Understanding Hypertension: A Metabolomic Perspective
Simple Summary
Abstract
1. Introduction
2. Metabolomics and Hypertension
| Sample Type | Techniques | Metabolites | Main Findings | Reference |
|---|---|---|---|---|
| Plasma | LC–MS/MS | ↑ Indole-3-carboxaldehyde; 4-aminohippuric acid; L-glutamic acid; saccharopine; DL-glutamate; L-kynurenine; L-valine; L-isoleucine; L-tyrosine; benzaldehyde; L-(-)-fucose; glyceraldehyde; pseudouridine; trans-aconitic acid; threonic acid; cis-aconitic acid; N-acetyl-L-alanine; 4-acetylbutyrate; citraconic acid; 2,4-undecadiene-8,10-diynoic acid isobutylamide; sphingomyelin (d18:1/18:1(9Z)); millefin; cis, cis-muconic acid; 2,4′,5,7-tetrhydroxyflavanone; L-cystine; acetylhomoserine; (3S,5S))—3,5-diaminohexanoate; N-acetylserine; pyrrolidonecarboxylic acid; 2-ocovaleric acid; 3,3,5-triiodo-L-thyronine-beta-D-glucuronoside; L-ribulose; L-glucose; (2R)-2-hydroxy-2-phenylethyl; 2-furoic acid; isosalsolidine; 3-propylidene-1(3H)-isobenzofuranone; piperidine; 2,4′,5,7-tetrahydroxyflavanone; N’-hydroxysaxitoxin; 3-(3,4-dihydroxyphenyl)prop-2-enoic acid; coumaric acid; bergapten; phenylacetaldehyde; 2-phenylacetamide; romucosine A; alpha-D-glucose; prostaglandin D2 ↓ (9S, 10E, 12Z, 15Z)-9-hydroxy-10,12,15-octadecatrienoic; falcarinolone; dolichosterone; deoxyribose 5-phosphate | L-cystine, L-glutamate acid, DL-glutamate, cis-aconitic acid, citraconic acid, and 2-furoic acid levels were positively associated with both SBP and DBP. | Chen et al. (2024) [34] |
| Plasma | LC–MS/MS | ↑ Uric acid; leucine ↓ LysoPC (18:2) | Sexual dimorphism of the metabolome may contribute to sex differences in hypertension. Higher levels of uric acid and leucine and lower levels of a LysoPC were associated with hypertension. | Couch et al. (2024) [35] |
| Serum | LC–MS/MS | ↑ Fatty acid 20:2; palmitoylcarnitine; palmitoleoylcarnitine; fatty acid 18; N-acetyleucine; PE (18:0_18:2); hydrocortisone; triglycerides (22:6_34:0); PC (14:0_20:5); phe-phe ↓ PC (15:0_18:2) | Results revealed the metabolic connectivity and specificity across multiple chronic metabolic diseases in terms of different metabolite classes. | Zhang et al. (2024) [36] |
| Serum | UPLC–MS | ↑ C2; butyric acid; ethanolamine ↓ Pyruvate | Hypertension plasma patients exhibited significantly higher levels of creatinine and LDL-C. | López-Romero et al. (2024) [37] |
| Plasma | high-throughput NMR | Related to SBP: ↑ ApoB; LDL cholesterol; total triglycerides ↓ ApoA1; HDL cholesterol Related to DBP: ↑ ApoA1; total triglycerides ↓ ApoB; HDL cholesterol; LDL cholesterol | HDL cholesterol and low-density lipoprotein cholesterol were found to be potential causal factors for pulse pressure (PP) elevation. Sub-lipoproteins, genetically predicted atherogenic lipoprotein particles had the most likely causal impact on increasing PP. | Liu et al. (2024) [38] |
| Plasma | UPLC–MS/MS coupled with a heated electrospray ionization (HESI-II) source | ↑ {stearidonate [18:4n3], hexadecadienoate [16:2n6]; 9 and 13-S-hydroxyoctadecadienoic acid (HODE); linolenate [alpha or gamma; (18:3n3 or 6)]}; N6-carbamoylthreonyladenosine; 2,3-dihydroxy-5-methylthio-4- pentenoate (DMTPA) | Six metabolites, including lipids, an amino acid and a nucleotide, were found to be associated with the increased risk of hypertension. | Al Ashmar et al. (2024) [39] |
| Plasma | flow-injection tandem MS | ↑ Alanine; valine; leucine; phenylalanine; proline; C2; C3; C3DC; C4; C4DC; C4OH; C5; C5OH; C5DC; C6; C8; C8:1; C10; C10:1; C12; C14; C14:1; C14:2; C14OH; C16; C16OH; C16:1OH; C16:1; C18; C18:1; C18OH; C18:1OH; C18:2OH ↓ Aspartic acid; glycine; serine | Five metabolites were identified as predictors of stage 2 hypertension: C0; C12; C14:1; C14:1 and glycine. | Arjmand et al. (2023) [40] |
| Plasma | UPLC–MS/MS | Related to SBP in women: ↑ dihomo-lineoylcarnitine; 4-hydroxyphenylacetateglutamine; vanillactate; DMTPA; 1-methyladenosine Related to SBP in Men: ↑ sphingomyelins; N-formylmethionine; conjugated bile acids; N-acyl amino acids | Plasma metabolite associated with BP in a sex-specific way: catecholamine derivatives are predictors for BP in women, while sphingomyelins were more important in men. | Verhaar et al. (2023) [41] |
| Plasma | LC–MS/MS | ES-: ↑ methadone-d9; N-dodecanoylshinganine; DL-arginine ↓ 4-methylcarbostyril; (E)-4-methoxycinnamic acid; guaietolin; (E)-3,4,5-trimethoxucinnamic acid; 3-hydroxy-3-(2,3,4-trimethoxyphenyl)propanic acid; 1-(1Z-hexadecenyl)-sn-glycero-3-phosphocholine; epi-jasmonic acid; (+-)-(2E)-abscisic acid; 2-amino-3-hydroxyoctadecyl dihydrogen phosphate; sphingosine 1-phosphate; umbelliferone; LU3453000; DL-lactic acid; hexitol; furaneol; sinaticin; 2-deoxyhexopyranose; chlorphentermine; [(4Z)-5-(methylsulfanyl)-4-penten-2-yn-1-yl]benzene; 1-O-arsonopentofuranose; 2,4-dichlorotoluene; S-3-oxodecanoyl cysteamine; lauramide; (2S)-2-amino-8-hydroxyoctanoic acid; crotonic acid; guvacine; glycine-leucine; 4-oxoproline ES+: ↑ 4-hydroxycyclophosphamine; methyl isoquinoline-3-carboxylate; benzothiazole; N-dodecanoylshinganine; DL-arginine ↓ 1-O-arsonopentofuranose; 2,4-dichlorotoluene; (2S)-2-amino-4-hexynoic acid; NLK; sinaticin; maltitol; 3,4,5-trihydroxy-6-(h)-oxan-2-yl-3-hydroxy-4,5-dimethoxybenzoate; [(4Z)-5-(methylsulfanyl)-4-penten-2-yn-1-yl]benzene; chlorphentermine; 6a,7,8,12-tetrahydroxy-3-methyl-(1,7),6a,12,12a-decahydrotetraphene-1,12-dione; (2Z)-3-{5-[(4Z)-5-(methylsulfanyl)-4-penten-2-yn-1-yl]-2-furyl acrylaldehyde; D-(+)-maltose; 3-methylglutarate; 5-acetylgidydro-2-(3H)-furanone; 2-deoxyhexopyranose; hexitol; S-3-oxodecanoyl cysteamine; lauramide; 4-hydroxycoumarin; 2-amino-3-hydroxyoctadecyl dihydrogen phosphate; shingosine 1-phosphate; hydroxytriazolam; 4-coumaric acid; guaietolin; 3-(6,7-dimethoxy-1,3-benzodioxol-5-yl)-2-propen-1-ol; P-Gal; 4-methylcarbostyril; epi-iasmonic acid; (+-)-(2E)-abscisic acid; 3-phenylpropanic acid; 1-[(1Z,9Z)-octadecadienyl]-sn-glycero-3-phosphocholine; 1-[(11Z)-octadecenoyl]-sn-glycero-3-phosphocholine; 1-(1Z-hexadecenyl)-sn-glycero-3-phosphocholine; 2-aminoethyl(2R)-3-[(1Z)-1-hexadecen-1-yloxy]-2-hydroxypropyl hydrogen phosphate | Correlations between plasma metabolites and microbiota suggested impairment of interactions between metabolites and microbes in patients with hypertension. | Chen et al. (2023) [42] |
| Plasma (from Chen et al. [43]) | UPLC–MS/MS (from Chen et al. [43]) | Related to DBP: ↑ N-alpha-acetylornithine; N-acetyl-2-aminoadipate; N-acetylarginine; N-acetylglutamine; N6-acetyllysine; N-acetylcitrulline; behenoyl dihydrosphingomyelin [d18:0/22:0] ↓ 2-hydroxyoctanoate; N2,N2-dimethylguanosine; alliin; N-delta-acetylornithine; 1-(1-enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4) Related to SBP: ↑ N1-methyl-2-pyridone-5-carboxamide; X-12847; X-12822; ferulic acid 4-sulfate; X-12839; X-11381; N-formylmethionine; X-15486 ↓ X-25420; Imidazole propionate; 5-hydroxy-2-methylpyridine sulfate; 3-hydroxy-2-methylpyridine sulfate; gamma-glutamylthreonine; mannonate; 1-methyl-4-imidazoleacetate; X-25419; CMPF; X-24531; X-21364; N-succinyl-phenylalanine; hydroxy-CMPF; phenylacetylglutamate | 12 metabolites had a causal effect on DBP, and 22 metabolites had a causal effect on SBP. | Dai et al. (2023) [44] |
| Plasma and Serum | MWAS/MS or NMR or other multianalyte analytical technology | ↑ Cortisol; cortisone; glycocholic acid; chenodeoxycholic acid glycine conjugate; 5-androstenediol; L-acetylcarnitine; tetradecanedioic acid; steraridonic acid; LysoPE (16:0/0:0); LysoPE (20:4(5Z,8Z,11Z,14Z)/(0:0); glycerylphosphorylethanolamine; homocitrulline; L-palmitoylcarnitine; L-isoleucine; L-leucine; N-acetyl-L-alanine; L-lactic acid; L-malic acid; Ketoleucine; 3-methyl-2-oxovaleric acid; myo-inositol; pantothenic acid; glycerol; erythritol; indolelactic acid; theophylline; caffeine; pyridoxine ↓ Glycine; hydrocinnamic acid; L-histidine; L-serine | Using a multi-ethnic cohort reported 5 novel hypertension-associated metabolites and confirmed 27 previous hypertension associations. Of these, 32 metabolite associations, predominantly lipid (steroids and fatty acyls) and organic acids (amino-, hydroxy-, and keto-acids) remained after further adjusting for comorbidities and dietary intake. | Louca et al. (2022) [45] |
| Serum | NMR | ↑ Acetoacetate; alanine; albumin; ApoB/Apo 1; ApoA1; ApoB; β-hydroxybutyrate; esterified-C; glucose; glycerol; glycoprotein acetyls; HDL cholesterol; HDL2 cholesterol; HDL3 cholesterol; isoleucine; LDL cholesterol; lactate; leucine; monounsaturated fatty acids; phenylalanine; PCs; phosphoglycerides; pyruvate; remnant-C; saturated fatty acids; sphingomyelins; triglycerides/phosphoglycerides; total cholesterol; total cholines; total fatty acids; total free cholesterol; total triglycerides; triglycerides in HDL; triglycerides in LDL; triglycerides in VLDL; VLDL cholesterol; VLDL size ↓ Acetate; unsaturation degree; glutamine; glycine; HDL size; linoleic acid%; LDL size; omega-3%; omega-6%; polyunsaturated fatty acids% | Among other findings, LDL-derived and VLDL-derived cholesterol and glucose metabolism abnormalities are associated with hypertension patients. | Palmu et al. (2022) [46] |
| Plasma | UPLC–MS/MS | ↓ Acetate; isobutyrate; butyrate; isovalerate | Microbiota and bacterial metabolites were used to classify non-treated hypertension in first-degree phase patients. | Calderón-Pérez et al. (2020) [47] |
| Plasma | LC–MS and GC–MS | Related to DBP: ↑ Ceramide (C18:1,C24:0); triacylglycerol (C16:0, C16:1); oleic acid (C18:cis [9]1); total glycerolipids ↓ Cholesterylester C16:0 | Ceramide, triacylglycerol, total glycerolipids, and oleic acid were positively associated with longitudinal diastolic BP change. Cholesterylester levels were inversely associated with longitudinal diastolic BP change. | Lin et al. (2020) [32] |
| Plasma | LC–MS | Related to Men: ↑ C4 acylcarnitine; LysoPC a C26:1 ↓ Ornithine; tryptophan; leucine; valine; histidine; threonine; methionine; lysine; spermidine Related to Women: ↑ Arginine; citrulline; tryptophan; histidine; tyrosine; phenylalanine; leucine; isoleucine; glutamine; methionine; lysine; kynurenine; taurine; alpha-AAA; C10, C12 and C12:1 acylcarnitines ↓ Spermine Both: ↑ Acetyl-ornithine; PC; LysoPC a C28:1; sphingomyelins; hydroxyproline; SDMA/total DMA ↓ LysoPC C16:0 | Both sexes showed a considerable increase in PCs, a decrease in C16:0 with an increase in C28:1 LysoPCs, an increase in sphingomyelins, as well as an increase in symmetric dimethylarginine (SDMA), acetyl-ornithine, and hydroxyproline. Twenty-nine metabolites, involved in phospholipidic and cardiac remodeling, arginine/nitric oxide pathway, and antihypertensive and insulin resistance mechanisms, discriminated the metabolic sexual dimorphism of hypertension. | Goïta et al. (2020) [48] |
| Serum | UPLC–MS/MS | ↑ Formiminoglutamate; 1-palmitoyl-2-linoleyl-GPE (16:0/18:2); 1-stearoyl-2-arachidonoyl-GPE (18:0/20:4); 1-stearoyl-2-linoleoyl-GPE (18:0/18:2); 1-stearoyl-2-oleoly-GPE (18:0/18:1); N-palmitoyl-sphinganine (d18:0/16:0); N-stearoyl-sphinganine (d18:0/18:0); fibrinopeptide B (1-13); gamma-glutamylisoleucine; ethyl glucuronide; X-24337; urate; glucose ↓ Oxalate; threonate; thromboxane B2; 5-methylutidine (ribothymide); fibrinopeptide B (1-11); fibrinopeptide B (1-12); tartronate (hydroxymalonate); X-17367; X-21752; serine | 24 novel metabolites were identified: 3 amino acid and nucleotide metabolites; 7 cofactor and vitamin or xenobiotic metabolites; bacterial/fungal, chemical, and food component sub-pathways; 10 lipid metabolites from the eicosanoid, PC, PE, and sphingolipid metabolism sub-pathways. | He et al. (2020) [49] |
| Platelet | ATR-FTIR and Raman Spectroscopy | ↓ cholesterol band area (2930 cm−1) Shift in: Phosphatidylinositol (589 cm−1) PE (760 cm−1) PC (720 cm−1) Phosphatidylserine (595 and 785 cm−1) | Significant modifications in the major lipid composition and cholesterol content of the plasma membrane in hypertension platelets were shown. | García-Rubio et al. (2019) [50] |
| Serum | UPLC/MS and GC/MS | ↑ Hexadecanoic acid; glycerol; hexadecenoic acid; Tetradecanoic acid; LysoPC (16:1); PC (14:0/18:1); PC (16:0/22:3); PC (18:0/20:4); DG (15:0/18:3); DG (15:0/18:4/0:0); DG (14:0/22:5); L-acetylcarnitine; cis-5-tetradecenoylcarnitine; C12; trans-hexadec-2-enoyl carnitine; 4,8 dimethylnonanoyl carnitine; palmitic amide; N-Acetylarylamide; 3-oxododecanoic acid; oleic acid; (±)-10-HDoHE; 4-hydroxybenzaldehyde; 3-hydroxyhippuric acid; hexadecanedioic acid | 26 metabolites were identified and are mainly involved in fatty acid metabolism, glycerophospholipid metabolism, alanine, aspartate, and glutamate metabolism, and are implicated in insulin resistance, vascular remodeling, macrophage activation, and oxidized LDL formation. | Ke et al. (2018) [51] |
| Blood (Dried Blood Spot) | ESI–MS | ↑ C4/C8; C5OH/C8; C3DC/C10; C10:2/C10; ornithine/citrulline; ornithine; C5DC/C8 ↓ Glycine; C8; C10; C12; glycine/alanine; phenylalanine/tyrosine; C14:1; C14:1/C16; C5DC; C10:1; C14OH; C14:2; C4DC | Glycine, ornithine, decanylcarnitine and the ratios Ornithine/citrulline; and phenylalanine/tyrosine and -hydroxyisovalerylcarnitine/octanoylcarnitine are characteristic of patients with hypertension. | Bai et al. (2018) [52] |
| Serum | NMR | ↑ Alanine; adenine; methionine; pyruvate; uracil ↓ Arginine | Alanine, arginine, methionine, pyruvate, adenine, and uracil showed excellent correlation in both isolated elevated DBP cases and combined elevated systolic–diastolic blood pressure cases. | Ameta et al. (2017) [53] |
| Serum | AbsolutelDQ p150 Kits based on flow injection analysis tandem MS | Lower risk of developing hypertension: ↑ serine; acyl-alkyl-PCs C42:4 and C44; glycine Higher risk of developing hypertension: ↑ diacyl-PCs C38:4 and C38:3 | Higher concentrations of serine, glycine, and acyl-alkyl-PCs C42:4 and C44:3 tended to be associated with higher predicted 10-year hypertension-free survival and diacyl-PCs C38:4 and C38:3 with lower predicted 10-year hypertension-free survival. | Dietrich et al. (2016) [8] |
| Serum | GC/MS | ↑ Talose; lyxose; methylmalonic acid; malonic acid; shikimic acid; glucose-1-phosphate ↓ Threonine; nicotinoyl glycine; glycine; phenylalanine; S-carboxylmethycysteine; tyrosine; Aspartic acid; glycine-proline; galactose; methyl-β-D-galactopyranoside; dihydroxyacetone; melezitose; oxalic acid; thymol; noradrenaline; 2-aminophenol; 2-methoxyestrone; alpha-tocopherol; octadecanol; 2-aminoethanethiol | Threonine and phenylalanine were negatively associated with the risk of future hypertension. A higher level of lyxose was associated with a higher risk of hypertension. | Hao et al. (2016) [54] |
| Plasma | MRM–MS | ↑ Oleic acid ↓ Myo-inositol | The results showed that oleic acid (OA) and myoinositol (MI) were the most important differential metabolites between the hypertensive plasma and the healthy plasma. | Yang et al. (2016) [15] |
| Serum and Plasma | MRM–MS | Related to DBP: ↓ Lactate; C8; hexadecanedioate; tetradecanedioate; 10-heptadecenoate (17:1n7); 5-dodecenoate (12:1n7); cortisol; caffeine Related to SBP: ↑ Lactate; C8; hexadecanedioate; tetradecanedioate; 10-heptadecenoate (17:1n7); 5-dodecenoate (12:1n7); cortisol; caffeine Both: ↑ Dihomo-linoleate (20:2n6); palmitate (16:0); 4-androsten-3β,17β-diol disulfate 1 ↓ Phenylacetylglutamine; stearoylcarnitine; nonadecanoate; HWESASXX | Hexadecanedioate showed a concordant direction of effect for both BP and mortality, while in contrast, the direct association between dihomo-linoleate(20:2n6) or caffeine and BP did not translate into increased mortality risk. | Menni et al. (2015) [55] |
| Plasma | GC–MS | ↑ oxalic acid; fumaric acid; glycerol; adenine; pyrophosphate; uric acid ↓ L-valine; L-isoleucine; glycine; L-threonine; L-methionine; ornithine; L-asparagine; L-glutamine; citrulline; L-lysine; L-tyrosine; L-tryptophan; L-cystine; capric acid | Disorders of amino acid metabolism might play an important role in predisposing young men to developing hypertension. | Wang et al. (2015) [56] |
2.1. Amino Acid Metabolism
2.2. Fatty Acid Metabolism
2.3. Inflammation
2.4. Oxidative Stress
3. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | Aromatic Amino Acids |
| ATR | Attenuated Total Reflectance |
| BP | Blood Pressure |
| BCAA | Branched-Chain Amino Acids |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| FTIR | Fourier Transform Infrared |
| GC | Gas Chromatography |
| LC | Liquid Chromatography |
| LysoPC | Lysophoshatidylcholine |
| MS | Mass Spectrometry |
| MUFA | Monounsaturated Fatty Acids |
| NMR | Nuclear Magnetic Resonance |
| NO | Nitric Oxide |
| PC | Phosphatidylcholine |
| RAS | Renin–Angiotensin System |
| ROS | Reactive Oxygen Species |
| SBP | Systolic Blood Pressure |
| SCFA | Short-Chain Fatty Acids |
References
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. Hypertension and Aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Aliani, M. Metabolomics Profiling in Hypertension and Blood Pressure Regulation: A Review. Clin. Hypertens. 2020, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Setters, B.; Holmes, H.M. Hypertension in the Older Adult. Prim. Care Clin. Off. Pract. 2017, 44, 529–539. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Dietrich, S.; Floegel, A.; Weikert, C.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H.; Drogan, D. Identification of Serum Metabolites Associated With Incident Hypertension in the European Prospective Investigation Into Cancer and Nutrition–Potsdam Study. Hypertension 2016, 68, 471–477. [Google Scholar] [CrossRef]
- Thongboonkerd, V. Genomics, Proteomics and Integrative ‘Omics’ in Hypertension Research. Curr. Opin. Nephrol. Hypertens. 2005, 14, 133–139. [Google Scholar] [CrossRef]
- Nikolic, S.B.; Sharman, J.E.; Adams, M.J.; Edwards, L.M. Metabolomics in Hypertension. J. Hypertens. 2014, 32, 1159–1169. [Google Scholar] [CrossRef]
- Au, A.; Cheng, K.-K.; Wei, L.K. Metabolomics, Lipidomics and Pharmacometabolomics of Human Hypertension. In Hypertension: From Basic Research to Clinical Practice; Springer: Cham, Switzerland, 2016; Volume 956, pp. 599–613. [Google Scholar]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in Metabolomics—A Review in Human Disease Diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tsiropoulou, S.; McBride, M.; Padmanabhan, S. Urine Metabolomics in Hypertension Research. In Hypertension; Humana Press: New York, NY, USA, 2017; Volume 1527, pp. 61–68. [Google Scholar]
- Onuh, J.O.; Qiu, H. Metabolic Profiling and Metabolites Fingerprints in Human Hypertension: Discovery and Potential. Metabolites 2021, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, Z.; Deng, S.; Chen, X.; Chen, L.; Guo, Z.; Zheng, H.; Chen, L.; Cai, D.; Wen, B.; et al. A Targeted Metabolomics MRM-MS Study on Identifying Potential Hypertension Biomarkers in Human Plasma and Evaluating Acupuncture Effects. Sci. Rep. 2016, 6, 25871. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H. Targeted Metabolomics for Biomarker Discovery. Angew. Chem. Int. Ed. 2010, 49, 5426–5445. [Google Scholar] [CrossRef]
- Wei, R.; Li, G.; Seymour, A.B. High-Throughput and Multiplexed LC/MS/MRM Method for Targeted Metabolomics. Anal. Chem. 2010, 82, 5527–5533. [Google Scholar] [CrossRef]
- Dudley, E.; Yousef, M.; Wang, Y.; Griffiths, W.J. Targeted Metabolomics and Mass Spectrometry. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 80, pp. 45–83. [Google Scholar]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The Apogee of the Omics Trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Alonso, A.; Marsal, S.; Julià, A. Analytical Methods in Untargeted Metabolomics: State of the Art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef]
- Dunn, W.B.; Bailey, N.J.C.; Johnson, H.E. Measuring the Metabolome: Current Analytical Technologies. Analyst 2005, 130, 606. [Google Scholar] [CrossRef]
- Bothwell, J.H.F.; Griffin, J.L. An Introduction to Biological Nuclear Magnetic Resonance Spectroscopy. Biol. Rev. 2011, 86, 493–510. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems Level Studies of Mammalian Metabolomes: The Roles of Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Advances in Metabolite Identification. Bioanalysis 2011, 3, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, G.; Gika, H.G.; Wilson, I.D. Mass Spectrometry-based Holistic Analytical Approaches for Metabolite Profiling in Systems Biology Studies. Mass. Spectrom. Rev. 2011, 30, 884–906. [Google Scholar] [CrossRef]
- Magalhães, S.; Goodfellow, B.J.; Nunes, A. FTIR Spectroscopy in Biomedical Research: How to Get the Most out of Its Potential. Appl. Spectrosc. Rev. 2021, 56, 869–907. [Google Scholar] [CrossRef]
- Neto, V.; Esteves-Ferreira, S.; Inácio, I.; Alves, M.; Dantas, R.; Almeida, I.; Guimarães, J.; Azevedo, T.; Nunes, A. Metabolic Profile Characterization of Different Thyroid Nodules Using FTIR Spectroscopy: A Review. Metabolites 2022, 12, 53. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, C.; Su, J.; Pan, C.-W.; Ke, C. Identification of Biomarkers for Essential Hypertension Based on Metabolomics. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 382–395. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Iliou, A.; Mikros, E.; Elliott, P. An Overview of Metabolic Phenotyping in Blood Pressure Research. Curr. Hypertens. Rep. 2018, 20, 78. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Salihovic, S.; Fall, T.; Hammar, U.; Ingelsson, E.; Ärnlöv, J.; Lind, L.; Sundström, J. Global Plasma Metabolomics to Identify Potential Biomarkers of Blood Pressure Progression. Arter. Thromb. Vasc. Biol. 2020, 40, e227–e237. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Li, Z.; Song, Y.; Cai, X.; Liu, Y.; Zhang, T.; Yang, L.; Li, L.; Gao, S.; et al. Identification of Essential Hypertension Biomarkers in Human Urine by Non-Targeted Metabolomics Based on UPLC-Q-TOF/MS. Clin. Chim. Acta 2018, 486, 192–198. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Y.; Zhou, H.; Wu, W.; Bao, J.; Cao, D.; Li, Y.; Feng, Y. Blood L-cystine Levels Positively Related to Increased Risk of Hypertension. J. Clin. Hypertens. 2024, 26, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Couch, C.A.; Ament, Z.; Patki, A.; Kijpaisalratana, N.; Bhave, V.; Jones, A.C.; Armstrong, N.D.; Cushman, M.; Kimberly, W.T.; Irvin, M.R. Sex-Associated Metabolites and Incident Stroke, Incident Coronary Heart Disease, Hypertension, and Chronic Kidney Disease in the REGARDS Cohort. J. Am. Heart Assoc. 2024, 13, e032643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Zhao, J.; Lv, W.; Jia, X.; Lu, X.; Zhao, X.; Xu, G. Quantified Metabolomics and Lipidomics Profiles Reveal Serum Metabolic Alterations and Distinguished Metabolites of Seven Chronic Metabolic Diseases. J. Proteome Res. 2024, 23, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, L.C.; Broseta, J.J.; Roca-Marugán, M.; Muñoz-Castañeda, J.R.; Lahoz, A.; Hernández-Jaras, J. Metabolomics of Plasma in XLH Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms. Int. J. Mol. Sci. 2024, 25, 3545. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, C.; Lei, F.; Huang, X.; Cai, J.; Chen, S.; She, Z.-G.; Li, H. Major Lipids and Lipoprotein Levels and Risk of Blood Pressure Elevation: A Mendelian Randomisation Study. EBioMedicine 2024, 100, 104964. [Google Scholar] [CrossRef]
- Al Ashmar, S.; Anwardeen, N.R.; Anlar, G.G.; Pedersen, S.; Elrayess, M.A.; Zeidan, A. Metabolomic Profiling Reveals Key Metabolites Associated with Hypertension Progression. Front. Cardiovasc. Med. 2024, 11, 1284114. [Google Scholar] [CrossRef]
- Arjmand, B.; Dehghanbanadaki, H.; Yoosefi, M.; Rezaei, N.; Mohammadi Fateh, S.; Ghodssi-Ghassemabadi, R.; Najjar, N.; Hosseinkhani, S.; Tayanloo-beik, A.; Adibi, H.; et al. Association of Plasma Acylcarnitines and Amino Acids with Hypertension: A Nationwide Metabolomics Study. PLoS ONE 2023, 18, e0279835. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Mosterd, C.M.; Collard, D.; Galenkamp, H.; Muller, M.; Rampanelli, E.; van Raalte, D.H.; Nieuwdorp, M.; van den Born, B.-J.H. Sex Differences in Associations of Plasma Metabolites with Blood Pressure and Heart Rate Variability: The HELIUS Study. Atherosclerosis 2023, 384, 117147. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Li, Y.-L.; Lin, W.-Z.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Zhang, J.; et al. Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort. Nutrients 2023, 15, 2074. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic Atlas of the Plasma Metabolome Prioritizes Metabolites Implicated in Human Diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Dai, N.; Deng, Y.; Wang, B. Association between Human Blood Metabolome and the Risk of Hypertension. BMC Genom. Data 2023, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Louca, P.; Nogal, A.; Moskal, A.; Goulding, N.J.; Shipley, M.J.; Alkis, T.; Lindbohm, J.V.; Hu, J.; Kifer, D.; Wang, N.; et al. Cross-Sectional Blood Metabolite Markers of Hypertension: A Multicohort Analysis of 44,306 Individuals from the COnsortium of METabolomics Studies. Metabolites 2022, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Palmu, J.; Tikkanen, E.; Havulinna, A.S.; Vartiainen, E.; Lundqvist, A.; Ruuskanen, M.O.; Perola, M.; Ala-Korpela, M.; Jousilahti, P.; Würtz, P.; et al. Comprehensive Biomarker Profiling of Hypertension in 36985 Finnish Individuals. J. Hypertens. 2022, 40, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J.; et al. Gut Metagenomic and Short Chain Fatty Acids Signature in Hypertension: A Cross-Sectional Study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef]
- Goïta, Y.; Chao de la Barca, J.M.; Keïta, A.; Diarra, M.B.; Dembélé, K.C.; Chabrun, F.; Dramé, B.S.I.; Kassogué, Y.; Diakité, M.; Mirebeau-Prunier, D.; et al. Sexual Dimorphism of Metabolomic Profile in Arterial Hypertension. Sci. Rep. 2020, 10, 7517. [Google Scholar] [CrossRef]
- He, W.J.; Li, C.; Mi, X.; Shi, M.; Gu, X.; Bazzano, L.A.; Razavi, A.C.; Nierenberg, J.L.; Dorans, K.; He, H.; et al. An Untargeted Metabolomics Study of Blood Pressure: Findings from the Bogalusa Heart Study. J. Hypertens. 2020, 38, 1302–1311. [Google Scholar] [CrossRef]
- García-Rubio, D.; Rodríguez-Varela, M.; Martínez-Vieyra, I.; de la Mora, M.B.; Méndez-Méndez, J.V.; Durán-Álvarez, J.C.; Cerecedo, D. Alterations to the Contents of Plasma Membrane Structural Lipids Are Associated with Structural Changes and Compartmentalization in Platelets in Hypertension. Exp. Cell Res. 2019, 385, 111692. [Google Scholar] [CrossRef]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic Characterization of Hypertension and Dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef]
- Bai, Q.; Peng, B.; Wu, X.; Cao, Y.; Sun, X.; Hong, M.; Na, R.; Liu, B.; Li, Q.; Li, Z.; et al. Metabolomic Study for Essential Hypertension Patients Based on Dried Blood Spot Mass Spectrometry Approach. IUBMB Life 2018, 70, 777–785. [Google Scholar] [CrossRef]
- Ameta, K.; Gupta, A.; Kumar, S.; Sethi, R.; Kumar, D.; Mahdi, A.A. Essential Hypertension: A Filtered Serum Based Metabolomics Study. Sci. Rep. 2017, 7, 2153. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Xi, L.; Li, G.; Zhao, F.; Qi, Y.; Liu, J.; Zhao, D. A Nested Case-Control Study of Association between Metabolome and Hypertension Risk. Biomed. Res. Int. 2016, 2016, 7646979. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Graham, D.; Kastenmüller, G.; Alharbi, N.H.J.; Alsanosi, S.M.; McBride, M.; Mangino, M.; Titcombe, P.; Shin, S.-Y.; Psatha, M.; et al. Metabolomic Identification of a Novel Pathway of Blood Pressure Regulation Involving Hexadecanedioate. Hypertension 2015, 66, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, E.; Wang, L.; Wang, Y.; Yang, L.; Zheng, X.; Xie, G.; Sun, Q.; Liang, M.; Tian, Z. Reconstruction and Analysis of Correlation Networks Based on GC–MS Metabolomics Data for Young Hypertensive Men. Anal. Chim. Acta 2015, 854, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Correia, M.; Martins, I.; Henriques, A.G.; Delgadillo, I.; da Cruz e Silva, O.; Nunes, A. FTIR and Raman Spectroscopy Applied to Dementia Diagnosis Through Analysis of Biological Fluids. J. Alzheimer’s Dis. 2016, 52, 801–812. [Google Scholar] [CrossRef]
- Al-Abbasi, F.A. Trend Analysis of the Correlation of Amino Acid Plasma Profile with Glycemic Status in Saudi Diabetic Patients. J. Adv. Res. 2012, 3, 305–313. [Google Scholar] [CrossRef][Green Version]
- Alqudah, A.; Qnais, E.; Wedyan, M.; Awali, A.; Bseiso, Y.; Gammoh, O. Amino Acid Profiles: Exploring Their Diagnostic and Pathophysiological Significance in Hypertension. Mol. Biol. Rep. 2024, 51, 200. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Hase, R.; Takahashi, H.; Ishimaru, Y.; Watanabe, R.; Saito, H.; Shimokawa, J.; Yamamoto, H.; Kikuchi, S.; et al. Plasma Branched-Chain and Aromatic Amino Acids in Relation to Hypertension. Nutrients 2020, 12, 3791. [Google Scholar] [CrossRef]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of Branched-Chain Amino Acids with Carotid Intima-Media Thickness and Coronary Artery Disease Risk Factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef]
- Harlan, S.M.; Guo, D.-F.; Morgan, D.A.; Fernandes-Santos, C.; Rahmouni, K. Hypothalamic MTORC1 Signaling Controls Sympathetic Nerve Activity and Arterial Pressure and Mediates Leptin Effects. Cell Metab. 2013, 17, 599–606. [Google Scholar] [CrossRef]
- Lightell, D.J.J.; Woods, T.C. Relative Resistance to Mammalian Target of Rapamycin Inhibition in Vascular Smooth Muscle Cells of Diabetic Donors. Ochsner J. 2013, 13, 56–60. [Google Scholar]
- Kumar, V.; Wollner, C.; Kurth, T.; Bukowy, J.D.; Cowley, A.W. Inhibition of Mammalian Target of Rapamycin Complex 1 Attenuates Salt-Induced Hypertension and Kidney Injury in Dahl Salt-Sensitive Rats. Hypertension 2017, 70, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Nomura, T.; Sumi-Ichinose, C. Metabolism of Tetrahydrobiopterin: Its Relevance in Monoaminergic Neurons and Neurological Disorders. Chem. Rec. 2008, 8, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and Blood Pressure Regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah, S.; Shirakawa, H.; Inagawa, Y.; Koseki, T.; Komai, M. Regulation of Blood Pressure and Glucose Metabolism Induced by L-Tryptophan in Stroke-Prone Spontaneously Hypertensive Rats. Nutr. Metab. 2011, 8, 45. [Google Scholar] [CrossRef]
- Khedr, S.; Deussen, A.; Kopaliani, I.; Zatschler, B.; Martin, M. Effects of Tryptophan-Containing Peptides on Angiotensin-Converting Enzyme Activity and Vessel Tone Ex Vivo and In Vivo. Eur. J. Nutr. 2018, 57, 907–915. [Google Scholar] [CrossRef]
- Böger, R.H.; Lentz, S.R.; Bode-Böger, S.M.; Knapp, H.R.; Haynes, W.G. Elevation of Asymmetrical Dimethylarginine May Mediate Endothelial Dysfunction during Experimental Hyperhomocyst(e)Inaemia in Humans. Clin. Sci. 2001, 100, 161–167. [Google Scholar] [CrossRef]
- Bishop, M.J.; Huang, T.; Cheney, F.W. Effect of Vasodilator Treatment on the Resolution of Oleic Acid Injury in Dogs. Am. Rev. Respir. Dis. 1985, 131, 421–425. [Google Scholar]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Álvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic Acid Content Is Responsible for the Reduction in Blood Pressure Induced by Olive Oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Nemecz, M.; Constantin, A.; Dumitrescu, M.; Alexandru, N.; Filippi, A.; Tanko, G.; Georgescu, A. The Distinct Effects of Palmitic and Oleic Acid on Pancreatic Beta Cell Function: The Elucidation of Associated Mechanisms and Effector Molecules. Front. Pharmacol. 2019, 9, 1554. [Google Scholar] [CrossRef]
- Alonso, Á.; Ruiz-Gutierrez, V.; Martínez-González, M.Á. Monounsaturated Fatty Acids, Olive Oil and Blood Pressure: Epidemiological, Clinical and Experimental Evidence. Public Health Nutr. 2006, 9, 251–257. [Google Scholar] [CrossRef]
- Nicklas, T.A.; Hampl, J.S.; Taylor, C.A.; Thompson, V.J.; Heird, W.C. Monounsaturated Fatty Acid Intake by Children and Adults: Temporal Trends and Demographic Differences. Nutr. Rev. 2004, 62, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.; Stamler, J.; Griep, L.M.O.; Daviglus, M.L.; Van Horn, L.; Elliott, P. An Update on Nutrients and Blood Pressure. J. Atheroscler. Thromb. 2016, 23, 276–289. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-Chain Fatty Acid Metabolism and Multiple Effects on Cardiovascular Diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.; Barati, M.; Ostadrahimi, A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 Induces Inflammation and Organ Damage by Alternative Inflammasome Activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Caillon, A.; Schiffrin, E.L. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr. Hypertens. Rep. 2016, 18, 21. [Google Scholar] [CrossRef]
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases A2 and Inflammatory Responses in the Central Nervous System. Neuromol. Med. 2010, 12, 133–148. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The Mechanisms of Lysophosphatidylcholine in the Development of Diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Liu, L.; Guo, Z.; Xiong, Y. Gene Transfer of Dimethylarginine Dimethylaminohydrolase-2 Improves the Impairments of DDAH/ADMA/NOS/NO Pathway in Endothelial Cells Induced by Lysophosphatidylcholine. Eur. J. Pharmacol. 2008, 584, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mota, G.P.; Navia-Pelaez, J.M.; Araujo-Souza, J.C.; Stergiopulos, N.; Capettini, L.S.A. Role of ERK1/2 Activation and NNOS Uncoupling on Endothelial Dysfunction Induced by Lysophosphatidylcholine. Atherosclerosis 2017, 258, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kozina, A.; Opresnik, S.; Wong, M.S.K.; Hallström, S.; Graier, W.F.; Malli, R.; Schröder, K.; Schmidt, K.; Frank, S. Oleoyl-Lysophosphatidylcholine Limits Endothelial Nitric Oxide Bioavailability by Induction of Reactive Oxygen Species. PLoS ONE 2014, 9, e113443. [Google Scholar] [CrossRef]
- Peng, Z.-Y.; Zhang, S.-D.; Liu, S.; He, B.-M. Protective Effect of Neferine on Endothelial Cell Nitric Oxide Production Induced by Lysophosphatidylcholine: The Role of the DDAH–ADMA Pathway. Can. J. Physiol. Pharmacol. 2011, 89, 289–294. [Google Scholar] [CrossRef]
- Xing, F.; Liu, J.; Mo, Y.; Liu, Z.; Qin, Q.; Wang, J.; Fan, Z.; Long, Y.; Liu, N.; Zhao, K.; et al. Lysophosphatidylcholine Up-regulates Human Endothelial Nitric Oxide Synthase Gene Transactivity by C-Jun N-terminal Kinase Signalling Pathway. J. Cell Mol. Med. 2009, 13, 1136–1148. [Google Scholar] [CrossRef]
- Kim, E.A.; Ae Kim, J.; Park, M.H.; Jung, S.C.; Suh, S.H.; Pang, M.-G.; Kim, Y.J. Lysophosphatidylcholine Induces Endothelial Cell Injury by Nitric Oxide Production through Oxidative Stress. J. Matern.-Fetal Neonatal Med. 2009, 22, 325–331. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid Metabolites in Inflammatory Disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Hammad, S.M.; Pierce, J.S.; Soodavar, F.; Smith, K.J.; Al Gadban, M.M.; Rembiesa, B.; Klein, R.L.; Hannun, Y.A.; Bielawski, J.; Bielawska, A. Blood Sphingolipidomics in Healthy Humans: Impact of Sample Collection Methodology. J. Lipid Res. 2010, 51, 3074–3087. [Google Scholar] [CrossRef]
- Norris, G.; Blesso, C. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on Autoimmunity and Oxidative Stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Rodríguez-Gómez, I.; Pérez-Abud, R.; Tendero, P.V.; Baca, Y.; Wangensteen, R. Cardiovascular and Renal Manifestations of Glutathione Depletion Induced by Buthionine Sulfoximine. Am. J. Hypertens. 2012, 25, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Girgih, A.T.; Nwachukwu, I.; Ievari-Shariati, S.; Raj, P.; Netticadan, T.; Aluko, R.E.; Aliani, M. A Metabolomics Approach for Investigating Urinary and Plasma Changes in Spontaneously Hypertensive Rats (SHR) Fed with Chicken Skin Protein Hydrolysates Diets. J. Funct. Foods 2016, 22, 20–33. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Salvi, P.; D’Addato, S.; Rosticci, M.; Borghi, C. Association between Serum Uric Acid, Hypertension, Vascular Stiffness and Subclinical Atherosclerosis. J. Hypertens. 2014, 32, 57–64. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty Acids as Modulators of the Cellular Production of Reactive Oxygen Species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef]
- Schönfeld, P.; Więckowski, M.R.; Lebiedzińska, M.; Wojtczak, L. Mitochondrial Fatty Acid Oxidation and Oxidative Stress: Lack of Reverse Electron Transfer-Associated Production of Reactive Oxygen Species. Biochim. Biophys. Acta 2010, 1797, 929–938. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty Acids Decrease Mitochondrial Generation of Reactive Oxygen Species at the Reverse Electron Transport but Increase It at the Forward Transport. Biochim. Biophys. Acta 2007, 1767, 1032–1040. [Google Scholar] [CrossRef]
- Korge, P.; Honda, H.M.; Weiss, J.N. Effects of Fatty Acids in Isolated Mitochondria: Implications for Ischemic Injury and Cardioprotection. Am. J. Physiol.-Heart Circ. Physiol. 2003, 285, H259–H269. [Google Scholar] [CrossRef]
- Di Paola, M.; Cocco, T.; Lorusso, M. Arachidonic Acid Causes Cytochrome c Release from Heart Mitochondria. Biochem. Biophys. Res. Commun. 2000, 277, 128–133. [Google Scholar] [CrossRef]
- Loskcovich, M.V.; Grivennikova, V.G.; Cecchini, G.; Vinogradov, A.D. Inhibitory Effect of Palmitate on the Mitochondrial NADH:Ubiquinone Oxidoreductase (Complex I) as Related to the Active–de-Active Enzyme Transition. Biochem. J. 2005, 387, 677–683. [Google Scholar] [CrossRef]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy Substrate Metabolism and Mitochondrial Oxidative Stress in Cardiac Ischemia/Reperfusion Injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; Bastos, M.L.; Guedes de Pinho, P. Metabolomics Analysis for Biomarker Discovery: Advances and Challenges. Curr. Med. Chem. 2013, 20, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Asnani, A.; Gerszten, R.E. Application of Metabolomics to Cardiovascular Biomarker and Pathway Discovery. J. Am. Coll. Cardiol. 2008, 52, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tuñón, J.; Barbas, C.; Blanco-Colio, L.; Burillo, E.; Lorenzo, Ó.; Martín-Ventura, J.L.; Más, S.; Rupérez, F.J.; Egido, J. Proteomics and Metabolomics in Biomarker Discovery for Cardiovascular Diseases: Progress and Potential. Expert. Rev. Proteom. 2016, 13, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-Based Metabolomics in Human Disease Diagnosis: Applications, Limitations, and Recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, Q.H.; Xu, Z.D.; Dou, J.J. Mass Spectrometry-Based Metabolomics in Health and Medical Science: A Systematic Review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Dona, A.C.; Coffey, S.; Figtree, G. Translational and Emerging Clinical Applications of Metabolomics in Cardiovascular Disease Diagnosis and Treatment. Eur. J. Prev. Cardiol. 2016, 23, 1578–1589. [Google Scholar] [CrossRef]
- Rhee, E.P.; Gerszten, R.E. Metabolomics and Cardiovascular Biomarker Discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef]
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and Other Tools (BEST) Resource in Neuro-Oncology. Neuro Oncol. 2018, 20, 1162–1172. [Google Scholar] [CrossRef]
- Shah, A.; Grimberg, D.C.; Inman, B.A. Classification of Molecular Biomarkers. Soc. Int. Urol. J. 2020, 1, 8–15. [Google Scholar] [CrossRef]
- Mujadzic, H.; Skeete, J.; DiPette, D.J. Historical, Present, and Emerging Biomarkers in Hypertension: A Narrative Review. J. Lab. Precis. Med. 2022, 7, 32. [Google Scholar] [CrossRef]
- Li, S.; Looby, N.; Chandran, V.; Kulasingam, V. Challenges in the Metabolomics-Based Biomarker Validation Pipeline. Metabolites 2024, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-Omics Integration in Biomedical Research—A Metabolomics-Centric Review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Barberis, E.; Khoso, S.; Sica, A.; Falasca, M.; Gennari, A.; Dondero, F.; Afantitis, A.; Manfredi, M. Precision Medicine Approaches with Metabolomics and Artificial Intelligence. Int. J. Mol. Sci. 2022, 23, 11269. [Google Scholar] [CrossRef]

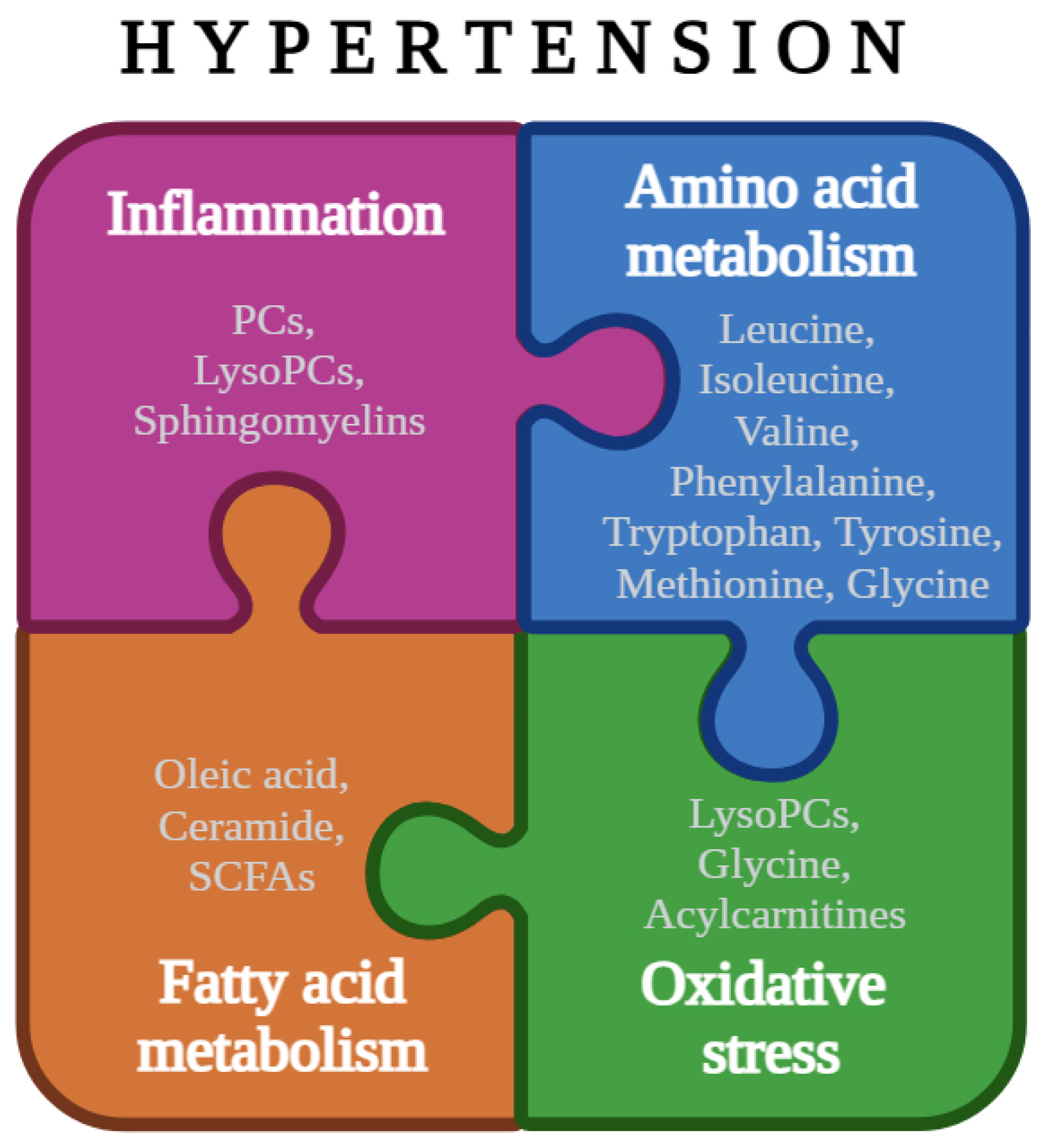
| Metabolic Pathway | Techniques | Metabolites | References |
|---|---|---|---|
| Amino Acid Metabolism * | MS and NMR | ↑ Leucine | [45,46,48] |
| ↓ Isoleucine | [45,46,48] | ||
| ↓ Valine | [48,56] | ||
| ↑ Phenylalanine | [40,46,48] | ||
| ↓ Tryptophan | [32,48,56] | ||
| ↓ Tyrosine | [48,54,56] | ||
| ↑ Methionine | [32,48,53,56] | ||
| ↓ Glycine | [8,40,45,46,52,54,56] | ||
| Fatty Acid Metabolism | MS and NMR | ↑ Oleic acid | [15,32,51] |
| ↑ Ceramide | [32] | ||
| ↓ SCFAs | [44,46,47] | ||
| Inflammation | MS, NMR and FTIR | ↑ PCs | [36,48,49,50,51] |
| ↑ LysoPCs | [32,35,48,51] | ||
| ↑ Sphingomyelins | [46,48] | ||
| Oxidative stress | MS and NMR | Acylcarnitines | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, I.C.R.; Martins, C.; Ribeiro, F.; Nunes, A. Understanding Hypertension: A Metabolomic Perspective. Biology 2025, 14, 403. https://doi.org/10.3390/biology14040403
Graça ICR, Martins C, Ribeiro F, Nunes A. Understanding Hypertension: A Metabolomic Perspective. Biology. 2025; 14(4):403. https://doi.org/10.3390/biology14040403
Chicago/Turabian StyleGraça, Inês C. R., Cláudia Martins, Fernando Ribeiro, and Alexandra Nunes. 2025. "Understanding Hypertension: A Metabolomic Perspective" Biology 14, no. 4: 403. https://doi.org/10.3390/biology14040403
APA StyleGraça, I. C. R., Martins, C., Ribeiro, F., & Nunes, A. (2025). Understanding Hypertension: A Metabolomic Perspective. Biology, 14(4), 403. https://doi.org/10.3390/biology14040403









