ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Protein Isolate from Bambara Groundnut
2.2. Preparation of Protein Hydrolysate
2.3. Determination of α-Amino Group Content and Degree of Hydrolysis
2.4. Separation of Peptides Using Ultrafiltration
2.5. Angiotensin-Converting Enzyme (ACE) Inhibition Assay
2.6. Dipeptidyl Peptidase IV (DPP-IV) Inhibition Assay
2.7. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.8. Bioactive Peptide Databases and Computational Analysis
2.9. Modeling of Peptide Structures and Molecular Docking
2.10. Statistical Analysis
3. Results
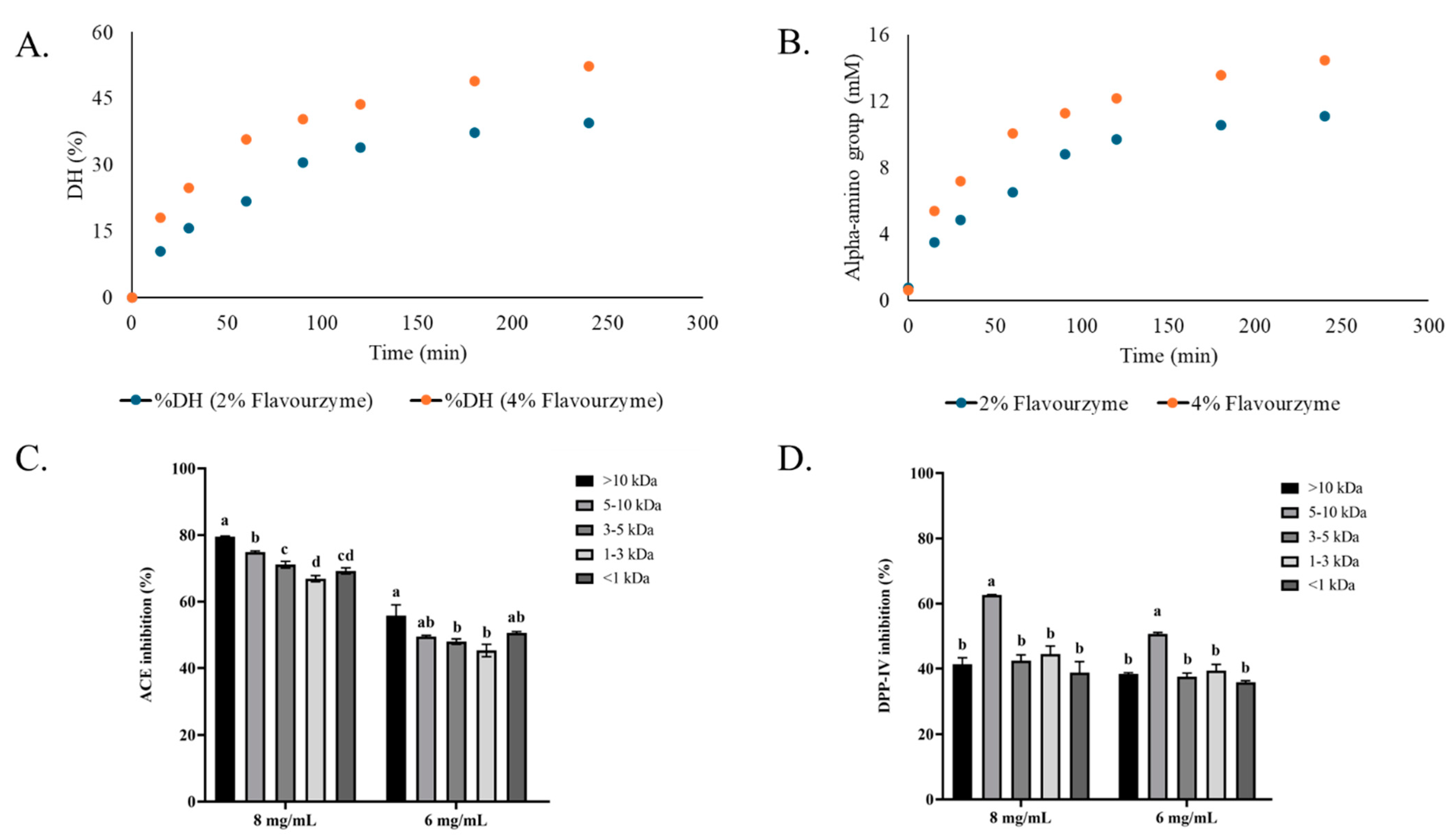
3.1. Degree of Hydrolysis and Inhibitory Activities of Bambara Groundnut Protein Hydrolysates
3.2. In Silico Screening and Characteristics of Bioactive Peptides
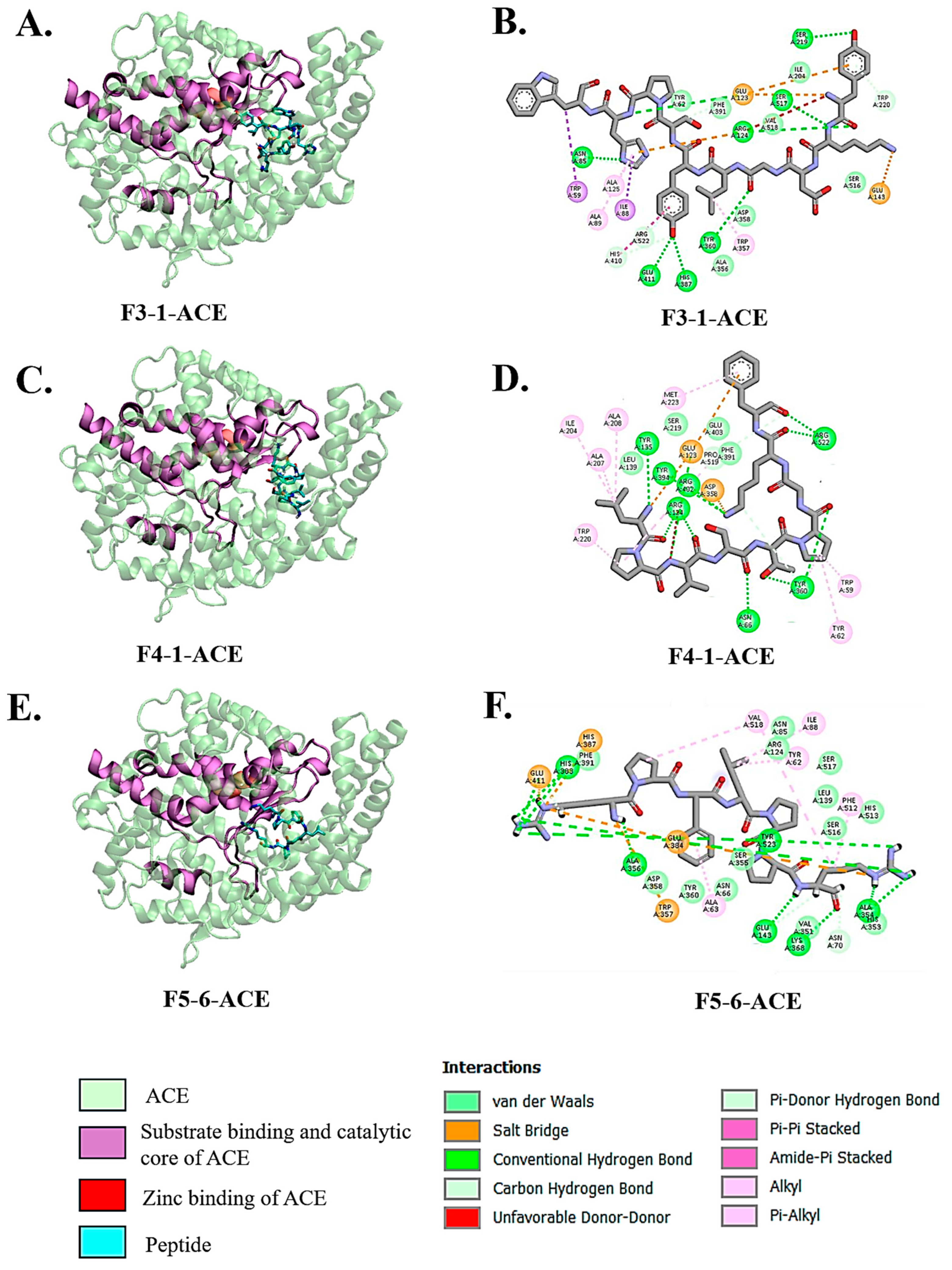
3.3. Molecular Docking Interactions of Peptides with ACE
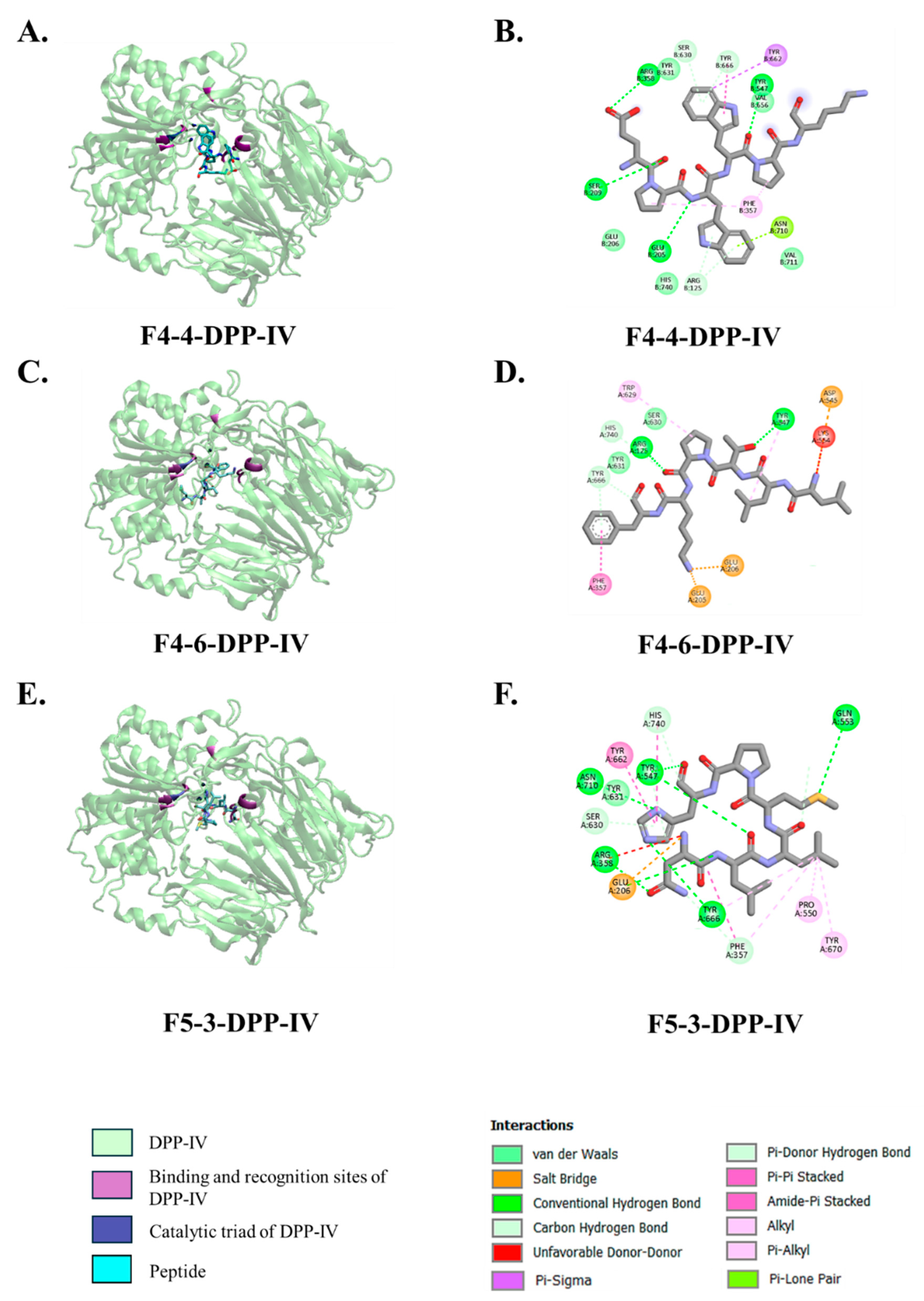
3.4. Molecular Docking Interactions of Peptides with DPP-IV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ignjacev-Lazich, I.; Kintsurashvili, E.; Johns, C.; Vitseva, O.; Duka, A.; Shenouda, S.; Gavras, I.; Gavras, H. Angiotensin-Converting Enzyme Regulates Bradykinin Receptor Gene Expression. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1814–H1820. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 Inhibitors for Treating T2DM—Hype or Hope? An Analysis Based on the Current Literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Q.; Zhong, J.; Liu, C.; Zheng, B.; Gong, Q. DPP-4 Inhibitors as Potential Candidates for Antihypertensive Therapy: Improving Vascular Inflammation and Assisting the Action of Traditional Antihypertensive Drugs. Front. Immunol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Montinaro, V.; Cicardi, M. ACE Inhibitor-Mediated Angioedema. Int. Immunopharmacol. 2020, 78, 106081. [Google Scholar] [CrossRef]
- Takuathung, M.N.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Avogaro, A.; Belcastro, S.; Bonora, B.M.; Croci, M.; Daniele, G.; Dauriz, M.; Dotta, F.; Formichi, C.; Frontoni, S.; et al. Ten Years of Experience with DPP-4 Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Acta Diabetol. 2019, 56, 605–617. [Google Scholar] [CrossRef]
- Zaresharifi, S.; Niroomand, M.; Borran, S.; Dadkhahfar, S. Dermatological Side Effects of Dipeptidyl Peptidase-4 Inhibitors in Diabetes Management: A Comprehensive Review. Clin. Diabetes Endocrinol. 2024, 10, 6. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the Mechanisms of Action Based on the Pathophysiology of Hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; Sosa-Espinoza, T.; Ruiz-Ruiz, J.; Segura-Campos, M.; Chel-Guerrero, L. Enzymatic Hydrolysis of Hard-to-Cook Bean (Phaseolus vulgaris L.) Protein Concentrates and Its Effects on Biological and Functional Properties. Int. J. Food Sci. Technol. 2014, 49, 2–8. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Mora-Melgem, J.A.; Arámburo-Gálvez, J.G.; Cárdenas-Torres, F.I.; Gonzalez-Santamaria, J.; Ramírez-Torres, G.I.; Arvizu-Flores, A.A.; Figueroa-Salcido, O.G.; Ontiveros, N. Dipeptidyl Peptidase IV Inhibitory Peptides from Chickpea Proteins (Cicer arietinum L.): Pharmacokinetics, Molecular Interactions, and Multi-Bioactivities. Pharmaceuticals 2023, 16, 1109. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Rivero-Pino, F.; Martin, M.E.; Gonzalez-de la Rosa, T.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Identification of the Bioavailable Peptidome of Chia Protein Hydrolysate and the In Silico Evaluation of Its Antioxidant and ACE Inhibitory Potential. J. Agric. Food Chem. 2024, 72, 3189–3199. [Google Scholar] [CrossRef]
- Kiersnowska, K.; Jakubczyk, A. Bioactive Peptides Obtained from Legume Seeds as New Compounds in Metabolic Syndrome Prevention and Diet Therapy. Foods 2022, 11, 3300. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional Quality of Fresh and Stored Legumes Sprouts—Effect of Lactobacillus plantarum 299v Enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef]
- Mayes, S.; Ho, W.K.; Chai, H.H.; Gao, X.; Kundy, A.C.; Mateva, K.I.; Zahrulakmal, M.; Hahiree, M.K.I.M.; Kendabie, P.; Licea, L.C.S.; et al. Bambara Groundnut: An Exemplar Underutilised Legume for Resilience under Climate Change. Planta 2019, 250, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Azman Halimi, R.; Barkla, B.J.; Mayes, S.; King, G.J. The Potential of the Underutilized Pulse Bambara Groundnut (Vigna subterranea (L.) Verdc.) for Nutritional Food Security. J. Food Compos. Anal. 2019, 77, 47–59. [Google Scholar] [CrossRef]
- Tan, X.L.; Azam-Ali, S.; Goh, E.V.; Mustafa, M.; Chai, H.H.; Ho, W.K.; Mayes, S.; Mabhaudhi, T.; Azam-Ali, S.; Massawe, F. Bambara Groundnut: An Underutilized Leguminous Crop for Global Food Security and Nutrition. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Steve Ijarotimi, O.; Ruth Esho, T. Comparison of Nutritional Composition and Anti-nutrient Status of Fermented, Germinated and Roasted Bambara Groundnut Seeds (Vigna subterranea). Br. Food J. 2009, 111, 376–386. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Ayedun, H.; Sanni, L.O. Chemical Composition and Functional Properties of Raw and Roasted Nigerian Benniseed (Sesamum indicum) and Bambara Groundnut (Vigna subterranean). Food Chem. 2008, 111, 277–282. [Google Scholar] [CrossRef]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant Activities of Bambara Groundnut (Vignas subterranea) Protein Hydrolysates and Their Membrane Ultrafiltration Fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of Protein Isolates from Black Bean and Mungbean on Proteolysis and Gel Properties of Surimi from Sardine (Sardinella albella). LWT—Food Sci. Technol. 2013, 50, 511–518. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de Novo Structure Prediction for Linear Peptides in Solution and in Complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A Web Server for Predicting the Binding Affinity of Protein–Protein Complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Romero-Durana, M.; Jiménez-García, B.; Fernández-Recio, J. pyDockEneRes: Per-Residue Decomposition of Protein–Protein Docking Energy. Bioinformatics 2020, 36, 2284–2285. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and Characterization of Antioxidative Hydrolysates and Peptides from Corn Gluten Meal Using Papain, Ficin, and Bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis). Molecules 2023, 28, 519. [Google Scholar] [CrossRef]
- Gui, M.; Gao, L.; Rao, L.; Li, P.; Zhang, Y.; Han, J.-W.; Li, J. Bioactive Peptides Identified from Enzymatic Hydrolysates of Sturgeon Skin. J. Sci. Food Agric. 2022, 102, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods 2021, 10, 2462. [Google Scholar] [CrossRef]
- Hayes, M.; Naik, A.; Mora, L.; Iñarra, B.; Ibarruri, J.; Bald, C.; Cariou, T.; Reid, D.; Gallagher, M.; Dragøy, R.; et al. Generation, Characterisation and Identification of Bioactive Peptides from Mesopelagic Fish Protein Hydrolysates Using In Silico and In Vitro Approaches. Mar. Drugs 2024, 22, 297. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Khan, F.N.; Yadava, P.; Alhazmi, A.; Mahmoud, S.F.; Saleh, D.I.; Zuan, A.T.K.; Kumar, A. In Silico Proteolysis and Analysis of Bioactive Peptides from Sequences of Fatty Acid Desaturase 3 (FAD3) of Flaxseed Protein. Saudi J. Biol. Sci. 2021, 28, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Packer, M.A.; Hayes, M. Identification of Bioactive Peptides from a Laminaria Digitata Protein Hydrolysate Using In Silico and In Vitro Methods to Identify Angiotensin-1-Converting Enzyme (ACE-1) Inhibitory Peptides. Mar. Drugs 2023, 21, 90. [Google Scholar] [CrossRef]
- Nugraha, R.; Kurniawan, F.; Abdullah, A.; Lopata, A.L.; Ruethers, T. Antihypertensive and Antidiabetic Drug Candidates from Milkfish (Chanos chanos)-Identification and Characterization through an Integrated Bioinformatic Approach. Foods 2024, 13, 2594. [Google Scholar] [CrossRef]
- Acharya, K.R.; Gregory, K.S.; Sturrock, E.D. Advances in the Structural Basis for Angiotensin-1 Converting Enzyme (ACE) Inhibitors. Biosci. Rep. 2024, 44, BSR20240130. [Google Scholar] [CrossRef]
- Masuyer, G.; Schwager, S.L.U.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. Molecular Recognition and Regulation of Human Angiotensin-I Converting Enzyme (ACE) Activity by Natural Inhibitory Peptides. Sci. Rep. 2012, 2, 717. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme–Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C. Structure and Function of Angiotensin Converting Enzyme and Its Inhibitors. Chin. J. Biotechnol. 2008, 24, 171–176. [Google Scholar] [CrossRef]
- Mathur, V.; Alam, O.; Siddiqui, N.; Jha, M.; Manaithiya, A.; Bawa, S.; Sharma, N.; Alshehri, S.; Alam, P.; Shakeel, F. Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents. Molecules 2023, 28, 5860. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mittal, A.; Mittal, A. A Review upon Medicinal Perspective and Designing Rationale of DPP-4 Inhibitors. Bioorg. Med. Chem. 2021, 46, 116354. [Google Scholar] [CrossRef] [PubMed]
- Pantaleão, S.Q.; Maltarollo, V.G.; Araujo, S.C.; Gertrudes, J.C.; Honorio, K.M. Molecular Docking Studies and 2D Analyses of DPP-4 Inhibitors as Candidates in the Treatment of Diabetes. Mol. Biosyst. 2015, 11, 3188–3193. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.B.; Branner, S.; Wiberg, F.C.; Wagtmann, N. Crystal Structure of Human Dipeptidyl Peptidase IV/CD26 in Complex with a Substrate Analog. Nat. Struct. Biol. 2003, 10, 19–25. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; Philot, E.A.; de Resende-Lara, P.T.; Lima, A.N.; Perahia, D.; Miteva, M.A.; Scott, A.L.; Honorio, K.M. Structural Dynamics of DPP-4 and Its Influence on the Projection of Bioactive Ligands. Molecules 2018, 23, 490. [Google Scholar] [CrossRef]
| Criteria | Peptide Size (kDa) | ||||
|---|---|---|---|---|---|
| <1 | 1–2 | 3–5 | 5–10 | >10 | |
| Top 26% abundance with de novo score >96% | 22 | 29 | 46 | 44 | 50 |
| Peptide Ranker score >0.5 | 5 | 5 | 4 | 10 | 9 |
| ACE-inhibitory peptide score >0.5 | 1 | 1 | 1 | 1 | 1 |
| DPP-IV-inhibitory peptide score >0.5 | 4 | 3 | 2 | 9 | 6 |
| Inhibitory Activities | Pep no f * | Peptide | Denovo Score | m/z | Chemical Formula | Bioranker Score | Activity Prediction Score | ΔG (kcal mol−1) | Kd (M) |
|---|---|---|---|---|---|---|---|---|---|
| ACE | F3-1 | YKDGLYSPHW | 98 | 633.30 | C61H80N14O16 | 0.59 | 0.70 | −10.5 | 4.0 × 10−8 |
| F4-1 | LPVSTPGKF | 98 | 473.27 | C45H72N10O12 | 0.65 | 0.67 | −10.2 | 6.3 × 10−8 | |
| F5-6 | RPFLPPR | 96 | 441.76 | C42H67N13O8 | 0.93 | 0.71 | −11.3 | 1.1 × 10−8 | |
| DPP-IV | F4-4 | EPWWPK | 98 | 421.71 | C43H55N9O9 | 0.92 | 0.83 | −8.7 | 7.1 × 10−7 |
| F4-6 | LLTPKF | 97 | 359.72 | C36H59N7O8 | 0.60 | 0.83 | −9.0 | 4.6 × 10−7 | |
| F5-3 | NLLMPH | 97 | 362.69 | C32H53N9O8S | 0.58 | 0.83 | −9.1 | 4.1 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saetang, J.; Haewphet, T.; Nilsuwan, K.; Benjakul, S. ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking. Biology 2025, 14, 511. https://doi.org/10.3390/biology14050511
Saetang J, Haewphet T, Nilsuwan K, Benjakul S. ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking. Biology. 2025; 14(5):511. https://doi.org/10.3390/biology14050511
Chicago/Turabian StyleSaetang, Jirakrit, Thaiyawat Haewphet, Krisana Nilsuwan, and Soottawat Benjakul. 2025. "ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking" Biology 14, no. 5: 511. https://doi.org/10.3390/biology14050511
APA StyleSaetang, J., Haewphet, T., Nilsuwan, K., & Benjakul, S. (2025). ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking. Biology, 14(5), 511. https://doi.org/10.3390/biology14050511








