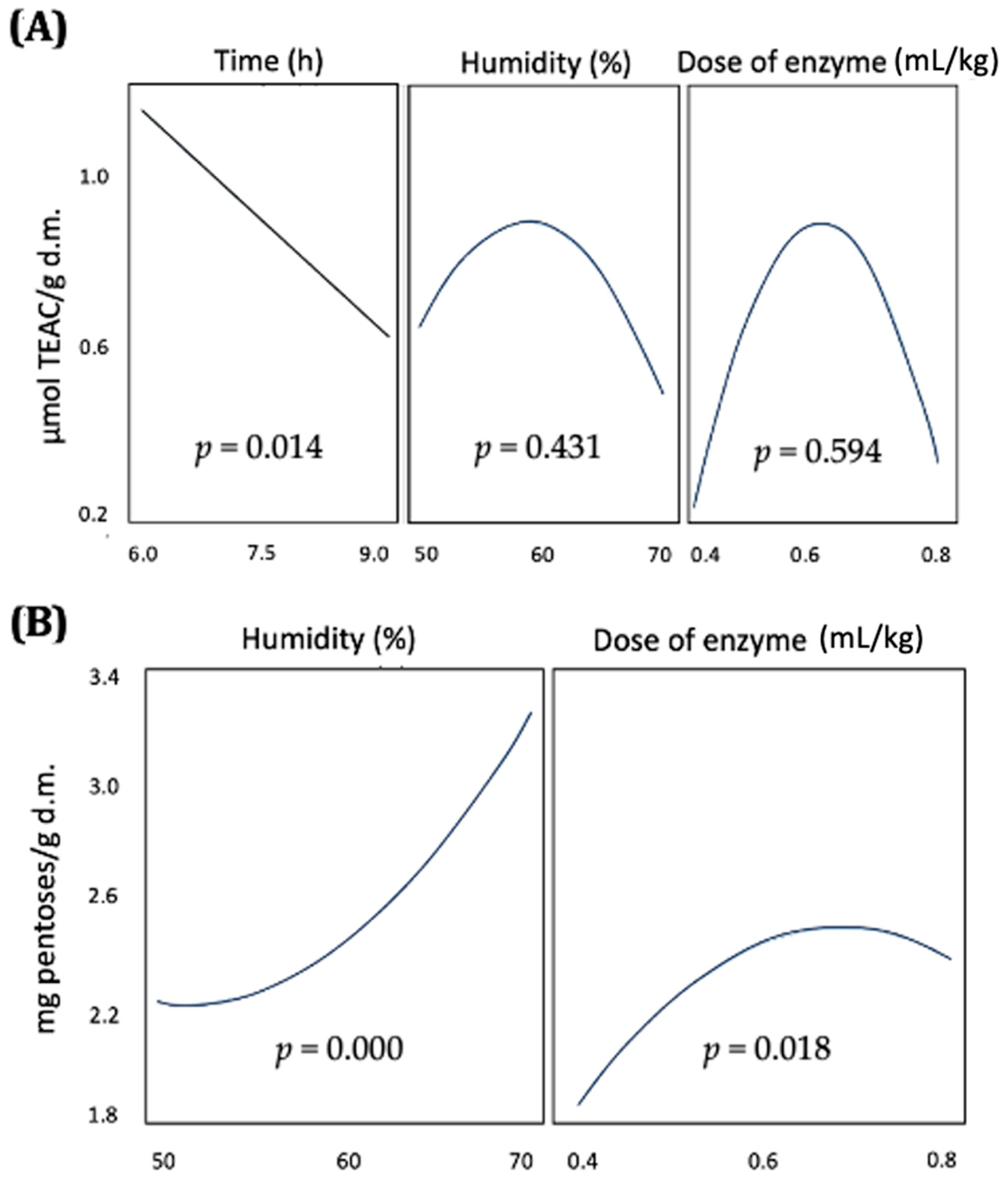
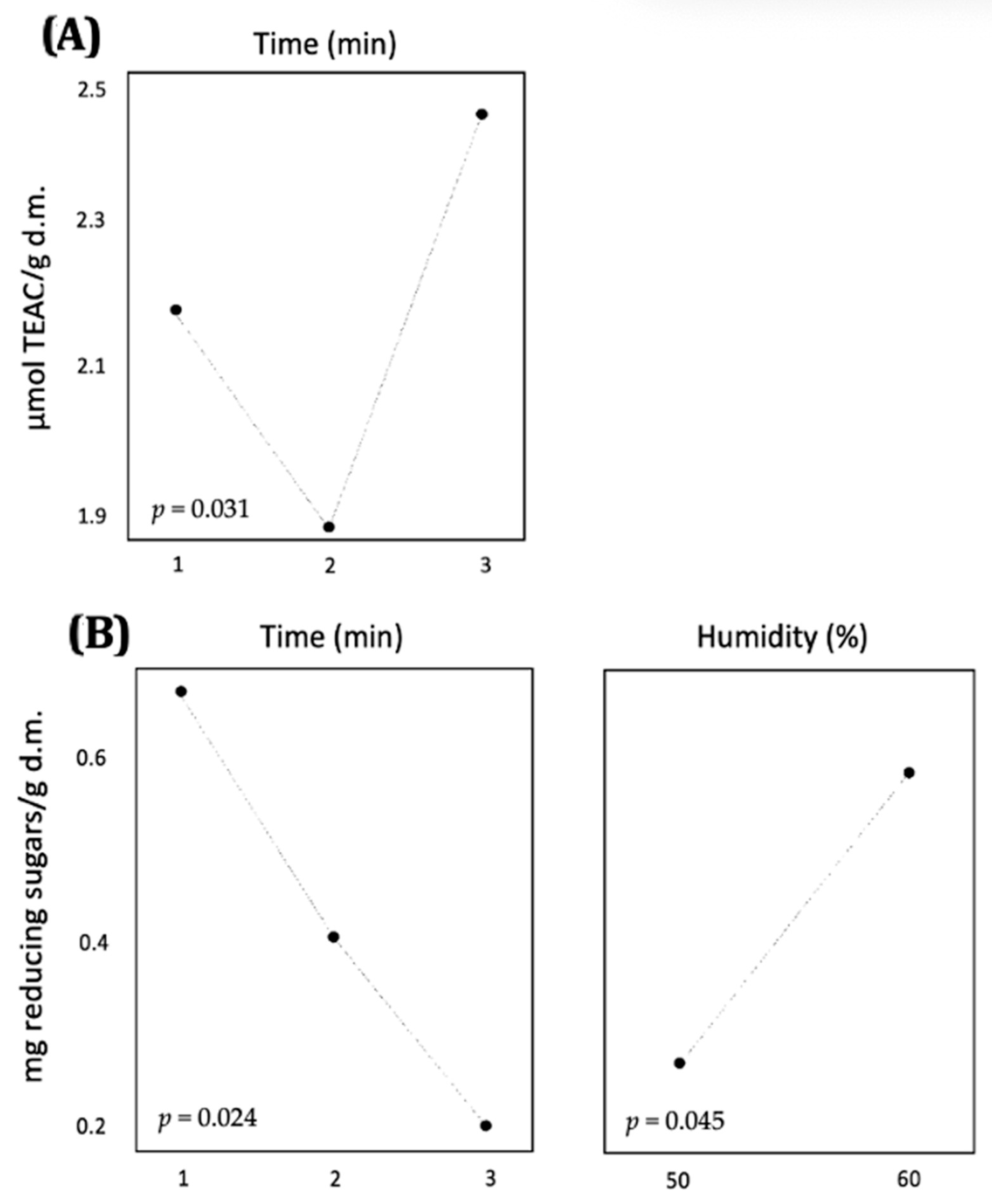
Biotechnological pretreatments for non-conventional ingredients require optimization to ensure the maximum bioaccessibility and bioavailability of nutrients and functional compounds. The results from this study indicate that the factors affecting enzyme activity differentially influence the release of antioxidant compounds and the products of carbohydrate hydrolysis. The assays were conducted at a fixed pH of 5.5, which is optimal for the enzyme activity present in the complex. Under these conditions, the release of pentoses from carbohydrate hydrolysis was significantly and positively influenced by both humidity and enzyme dose. These optimal conditions are consistent with those reported by San Martin et al. [
17] for other commercial enzymes, involving a temperature of 55 °C, pH 6, and a fixed solid-to-liquid ratio of 1:1 with water. It is important to note that the conditions used in this enzymatic pretreatment differ considerably from those employed when the goal is to maximize carbohydrate hydrolysis or phenolic extraction from BSG in a biorefinery context [
7,
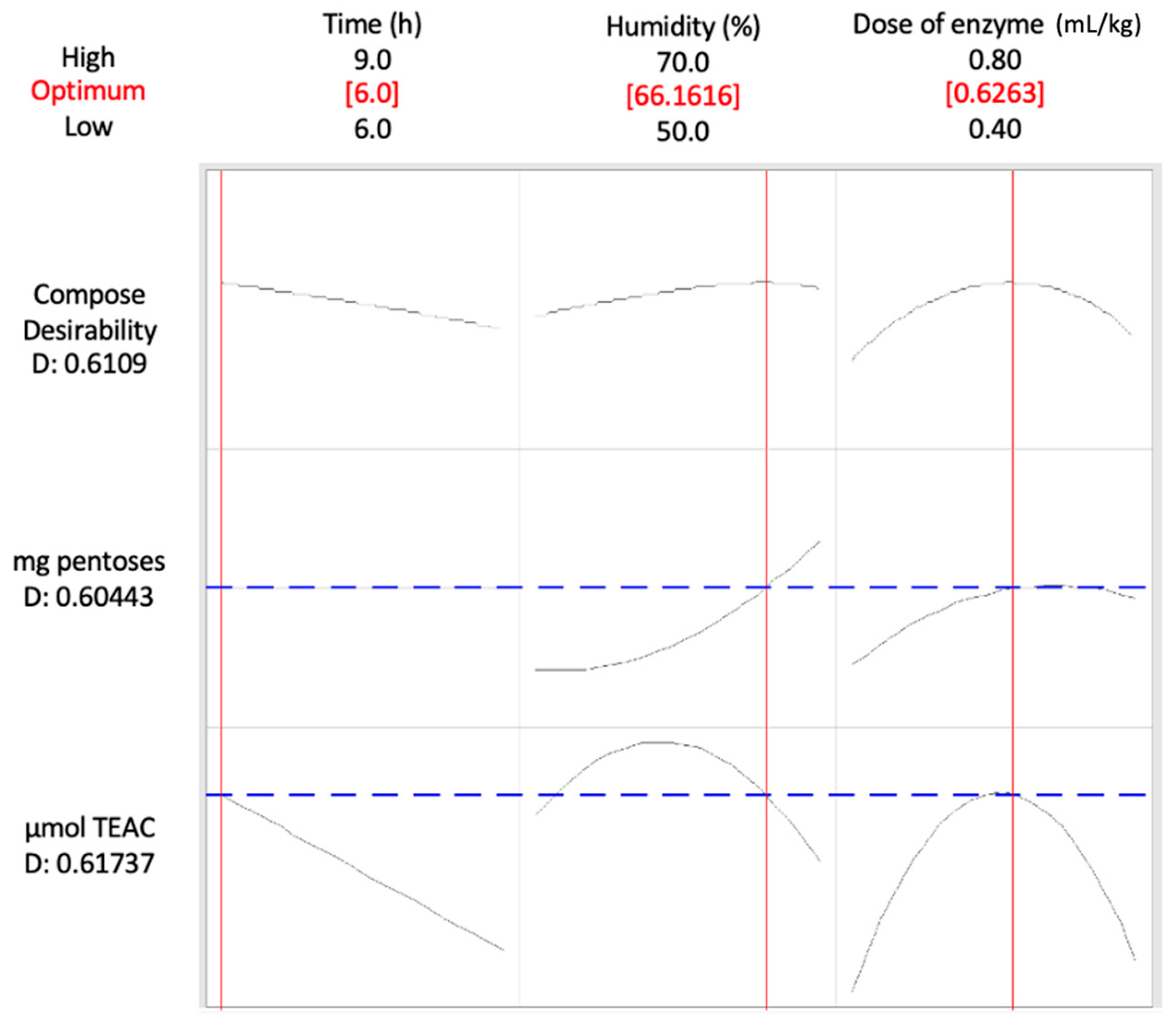
41]. In such scenarios, more extreme conditions are typically used, resulting in higher total consumption of time, energy, and enzyme products. Nevertheless, the conditions determined in the present study, using the desirability function, represent a compromise between optimizing carbohydrate hydrolysis and preserving high levels of antioxidant compounds. These conditions included a short reaction time of 6 h, a relatively high humidity of 66%, and a moderate enzyme dose of 0.63 mL/kg dry weight.
Given their potential, numerous studies have focused on maximizing the extraction of compounds from BSG utilizing microwave heat pretreatment. Effective conditions for extracting bioactive compounds typically involve brief treatment durations of less than 10 min [
44], the predominant use of water as the solvent [
45], high solvent-to-solid ratios approaching 90% [
44,
45], and power levels not exceeding 1000 W [
45,
46,
47]. Conditions for optimizing carbohydrate hydrolysis also encompass diverse ranges of these parameters. Previous research has demonstrated the positive influence of microwave irradiation on carbohydrate bioavailability in various feed ingredients [
48,
49,
50,
51,
52]. López-Linares et al. [
53] achieved high sugar yields from BSG using temperatures exceeding 190 °C and employing acidic and alkaline solvents. However, as observed with enzymatic pretreatment, the conditions described in these studies were not primarily intended to enhance the nutritional value of BSG as a feed ingredient but rather aimed at maximizing the yields of various compounds. The objective of the present study diverged from this, focusing on optimizing the simultaneous release of both sugars and antioxidant compounds. Consequently, milder conditions were employed, and the utilization of a factorial design facilitated the identification of the optimal combination of time and moisture content for achieving the maximum release of these compounds.
4.2. In Vivo Assay
As outlined in the Introduction, the primary objective of this study was not to formulate a practical diet incorporating BSG to maximize seabream growth. Indeed, a previous study by Estévez et al. [
20] demonstrated that incorporating 20% BSG, regardless of whether it was simply dried or enzymatically hydrolyzed, significantly impaired seabream growth and feed digestibility due to its high fiber content. Similar limitations have been observed in other species, such as
Oreochromis niloticus and
Icatalurus punctatus, where high inclusion levels of BSG have been shown to reduce growth performance [
60]. Nevertheless, this high inclusion level was selected for the present study to investigate whether or not different pretreatments could positively influence the nutritional value of BSG. Despite the anticipated compromise in fish growth performance compared to a standard diet, notable differences emerged between the two processing methods tested. The results confirmed suboptimal growth rates in the fish but also revealed significant disparities between the two pretreatment methods. While the enzymatically treated feed (H) exhibited an improvement in nutritional value, no significant effects were observed on the zootechnical parameters of fish fed this diet. Conversely, fish fed the MW diet demonstrated a significant enhancement in feed efficiency (FE) and specific growth rate (SGR). These findings suggest that microwave heat pretreatment holds considerable promise for enhancing growth performance in animal feed [
21,
22].
Surprisingly, the in vivo experimental outcomes did not fully corroborate the findings of the in vitro assays. In vitro, the MW diet demonstrated significantly enhanced bioavailability of sugars but exhibited a diminished bioavailability of amino acids compared to the control. This apparent contradiction may be explained by the thermally induced degradation of some amino acids during microwave heat pretreatment, a phenomenon previously reported in studies on heat-processed protein sources [
61]. Nonetheless, the increased release of reducing sugars could have compensated for this loss by providing an additional readily available energy source, thereby supporting growth performance [
62]. Furthermore, the observed improvement in nutrient absorption, as evidenced by the increased intestinal length in the MW group (
Table 5), could also contribute to the superior growth observed in fish fed the MW diet. However, further investigations are warranted to confirm this hypothesis and fully elucidate the underlying mechanisms.
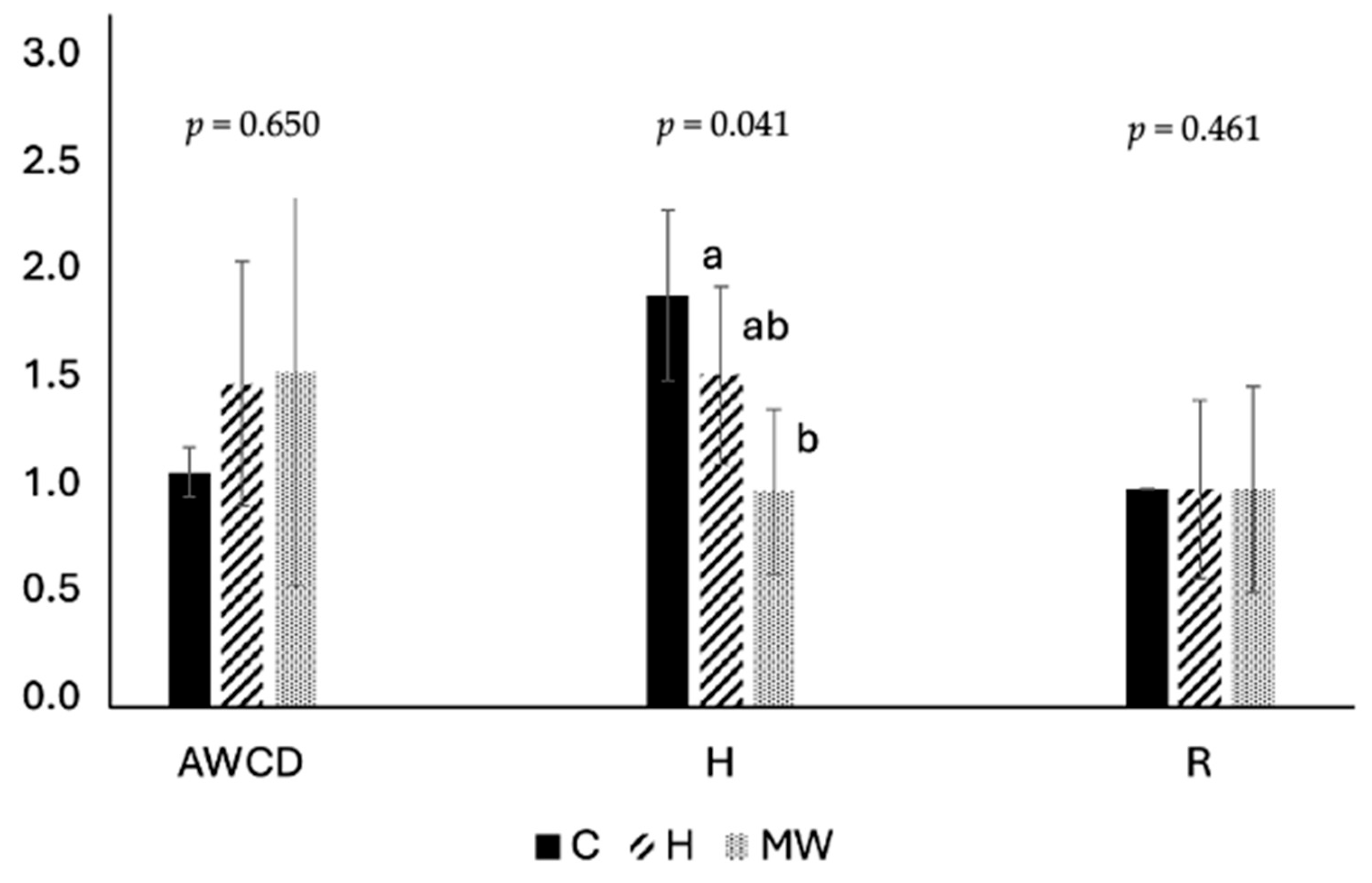
As previously noted, a significant increase of approximately 18% in the intestinal length index (ILI) was observed in fish fed the MW diet compared to those receiving untreated BSG (C). This enhancement in ILI may be associated with the significant reduction in intestinal microbial diversity also observed in fish fed the MW diet. While it is well-established that dietary treatments can significantly influence the intestinal microbiota of aquaculture species [
63], there is currently a dearth of research specifically investigating the impact of microwave heat pretreatment on this aspect. Microwave heat pretreatment of BSG has been demonstrated to increase the bioavailability of both reducing sugars and phenolic compounds within the intestinal milieu. Both of these factors could exert a significant influence on the microbial profile. Villasante et al. [
64] reported that carbohydrate-rich diets for Atlantic salmon resulted in a reduction in microbial diversity while simultaneously improving diet utilization. Similarly, Gericke et al. [
65] observed that enzymatically treated BSG incorporated into the diet of African catfish significantly reduced microbial diversity within the intestinal tract.
Furthermore, dietary polyphenols are well-known to exert a significant influence on the diversity and functional activity of the gut microbiota through both direct and indirect interactions [
66,
67]. Polyphenols can stimulate or inhibit bacterial growth, while the gastrointestinal microbiota is capable of metabolizing these compounds. Conversely, polyphenols can significantly alter the composition, diversity, and metabolic activity of intestinal bacterial communities [
68]. A reduction in microbial diversity can be associated with a diminished intestinal absorptive capacity. Li et al. [
69] demonstrated in rhesus macaques that the dysbiosis of commensal bacteria induced by antibiotics adversely affects the epithelial cells of the intestinal mucosa. In this context, the observed increase in intestinal size in fish fed the MW diet may represent a physiological compensatory mechanism to counteract the reduced absorptive capacity resulting from alterations in the gut microbiota’s functional profile. This compensatory mechanism exhibits similarities to that described when increasing the dietary proportion of vegetable ingredients in feeds for carnivorous species [
70]. Although variations in the composition of microbial populations in fish fed after MW pretreatment can only be presumed, as they were not directly assessed using a genomic approach, some indirect effects may still be considered. Among these, it cannot be entirely discounted that the reduction in microbial diversity may reflect the predominance of bacterial groups that effectively utilize the substrates whose bioavailability was enhanced by the pretreatments. In this context, such microbial predominance could be related to modifications in intestinal absorptive capacity.
Significantly lower plasma lactate levels were observed in fish fed both enzyme-treated (H) and microwave-treated (MW) BSG diets compared to the control group. This reduction can be attributed to several factors, including decreased lactate production through a reduction in anaerobic metabolism and enhanced lactate clearance. Lactate can be effectively utilized in various organs, such as the liver, for conversion to pyruvate via the Cori cycle [
70]. Furthermore, lower plasma lactate levels may also serve as an indicator of more efficient energy metabolism, characterized by a closer alignment between the energy derived from food intake and the physiological demands for growth and other essential metabolic processes, including swimming and immune function [
28].
Conversely, the lower plasma protein and cholesterol levels observed in fish fed the enzyme-treated BSG (H) diet may be indicative of enhanced nutrient digestibility and utilization, leading to the greater absorption of dietary proteins and lipids [
71]. This suggests that lower circulating levels of these metabolites may be associated with more efficient nutrient utilization for growth and overall fish welfare. While elevated plasma protein levels might reflect increased protein anabolism and a more active metabolic state [
72], potentially indicating greater growth potential in fish fed the MW diet or, at the very least, improved feed efficiency, further investigation is warranted to confirm this hypothesis.
Collectively, fish fed the enzyme-treated BSG (H) diet exhibited lower plasma levels of cortisol, cholesterol, and protein, and a tendency towards reduced muscle TAG content, potentially suggesting a less active or reduced metabolic rate compared to fish fed the microwave-treated BSG (MW) diet [
72]. These findings underscore the differential effects of the two BSG pretreatments on fish physiology and metabolism.
Significantly reduced levels of triacylglycerols (TAG) were observed in the muscle tissue of fish fed the MW diet, suggesting enhanced lipid bioavailability and utilization within the fish. Previous studies have demonstrated that microwave heat pretreatment can significantly enhance the extraction and digestibility of lipids from various feed ingredients, including BSG. This process potentially increases the availability of fatty acids and triacylglycerols for absorption within the fish’s digestive tract [
73]. This improved lipid utilization, reflected by reduced muscle TAG accumulation, has important implications for fish growth, performance, and overall health. Lipids serve as a crucial energy source and play a vital role in numerous physiological processes, including growth, reproduction, and immune function in aquatic organisms [
74]. Moreover, reduced muscle TAG levels may also contribute to an extended shelf life of the fillets by minimizing lipid oxidation and enhancing fillet quality, a highly desirable attribute within the aquaculture industry [
75].
A significant increase in the activity of mucus alkaline phosphatase was observed in gilthead seabream fed both enzyme-treated (H) and microwave-treated (MW) BSG diets. This finding aligns with the review by Lallès [
76], which emphasizes that alkaline phosphatase activity in fish skin mucus is significantly influenced by multiple factors. These include environmental conditions, nutritional status, and dietary supplementation. Lallès [
76] concludes that the modulation of alkaline phosphatase activity is a complex process influenced by various factors. This supports the idea that dietary pretreatments, such as the BSG pretreatments used in this study, may positively impact the fish immune response by enhancing enzyme activity in the mucus.
Maintaining elevated levels of alkaline phosphatase activity in skin mucus is essential for optimal fish health. Several studies have shown a strong positive correlation between increased alkaline phosphatase activity and improved fish welfare. This positive association has been substantiated by research investigating dietary interventions such as the inclusion of bioactive compounds or plant extracts [
77,
78,
79,
80] as well as the utilization of probiotics and prebiotics [
81,
82] in aquaculture feeds. Endogenous antioxidant systems, comprising enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), are crucial for cellular defense against oxidative damage. SOD catalyzes the dismutation of superoxide radicals into molecular oxygen and hydrogen peroxide, which are subsequently detoxified by CAT or GPx [
83]. The activation of these antioxidant enzymes in response to oxidative challenges, such as increased metabolic activity or environmental stressors, is well-documented [
84].
Although this study did not specifically induce oxidative stress, it revealed notable differences in antioxidant enzyme activities. Fish fed the enzyme-treated (H) and microwave-treated (MW) BSG diets exhibited significantly lower levels of catalase (CAT) and glutathione peroxidase (GPx) compared to the control group. These findings suggest that both pretreatments may enhance the bioavailability of antioxidant compounds within BSG [
85], thereby potentially mitigating the oxidative stress [
86] generated during normal metabolic processes more effectively than untreated BSG. Consequently, this increased antioxidant capacity derived from the diet may result in a reduced reliance on the activity of endogenous antioxidant enzymes, such as CAT and GPx, to maintain cellular redox homeostasis. Such a decrease in antioxidant enzyme activity aligns with a reduction in oxidative stress, as supported by various studies in different organisms [
87,
88,
89].
Furthermore, dietary supplementation with antioxidants, such as alpha-tocopheryl acetate [
90] or carotenoids derived from
Dunaliella salina [
91], has been shown to elicit a reduction in hepatic catalase activity in rainbow trout, alongside other notable alterations in antioxidant defense mechanisms, such as an upregulation in the expression of antioxidant genes. Given these findings, future research endeavors should prioritize investigating the effects of maximizing the bioavailability of antioxidant compounds within BSG under conditions that stimulate the production of reactive oxygen species (ROS). This research should not only encompass the assessment of the activity of the aforementioned antioxidant enzymes (SOD, CAT, and GPx) but also encompass the evaluation of Nrf2 (nuclear factor erythroid 2-related factor 2) activation. Nrf2 is a pivotal transcriptional regulator of cellular responses to oxidative stress [
92,
93].