1. Introduction
Egg yolk contains a major source of active principle usable in medical, pharmaceutical, cosmetic, nutritional, and biotechnological industries [
1]. A recent survey carried out among farmers and buyers of quails and its products showed that even though there is no scientific backing concerning the use of quail egg and meat, all the people interviewed agreed that quail eggs and meat have medicinal value and are very effective against hypertension (100%) and diabetes (95.2%). Seventy-six percent of participants claimed the medicinal effects were more potent when the quail eggs were consumed raw. The majority (96.2%) of quail farmers reared these birds for their perceived medicinal properties [
2]. In the past, recommendations were made to decrease egg consumption to limit the risk of cardiovascular diseases. However, recent research has shown that there was no correlation between egg consumption and an increase in plasma total cholesterol [
3]. Quail eggs have the potential to be used in adjuvant therapies in many pharmaceutical preparations. It is gaining momentum in research on the management of various ailments, but there is a dearth of information regarding its toxicity. Thus, screening for toxicity of compounds intended for medicinal, pharmaceutical, or nutraceutical purposes is an important step in every biomedical research to ensure safety.
Egg yolk oil is also called ovum oil. It is generally derived from the yolk of chicken eggs, although it can be obtained from goose, duck, and other avian eggs [
4]. Folkloric reports showed that chicken egg oil has many medicinal uses, and it has been used for its analgesic effects for several years [
5]. Historically, the consumption of quail has been linked to curing tuberculosis, which led to the domestication and consumption of quail meat and eggs in the later part of the nineteen century in Japan [
6]. Quail egg oils have also been used to heal surgical wounds in rabbits [
7].
Egg yolk contains amino acids such as tryptophan and tyrosine [
8]; bioactive compounds such as choline and gamma linoleic acid (GLA) are also present [
9]. There are also small amounts of micronutrients such as vitamins A, D, and E [
10]. Zeisel et al. [
11] discovered that the proteins in egg yolk could act as potent inhibitors of human platelet aggregation. Chicken yolk oil has been observed to have anti-inflammatory, analgesic, and nociceptive properties [
5], and another study showed that this oil has wound-healing properties in rats [
12].
Hypertension is the persistent increase in blood pressure (BP) above 140/90 mmHg. Blood pressure in the arteries is persistently elevated. Blood pressure is expressed by two measurements, systolic and diastolic, which are the maximum and minimum pressures, respectively, in the arterial system [
13]. Elevated blood pressure is a major risk factor for the development of cardiovascular disease, including stroke, myocardial infarction, renal diseases, and cardiac mortality [
14]. Persistent elevation of blood pressure is an important public health issue globally because, even though it is readily detectable by routine blood pressure measuring, it can result in lethal complications if left untreated.
New drugs such as aprocitentan have been used to treat hypertension but possess adverse effects such as edema [
15]. Lately, attention has been focused on herbs and minerals as potential therapeutic agents for the prevention and management of cardiovascular disease [
16]. The objectives of this study were to assess the anti-hypertensive effect of QEYO extract whilst monitoring its toxicity using in vivo and in vitro techniques.
4. Discussion
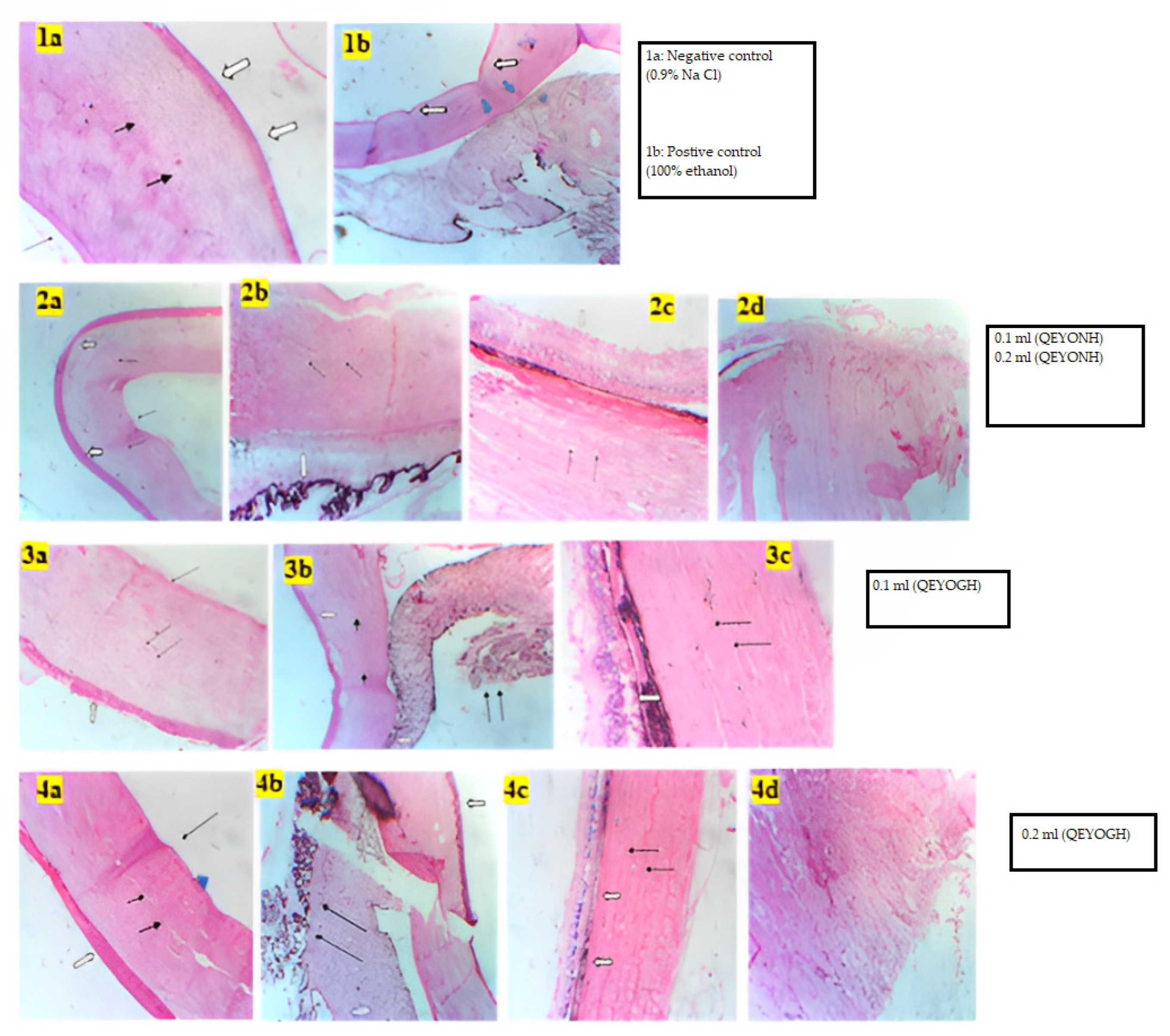
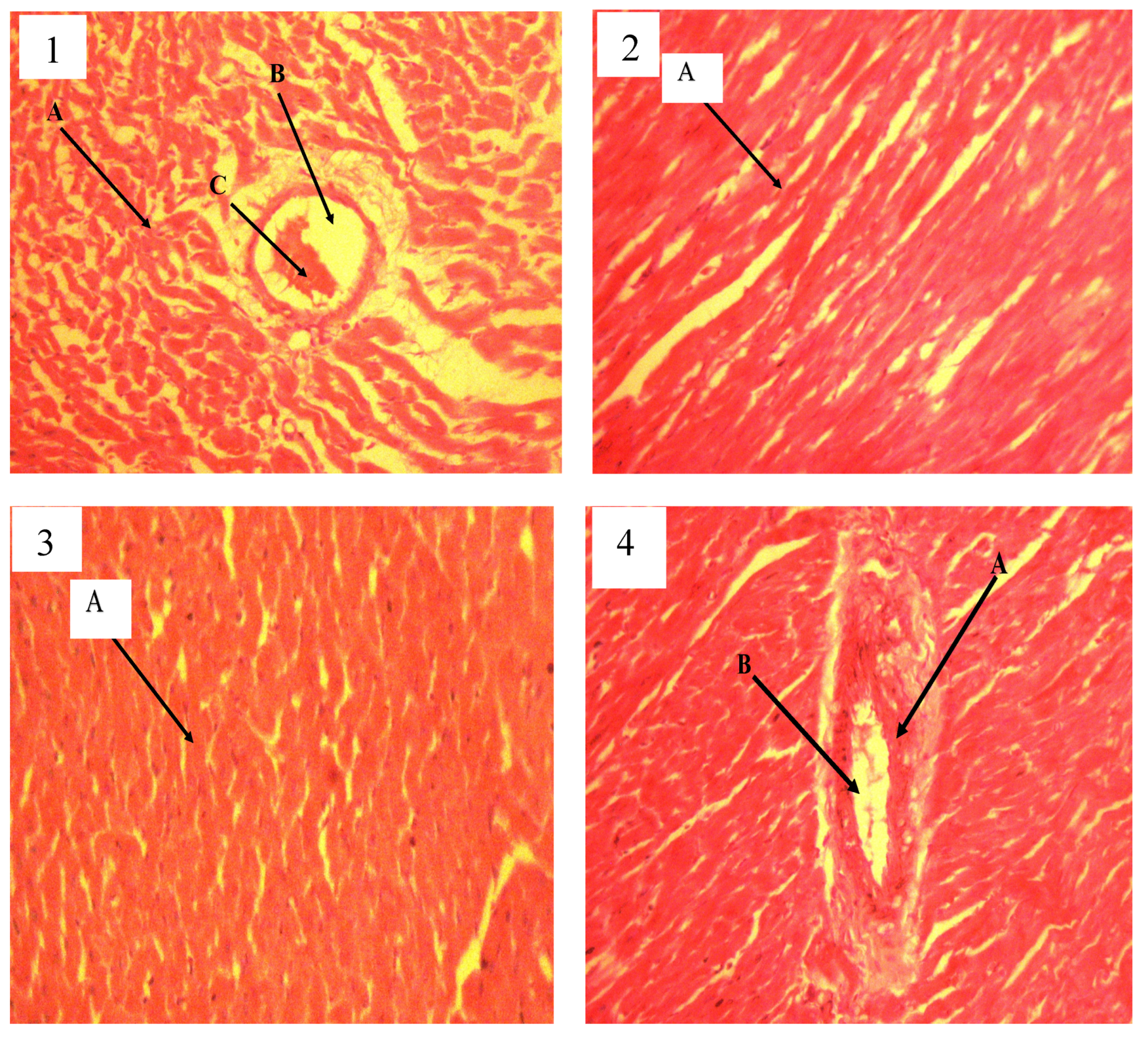
In drug discovery, toxicity studies play a very critical role. Screening for toxicity in any substance that is intended for medicinal, cosmeceutical, or nutraceutical use is an important factor that can never be neglected. In vitro toxicity testing serves as a fundamental stage for testing the toxicity of compounds intended for medicinal or cosmeceutical purposes. In this research, both the HET-CAM and bovine histology evaluation of the toxicity level were used due to their rapid and accurate significance for measuring toxicity levels. The BCOP test, which assesses opacity and permeability endpoints, can be used to determine the damage to the corneal tissue caused by chemicals. However, it is challenging to identify chemicals that react with cellular targets such as nucleic acids or mitochondrial proteins without causing immediate loss of cellular integrity or protein precipitation. Histological evaluation of treated corneas is necessary for the identification of such chemicals [
33,
34]. Thus, evaluating the corneal tissue histologically provides a direct measurement of injury depth [
35].
Only two out of four types of in vitro irritation toxicity tests were used in this study: HET-CAM and bovine corneal histology test [
36,
37]. Teixeira [
38] reported that the method of toxicity assessment used for a substance is appropriate for general assessment and can be a useful tool to achieve the objectives of the 3Rs program (Refine, Reduce, and Replacement). Both BCOP and HET-CAM methods are valid and can be reliably applied in research on potential toxic substances. Our study found that QEYO did not show any signs of toxicity or irritation on the CAM, and the histology of the bovine eye revealed no irritation after treatment with the two different extracts of QEYO. L-NAME-induced hypertension is a method of inducing hypertension in animal research models. It is characterized by a generalized deficiency of NO and a progressive increase in BP when prolonged [
39]. The L-NAME model is a good representation of hypertension in humans, making it ideal for studying the cardiovascular effects of new treatments. Prior research on quail egg yolk oil (QEYO) found that it contains sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), and phosphorus (P). The concentration of potassium, sodium, and phosphorus was slightly higher than that of calcium and magnesium when QEYO was obtained via gentle heating as compared to n-hexane extraction [
40]. Potassium supplementation has been proven to reduce blood pressure and mitigate complications in individuals with hypertension [
41]. Thus, QEYO could be recommended as an anti-hypertensive agent. Several studies have shown that a reduced sodium intake is effective in lowering blood pressure and protecting the heart against various cardiovascular diseases. This may explain why QEYO has cardio-protective properties due to its low sodium concentration. Magnesium and, more recently, calcium levels have been found to have significant cardio-protective effects on the heart and lower blood pressure [
42].
The production of nitric oxide (NO) by endothelial cells is responsible for the vasodilatory effect that regulates blood pressure. This process is dependent on factors that increase intracellular calcium concentration [
43]. Previous research has shown that quail egg yolk oil extracted using gentle heating has a higher concentration of calcium (0.90 mg/L), which could be the reason for the observed decrease in blood pressure. A high calcium diet has plausible antihypertensive mechanisms, including decreased α1-adrenoceptor responsiveness [
44,
45], improved function of the cell membrane Na
+-K
+-ATPase, and reduced voltage-dependent calcium entry in arterial smooth muscle [
46].
Ca supplementation may also augment arterial sensitivity to nitric oxide (NO) and enhance hyperpolarization of vascular smooth muscle [
47], which may be one of the reasons for the lowered blood pressure observed in the QEYO treatment groups.
Saponins were reported to be obtained in significant amount in QEYO [
40]. Saponins aid in reducing cholesterol levels by forming complexes with cholesterol and bile acids, which prevent them from being absorbed through the small intestine, thus lowering the cholesterol level in the blood and liver [
48]. Saponins have been shown to be useful in maintaining high-density lipoprotein cholesterol (HDL-C) levels and lowering low-density lipoprotein cholesterol (LDL-C) levels, as reported earlier [
49,
50].
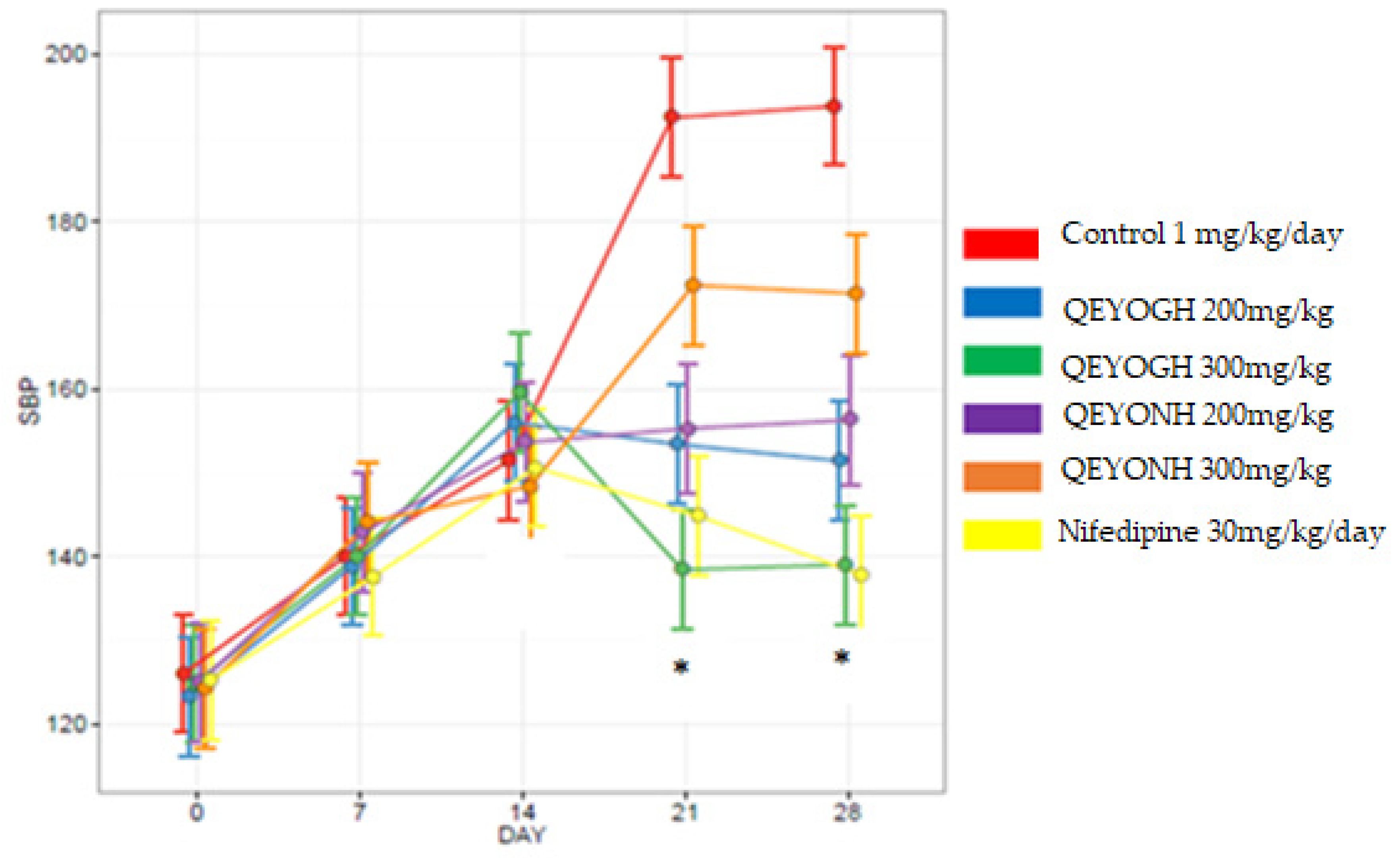
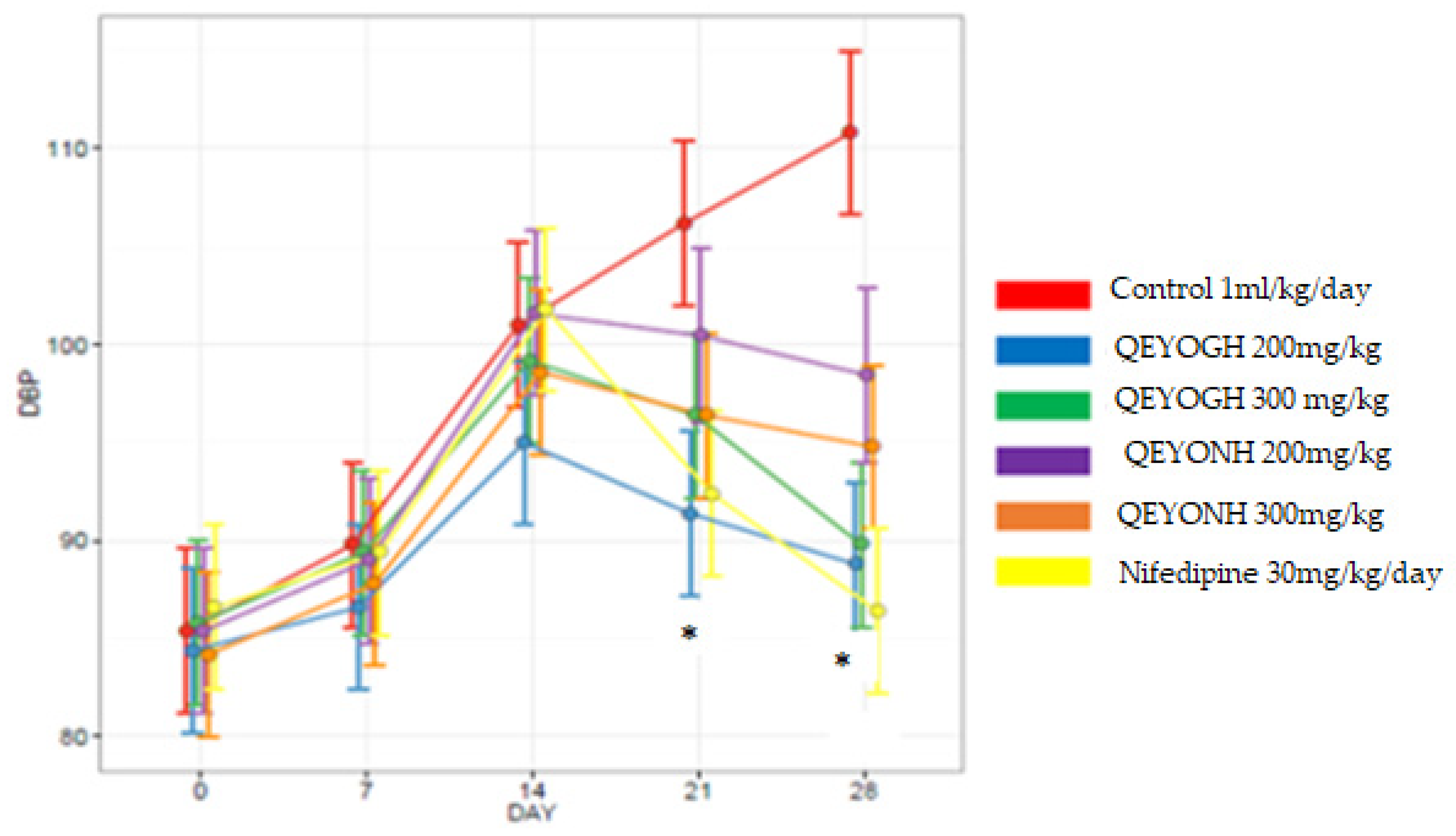
This study has shown that QEYO has the potential to lower blood pressure. This effect may be due to the high levels of antioxidants found in QEYO [
51]. Previous research has shown that these antioxidants can improve the activity of antioxidant enzymes and up-regulate antioxidant genes in fruit flies [
51]. This study further confirmed the positive effect of QEYO on antioxidants, as the treated group showed a significant increase in antioxidant biomarkers compared to the control group. These biomarkers help to boost nitrous oxide production [
52], which contributes to the regulation of blood flow and pressure from the non-adrenergic, non-cholinergic terminals [
53,
54]. The mean decrease in SBP for all QEYOGH-treated groups was compared to the mean decrease in all SBP QEYONH groups, and there was no statistically significant difference (
p > 0.05) between the groups. This shows that QEYO extracted through either the gentle heating method or n-hexane method could reduce blood pressure. These findings partially contradict the findings that quail eggs, either raw, fried, or boiled, consumed by anemic female hypertensive patients did not reduce blood pressure but rather increased the lipid profile in blood serum in humans [
54].
High cholesterol levels in the blood are linked to cardiovascular diseases such as atherosclerosis, coronary heart diseases, and hypertension. The level to which elevated blood lipids contribute to these heart conditions is dependent on the distribution of the various types of lipoprotein classes in blood serum [
55]. High levels of lipids except for high-density lipoproteins are associated with hypertension and atherosclerosis, while high levels of triglycerides and LDLP are associated with coronary artery diseases [
55].
The significant levels of high-density lipoprotein (HDLP) in the treated rats show that QEYO has some cardio-protective abilities as it has been shown that high levels of HDLP in blood serum may exact some protective effects against atherosclerosis and hypertension and promote the mobilization and metabolism of cholesterol, thus reducing the deposition of cholesterol in the arterial blood vessels. Similarly, HDLP has also been shown in vitro to have some form of mechanism for the removal of peripheral cholesterol as well as very low-density lipoprotein (VLDL) and LDLP [
55].
In this study, HDLP levels were observed to be significantly (
p < 0.05) elevated in all the treated groups compared to the control group that did not receive QEYO, while the TC, TG, and LDLP levels were elevated compared to the control groups, but these increases were not significant (
p < 0.05). This shows that QEYO does not totally increase the risk of cardiovascular diseases and hypertension, as opposed to early reports [
52]. This study also reveals that QEYO has some anticholesteremic and antilipidemic effects. Several epidemiological studies have linked low intake of dietary antioxidants to an increased frequency of hypertension; this is in addition to the inverse relationship between heart disease and plasma antioxidants levels [
54]. Endothelial cells of the vascular smooth muscles are particularly susceptible to oxidative stress, not only through ROS-mediated cell death but also because of the bioavailability of the normally protective mediator such as nitric oxide. Our previous study [
51] reveals that QEYO contains oleic acid and diltiazem. Oleic acid has been shown to decrease the myocardial infarction rate, platelet aggregation, and secretion of TXA2, reduce systolic blood pressure, and improve immunity [
56]. On the other hand, diltiazem is a calcium channel blocker that is clinically used as an antihypertensive, anti-arrhythmic, and anti-anginal agent for the management of cardiovascular conditions such as hypertension, chronic stable angina, atrial fibrillation, and atrial flutter [
57]. Studies have revealed that egg yolk contains proline and arginine, both of which may decrease blood pressure, which may be through the inhibition of angiotensin-converting enzyme [
3,
58,
59].