The LIFE STREAMS Project for the Recovery of the Native Mediterranean Trout in Six Italian Pilot Areas: Planning and Adoption of Conservation Actions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Data Collection
2.3. Environmental and Demographic Assessment of Wild Populations
- Density (ind m−2) and standing crop (g m−2);
- Age structure (age individually attributed by the scalimetric method);
- Average individual total length;
- Average individual relative weight (Wr), a measure of body condition whose values in the range 95–105 indicate optimal conditions [43];
- Proportional stock density index (PSD), ranging between 0 and 100, that estimates population structure deviations from a hypothetical balanced population, i.e., 35 ≤ PSD ≤ 65 [44]. Further details on PSD and Wr can be seen in the work of Carosi et al. [15] and in Supplementary Material File S1.
2.4. Preliminary Genetic Assessment of Wild Populations
2.5. Advanced Genetic Characterization to Define Population Structure and Identify Putative Spawners
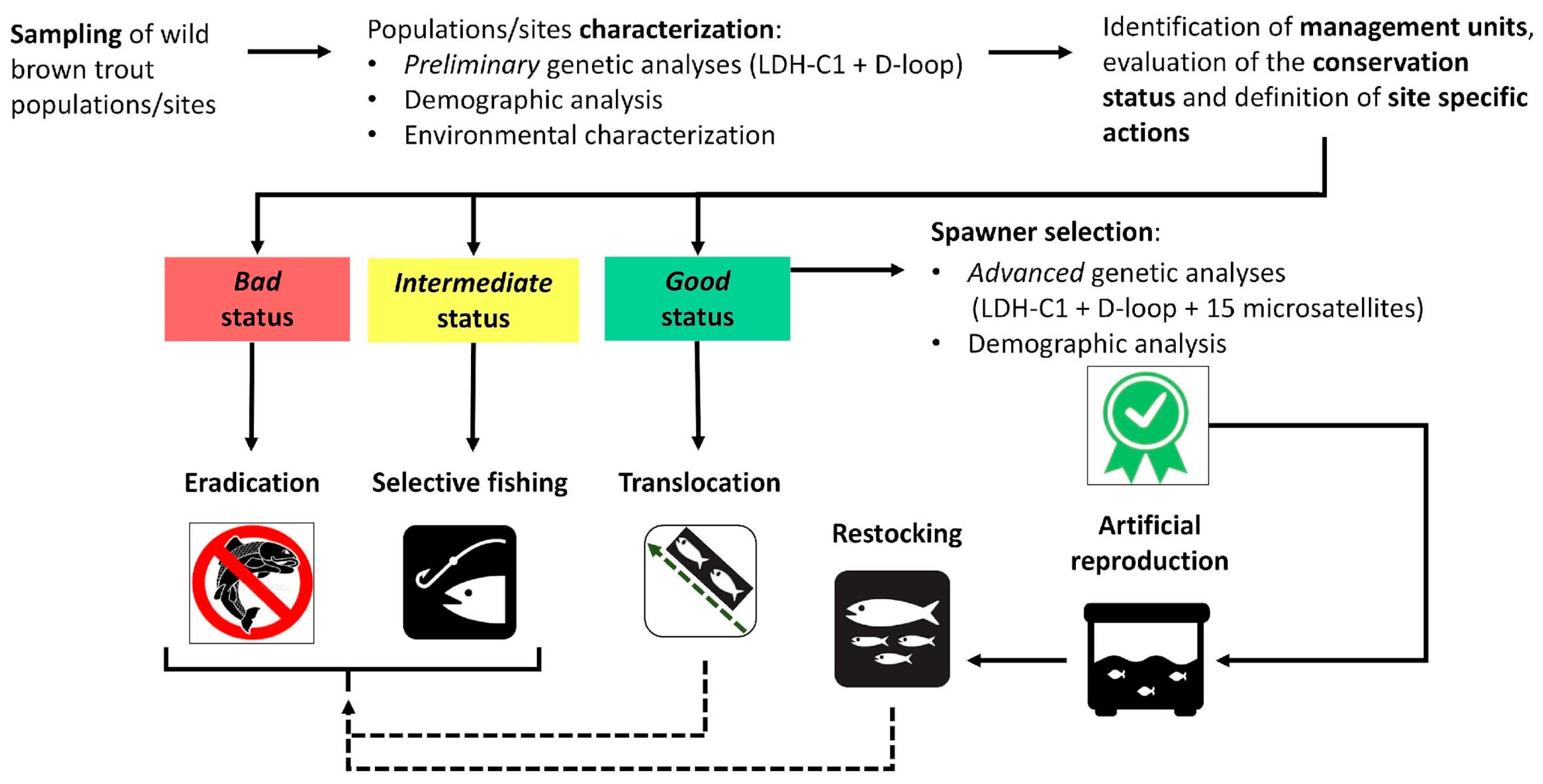
2.6. Decisional Workflow for Conservation Actions
2.7. Planned Conservation Strategies
- The artificial reproduction of wild pure spawners (selected by the above-mentioned “advanced” genetic analysis) in temporary or stable hatcheries, to produce genetically pure fertilized eggs and/or alevins for restocking and reintroduction activities. Each hatchery was designated for a specific basin corresponding to the origin of the breeders, which were to be captured annually from different selected locations.
- Trout populations showing the highest levels of genetic introgression (approximately >80%) were selected for the total eradication of all alien Atlantic individuals through repeated electrofishing removal actions. The restoration of native populations, through reintroductions, started at the end of the eradication phases. To optimize effectiveness, eradication actions were carried out several times a year between August and November, when environmental and biological conditions facilitate catchability.
- Trout populations characterized by moderate levels of genetic introgression (around 50%) were subject to experimental selective fishing. Based on the morphological distinction between alien and native phenotypes, specifically trained anglers should selectively remove alien specimens, hence improving the genetic integrity of trout populations over time [52]. Involved anglers were asked to collect a fin clip to genetically assess the effectiveness of this action.
- Contrasting illegal stocking is a wide-range strategy based on the consideration that recent worsening of genetic integrity in wild Mediterranean trout populations could also be due to this bad practice. The aim was to create a mapping and recording system of the illegal stocking actions in the pilot areas and quantify potential impacts. The implementation of this action included the improvement of the surveillance system, also through the installation of camera traps in the sites selected for conservation actions.
- Enhancement of freshwater habitats. In accordance with the Habitat Directive 92/43/EEC and the Water Framework Directive 2000/60/EC, this action was aimed at addressing the primary factors contributing to degradation of river ecosystems and biodiversity loss: river fragmentation, reduced water flow, and pollution. The Minimum Instream Flow (MIF) monitoring [53], and the selection and removal of physical and hydraulic barriers for each pilot area were included in the project. We georeferenced both natural and artificial physical barriers on field that interrupt river continuity and prevent the movement of fish fauna—only those preventing connectivity between native populations were selected for removal.
- The project aimed to develop Guidelines for the conservation and management of the Mediterranean trout and its habitat, intending to extend this model to all Italian Natura 2000 sites and protected areas where trout conservation efforts are required. The process of creating these guidelines involved a participatory approach, which included organizing roundtable discussions across Italy and a public consultation initiative. These discussions engaged various national authorities and stakeholders, including the Ministry of Environment, regional governments, Basin District Authorities, as well as environmental, scientific, and sport fishing associations.
2.8. Production of Native Eggs and Alevins in Captive Condition
3. Results
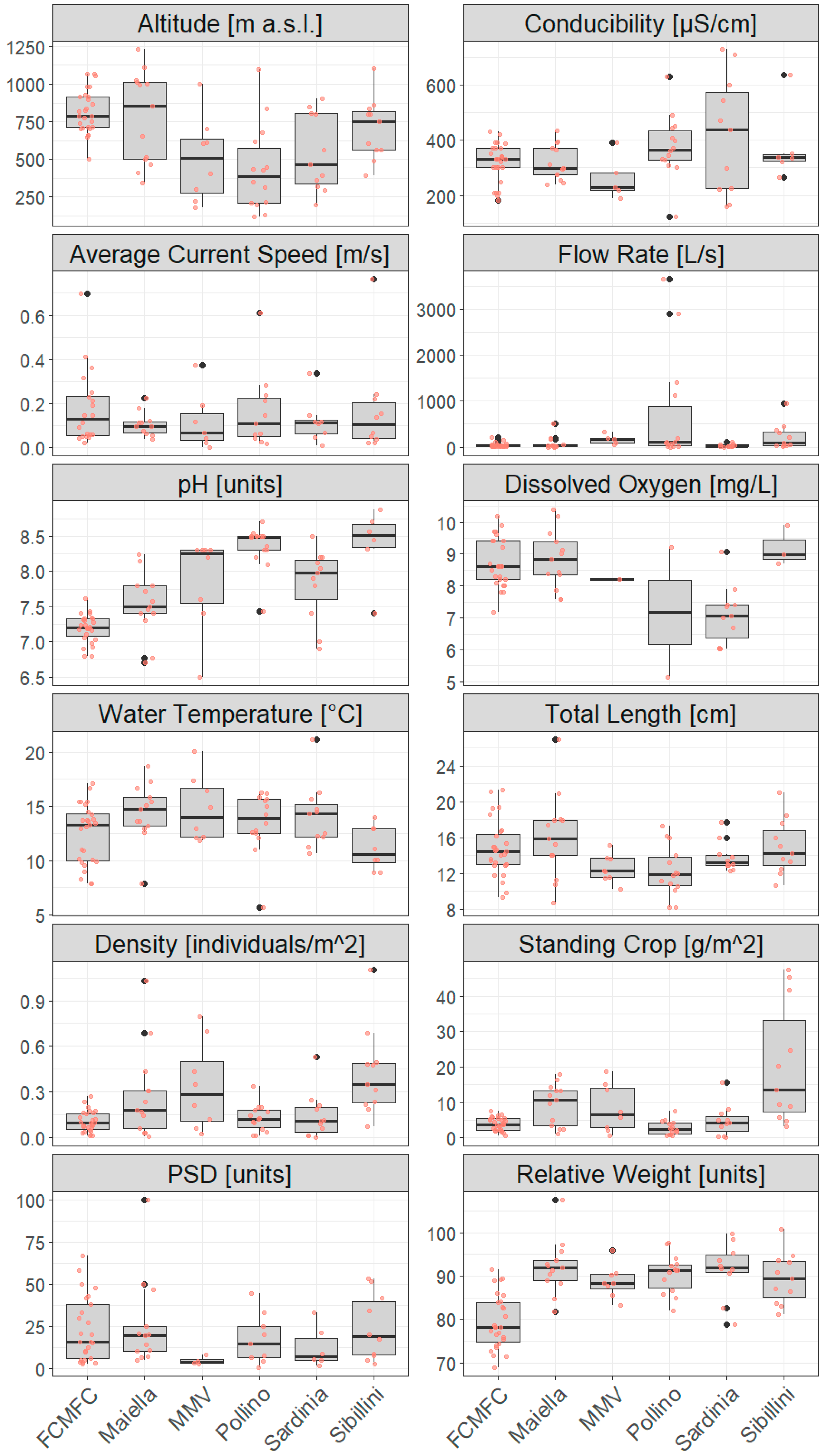
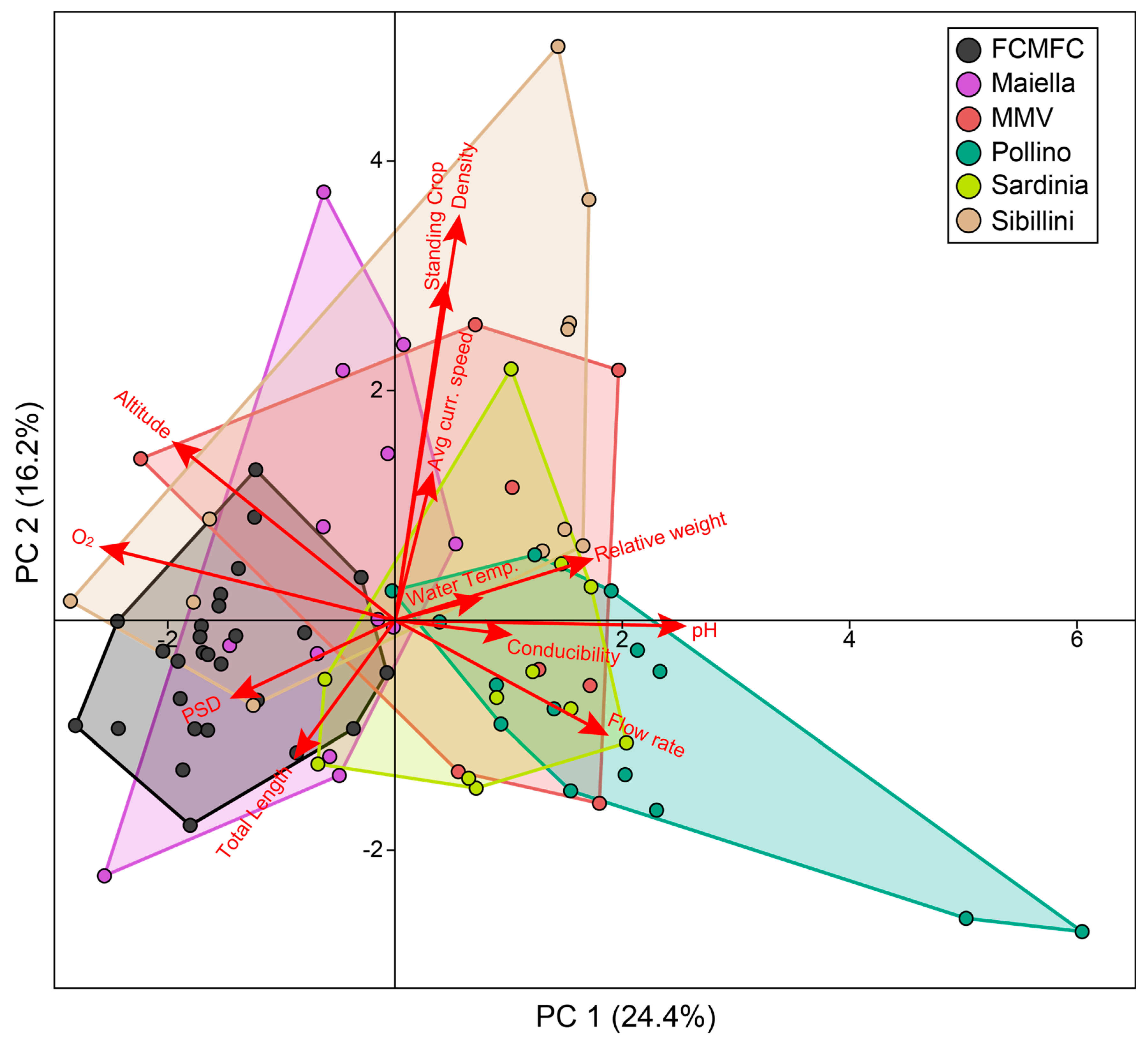
3.1. Environmental and Demographic Characterization of Wild Populations in Pilot Areas
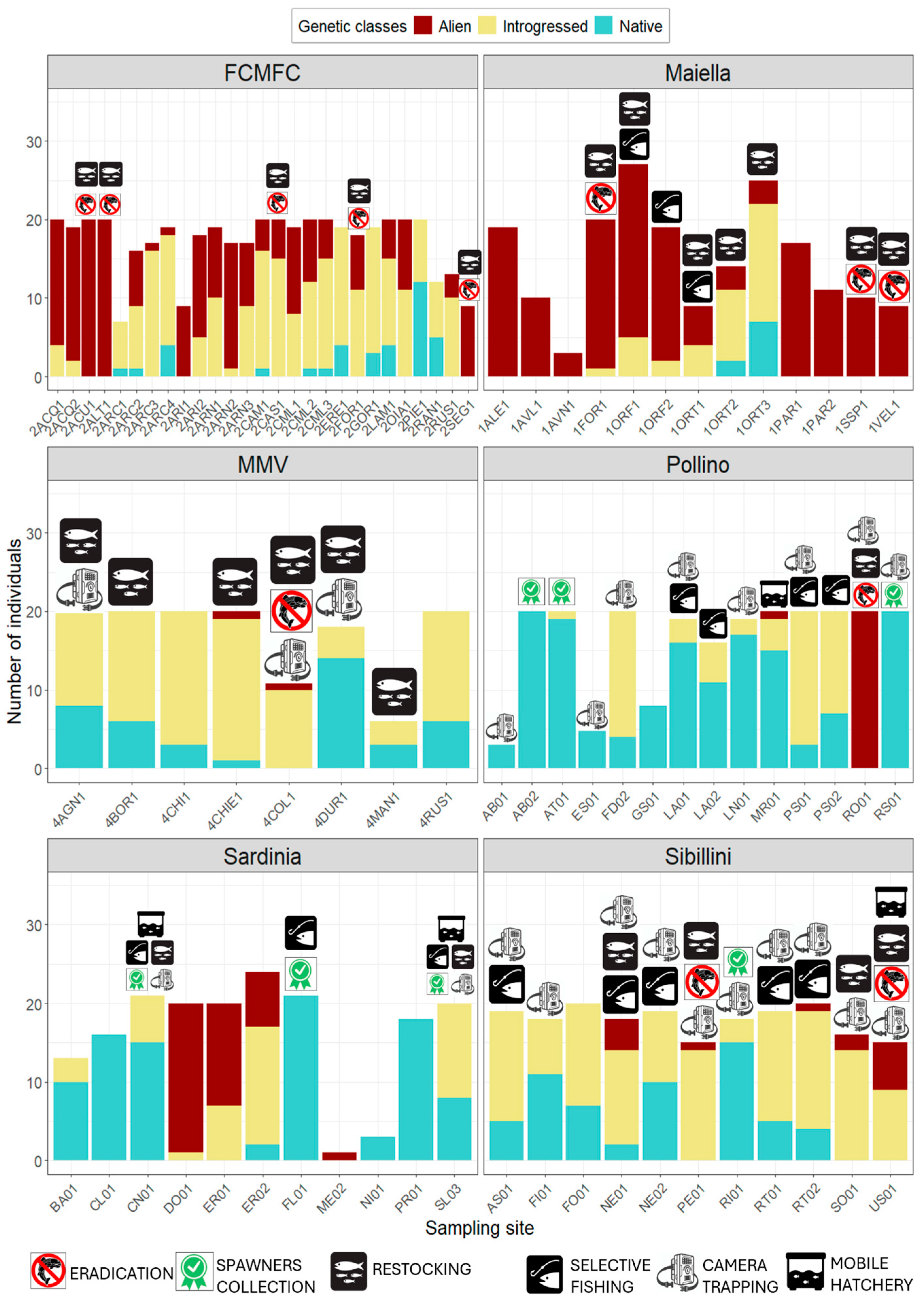
3.2. Preliminary Genetic Characterization of Wild Populations
3.3. Advanced Genetic Characterization and Spawners Selection from Wild Populations and Hatcheries
- “Experimental Ichthyogenic and Hydrobiology Centre” of L’Aquila plus individuals from Aterno to supply Maiella populations.
- “Premilcuore” hatchery for FCMFC along with wild individuals from Fosso delle Cortine population (Bidente di Pietrapazza drainage).
- “Maresca” private hatchery (that maintained, on behalf of the Liguria Region, spawners from wild individuals caught in previous years in the local drainage of Val di Vara) plus few individuals from 4DUR1, Usurana and Malacqua rivers for MMV.
- “Borgo Cerreto” and “Cantiano” hatcheries—already successfully managed for local Mediterranean trout conservation actions within the LIFE19 IPE/IT/00015 IMAGINE and LIFE12 NAT/IT/000940 TROTA project frameworks, respectively, along with RI01 trout.
3.4. Reproduction of Wild Pure Spawners and Restocking Activities
3.5. Alien Trout Removal Activities and Native Trout Reintroduction
3.6. Selective Fishing and Actions Against Illegal Stocking
3.7. Freshwater Habitat Improvement
3.8. Guidelines for the Conservation and Management of the Mediterranean Trout and Its Habitat
4. Discussion
4.1. Project Challenges
- The identification of native populations: The major issue was the (partly) unexpected absence of residual and viable native populations in many areas to sustain supportive artificial breeding. Given the general scarcity of pure-native populations, extended sampling may be provided for in some situations only, while spawner integration from hatchery stocks of Mediterranean trout should be minimized because of trout domestication and genetic diversity erosion [72,73].
- Translocations and supportive breeding: When pure-native populations are abundant and/or supportive breeding via hatcheries is inapplicable, translocations provide a more cost-effective approach for restoration of moderately depauperated populations. Conversely, to enhance partly introgressed and isolated wild populations for which genetically suitable and demographically adequate donor populations are lacking, any intervention could be avoided except for habitat protection, relying on the restorative power of natural selection [6,74]. Depending on the level of introgression, the institution of genetic refugees [71] may represent another practical conservation solution.
- Temporary vs. stable hatchery: Mobile hatcheries appear to be advantageous for minimizing spawners domestication and genetic diversity erosion through inbreeding [67,71], while reducing management costs. On the other hand, egg/alevin production was found to be much lower than in stable hatcheries, likely because of higher mortality and reduced adaptability to captive conditions of wild-caught spawners, coupled with their usually smaller size compared to hatchery-reared individuals [75]. As a suggestion for future research, the application of alternative techniques of semen cryopreservation, developed within the LIFE Nat.Sal.Mo. project [28] could be extremely useful in rationalizing breeding practices in hatcheries.
4.2. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUCN | International Union for Conservation of Nature |
| MIF | Minimum Instream Flows |
| LDH-C1 | Lactate Dehydrogenase chain-1 gene |
| SACs | Special Areas of Conservation |
| SPAs | Special Protection Areas |
| STREAMS | Salmo ceTtii REcovery Actions in Mediterranean Streams |
| PCA | Principal Components Analysis |
References
- Bernatchez, L. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution 2001, 55, 351–379. [Google Scholar] [CrossRef]
- Gratton, P.; Allegrucci, G.; Sbordoni, V.; Gandolfi, A. The evolutionary jigsaw puzzle of the surviving trout (Salmo trutta L. complex) diversity in the Italian region. A multilocus Bayesian approach. Mol. Phylogenet. Evol. 2014, 79, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Tougard, C.; Justy, F.; Guinand, B.; Douzery, E.J.P.; Berrebi, P. Salmo macrostigma (Teleostei, Salmonidae): Nothing more than a brown trout (S. trutta) lineage? J. Fish Biol. 2018, 93, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lobón-Cerviá, J.; Esteve, M.; Berrebi, P.; Duchi, A.; Lorenzoni, M.; Young, K.A. Trout and char of central and southern Europe and Northern Africa. In Trout And Char of the World; Kershner, J., Williams, J., Lobón-Cerviá, J., Gresswell, B., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 379–410. [Google Scholar]
- Lorenzoni, M.; Borghesan, F.; Carosi, A.; Ciuffardi, L.; De Curtis, O.; Delmastro, G.B.; Di Tizio, L.; Franzoi, P.; Maio, G.; Mojetta, A.; et al. Check-list dell’ittiofauna delle acque dolci italiane [The check-list of the Italian freshwater fish fauna]. Ital. J. Freshw. Ichthyol. 2019, 5, 239–254. [Google Scholar]
- Splendiani, A.; Giovannotti, M.; Righi, T.; Fioravanti, T.; Nisi Cerioni, P.; Lorenzoni, M.; Carosi, A.; La Porta, G.; Barucchi, V.C. Introgression despite protection: The case of native brown trout in Natura 2000 network in Italy. Conserv. Genet. 2019, 20, 343–356. [Google Scholar] [CrossRef]
- Marchi, A.; Bertaccini, A.; Fan, W.; Zuffi, G.; Sacchetti, S.; Nanetti, M.; Lee, C.; Agostini, A.; Lucchini, D.; Bianconcini, S.; et al. Refinement of the NISECI ecological index reference conditions for Italian freshwater fish communities in the eastern Emilia-Romagna region. Ecol. Indic. 2023, 155, 111070. [Google Scholar] [CrossRef]
- Ayllón, D.; Almodóvar, A.; Nicola, G.G.; Parra, I.; Elvira, B. A new biological indicator to assess the ecological status of Mediterranean trout type streams. Ecol. Indic. 2012, 20, 295–303. [Google Scholar] [CrossRef]
- Dauwalter, D.C.; Duchi, A.; Epifanio, J.; Gandolfi, A.; Gresswell, R.; Juanes, F.; Kershne, J.; Lobón-Cerviá, J.; McGinnity, P.; Meraner, A.; et al. A call for global action to conserve native trout in the 21st century and beyond. Ecol. Freshw. Fish 2020, 29, 429–432. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species, Version 2025-1. 2025. Available online: https://www.iucnredlist.org (accessed on 25 April 2025).
- Rondinini, C.; Battistoni, A.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Rome, Italy, 2022.
- Stoch, F.; Grignetti, A. IV Report Direttiva Habitat: Specie animali. In Rapporti Direttive Natura (2013–2018). Sintesi dello Stato di Conservazione delle Specie e Degli Habitat di Interesse Comunitario e delle Azioni di Contrasto Alle Specie Esotiche di Rilevanza Unionale in Italia, Serie Rapporti 349/2021; Ercole, S., Angelini, P., Carnevali, L., Casella, L., Giacanelli, V., Grignetti, A., La Mesa, G., Nardelli, R., Serra, L., Stoch, F., et al., Eds.; Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA): Rome, Italy, 2021; pp. 39–68. [Google Scholar]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Nisi Cerioni, P.; Caputo Barucchi, V. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Invasions 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Splendiani, A.; Palmas, F.; Sabatini, A.; Caputo Barucchi, V. The name of the trout: Considerations on the taxonomic status of the Salmo trutta L.; 1758 complex (Osteichthyes: Salmonidae) in Italy. Eur. Zool. J. 2019, 86, 432–442. [Google Scholar] [CrossRef]
- Carosi, A.; Ghetti, L.; Padula, R.; Lorenzoni, M. Population status and ecology of the Salmo trutta complex in an Italian river basin under multiple anthropogenic pressures. Ecol. Evol. 2020, 10, 7320–7333. [Google Scholar] [CrossRef]
- Talarico, L.; Marta, S.; Rossi, A.R.; Crescenzo, S.; Petrosino, G.; Martinoli, M.; Tancioni, L. Balancing selection, genetic drift, and human-mediated introgression interplay to shape MHC (functional) diversity in Mediterranean brown trout. Ecol. Evol. 2021, 11, 10026–10041. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.R.; Talarico, L.; Petrosino, G.; Crescenzo, S.; Tancioni, L. Conservation genetics of Mediterranean brown trout in central Italy (Latium): A multi-marker approach. Water 2022, 14, 937. [Google Scholar] [CrossRef]
- Rossi, A.R.; Petrosino, G.; Milana, V.; Martinoli, M.; Rakaj, A.; Tancioni, L. Genetic identification of native populations of Mediterranean brown trout Salmo trutta L. complex (Osteichthyes: Salmonidae) in central Italy. Eur. Zool. J. 2019, 86, 424–431. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Polgar, G.; Iaia, M.; Righi, T.; Volta, P. The Italian Alpine and Subalpine trouts: Taxonomy, evolution, and conservation. Biology 2022, 11, 576. [Google Scholar] [CrossRef]
- Abell, R.; Allan, J.D.; Lehner, B. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 2007, 134, 48–63. [Google Scholar] [CrossRef]
- Thieme, M.L.; Rudulph, J.; Higgins, J.; Takats, J.A. Protected areas and freshwater conservation: A survey of protected area managers in the Tennessee and Cumberland River Basins, USA. J. Environ. Manag. 2012, 109, 189–199. [Google Scholar] [CrossRef]
- Hermoso, V.; Abell, R.; Linke, S.; Boon, P. The role of protected areas for freshwater biodiversity conservation: Challenges and opportunities in a rapidly changing world. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Crivelli, A.J. The role of protected areas in freshwater fish conservation. In Conservation of Freshwater Fishes: Options for the Future; Collares-Pereira, M.J., Cowx, I.G., Coelho, M.M., Eds.; Fishing News Books: Oxford, UK, 2002; pp. 373–388. [Google Scholar]
- Sabatini, A.; Podda, C.; Frau, G.; Cani, M.V.; Musu, A.; Serra, M.; Palmas, F. Restoration of native Mediterranean brown trout Salmo cettii Rafinesque, 1810 (Actinopterygii: Salmonidae) populations using an electric barrier as a mitigation tool. Eur. Zool. J 2018, 85, 137–149. [Google Scholar] [CrossRef]
- Tiberti, R.; Bogliani, G.; Brighenti, S.; Iacobuzio, R.; Liautaud, K.; Rolla, M.; von Hardenberg, A.; Bassano, B. Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol. Invasions 2019, 21, 875–894. [Google Scholar] [CrossRef]
- Palombo, V.; De Zio, E.; Salvatore, G.; Esposito, S.; Iaffaldano, N.; D’Andrea, M. Genotyping of two Mediterranean trout populations in central-southern Italy for conservation purposes using a rainbow-trout-derived SNP array. Animals 2021, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Rusco, G.; Esposito, S.; D’Andrea, M.; Roncarati, A.; Iaffaldano, N. The role of semen cryobanks for protecting endangered native salmonids: Advantages and perspectives as outlined by the LIFE Nat. Sal. Mo. project on Mediterranean brown trout (Molise region–Italy). Front. Mar. Sci. 2023, 9, 1075498. [Google Scholar] [CrossRef]
- Polgar, G.; Iaia, M.; Sala, P.; Khang, T.F.; Galafassi, S.; Zaupa, S.; Volta, P. Size-age population structure of an endangered and anthropogenically introgressed northern Adriatic population of marble trout (Salmo marmoratus Cuv.): Insights for its conservation and sustainable exploitation. PeerJ 2023, 11, e14991. [Google Scholar] [CrossRef]
- Caudron, A.; Champigneulle, A.; Vigier, L.; Hamelet, V.; Guyomard, R. Early effects of the strategies of creating a genetic refuge and direct translocation for conserving and restoring populations of native brown trout. Freshw. Biol. 2012, 57, 1702–1715. [Google Scholar] [CrossRef]
- Gil, J.; Labonne, J.; Caudron, A. Evaluation of strategies to conserve and restore intraspecific biodiversity of brown trout: Outcomes from genetic monitoring in the French Alps. Rev. Fish Biol. Fish. 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Splendiani, A.; Righi, T.; Fioravanti, T.; Sabatini, A.; Palmas, F.; Tougard, C.; Berrebi, P.; Talarico, L.; Caputo Barucchi, V. Population genetics, demography and conservation of Mediterranean brown trout from Sardinia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4099. [Google Scholar] [CrossRef]
- Righi, T.; Fasola, E.; Iaia, M.; Stefani, F.; Volta, P. Limited contribution of hatchery-produced individuals to the sustainment of wild marble trout (Salmo marmoratus Cuvier, 1829) in an Alpine basin. Sci. Total Environ. 2023, 892, 164555. [Google Scholar] [CrossRef]
- Nonnis Marzano, F.; Corradi, N.; Papa, R.; Tagliavini, J.; Gandolfi, G. Molecular evidence for introgression and loss of genetic variability in Salmo (trutta) macrostigma as a result of massive restocking of Apennine populations (Northern and Central Italy). Environ. Biol. Fishes 2003, 68, 349–356. [Google Scholar] [CrossRef]
- Baratti, M.; Nonnis-Marzano, F.; Fratini, S.; Piccinini, A.; Patarnello, T.; Dessì-Fulgheri, F.; Gandolfi, G. Caratterizzazione genetica delle popolazioni di Trota fario del Parco delle Foreste Casentinesi [Genetic characterization of brown trout populations living in the Foreste casentinesi national Park]. Biol. Ambient. 2006, 20, 237–240. [Google Scholar]
- Ketmaier, V.; Bianco, P.G. Monitoraggio genetico e ibridazione tra popolazioni atlantiche e mediterranee di Salmo trutta in Abruzzo e Campania. In Etica, Economia, Ecologia: Sguardi sulla Complessità, Proceedings of the Atti del XIII Congresso Nazionale della Societa’ Italiana di Ecologia, Como, Italy, 8–10 Settembre 2003; Casagrandi, R., Melia, P., Eds.; Ecologia Aracne Editrice: Rome, Italy, 2003; Available online: https://www.researchgate.net/publication/262198836_Monitoraggio_genetico_e_ibridazione_tra_popolazioni_Atlantiche_e_Mediterranee_di_Salmo_trutta_in_Abruzzo_e_Campania (accessed on 19 January 2025).
- Zaccara, S.; Trasforini, S.; Antognazza, C.M.; Puzzi, C.; Britton, J.R.; Crosa, G. Morphological and genetic characterization of Sardinian trout Salmo cettii Rafinesque, 1810 and their conservation implications. Hydrobiologia 2015, 760, 205–223. [Google Scholar] [CrossRef]
- Nonnis Marzano, F.; Tagliavini, J.; Papa, R.; Vaghi, M.; Pascale, M.; Maio, G.; Gandolfi, G. Caratterizzazione genetica di popolazioni appenniniche di trota fario: Aspetti tassonomici e conservazionistici. Biol. Ambient. 2002, 18, 19–24. [Google Scholar]
- Sabatini, A.; Cannas, R.; Follesa, M.C.; Palmas, F.; Manunza, A.; Matta, G.; Pendugiu, A.A.; Serra, P.; Cau, A. Genetic characterization and artificial reproduction attempt of endemic Sardinian trout Salmo trutta L.; 1758 (Osteichthyes, Salmonidae): Experiences in captivity. Ital. J. Zool. 2011, 78, 20–26. [Google Scholar] [CrossRef]
- Moran, P.A.P. A mathematical theory of animal trapping. Biometrika 1951, 38, 307–311. [Google Scholar] [CrossRef]
- Zippin, C. An evaluation of the removal method of estimating animal populations. Biometrics 1956, 12, 163–189. [Google Scholar] [CrossRef]
- Scardi, M.; Tancioni, L.; Martone, C. Protocollo di Campionamento e Analisi della Fauna Ittica dei Sistemi Lotici; APAT/MATTM: Torino, Italy, 2007; pp. 1–31. [Google Scholar]
- Anderson, R.O.; Neumann, R.M. Length, weight and associated structural indices. In Fisheries Techniques; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 447–482. [Google Scholar]
- Gabelhouse, D.W., Jr. A length-categorization system to assess fish stocks. N. Am. J. Fish. Manag. 1984, 4, 273–285. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron 2001, 4, 1–9. [Google Scholar]
- Padula, A.; Greco, C.; Talarico, L.; Caniglia, R.; Antognazza, C.M.; D’Antoni, S.; Lorenzoni, M.; Vanetti, I.; Zaccara, S.; Mucci, N. A rapid and reliable detection procedure of Atlantic trout introgression at the diagnostic lactate dehydrogenase chain-1 gene. Aquac. Fish Fish. 2023, 3, 388–392. [Google Scholar] [CrossRef]
- Talarico, L.; Caniglia, R.; Carosi, A.; Lorenzoni, M.; Greco, C.; Padula, A.; D’Antoni, S.; Alberti, D.; De Paoli, A.; Casali, L.; et al. Population structure, genetic diversity and demographic patterns unveil massive Mediterranean brown trout manipulations in a protected area of the northern Apennines (Italy). Eur. Zool. J. 2023, 90, 470–486. [Google Scholar] [CrossRef]
- Berrebi, P.; Caputo Barucchi, V.; Splendiani, A.; Muracciole, S.; Sabatini, A.; Palmas, F.; Tougard, C.; Arculeo, M.; Marić, S. Brown trout (Salmo trutta L.) high genetic diversity around the Tyrrhenian Sea as revealed by nuclear and mitochondrial markers. Hydrobiologia 2019, 826, 209–231. [Google Scholar] [CrossRef]
- Palsbøll, P.J.; Bérubé, M.; Allendorf, F.W. Identification of management units using population genetic data. Trends Ecol. Evol. 2007, 22, 11–16. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Carosi, A.; Bonomo, G.; Lorenzoni, M. Effectiveness of alien brown trout Salmo trutta L. removal activities for the native trout conservation in Mediterranean streams. J. Appl. Ichthyol. 2020, 36, 461–471. [Google Scholar] [CrossRef]
- Härkönen, L.; Hyvärinen, P.; Paappanen, J.; Vainikka, A. Explorative behavior increases vulnerability to angling in hatchery-reared brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2014, 71, 1900–1909. [Google Scholar] [CrossRef]
- Ubertini, L.; Manciola, P.; Casadei, S. Evaluation of the minimum instream flow of the Tiber River Basin. Environ. Monit. Assess. 1996, 41, 125–136. [Google Scholar] [CrossRef] [PubMed]
- D’Antoni, S.; Longobardi, A.; Lorenzoni, M.; Macchio, S.; Mucci, N.; Talarico, L. Linee Guida per la Conservazione della Trota Mediterranea e del Suo Habitat nelle Aree Protette e nei Siti Natura 2000. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/linee-guida-per-la-conservazione-della-trota-mediterranea-e-del-suo-habitat-nelle-aree-protette-e-nei-siti-natura-2000 (accessed on 19 January 2025). (In Italian)
- Matono, P.; Bernardo, J.M.; Oberdorff, T.; Ilhéu, M. Effects of natural hydrological variability on fish assemblages in small Mediterranean streams: Implications for ecological assessment. Ecol. Indic. 2012, 23, 467–481. [Google Scholar] [CrossRef]
- Ayllón, D.; Nicola, G.G.; Parra, I.; Elvira, B.; Almodóvar, A. Spatiotemporal habitat selection shifts in brown trout populations under contrasting natural flow regimes. Ecohydrology 2014, 7, 569–579. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Carosi, A.; Giovannotti, M.; La Porta, G.; Splendiani, A.; Barucchi, V.C. Ecology and conservation of the Mediterranean trout in the central Apennines (Italy). J. Limnol. 2019, 78, 1–13. [Google Scholar] [CrossRef]
- MacCrimmon, H.R.; Marshall, T.L. World Distribution of Brown Trout, Salmo trutta. J. Fish. Res. Board Can. 1968, 25, 2527–2548. [Google Scholar] [CrossRef]
- Aparicio, E.; Rocaspana, R.; de Sostoa, A.; Palau-Ibars, A.; Alcaraz, C. Movements and dispersal of brown trout (Salmo trutta Linnaeus, 1758) in Mediterranean streams: Influence of habitat and biotic factors. PeerJ 2018, 6, e5730. [Google Scholar] [CrossRef]
- Elliott, J.M. Quantitative Ecology and the Brown Trout; Oxford University Press: Oxford, UK, 1994; pp. 1–286. [Google Scholar]
- Caballero, P.; Morán, P.; Marco-Rius, F. A review of the genetic and ecological basis of phenotypic plasticity in brown trout. In Trout: From Physiology to Conservation; Polakof, S., Moon, T.W., Eds.; Nova Science: Hauppauge, NY, USA, 2013; pp. 9–26. [Google Scholar]
- Lionello, P.; Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Almodóvar, A.; Nicola, G.G.; Ayllón, D.; Elvira, B. Global warming threatens the persistence of Mediterranean brown trout. Glob. Change Biol. 2012, 18, 1549–1560. [Google Scholar] [CrossRef]
- Coles, T.F.; Extence, C.A.; Bates, A.J.; Oglanby, G.T.; Mason, C. Surveying the entire ecosystem. Pol. Arch. Hydrobiol. 1988, 35, 563–575. [Google Scholar]
- Carosi, A.; Ghetti, L.; Soresina, A.; Lorenzoni, M. Catch and release angling: Implications for the management and conservation of the Mediterranean trout in central Italy. Fish. Res. 2022, 250, 106285. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J.; Sanz, N. Brown Trout: Biology, Ecology and Management; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–851. [Google Scholar] [CrossRef]
- Casanova, A.; Heras, S.; Abras, A.; Roldán, M.I.; Bouza, C.; Vera, M.; García-Marín, J.L.; Martínez, P. Genomic Hatchery Introgression in Brown Trout (Salmo trutta): Development of a Diagnostic SNP Panel for Monitoring the Impacted Mediterranean Rivers. Genes 2022, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Araguas, R.M.; Vera, M.; Aparicio, E.; Sanz, N.; Fernández-Cebrián, R.; Marchante, C.; García-Marín, J.L. Current status of the brown trout (Salmo trutta) populations within eastern Pyrenees genetic refuges. Ecol. Freshw. Fish 2017, 26, 120–132. [Google Scholar] [CrossRef]
- Araguas, R.M.; Sanz, N.; Pla, C.; Garcia-Marin, J.L. Breakdown of the brown trout evolutionary history due to hybridization between native and cultivated fish. J. Fish Biol. 2004, 65, 28–37. [Google Scholar] [CrossRef]
- Araguas, R.M.; Sanz, N.; Fernandez, R.; Utter, F.M.; Pla, C.; García-Marín, J.L. Role of genetic refuges in the restoration of native gene pools of brown trout. Conserv. Biol. 2009, 23, 871–878. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Population enhancement and population restoration. In Ecology of Atlantic Salmon and Brown Trout; Fish & Fisheries Series; Springer: Dordrecht, The Netherlands, 2011; Volume 33, pp. 567–632. [Google Scholar] [CrossRef]
- Pinter, K.; Epifanio, J.; Unfer, G. Release of hatchery-reared brown trout (Salmo trutta) as a threat to wild populations? A case study from Austria. Fish. Res. 2019, 219, 105296. [Google Scholar] [CrossRef]
- Araki, H.; Berejikian, B.A.; Ford, M.J.; Blouin, M.S. SYNTHESIS: Fitness of hatchery-reared salmonids in the wild. Evol. Appl. 2008, 1, 342–355. [Google Scholar] [CrossRef]
- Forneris, G. Gli Incubatoi di Valle; Amministrazione Provinciale di Torino: Turin, Italy, 1989; pp. 1–57. [Google Scholar]
- Fleming, I.A.; Petersson, E. The ability of released, hatchery salmonids to breed and contribute to the natural productivity of wild populations. Nord. J. Freshw. Res. 2001, 75, 71–98. [Google Scholar]

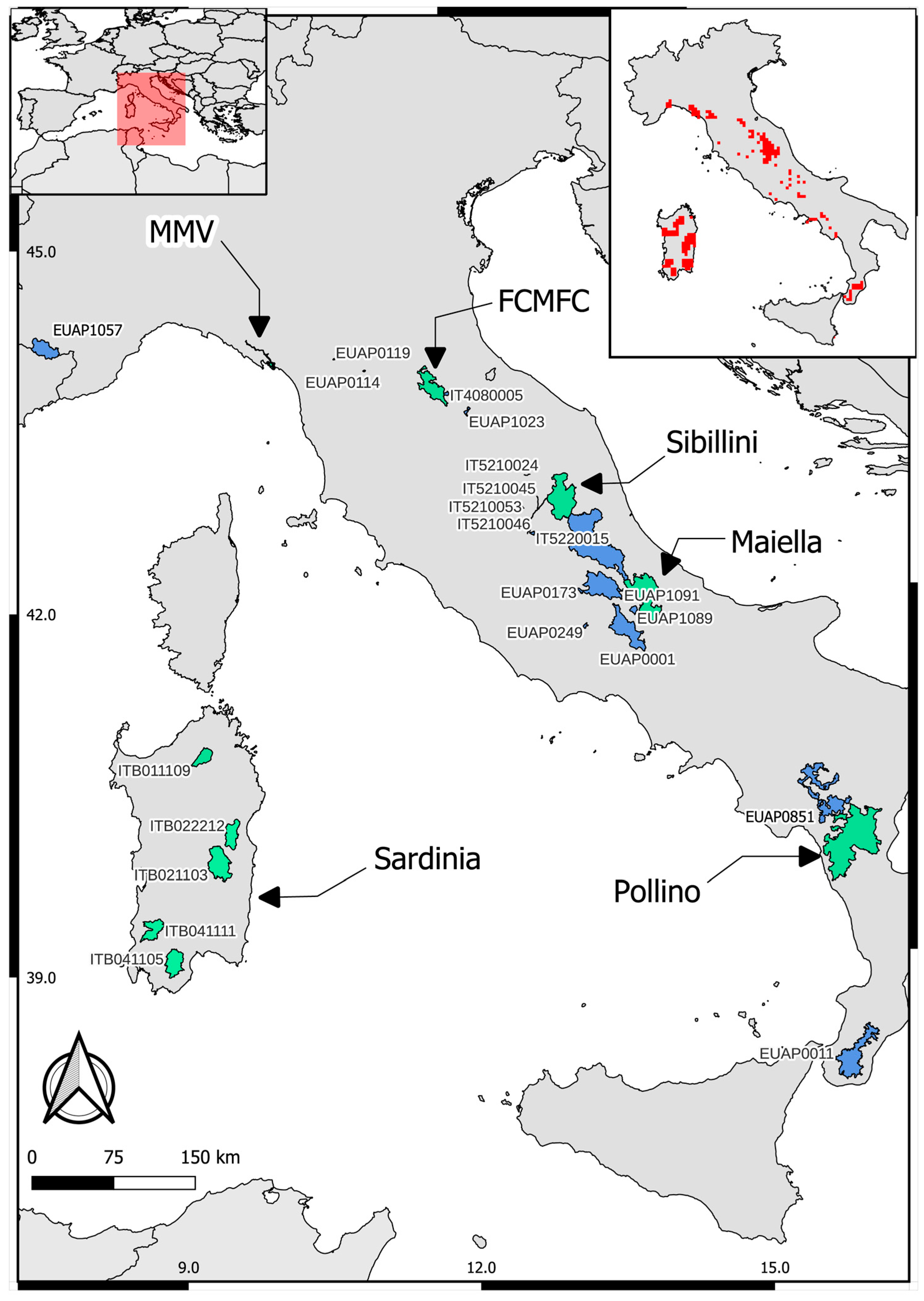
| Pilot Area (Abbreviation) | Extension (ha) | Ecoregion | Elevation Range (m a.s.l.) | Climate | Prevailing Land Type | Main Hydrographic Systems | Background on Genetic Conservation status | Main Threats to Trout Populations |
|---|---|---|---|---|---|---|---|---|
| Foreste Casentinesi national Park (FCMFC) | 36,843 | Temperate | 400–1658 | Warm temperate–hot summers | Semi-natural forest habitats | Watercourses draining into either the Tyrrhenian or Adriatic Sea | Low occurrence of native trout [33,34] | Alien trout |
| Maiella National Park (Maiella) | 74,082 | Temperate | 130–2793 | Warm temperate–hot summers | Mountainous, Apennine deciduous and semi-deciduous forests | Orta, Orfento, Gizio (Aterno-Pescara Adriatic basin) | Pure and partially introgressed populations [35] | Alien trout, water abstraction, hydrological alterations |
| Montemarcello-Magra-Vara Natural Regional Park (MMV) | 4597 | Mediterranean | 0–1639 | Warm temperate–hot summers | Residual alluvial forests, rural areas | Vara and Magra rivers (Ligurian Sea) | Pure, almost pure and moderately introgressed trout populations [33,36] | Alien trout, water abstraction for agricultural purposes |
| Pollino National Park (Pollino) | 192,565 | Temperate | 200–2000 | Warm temperate–hot summers | Mountainous, minimal urban territory | River Sinni (Ionian basin), River Mercure-Lao (Tyrrhenian basin) | Pure Mediterranean trout (river Abatemarco) medium-low level of genetic introgression (River Lao) [13] | Water abstraction for agricultural purposes |
| 2 SACs of Sardinia region: ITB041111, ITB011109, and 3 SPAs: ITB041105, ITB021103, ITB022212 (Sardinia) | 138,873 | Mediterranean | 62–1829 | Temperate with low level of continentality, prolonged Mediterranean summer droughts | Broad-leaved forest and natural grasslands. with a rugged landscape of deep valleys and rocky outcrops | Intermittently flowing Mediterranean rivers | Hybrid and native trout populations [37,38,39] | Alien trout, overfishing, water withdrawal, habitat fragmentation |
| Sibillini Mountains National Park (Sibillini) | 71,400 | Temperate | 400–2476 | Warm temperate–hot summers | Mountainous, Apennine deciduous and semi-deciduous forests | Watercourses draining into either the Tyrrhenian or Adriatic Sea | Residual nearly pure native trout populations [6] | Alien trout, river fragmentation, presence of hatcheries |
| Pilot Area | Elevation (m a.s.l.) | Conductivity (µS cm−1) | Average Current Speed (m s−1) | Flow Rate (L s−1) | pH (Units) | Dissolved Oxygen (mg L−1) | Water Temperature (°C) | Total Length (cm) | Density (ind m 2) | Standing Crop (g m−2) | PSD (Units) | Wr (Units) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCMFC | 814.3 ± 142.0 | 318.6 ± 68.8 | 0.18 ± 0.17 | 39.5 ±47.6 | 7.2 ± 0.2 | 8.7 ± 0.8 | 12.5 ± 2.8 | 14.77 ± 3.12 | 0.11 ± 0.07 | 3.91 ± 1.84 | 23.4 ± 19.1 | 79.47 ± 6.2 |
| Maiella | 776.7 ± 304.2 | 319.2 ± 64.7 | 0.11 ± 0.05 | 80.0 ± 142.7 | 7.5 ± 0.4 | 8.8 ± 0.9 | 14.4 ± 2.7 | 16.09 ± 4.75 | 0.28 ± 0.30 | 9.37 ± 5.79 | 25.8 ± 26.5 | 92.01 ± 6.3 |
| MMV | 503.1 ± 279.8 | 262.0 ± 78.5 | 0.12 ± 0.13 | 167.2 ± 102.3 | 7.9 ± 0.7 | 8.2 | 14.8 ± 3.0 | 12.54 ± 1.55 | 0.28 ± 0.30 | 8.27 ± 6.71 | 4.65 ± 2.55 | 88.73 ± 3.8 |
| Pollino | 433.1 ± 286.4 | 377.2 ± 114.4 | 0.16 ± 0.17 | 696.0 ± 1185.8 | 8.4 ± 0.3 | 7.2 ± 2.9 | 13.5 ± 2.8 | 12.27 ± 2.80 | 0.13 ± 0.09 | 2.82 ± 2.04 | 17.4 ± 14.7 | 90.51 ± 4.5 |
| Sardinia | 540.5 ± 254.8 | 414.4 ± 212.9 | 0.12 ± 0.10 | 28.4 ± 33.9 | 7.8 ± 0.5 | 7.1 ± 0.9 | 14.1 ± 3.0 | 13.87 ± 1.72 | 0.14 ± 0.15 | 4.79 ± 4.42 | 12.4 ±1 2.3 | 91.48 ± 6.5 |
| Sibillini | 704.9 ± 204.0 | 374.8 ± 131.8 | 0.17 ± 0.22 | 230.9 ± 281.9 | 8.4 ± 0.5 | 9.2 ± 0.6 | 11.1 ± 2.0 | 14.95 ± 3.08 | 0.42 ± 0.29 | 20.4 ± 17.0 | 24.2 ± 19.5 | 89.44 ± 5.9 |
| Protected Area | Years | Spawners | Genetically Suitable Spawners (Relative Ratio) | Fertilized Eggs | Released Material (Type) |
|---|---|---|---|---|---|
| FCMFC | 2021, 2023 | 106 | 82 (77.4%) | 6309 | 3994 (alevins) |
| Maiella | 2021, 2022, 2023 | 638 | 298 (46.7%) | 319,524 | 3500 (eggs), 5150 (alevins) |
| MMV | 2021, 2022, 2024 | 181 | 139 (76.8%) | 203,025 | 34,000 (eggs), 122,700 (alevins) |
| Pollino | 2022, 2024 | 135 | 125 (92.6%) | 1380 | 578 (alevins) |
| Sardinia | 2022, 2024 | 146 | 105 (71.9%) | 2046 | 254 (eggs) |
| Sibillini | 2021, 2023 | 225 | 199 (88.4%) | n.a. | 23,000 (eggs), 5750 (alevins) |
| Overall | 2021–2024 | 1431 | 948 (66.2%) | 532,284 | 60,754 (eggs), 138,172 (alevins) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carosi, A.; Talarico, L.; Greco, C.; Vecchiotti, A.; D’Antoni, S.; Longobardi, A.; Macchio, S.; Carafa, M.; Casula, P.; Perfetti, A.; et al. The LIFE STREAMS Project for the Recovery of the Native Mediterranean Trout in Six Italian Pilot Areas: Planning and Adoption of Conservation Actions. Biology 2025, 14, 573. https://doi.org/10.3390/biology14050573
Carosi A, Talarico L, Greco C, Vecchiotti A, D’Antoni S, Longobardi A, Macchio S, Carafa M, Casula P, Perfetti A, et al. The LIFE STREAMS Project for the Recovery of the Native Mediterranean Trout in Six Italian Pilot Areas: Planning and Adoption of Conservation Actions. Biology. 2025; 14(5):573. https://doi.org/10.3390/biology14050573
Chicago/Turabian StyleCarosi, Antonella, Lorenzo Talarico, Claudia Greco, Antonia Vecchiotti, Susanna D’Antoni, Alessandro Longobardi, Stefano Macchio, Marco Carafa, Paolo Casula, Antonio Perfetti, and et al. 2025. "The LIFE STREAMS Project for the Recovery of the Native Mediterranean Trout in Six Italian Pilot Areas: Planning and Adoption of Conservation Actions" Biology 14, no. 5: 573. https://doi.org/10.3390/biology14050573
APA StyleCarosi, A., Talarico, L., Greco, C., Vecchiotti, A., D’Antoni, S., Longobardi, A., Macchio, S., Carafa, M., Casula, P., Perfetti, A., Amprimo, P., Rossetti, A., Morandi, F., Alberti, D., Serroni, P., Raimondi, S., Mattioli, D., Mucci, N., & Lorenzoni, M. (2025). The LIFE STREAMS Project for the Recovery of the Native Mediterranean Trout in Six Italian Pilot Areas: Planning and Adoption of Conservation Actions. Biology, 14(5), 573. https://doi.org/10.3390/biology14050573









