Application of Biochar on Soil Improvement and Speciation Transformation of Heavy Metal in Constructed Wetland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of SMB
2.3. Pot Experiments
2.4. Soil Properties Analysis
2.5. Statistics Analysis
3. Results and Discussion
3.1. Properties of Sewage Sludge and Maize Straw
3.2. Properties of SMB
3.2.1. Physicochemical Characteristics of SMB
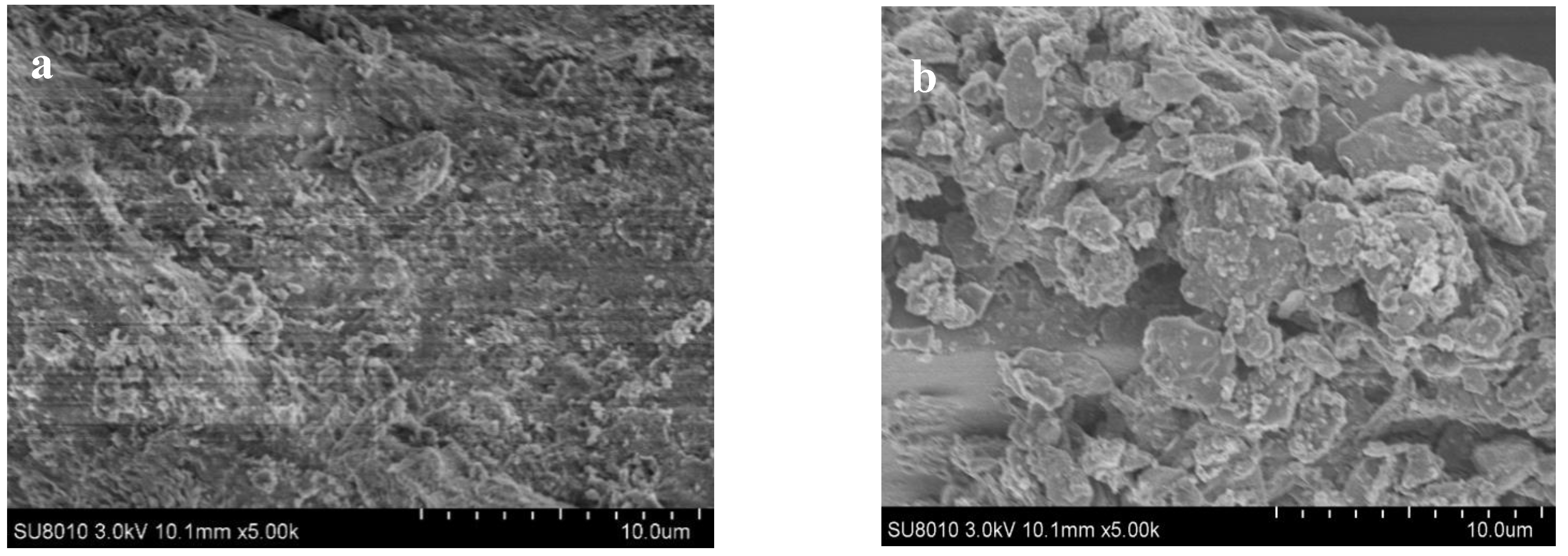
3.2.2. SEM Images of SMB
3.2.3. Functional Groups of SMB
3.3. Influence of SMB on Soil Properties
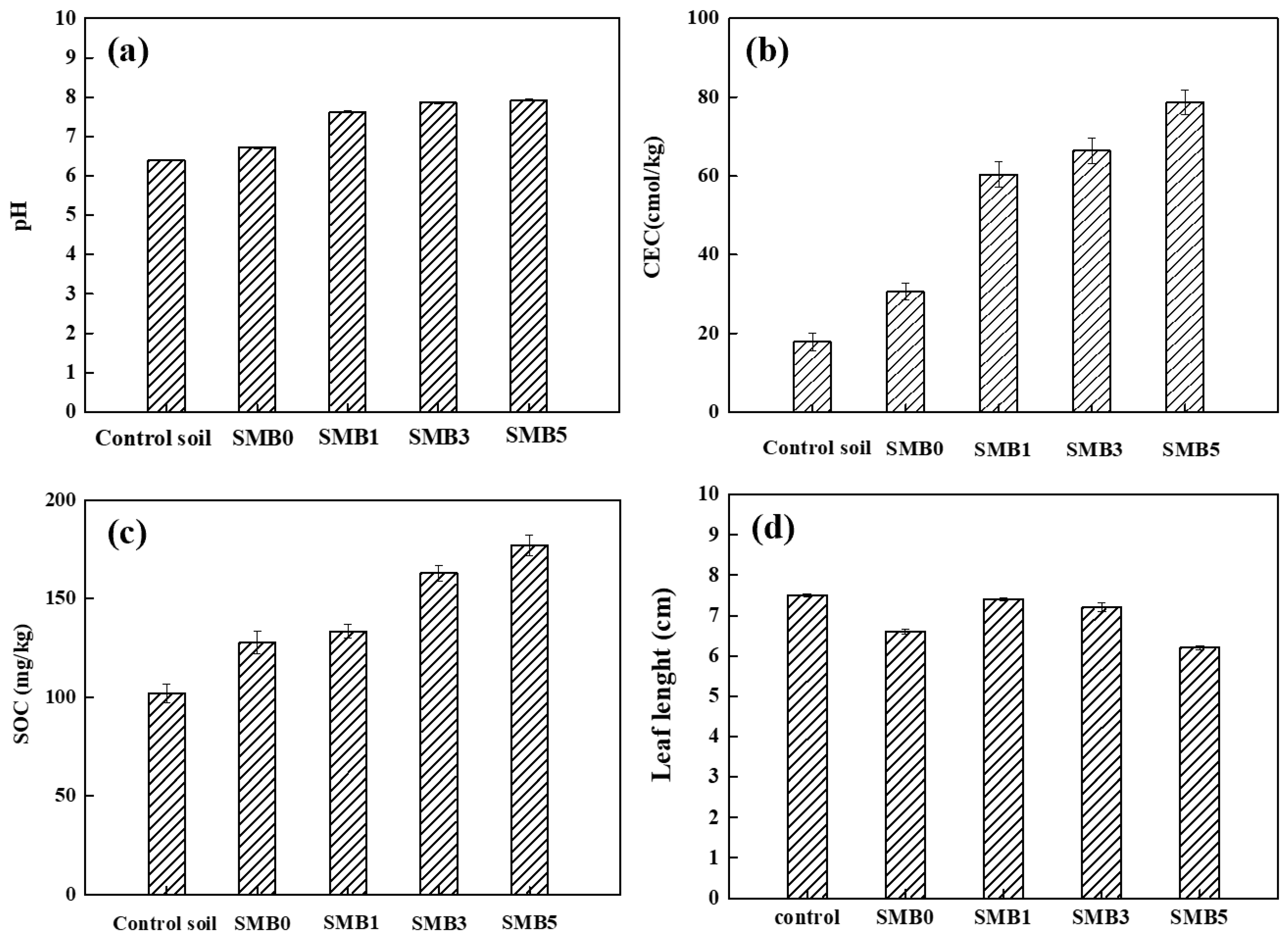
3.3.1. Influence of SMB on Soil pH and CEC
3.3.2. Influence of SMB on Soil Organic Carbon and Nutrient Contents
3.3.3. Mechanism of the SMB on Affecting Soil Properties
- (i)
- The alkaline minerals (e.g., CaCO3 and MgO) and soluble cations (K+ and Ca2+) derived from sewage sludge ash elevated the soil pH (from 6.40 to 7.93) and boosted CEC, facilitating Pb2+ immobilization via electrostatic adsorption and hydroxide precipitation.
- (ii)
- Maize straw-derived aromatic carbon structures enhanced soil organic carbon (SOC: 176.79 mg/kg at 5% SMB) while forming microporous networks (pore size: 20–150 nm) that adsorbed nutrients (N, P, K) and stabilized labile organic matter.
- (iii)
- The hybrid mineral-organic matrix of SMB, such as sludge-originated oxides, immobilized Pb through ion exchange, while the induced functional groups (i.e., -COOH, -C-H) promoted microbial enrichment. The prominent C-O stretching band (1033 cm−1) from hydroxyl/phenolic groups contributed to elevated soil pH through OH− release, while CEC was via negatively charged surfaces for electrostatic Pb2+ adsorption. These groups facilitated Pb immobilization via coordination bonding and phosphate precipitation. The disappearance of maize straw’s C=C (1250 cm−1) and sludge’s -CH2 (2975 cm−1) confirmed carbonization-driven aromaticity, which stabilized soil organic carbon via hydrophobic interactions.
3.4. Influence of SMB on Plant Growth
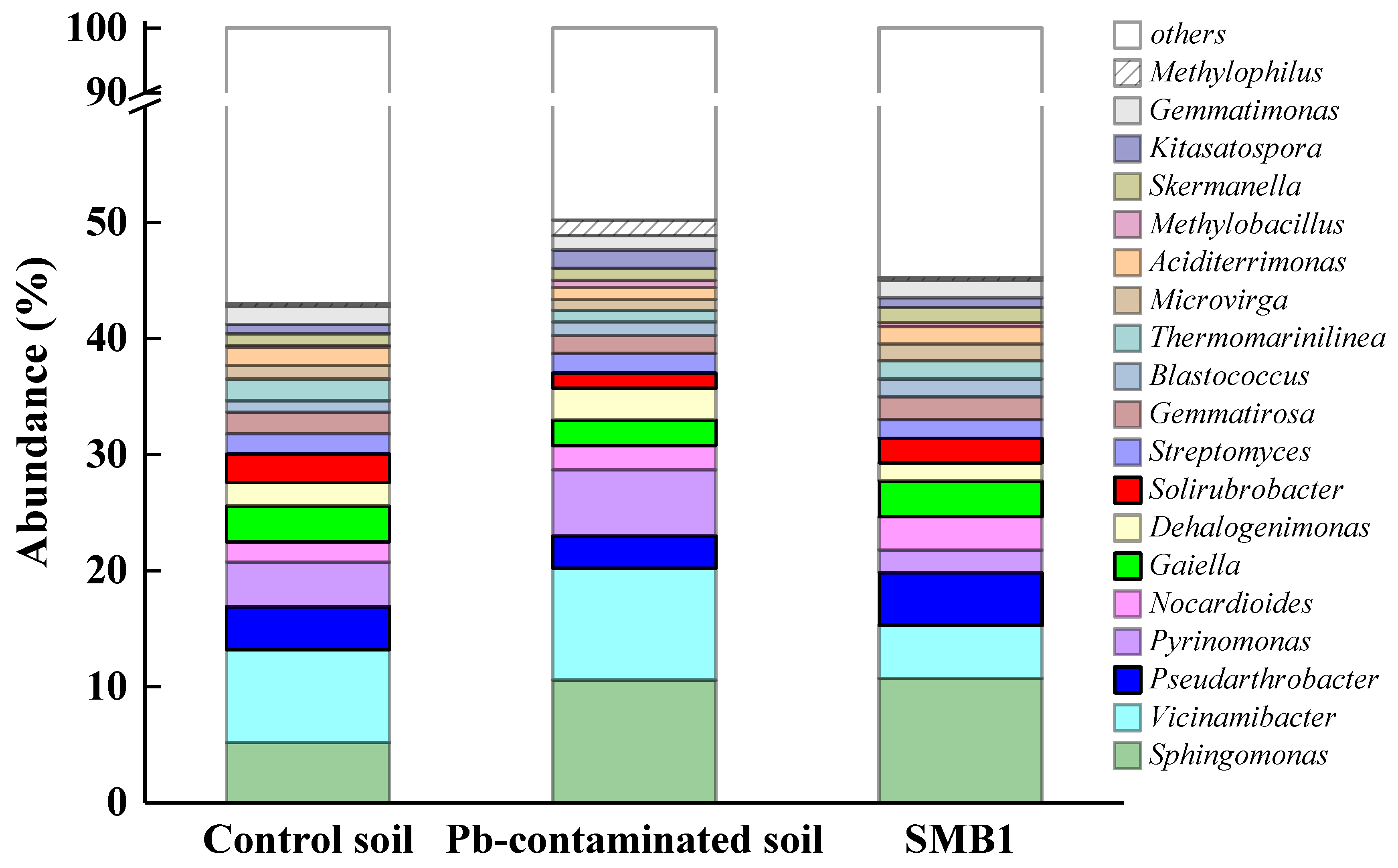
3.5. Influence of SMB on Microbial Community Structure
3.6. Influence of SMB on Pb Content in Soil
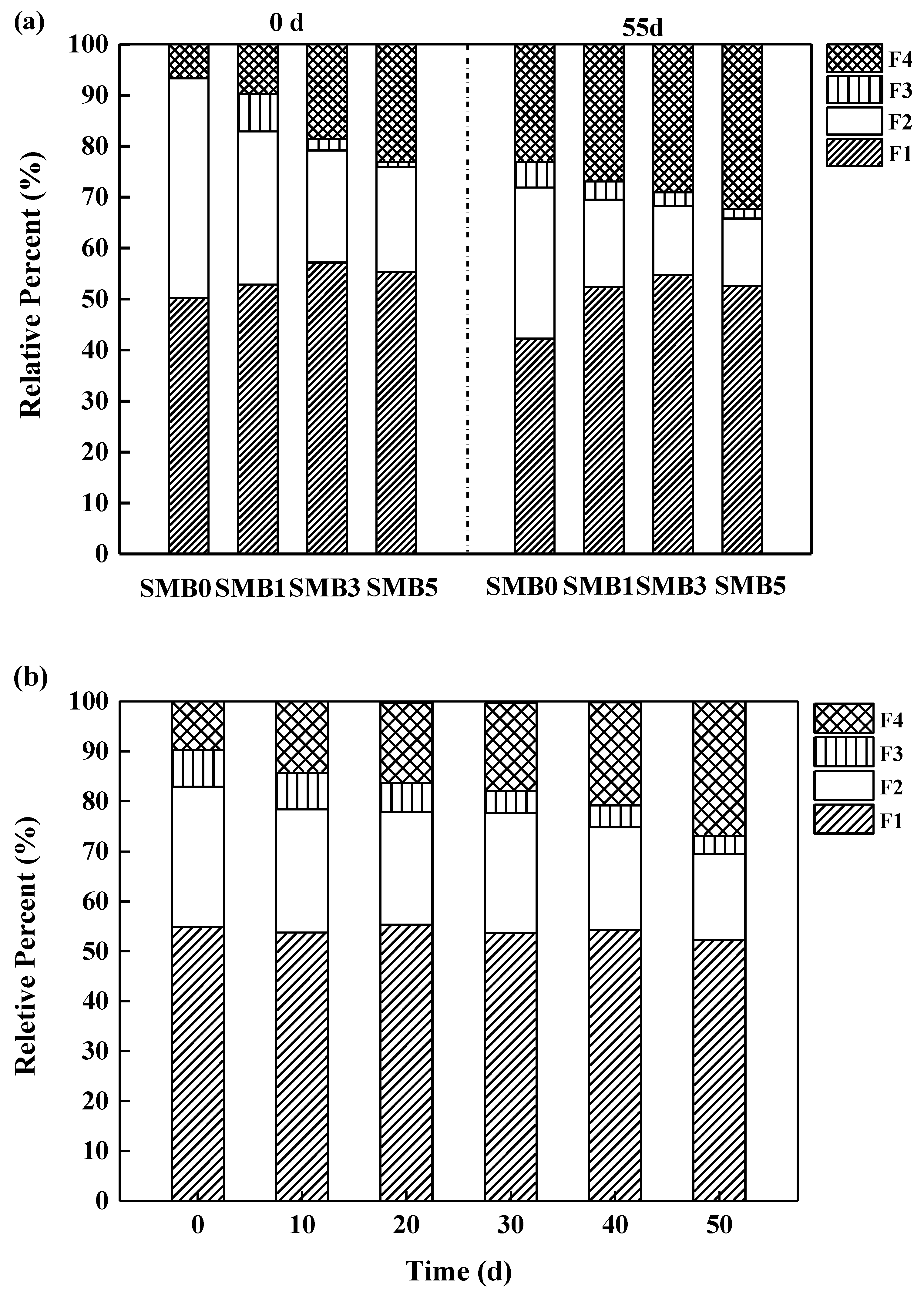
3.7. Influence of SMB on Pb Speciation Transformation in Soil
4. Engineering Implications of SMB Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Wang, F.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Li, C.; Zeng, G. Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil. Chemosphere 2017, 189, 627–633. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, Y.; Zhang, Q.; Zhang, Z.; Wang, C.; Zhai, S.; Li, Y.; Sun, H. Biochar derived from hydrolysis of sewage sludge influences soil properties and heavy metals distributed in the soil. J. Hazard. Mater. 2023, 442, 130053. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.C.; Li, Z.; Song, Y.; Li, C.; Wang, Y. Co-pyrolysis of sewage sludge and food waste digestate to synergistically improve biochar characteristics and heavy metals immobilization. Waste Manag. 2022, 141, 231–239. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Liu, H.; Zhao, Y.; Li, L. Effects of pyrolysis temperature on biochar’s characteristics and speciation and environmental risks of heavy metals in sewage sludge biochars. Environ. Technol. Innov. 2022, 26, 102288. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Jiang, W.; Shao, L.; Zhang, L.; Feng, L. Effects of pyrolysis temperature and addition proportions of corncob on the distribution of products and potential energy recovery during the preparation of sludge activated carbon. Chemosphere 2019, 221, 175–183. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Sultana, T.; Akram, M.; Shoumik, B.A.A.; Khan, M.Z.; Farooq, M.A. Utilization of sewage sludge to manage saline–alkali soil and increase crop production: Is it safe or not? Environ. Technol. Innov. 2023, 32, 103266. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y. Biofuel production by co-pyrolysis of sewage sludge and other materials: A review. Environ. Chem. Lett. 2023, 21, 153–182. [Google Scholar] [CrossRef]
- Chen, G.; Tian, S.; Liu, B.; Hu, M.; Ma, W.; Li, X. Stabilization of heavy metals during co-pyrolysis of sewage sludge and excavated waste. Waste Manag. 2020, 103, 268–275. [Google Scholar] [CrossRef]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage sludge and wetland biomass waste for biochar production: Behaviors of phosphorus and heavy metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef]
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Bao, Z.; Shi, C.; Tu, W.; Li, L.; Li, Q. Recent developments in modification of biochar and its application in soil pollution control and ecoregulation. Environ. Pollut. 2022, 313, 120184. [Google Scholar] [CrossRef]
- Gondek, K.; Hersztek, M.M.; Kopec, M. Mobility of heavy metals in sandy soil after application of composts produced from maize straw, sewage sludge and biochar. J. Environ. Manag. 2018, 210, 87–95. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, X.; Zhao, Z.; He, Q.; Wang, S. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ. Pollut. 2016, 218, 513–522. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Kwon, E.E.; Vithanage, M.; Rinklebe, J.; Moon, D.H.; Meers, E.; Tsang, D.C.W.; Ok, Y.S. Soil lead immobilization by biochars in short-term laboratory incubation studies. Environ. Int. 2019, 127, 190–198. [Google Scholar] [CrossRef]
- Rice, E.W.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; pp. 53–58. [Google Scholar]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China Environmental Science Press: Beijing, China, 2018.
- Xu, C.; Xiang, Q.; Zhu, H.; Wang, S.; Zhu, Q.; Huang, D.; Zhang, Y. Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soil. Ecotoxicol. Environ. Saf. 2018, 164, 554–561. [Google Scholar]
- Kongthod, T.; Thanachit, S.; Anusontpornperm, S.; Wiriyakitnateekul, W. Effects of biochars and other organic soil amendments on plant nutrient availability in an ustoxic quartzipsamment. Pedosphere 2015, 25, 790–798. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, D.; Dang, Y.; Lei, Y.; Ji, J.; Lv, T.; Bian, R.; Xiao, Z.; Yan, L.; Holmes, D.E. Enhancing biomethanogenic treatment of fresh incineration leachate using single chambered microbial electrolysis cells. Bioresour. Technol. 2017, 231, 129–137. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, L.; Liu, K.; Wang, J.; Zhu, H.; Song, Q.; Shu, X. Co-pyrolysis of sewage sludge and cotton stalks. Waste Manag. 2019, 89, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, T.; Lai, F.; Wu, G. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. China Environmental Science Press: Beijing, China, 2007.
- Liu, Y.; Song, Y.; Fu, J.; Ao, W.; Siyal, A.A.; Zhou, C.; Liu, C.; Yu, M.; Zhang, Y.; Dai, J.; et al. Co-pyrolysis of sewage sludge and lignocellulosic biomass: Synergistic effects on products characteristics and kinetics. Energy Convers. Manag. 2022, 268, 116061. [Google Scholar] [CrossRef]
- Conti, R.; Fabbri, D.; Vassura, I.; Ferroni, L. Comparison of chemical and physical indices of thermal stability of biochars from different biomass by analytical pyrolysis and thermogravimetry. J. Anal. Appl. Pyrolysis 2016, 122, 160–168. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2011, 41, 1001–1013. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, B.; Sun, M.; Chen, X.; Zhang, M.; Li, H.; Xu, S. Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis. Waste Manag. 2017, 62, 91–100. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Munoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Wang, S.; Guo, W.; Gao, F.; Yang, R. Characterization and Pb(II) removal potential of corn straw- and municipal sludge-derived biochars. R. Soc. Open Sci. 2017, 4, 170402. [Google Scholar] [CrossRef]
- Alvarez-Rogel, J.; Tercero Gomez, M.D.C.; Conesa, H.M.; Párraga-Aguado, I.; González-Alcaraz, M.N. Biochar from sewage sludge and pruning trees reduced porewater Cd, Pb and Zn concentrations in acidic, but not basic, mine soils under hydric conditions. J. Environ. Manag. 2018, 223, 554–565. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Ming, Z.; Bolan, N.; Mohan, D.; Vithanage, M.; Sang, S.L.; Yong, S.O. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manage. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L., Jr.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Qin, P.; Wang, H.; Yang, X.; He, L.; Muller, K.; Shaheen, S.M.; Xu, S.; Rinklebe, J.; Tsang, D.C.M.; Ok, Y.S. Bamboo-and pig-derived biochars reduce leaching losses of dibutyl phthalate, cadmium, and lead from co-contaminated soils. Chemosphere 2018, 198, 450–459. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Huang, H.; Chen, Y. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, X.; Zhu, Y.; Han, Z.; He, Q.; Zhang, F. Effect of biochar on the presence of nutrients and ryegrass growth in the soil from an abandoned indigenous coking site: The potential role of biochar in the revegetation of contaminated site. Sci. Total Environ. 2017, 601, 469–477. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338. [Google Scholar] [CrossRef]
- Jones, S.; Bardos, R.P.; Kidd, P.S.; Mench, M.; Leij, F.; Hutchings, T.; Cundy, A.; Joyce, C.; Soja, G.; Hanl, W.F.; et al. Biochar and compost amendments enhance copper immobilisation and support plant growth in contaminated soils. J. Environ. Manag. 2016, 171, 101–112. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Kim, H.J.; Yoon, J.H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.H. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Feng, G.D.; Yang, S.-Z.; Wang, Y.-H.; Zhao, G.Z.; Deng, M.R.; Zhu, H.H. Sphingomonas gimensis sp. nov., a novel Gram-negative bacterium isolated from abandoned lead–zinc ore mine. Antonie Van Leeuwenhoek 2014, 105, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Z.; Gao, Q.; Ma, Y. Isolation and characterization of aerobic, culturable, arsenic-tolerant bacteria from lead–zinc mine tailing in southern China. World J. Microbiol. Biotechnol. 2018, 34, 177. [Google Scholar] [CrossRef]
- Fekih, B.I.; Ma, Y.; Herzberg, M.; Zhang, C.; Li, Y.; Mazhar, S.; Bello, S.; Yang, N.; Su, J.; Xu, J.; et al. Draft genome sequence of Pseudarthrobacter sp. strain AG30, isolated from a gold and copper mine in China. Microbiol. Resour. Announc. 2018, 7, 01329-18. [Google Scholar]
- Yuan, L.; Hu, X.F. Distribution Characteristics and Pollution Assessment of Heavy Metals in Soil of Coal Gangue Leachate Polluted Area in Guizhou. Adv. Environ. Prot. 2022, 12, 176–184. [Google Scholar] [CrossRef]
- Wang, T.-J.; Su, N.-N.; Lei, P. Community structure of heavy metal immobilized bacteria in the lettuce (Lactuca sativa L.) rhizosphere in soil polluted by heavy metals and its effects on reducing heavy metal accumulation in lettuce. Huanjing Kexue 2019, 40, 5133–5141. [Google Scholar]
- Saurav, K.; Kannabiran, K. Biosorption of Cd (II) and Pb (II) ions by aqueous solutions of novel alkalophillic Streptomyces VITSVK5 spp. biomass. J. Ocean Univ. China 2011, 10, 61–66. [Google Scholar] [CrossRef]
- Rodrigues, V.D.; Torres, T.T.; Ottoboni, L.M. Bacterial diversity assessment in soil of an active Brazilian copper mine using high-throughput sequencing of 16S rDNA amplicons. Antonie Van Leeuwenhoek 2014, 106, 879–890. [Google Scholar] [CrossRef]
- Hemmat-Jou, M.H.; Safari-Sinegani, A.A.; Mirzaie-Asl, A.; Tahmourespour, A. Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 2018, 27, 1281–1291. [Google Scholar] [CrossRef]
- Dudnikova, T.; Wong, M.H.; Minkina, T.; Sushkova, S.; Bauer, T.; Khroniuk, O.; Barbashev, A.; Shuvaev, E.; Nemtseva, A.; Kravchenko, E. Effects of pyrolysis conditions on sewage sludge-biochar properties and potential risks based on PAH contents. Environ. Res. 2025, 266, 120444. [Google Scholar] [CrossRef]


| Sewage Sludge | Maize Straw | SMB | Sandy Soil | Unit | |
|---|---|---|---|---|---|
| Moisture | 2.25 | 4.59 | 2.33 | 22.11 | wt% |
| Ash | 29.53 | 1.69 | 54.44 | – | wt% |
| VM a | 61.52 | 78.08 | 14.65 | – | wt% |
| FC b | 6.70 | 15.64 | 28.58 | – | wt% |
| C | 53.21 | 49.20 | 21.18 | 1.21 | wt% |
| H | 7.53 | 6.31 | 2.73 | 0.91 | wt% |
| O | 30.89 | 43.74 | 73.51 | 50.34 | wt% |
| N | 6.39 | 0.45 | 1.48 | 0.12 | wt% |
| pH | 5.9–6.0 | – | 8.1–8.2 | 6.3–6.4 | – |
| CEC c | – | – | 29.93 ± 0.55 | 17.32 ± 0.13 | cmol/kg |
| Surface area | – | – | 163.11 | – | m2/g |
| Pb | 5.04 ± 0.79 | 8.75 ± 0.02 | 32.56 ± 0.41 | 17.83 ± 0.62 | mg/kg |
| Cd | ND d | ND d | 0.06 ± 0.01 | 0.09 ± 0.15 | mg/kg |
| Mg | 0.63 ± 0.15 | 3.30 ± 0.02 | 4.83 ± 0.46 | 4.56 ± 1.31 | g/kg |
| Na | 0.38 ± 0.08 | 0.90 ± 0.09 | 1.01 ± 0.08 | 0.18 ± 0.06 | g/kg |
| K | 0.49 ± 0.09 | 11.87 ± 1.30 | 5.09 ± 0.07 | 0.88 ± 0.15 | g/kg |
| Al | 8.55 ± 0.64 | 3.62 ± 1.04 | 35.26 ± 0.67 | 4.26 ± 0.41 | g/kg |
| Cu | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.21 ± 0.09 | 0.02 ± 0.10 | g/kg |
| Fe | 2.32 ± 0.23 | 3.45 ± 1.42 | 20.76 ± 0.21 | 9.06 ± 0.79 | g/kg |
| Mn | 0.04 ± 0.01 | 0.12 ± 0.20 | 0.33 ± 0.07 | 0.24 ± 0.09 | g/kg |
| Zn | 0.28 ± 0.05 | 0.18 ± 0.03 | 1.35 ± 0.03 | 0.08 ± 0.10 | g/kg |
| Ca | 7.38 ± 0.21 | 15.32 ± 1.26 | 44.11 ± 0.26 | 15.80 ± 1.94 | g/kg |
| SMB Proportion | pH | SOC | CEC | Ryegrass Weight | Leaf Length | Microbial Community | |
|---|---|---|---|---|---|---|---|
| pH | 0.818 | ||||||
| SOC | 0.987 * | 0.794 | |||||
| CEC | 0.898 | 0.978 * | 0.856 | ||||
| Ryegrass weight | −0.010 | 0.559 | −0.009 | 0.389 | |||
| Leaf length | −0.450 | 0.125 | −0.427 | −0.071 | 0.891 | ||
| Microbial community | −0.300 | −0.074 | −0.446 | −0.032 | 0.151 | 0.169 | |
| Soil Pb | 0.812 | 0.997 ** | 0.775 | 0.983 * | 0.551 | 0.113 | −0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Nie, X.; Zhao, Y.; Zhang, L.; Cheng, Y.; Jiang, C.; Zhao, W.; Wang, X.; Yang, C. Application of Biochar on Soil Improvement and Speciation Transformation of Heavy Metal in Constructed Wetland. Biology 2025, 14, 515. https://doi.org/10.3390/biology14050515
Zhou Y, Nie X, Zhao Y, Zhang L, Cheng Y, Jiang C, Zhao W, Wang X, Yang C. Application of Biochar on Soil Improvement and Speciation Transformation of Heavy Metal in Constructed Wetland. Biology. 2025; 14(5):515. https://doi.org/10.3390/biology14050515
Chicago/Turabian StyleZhou, Yuan, Xiaoqin Nie, Yao Zhao, Liqiu Zhang, Yatian Cheng, Cancan Jiang, Wenbin Zhao, Xiangchun Wang, and Chao Yang. 2025. "Application of Biochar on Soil Improvement and Speciation Transformation of Heavy Metal in Constructed Wetland" Biology 14, no. 5: 515. https://doi.org/10.3390/biology14050515
APA StyleZhou, Y., Nie, X., Zhao, Y., Zhang, L., Cheng, Y., Jiang, C., Zhao, W., Wang, X., & Yang, C. (2025). Application of Biochar on Soil Improvement and Speciation Transformation of Heavy Metal in Constructed Wetland. Biology, 14(5), 515. https://doi.org/10.3390/biology14050515








