sci. Adv. 2022, 8, eabj5908. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.-Z.; Zheng, Y.-W.; Miyakawa, K.; Murata, S.; Zhang, R.-R.; Sekine, K.; Ueno, Y.; Takebe, T.; Wakita, T.; Ryo, A.; et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine 2018, 35, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Serras, A.S.; Rodrigues, J.S.; Cipriano, M.; Rodrigues, A.V.; Oliveira, N.G.; Miranda, J.P. A Critical Perspective on 3D Liver Models for Drug Metabolism and Toxicology Studies. Front. Cell Dev. Biol. 2021, 9, 626805. [Google Scholar] [CrossRef] [PubMed]
- Abady, M.; Zahran, I.A.; Elmokhtar, Y. The liver organoid’s past, present and future: A personalized medicine strategy. Int. J. Health Sci. 2024, 8, 972–998. [Google Scholar] [CrossRef]
- Hendriks, D.; Brouwers, J.F.; Hamer, K.; Geurts, M.H.; Luciana, L.; Massalini, S.; López-Iglesias, C.; Peters, P.J.; Rodríguez-Colman, M.J.; de Sousa Lopes, S.C.; et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 2023, 41, 1567–1581. [Google Scholar] [CrossRef]
- Siller, R.; Sullivan, G.J. Rapid Screening of the Endodermal Differentiation Potential of Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2017, 43, 1G.7.1–1G.7.23. [Google Scholar] [CrossRef]
- Kondrateva, E.; Grigorieva, O.; Panchuk, I.; Bychkov, I.; Zakharova, E.; Tabakov, V.; Pozhitnova, V.; Voronina, E.; Shchagina, O.; Lavrov, A.; et al. Generation of induced pluripotent stem cell line (RCMGi012-A) from fibroblasts of patient with mucopolysaccharidosis type VI. Stem Cell Res. 2023, 73, 103259. [Google Scholar] [CrossRef]
- Panchuk, I.O.; Grigorieva, O.V.; Kondrateva, E.V.; Kurshakova, E.V.; Tabakov, V.Y.; Bychkov, I.O.; Zakharova, E.Y.; Orlova, M.D.; Voronina, E.S.; Pozhitnova, V.O.; et al. Generation of two iPSC lines from patient with Mucopolysaccharidosis IV B type and autosomal recessive non-syndromic hearing loss 12. Stem Cell Res. 2023, 71, 103183. [Google Scholar] [CrossRef]
- Panchuk, I.O.; Grigorieva, O.V.; Kondrateva, E.V.; Kurshakova, E.V.; Petrova, I.O.; Voronina, E.S.; Pozhitnova, V.O.; Shchagina, O.A.; Tabakov, V.Y.; Strokova, T.V.; et al. Generation of iPSC Lines from Family with Glycogen Storage Disease Type Ia. Russ. J. Dev. Biol. 2025. [Google Scholar] [CrossRef]
- Belova, L.; Demchenko, A.; Kochergin-Nikitsky, K.; Kondrateva, E.; Slesarenko, Y.; Salikhova, D.; Lavrov, A.; Efremova, A.; Bukharova, T.; Goldshtein, D.; et al. Recombinant Adeno-associated Viral Vectors Serotypes 6 and 9 are Able to Transduce Human Tracheal Epithelial Cells but Not Human Induced Pluripotent Stem Cells. Mol. Biotechnol. 2023, 65, 1539–1546. [Google Scholar] [CrossRef]
- Beskorovainiy, N.S. [NGSDATA], Computer software. Certificate No. 2021614055; Research Centre for Medical Genetics: Moscow, Russia, 2021. [Google Scholar]
- Senate, S. 5002-FDA Modernization Act 2.0, A Bill to Allow for Alternatives to Animal Testing for Purposes of Drug and Biological Product Applications. 2022. Available online: https://www.congress.gov/bill/117th-congress/senate-bill/5002 (accessed on 10 January 2025).
- Graffmann, N.; Ncube, A.; Wruck, W.; Adjaye, J. Cell fate decisions of human iPSC-derived bipotential hepatoblasts depend on cell density. PLoS ONE 2018, 13, e0200416. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.M.; Palaria, A.; Ang, L.T. Efficient Differentiation of Human Pluripotent Stem Cells into Liver Cells. J. Vis. Exp. 2019, 148, e58975. [Google Scholar]
- Du, C.; Feng, Y.; Qiu, D.; Xu, Y.; Pang, M.; Cai, N.; Xiang, A.P.; Zhang, Q. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res. Ther. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef]
- Engert, S.; Burtscher, I.; Liao, W.P.; Dulev, S.; Schotta, G.; Lickert, H. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development 2013, 140, 3128–3138. [Google Scholar] [CrossRef]
- Gao, X.; Li, R.; Cahan, P.; Zhao, Y.; Yourick, J.J.; Sprando, R.L. Hepatocyte-like cells derived from human induced pluripotent stem cells using small molecules: Implications of a transcriptomic study. Stem Cell Res. Ther. 2020, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.P.; Siller, R.; Tanaka, Y.; Chollet, M.E.; de la Morena-Barrio, M.E.; Xiang, Y.; Patterson, B.; Andersen, E.; Bravo-Pérez, C.; Kempf, H.; et al. Scalable production of tissue-like vascularized liver organoids from human PSCs. Exp. Mol. Med. 2023, 55, 2005–2024. [Google Scholar] [CrossRef]
- Song, Z.; Cai, J.; Liu, Y.; Zhao, D.; Yong, J.; Duo, S.; Song, X.; Guo, Y.; Zhao, Y.; Qin, H.; et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009, 19, 1233–1242. [Google Scholar] [CrossRef]
- Suominen, S.; Hyypijev, T.; Venäläinen, M.; Yrjänäinen, A.; Vuorenpää, H.; Lehti-Polojärvi, M.; Räsänen, M.; Seppänen, A.; Hyttinen, J.; Miettinen, S.; et al. Improvements in Maturity and Stability of 3D iPSC-Derived Hepatocyte-like Cell Cultures. Cells 2023, 12, 2368. [Google Scholar] [CrossRef]
- Zhang, R.; Takebe, T.; Sekine, K.; Koike, H.; Zheng, Y.; Taniguchi, H. Identification of Proliferating Human Hepatic Cells from Human Induced Pluripotent Stem Cells. Transpl. Proc. 2014, 46, 1201–1204. [Google Scholar] [CrossRef]
- Koui, Y.; Himeno, M.; Mori, Y.; Nakano, Y.; Saijou, E.; Tanimizu, N.; Kamiya, Y.; Anzai, H.; Maeda, N.; Wang, L.; et al. Development of human iPSC-derived quiescent hepatic stellate cell-like cells for drug discovery and in vitro disease modeling. Stem Cell Rep. 2021, 16, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Narumiya, S. Roles of hepatic stellate cells in liver inflammation: A new perspective. Inflamm. Regen. 2016, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú, J.; de la Torre, R.A.M.G.; Mannaerts, I.; Verhulst, S.; Smout, A.; Coll, M.; Ariño, S.; Rubio-Tomás, T.; Aguilar-Bravo, B.; Martínez-Sánchez, C.; et al. Directed differentiation of human induced pluripotent stem cells to hepatic stellate cells. Nat. Protoc. 2021, 16, 2542–2563. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen; Martinez, M.A.; Stokowiec, J.; Wang, C.; Aizenshtadt, A.; Krauss, S. Characterization of human stem cell-derived hepatic stellate cells and liver sinusoidal endothelial cells during extended in vitro culture. Front. Bioeng. Biotechnol. 2023, 11, 1223737. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, Y.; Xiao, X.; Hu, L.; Xu, J.; Zheng, D.; Koc, H.C.; Chan, U.I.; Meng, Y.; Lu, L.; et al. Definitive Endodermal Cells Supply an in vitro Source of Mesenchymal Stem/Stromal Cells. Commun. Biol. 2023, 6, 476. [Google Scholar] [CrossRef]
- Brees, C.; Fransen, M. A cost-effective approach to microporate mammalian cells with the Neon Transfection System. Anal. Biochem. 2014, 466, 49–50. [Google Scholar] [CrossRef]
- Palaschak, B.; Herzog, R.W.; Markusic, D.M. AAV-Mediated Gene Delivery to the Liver: Overview of Current Technologies and Methods. Methods Mol. Biol. 2019, 1950, 333–360. [Google Scholar]
- Datsomor, A.K.; Wilberg, R.; Torgersen, J.S.; Sandve, S.R.; Harvey, T.N. Efficient transfection of Atlantic salmon primary hepatocyte cells for functional assays and gene editing. G3 Genes Genomes Genet. 2023, 13, jkad039. [Google Scholar] [CrossRef]
- Castle, M.J. (Ed.) Adeno-Associated Virus Vectors; Springer: New York, NY, USA, 2019. [Google Scholar]
- Jacobs, F.; Gordts, S.; Muthuramu, I.; De Geest, B. The Liver as a Target Organ for Gene Therapy: State of the Art, Challenges, and Future Perspectives. Pharmaceuticals 2012, 5, 1372–1392. [Google Scholar] [CrossRef]
- Pillay, S.; Zou, W.; Cheng, F.; Puschnik, A.S.; Meyer, N.L.; Ganaie, S.S.; Deng, X.; Wosen, J.E.; Davulcu, O.; Yan, Z.; et al. Adeno-associated Virus (AAV) Serotypes Have Distinctive Interactions with Domains of the Cellular AAV Receptor. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Westhaus; Cabanes-Creus, M.; Dilworth, K.L.; Zhu, E.; Gómez, D.S.; Navarro, R.G.; Amaya, A.K.; Scott, S.; Kwiatek, M.; McCorkindale, A.L.; et al. Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors. Hum. Gene Ther. 2023, 34, 273–288. [Google Scholar] [CrossRef] [PubMed]


| Antibodies | Source | Identifier |
|---|---|---|
| Rabbit anti-SOX17 | Abcam, UK | Cat#ab224637; RRID: AB_2801385 |
| Rabbit anti-HNF1A | Abclonal, USA | Cat#A20865; RRID: AB_2728751 |
| Rabbit anti-FOXA2 | Abcam, UK | Cat#ab108422; RRID:AB_11157157 |
| Mouse Anti-Alpha-Fetoprotein (AFP) | Abclonal, USA | Cat#A17898; RRID:AB_2861748 |
| Rabbit Anti-Albumin | Abcam, UK | Cat#ab106582;RRID:AB_10888110 |
| Rabbit Anti-Cytokeratin 18 | Abcam, UK | Cat#ab133263; RRID:AB_11155892 |
| Rabbit Anti-Cytokeratin 7 | Abcam, UK | Cat#ab181598; RRID:AB_2783822 |
| Anti-CD271 (NGF Receptor)-PE | Invitrogen, USA | Cat#12-9400-42; RRID:AB_2572710 |
| a-SMA, alpha smooth muscle Actin Rabbit mAb | Abclonal, USA | Cat#A17910; RRID:AB_2861755 |
| ZO-1 | Abcam, UK | Cat#ab216880; RRID:AB_2909434 |
| Goat anti-Mouse IgG (H + L), Alexa Fluor 594 | Thermo Fisher Scientific, USA | Cat#A-11032; RRID:AB_2534091 |
| Goat anti-Rabbit IgG (H + L), Alexa Fluor 594 | Thermo Fisher Scientific, USA | Cat#A-11037; RRID:AB_2534095 |
| Goat anti-Rat IgG (H + L), Alexa Fluor 488 | Abcam, UK | Cat#ab150113; RRID:AB_2576208 |
| Anti-Rabbit IgG H&L, Alexa Fluor 488 | Abcam, UK | Cat#ab150077; RRID:AB_2630356 |
| Cell Line | Neon Protocol Electroporation |
|---|---|
| ChangX-31 | 1050 V−50 ms—1 pulse |
| HepG2 | 1200 V−20 ms—3 pulse |
| SK-Hep-1 | 870 V−35 ms—2 pulse |
| H-4-II-E | 1600 V−20 ms—1 pulse 1300 V−30 ms—1 pulse |
| Publications | S. Altmaier et al. [1] | Y. Ao et al. [2] | X. Gao et al. [29] | N. Graffman et al. [24] | C. Du et al. [26] | Present Article | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definitive endoderm | ||||||||||||
| Medium | MCDB131 | RPMI | RPMI 1640 B27 supplement minus insulin | RPMI 1640 B27 supplement minus insulin | RPMI 1640 1% B27 without vitamin A | RPMI 1640 1% B27 without vitamin A | ||||||
| Small molecules | Day 1 | 0.5% BSA 1.5 g/L NaHCO3 10 mM Glucose 1% Glutamax 0.1% Pen/Strep 100 ng/mL Activin A 3 µM CHIR99021 | Day 1 | 100 ng/mL Activin A 25 ng/mL Wnt 3a | Day 1 | 3 μM CHIR99021 | Day 1 | 1% Glutamax 1% Pen/Strep 100 ng/mL Activin A 2.5 μM CHIR99021 | Day 1 | 3 μM CHIR99021 | Day 1 | 1% Glutamax 1% sodium pyruvate 100 ng/mL Activin A 3 μM CHIR99021 |
| Day 2–3 | 0.5% BSA 1.5 g/L NaHCO3 10 mM Glucose, 1% Glutamax 0.1% Pen/Strep 100 ng/mL Activin A | Day 2–5 | 100 ng/mL Activin A 10 ng/mL bFGF | Day 2 | - | Day 2–5 | 1% Glutamax 1% Pen/Strep 100 ng/mL Activin A | Day 2 | Day 2–3 | 1% Glutamax 1% sodium pyruvate 100 ng/mL Activin A 10 ng/mL FGFb | ||
| Hepatocyte progenitors and specification (hepatoblast cells) | ||||||||||||
| DMEM/F12 | SFD | DMEM | Knockout DMEM | DMEM/F12 1% B27 Serum-Free-Supplement | RPMI1640 1% B27 without vitamin A | |||||||
| Small molecules | Day 4–9 | 10% KOSR 1% Glutamax 1% Non-essential amino acids (NEAAs) 1% Pen/Strep 1% DMSO | Day 6 –8 | 10 ng/mL bFGF 50 ng/mL bone morphogenetic protein 4 (BMP4) 10 ng/mL epidermal growth factor (EGF) 100 ng/mL hepatic growth factor (HGF) | Day 3–8 | 1% DMSO 20% knockout serum replacement 2 mM Glutamax 1× MEM non-essential amino acids 100 μM 2-mercaptoethanol | Day 6–10 | 20% Knockout serum replacement 0.5% Glutamax 1% P/S, 0.01% 2-Mercaptoethanol 1% DMSO | Day 3–8 | 1% KOSR 1% Glutamax 1% NEAA 0.5 µM A83-01 250 nM sodium butyrate 0.5% DMSO | Day 4–6 | 1% Glutamax 1% sodium pyruvate 50 ng/mL FGF-10 10 µM Retinoid acid 10 µM SB431542 |
| Day 7–9 | 1%Glutamax 1% sodium pyruvate 50 ng/mL FGF-10 10 µM BMP4 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchuk, I.; Kovalskaia, V.; Balinova, N.; Ryzhkova, O.; Smirnikhina, S. The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes. Biology 2025, 14, 586. https://doi.org/10.3390/biology14060586
Panchuk I, Kovalskaia V, Balinova N, Ryzhkova O, Smirnikhina S. The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes. Biology. 2025; 14(6):586. https://doi.org/10.3390/biology14060586
Chicago/Turabian StylePanchuk, Irina, Valeriia Kovalskaia, Natalia Balinova, Oxana Ryzhkova, and Svetlana Smirnikhina. 2025. "The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes" Biology 14, no. 6: 586. https://doi.org/10.3390/biology14060586
APA StylePanchuk, I., Kovalskaia, V., Balinova, N., Ryzhkova, O., & Smirnikhina, S. (2025). The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes. Biology, 14(6), 586. https://doi.org/10.3390/biology14060586