A Single Intraperitoneal Secreted Protein Acidic and Rich in Cysteine Injection in Mice Is Towards an Exercise-like Phenotype
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
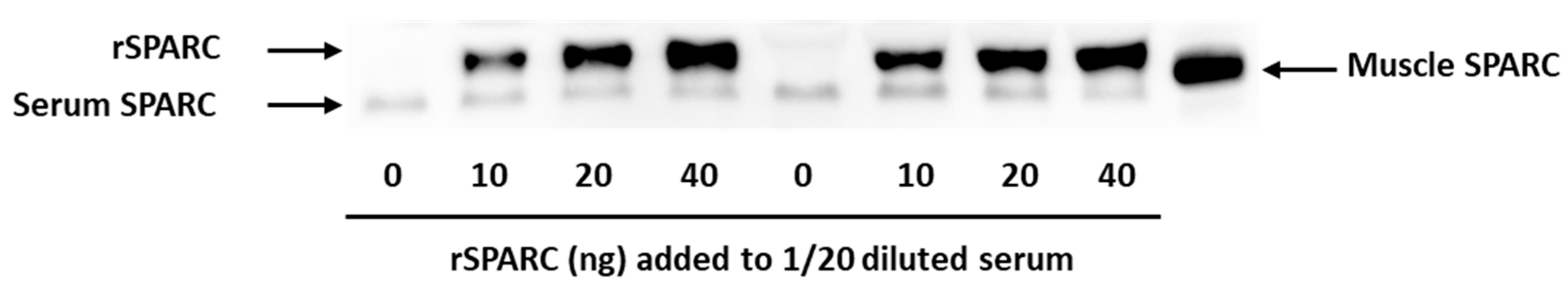
2.1. Validation of Western Blot Method to Measure the Injected rSPARC in Serum
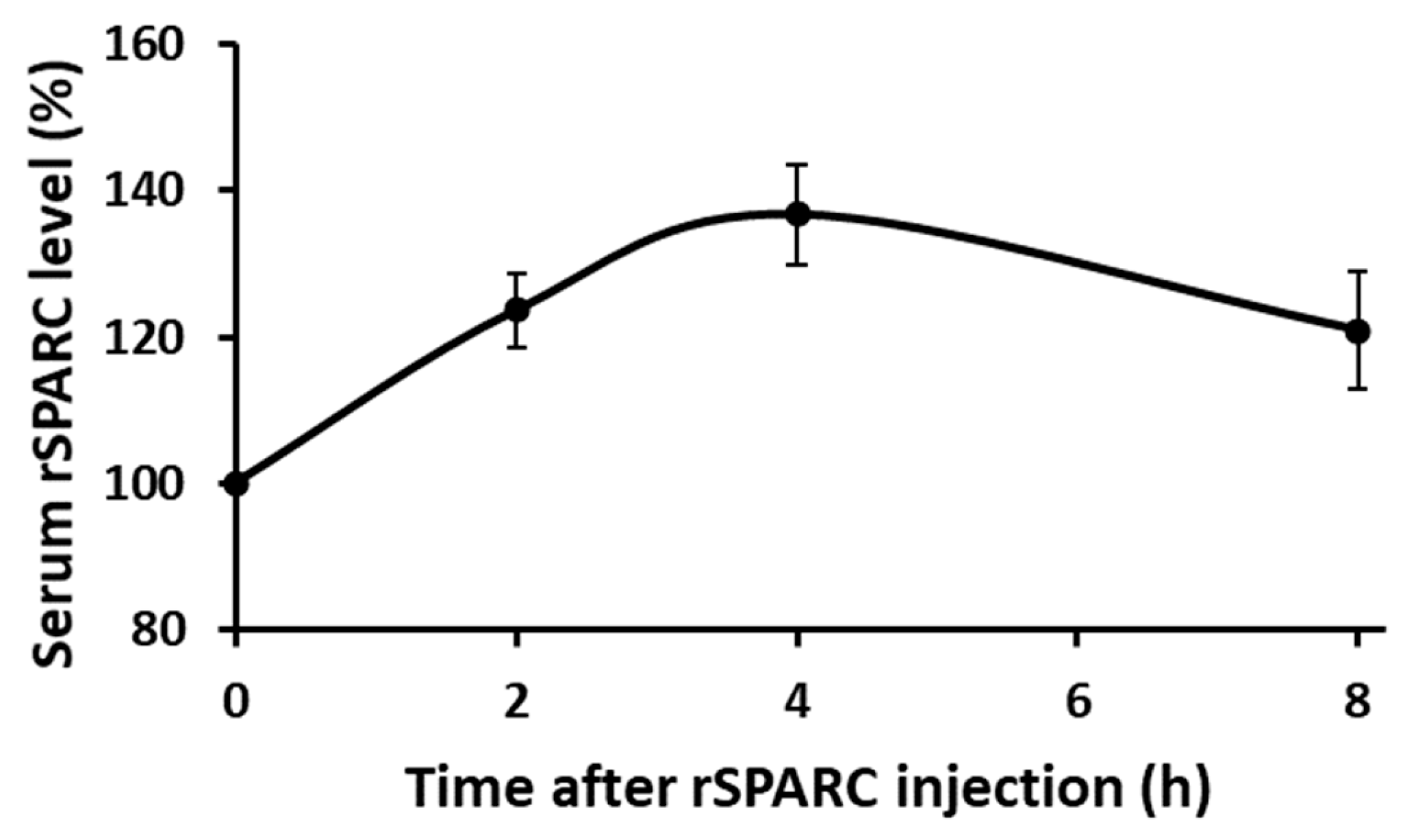
2.2. Finding How Long Following Intraperitoneal rSPARC Injection Does It Take (T Max) to Reach the Maximum Serum Concentration
2.3. Experimental Design and rSPARC Injection to Mice
2.3.1. Blood Glucose (Glycemia)
2.3.2. Tissues’ Weights
2.3.3. Muscle Proteins Expression (Western Blot)
2.3.4. Statistical Analyses
3. Results
3.1. Validation of rSPARC Detection by Western Blot
3.2. Determination of the Optimum Timepoint Tmax
3.3. Post-Injection Analyses
3.3.1. Body Weights and Tissues Weights and Injection Volumes (Table 1)
| Male | Female | 2-way ANOVA | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | rSPARC | Saline | rSPARC | p Value | Effect Size (h2) | |||||||||||||||||
| Treatment | Sex | Interaction | Treatment | Sex | Interaction | |||||||||||||||||
| Body weight (g) | 31.7 | ± | 2.0 | 31.2 | ± | 1.3 | 22.9 | ± | 1.3 | 23.4 | ± | 2.7 | 0.99 | 0.0001 | 0.65 | 0.00 | 0.88 | 0.03 | ||||
| Injection volume (mL) | 190 | ± | 12 | 187 | ± | 8 | 137 | ± | 8 | 140 | ± | 17 | 1.00 | 0.0001 | 0.67 | 0.00 | 0.87 | 0.02 | ||||
| Blood glucose (mM) | 9.7 | ± | 0.5 | 9.4 | ± | 2.3 | 6.0 | ± | 1.2 | 6.2 | ± | 0.6 | 0.97 | 0.002 | 0.75 | 0.00 | 0.70 | 0.01 | ||||
| Organ/Tissue weight (mg) | ||||||||||||||||||||||
| Liver | 1.14 | ± | 0.09 | 1.05 | ± | 0.15 | 0.75 | ± | 0.07 | 0.82 | ± | 0.09 | 0.88 | 0.001 | 0.21 | 0.00 | 0.77 | 0.19 | ||||
| Heart | 0.133 | ± | 0.012 | 0.146 | ± | 0.011 | 0.109 | ± | 0.020 | 0.100 | ± | 0.002 | 0.87 | 0.001 | 0.17 | 0.00 | 0.74 | 0.22 | ||||
| Adipose tissue | ||||||||||||||||||||||
| Retroperi-toneal | 0.235 | ± | 0.076 | 0.139 | ± | 0.035 | 0.056 | ± | 0.040 | 0.101 | ± | 0.097 | 0.52 | 0.02 | 0.11 | 0.05 | 0.49 | 0.29 | ||||
| Inguinal | 0.364 | ± | 0.090 | 0.295 | ± | 0.046 | 0.215 | ± | 0.085 | 0.288 | ± | 0.130 | 0.97 | 0.18 | 0.22 | 0.00 | 0.21 | 0.18 | ||||
| Para-ovary | 0.814 | ± | 1.146 | 0.967 | ± | 0.913 | 0.87 * | - | - | 0.15 ** | - | - | ||||||||||
| Epidi-dymal | 0.838 | ± | 0.198 | 0.616 | ± | 0.103 | 0.16 * | - | - | 1.41 ** | - | - | ||||||||||
| Skeletal muscle | ||||||||||||||||||||||
| Soleus | 0.033 | ± | 0.010 | 0.034 | ± | 0.009 | 0.021 | ± | 0.006 | 0.024 | ± | 0.008 | 0.76 | 0.05 | 0.90 | 0.01 | 0.39 | 0.00 | ||||
| Gastro-cnemius | 0.334 | ± | 0.023 | 0.330 | ± | 0.040 | 0.244 | ± | 0.010 | 0.233 | ± | 0.036 | 0.67 | 0.001 | 0.85 | 0.02 | 0.79 | 0.00 | ||||
| Tibialis anterior | 0.120 | ± | 0.018 | 0.130 | ± | 0.012 | 0.082 | ± | 0.004 | 0.084 | ± | 0.003 | 0.40 | 0.0002 | 0.56 | 0.09 | 0.85 | 0.04 | ||||
3.3.2. Western Blot Analyses (Table 2)
| Male | Female | 2-Way ANOVA | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | rSPARC | Saline | rSPARC | p Value | Effect Size (h2) | dCohen Effect Size | ||||||||||||||||
| Treatment | Sex | Interaction | Male | Female | Treatment | Sex | Interaction | Male | Female | |||||||||||||
| ILK | 0.52 | ± | 0.05 | 0.99 | ± | 0.24 | 1.09 | ± | 0.13 | 1.32 | ± | 0.14 | 0.01 | 0.003 | 0.31 | - | - | 0.56 | 0.68 | 0.13 | - | - |
| GSK-3b | 0.95 | ± | 0.11 | 0.87 | ± | 0.04 | 1.04 | ± | 0.27 | 1.13 | ± | 0.33 | 0.99 | 0.28 | 0.61 | - | - | 0.00 | 0.14 | 0.03 | - | - |
| pGSK-3b | 0.95 | ± | 0.11 | 0.87 | ± | 0.04 | 1.04 | ± | 0.27 | 1.13 | ± | 0.33 | 0.02 | 0.70 | 0.05 | 0.01 | 0.69 | 0.51 | 0.02 | 0.40 | 1.0 | 0.3 |
| COL1A2 | 0.41 | ± | 0.17 | 1.11 | ± | 0.24 | 1.50 | ± | 0.46 | 0.83 | ± | 0.29 | 0.95 | 0.10 | 0.01 | 0.05 | 0.06 | 0.00 | 0.30 | 0.55 | 3.4 | 1.7 |
| pAkt1 | 0.79 | ± | 0.33 | 2.21 | ± | 0.86 | 0.33 | ± | 0.05 | 0.66 | ± | 0.16 | 0.03 | 0.02 | 0.14 | - | - | 0.46 | 0.53 | 0.25 | - | - |
| pS6K | 1.15 | ± | 0.54 | 0.88 | ± | 0.43 | 0.94 | ± | 0.27 | 1.08 | ± | 0.20 | 0.82 | 1.00 | 0.46 | - | - | 0.01 | 0.00 | 0.07 | - | - |
| p4EBP1 | 0.53 | ± | 0.08 | 0.75 | ± | 0.34 | 1.10 | ± | 0.25 | 1.40 | ± | 0.34 | 0.22 | 0.01 | 0.83 | - | - | 0.18 | 0.55 | 0.01 | - | - |
| 4EBP1 | 0.59 | ± | 0.28 | 1.08 | ± | 0.31 | 1.48 | ± | 0.10 | 0.74 | ± | 0.41 | 0.58 | 0.23 | 0.02 | 0.14 | 0.04 | 0.04 | 0.17 | 0.51 | 1.7 | 2.5 |
| IL6 | 1.14 | ± | 0.13 | 1.02 | ± | 0.19 | 0.89 | ± | 0.01 | 0.98 | ± | 0.05 | 0.85 | 0.12 | 0.23 | - | - | 0.00 | 0.28 | 0.17 | - | - |
| pAMPK | 0.45 | ± | 0.10 | 0.43 | ± | 0.16 | 1.01 | ± | 0.05 | 1.92 | ± | 0.41 | 0.0002 | 0.02 | 0.02 | 0.93 | 0.004 | 0.49 | 0.84 | 0.51 | 0.1 | 3.1 |
| GLUT4 | 0.98 | ± | 0.11 | 1.39 | ± | 0.47 | 0.66 | ± | 0.09 | 1.04 | ± | 0.18 | 0.11 | 0.07 | 0.93 | - | - | 0.36 | 0.29 | 0.00 | - | - |
| PGC1a | 0.24 | ± | 0.12 | 0.81 | ± | 0.21 | 1.11 | ± | 0.22 | 1.64 | ± | 0.19 | 0.003 | 0.0002 | 0.89 | - | - | 0.68 | 0.83 | 0.00 | - | - |
| NRF1 | 0.64 | ± | 0.23 | 1.32 | ± | 0.44 | 1.15 | ± | 0.10 | 0.91 | ± | 0.32 | 0.33 | 0.82 | 0.06 | 0.05 | 0.43 | 0.12 | 0.01 | 0.37 | 1.9 | 0.3 |
| COX2 | 0.87 | ± | 0.22 | 1.17 | ± | 0.10 | 0.91 | ± | 0.18 | 1.06 | ± | 0.01 | 0.07 | 0.72 | 0.50 | - | - | 0.36 | 0.02 | 0.06 | - | - |
| SDHB | 0.90 | ± | 0.36 | 1.11 | ± | 0.21 | 1.02 | ± | 0.10 | 0.98 | ± | 0.17 | 0.63 | 0.97 | 0.44 | - | - | 0.03 | 0.00 | 0.07 | - | - |
| pSmad3 | 1.21 | ± | 0.07 | 0.90 | ± | 0.06 | 0.99 | ± | 0.16 | 0.91 | ± | 0.05 | 0.02 | 0.14 | 0.14 | - | - | 0.50 | 0.25 | 0.25 | - | - |
| pFOXO1 | 1.33 | ± | 0.61 | 0.48 | ± | 0.19 | 1.34 | ± | 0.50 | 0.81 | ± | 0.13 | 0.04 | 0.58 | 0.59 | - | - | 0.41 | 0.04 | 0.04 | - | - |
| TRIM63 | 1.49 | ± | 0.82 | 0.83 | ± | 0.38 | 0.82 | ± | 0.17 | 0.89 | ± | 0.32 | 0.42 | 0.39 | 0.32 | - | - | 0.08 | 0.09 | 0.12 | - | - |
| Fbx32 | 0.65 | ± | 0.28 | 0.71 | ± | 0.06 | 1.54 | ± | 0.27 | 1.02 | ± | 0.23 | 0.19 | 0.01 | 0.10 | 0.80 | 0.05 | 0.20 | 0.63 | 0.29 | 0.3 | 2.1 |
| Myod1 | 1.04 | ± | 0.24 | 0.93 | ± | 0.12 | 0.94 | ± | 0.07 | 1.09 | ± | 0.18 | 0.90 | 0.81 | 0.30 | - | - | 0.00 | 0.01 | 0.13 | - | - |
| Myogenin | 0.21 | ± | 0.15 | 0.36 | ± | 0.15 | 1.12 | ± | 0.53 | 2.32 | ± | 0.23 | 0.01 | 0.0002 | 0.04 | 0.63 | 0.005 | 0.55 | 0.85 | 0.42 | 1.0 | 2.9 |
4. Discussion
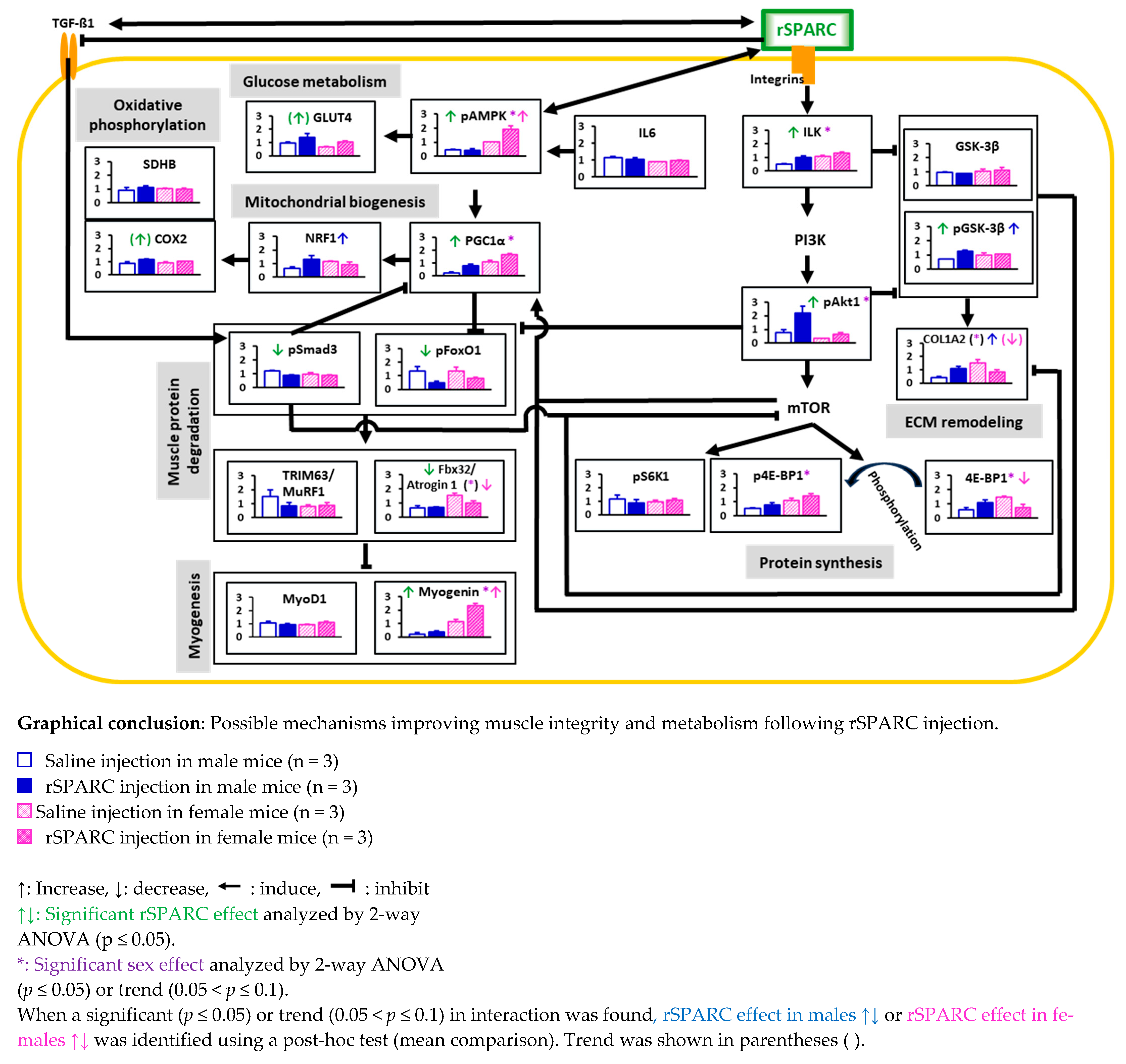 |
- Abbreviations: COL1A2: collagen type I alpha 2 chain; COX2: mitochondrial cytochrome c oxidase 2; ECM: extracellular matrix; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein 1; Fbx32/Atrogin 1: F-box protein 32/atrogin 1; GLUT4: glucose transporter 4; GSK-3β: glycogen synthase kinase 3 beta; IL6: interleukin 6; ILK: integrin-linked kinase; mTOR: mammalian target of rapamycin; MyoD1: myogenic differentiation 1; NRF-1: nuclear respiratory factor 1; p4E-BP1: phosphorylated (Ser65) eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; pAkt1/PKB: phosphorylated (Ser473) Ak strain transforming/protein kinase B; pAMPK: phosphorylated (α1-Thr183 and α2-Thr172) AMP-activated protein kinase; pFoxO1: phosphorylated (Ser256) forkhead box protein O1; PGC1α: peroxisome proliferator-activated receptor gamma coactivator-1α; PI3K: phosphoinositide 3; pGSK-3β: phosphorylated (Ser-9) glycogen synthase kinase 3 beta; PI3K: phosphoinositide 3; pS6K1: phosphorylated (Thr389) ribosomal protein S6 kinase beta-1; pSmad3: phosphorylated (Ser423/Ser425) SMAD family member 3; SDHB: succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial; rSPARC: recombinant secreted protein acidic and rich in cysteine; TGF-β1: transforming growth factor beta 1; TRIM63/MuRF1: tripartite motif-containing 63/muscle-specific RING finger protein 1.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riedl, I.; Yoshioka, M.; Nishida, Y.; Tobina, T.; Paradis, R.; Shono, N.; Tanaka, H.; St-Amand, J. Regulation of skeletal muscle transcriptome in elderly men after 6 weeks of endurance training at lactate threshold intensity. Exp. Gerontol. 2010, 45, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020, 10, 9108. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Leads to an Accelerated Ageing Phenotype Which Is Improved by Exercise Whereas SPARC Overexpression Mimics Exercise Effects in Mice. Metabolites 2022, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Delany, A.M.; Kalajzic, I.; Bradshaw, A.D.; Sage, E.H.; Canalis, E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology 2003, 144, 2588–2596. [Google Scholar] [CrossRef]
- Motamed, K. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol. 1999, 31, 1363–1366. [Google Scholar] [CrossRef]
- Scavelli, K.; Chatterjee, A.; Rhee, D.J. Secreted Protein Acidic and Rich in Cysteine in Ocular Tissue. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2015, 31, 396–405. [Google Scholar] [CrossRef]
- Sage, H.; Johnson, C.; Bornstein, P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 1984, 259, 3993–4007. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biol. J. Int. Soc. Matrix Biol. 2000, 19, 569–580. [Google Scholar] [CrossRef]
- Norose, K.; Clark, J.I.; Syed, N.A.; Basu, A.; Heber-Katz, E.; Sage, E.H.; Howe, C.C. SPARC deficiency leads to early-onset cataractogenesis. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2674–2680. [Google Scholar]
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1999, 47, 1495–1506. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.Y.; Li, C.Y.; Wu, J.Y.; Zhang, Y.T.; Pang, K.P.; Wei, Y.; Du, L.Q.; Liu, M.; Wu, X.Y. SPARC promotes self-renewal of limbal epithelial stem cells and ocular surface restoration through JNK and p38-MAPK signaling pathways. Stem Cells 2020, 38, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, H.; Santhanam, R.; Maharry, K.; Metzeler, K.H.; Huang, X.; Kohlschmidt, J.; Mendler, J.H.; Benito, J.M.; Hickey, C.; Neviani, P.; et al. SPARC promotes leukemic cell growth and predicts acute myeloid leukemia outcome. J. Clin. Investig. 2014, 124, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000, 105, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Baneyx, G.; Cardó-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005, 280, 36483–36493. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 2009, 3, 239–246. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. Biochem. Cell Biol. 2019, 117, 105627. [Google Scholar] [CrossRef]
- M Onorato, A.; Fiore, E.; Bayo, J.; Casali, C.; Fernandez-Tomé, M.; Rodríguez, M.; Domínguez, L.; Argemi, J.; Hidalgo, F.; Favre, C.; et al. SPARC inhibition accelerates NAFLD-associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 1677–1693. [Google Scholar] [CrossRef]
- Song, H.; Ding, L.; Zhang, S.; Wang, W. MiR-29 family members interact with SPARC to regulate glucose metabolism. Biochem. Biophys. Res. Commun. 2018, 497, 667–674. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020, 10, 2388. [Google Scholar] [CrossRef]
- McCurdy, S.; Baicu, C.F.; Heymans, S.; Bradshaw, A.D. Cardiac extracellular matrix remodeling: Fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J. Mol. Cell. Cardiol. 2010, 48, 544–549. [Google Scholar] [CrossRef]
- Petersson, S.J.; Jørgensen, L.H.; Andersen, D.C.; Nørgaard, R.C.; Jensen, C.H.; Schrøder, H.D. SPARC is up-regulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol. Histopathol. 2013, 28, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Galvão, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-induced myokines and their effect on prostate cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Melouane, A.; Yoshioka, M.; Kanzaki, M.; St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019, 229, 277–287. [Google Scholar] [CrossRef]
- Melouane, A.; Carbonell, A.; Yoshioka, M.; Puymirat, J.; St-Amand, J. Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE 2018, 13, e0192714. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Genetic Expression between Ageing and Exercise: Secreted Protein Acidic and Rich in Cysteine as a Potential “Exercise Substitute” Antiageing Therapy. Genes 2022, 13, 950. [Google Scholar] [CrossRef]
- Rowlatt, C.; Chesterman, F.C.; Sheriff, M.U. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab. Anim. 1976, 10, 419–442. [Google Scholar] [CrossRef]
- Kunstyr, I.; Leuenberger, H.G. Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. J. Gerontol. 1975, 30, 157–162. [Google Scholar] [CrossRef]
- Available online: https://insights.envigo.com/hubfs/resources/data-sheets/2018s-datasheet-0915.pdf (accessed on 13 December 2020).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. Biomed. Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Spadaro, O.; Sidorov, S.; Lee, A.H.; Caprio, S.; Morrison, C.; Smith, S.R.; Ravussin, E.; Shchukina, I.; Artyomov, M.N.; et al. Reduction of SPARC protects mice against NLRP3 inflammasome activation and obesity. J. Clin. Investig. 2023, 133, e169173. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Aoi, W.; Sakuma, K. Skeletal muscle: Novel and intriguing characteristics as a secretory organ. BioDiscovery 2013, 7, e8942. [Google Scholar] [CrossRef]
- Usach, I.; Martinez, R.; Festini, T.; Peris, J.E. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv. Ther. 2019, 36, 2986–2996. [Google Scholar] [CrossRef]
- Mathaes, R.; Koulov, A.; Joerg, S.; Mahler, H.C. Subcutaneous Injection Volume of Biopharmaceuticals-Pushing the Boundaries. J. Pharm. Sci. 2016, 105, 2255–2259. [Google Scholar] [CrossRef]
- Shah, O.J.; Anthony, J.C.; Kimball, S.R.; Jefferson, L.S. 4E-BP1 and S6K1: Translational integration sites for nutritional and hormonal information in muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E715–E729. [Google Scholar] [CrossRef]
- Greiwe, J.S.; Kwon, G.; McDaniel, M.L.; Semenkovich, C.F. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E466–E471. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S.; Fadden, P.; Haystead, T.A.; Lawrence, J.C., Jr. Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am. J. Physiol. 1996, 270, C705–C709. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Hudson, C.C.; Sekulic, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C., Jr.; Abraham, R.T. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Marshall, C.A.; Lin, T.A.; Kwon, G.; Munivenkatappa, R.B.; Hill, J.R.; Lawrence, J.C., Jr.; McDaniel, M.L. Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic beta cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J. Biol. Chem. 1998, 273, 4485–4491. [Google Scholar] [CrossRef]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef]
- Long, W.; Saffer, L.; Wei, L.; Barrett, E.J. Amino acids regulate skeletal muscle PHAS-I and p70 S6-kinase phosphorylation independently of insulin. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E301–E306. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Winder, W.W.; Hardie, D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. 1996, 270, E299–E304. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Tando, T.; Hirayama, A.; Furukawa, M.; Sato, Y.; Kobayashi, T.; Funayama, A.; Kanaji, A.; Hao, W.; Watanabe, R.; Morita, M.; et al. Smad2/3 Proteins Are Required for Immobilization-induced Skeletal Muscle Atrophy. J. Biol. Chem. 2016, 291, 12184–12194. [Google Scholar] [CrossRef]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Chunthorng-Orn, J.; Lord, S.; Musa, I.; Dawson, P.; Holm, L.; Lai, Y.C. Ubiquitin E3 ligase Atrogin-1 protein is regulated via the rapamycin-sensitive mTOR-S6K1 signaling pathway in C2C12 muscle cells. Am. J. Physiol. Cell Physiol. 2022, 323, C215–C225. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sage, E.H. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J. Biol. Chem. 2009, 284, 1279–1290. [Google Scholar] [CrossRef]
- Konigshoff, M.; Balsara, N.; Pfaff, E.M.; Kramer, M.; Chrobak, I.; Seeger, W.; Eickelberg, O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008, 3, e2142. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, X.; Jiang, Y.; Chen, L.; Sheng, M.; Chen, Y. SPARC knockdown attenuated TGF-beta1-induced fibrotic effects through Smad2/3 pathways in human pterygium fibroblasts. Arch. Biochem. Biophys. 2021, 713, 109049. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef]
- Rivera, L.B.; Brekken, R.A. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-beta1 activity. J. Cell Biol. 2011, 193, 1305–1319. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakano, S.; Miyoshi, T.; Yamanouchi, K.; Nishihara, M. Loss of SPARC in mouse skeletal muscle causes myofiber atrophy. Muscle Nerve 2013, 48, 791–799. [Google Scholar] [CrossRef]
- Song, H.; Guan, Y.; Zhang, L.; Li, K.; Dong, C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem. Biophys. Res. Commun. 2010, 396, 961–966. [Google Scholar] [CrossRef]
- Theeuwes, W.F.; Gosker, H.R.; Langen, R.C.J.; Pansters, N.A.M.; Schols, A.; Remels, A.H.V. Inactivation of glycogen synthase kinase 3beta (GSK-3beta) enhances mitochondrial biogenesis during myogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes. Dev. 1999, 13, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Cooke, M.B.; Willoughby, D.S. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur. J. Appl. Physiol. 2009, 107, 463–471. [Google Scholar] [CrossRef]
- Jung, S.; Kim, K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr. Med. Res. 2014, 3, 155–160. [Google Scholar] [CrossRef]
- Mavropalias, G.; Wu, Y.F.; Boppart, M.D.; Blazevich, A.J.; Nosaka, K. Increases in Integrin-ILK-RICTOR-Akt Proteins, Muscle Mass, and Strength after Eccentric Cycling Training. Med. Sci. Sports Exerc. 2022, 54, 89–97. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005, 135, 181–193. [Google Scholar] [CrossRef]
- Yang, Y.; Creer, A.; Jemiolo, B.; Trappe, S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J. Appl. Physiol. 2005, 98, 1745–1752. [Google Scholar] [CrossRef]
- Nikooie, R.; Jafari-Sardoie, S.; Sheibani, V.; Nejadvaziri Chatroudi, A. Resistance training-induced muscle hypertrophy is mediated by TGF-β1-Smad signaling pathway in male Wistar rats. J. Cell Physiol. 2020, 235, 5649–5665. [Google Scholar] [CrossRef]
- Aoi, W.; Hirano, N.; Lassiter, D.G.; Björnholm, M.; Chibalin, A.V.; Sakuma, K.; Tanimura, Y.; Mizushima, K.; Takagi, T.; Naito, Y.; et al. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 10551–10562. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Garneau, L.; Parsons, S.A.; Smith, S.R.; Mulvihill, E.E.; Sparks, L.M.; Aguer, C. Plasma Myokine Concentrations After Acute Exercise in Non-obese and Obese Sedentary Women. Front. Physiol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Yamada, M.; Moriguch, Y.; Mitani, T.; Aoyama, T.; Arai, H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 8–14. [Google Scholar] [CrossRef]
- Miyamoto, T.; Shimizu, Y.; Matsuo, Y.; Otaru, T.; Kanzawa, Y.; Miyamae, N.; Yamada, E.; Katsuno, T. Effects of exercise intensity and duration on a myokine, secreted protein acidic and rich in cysteine. Eur. J. Sport Sci. 2021, 22, 1401–1410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Yoshioka, M.; St-Amand, J. A Single Intraperitoneal Secreted Protein Acidic and Rich in Cysteine Injection in Mice Is Towards an Exercise-like Phenotype. Biology 2025, 14, 398. https://doi.org/10.3390/biology14040398
Ghanemi A, Yoshioka M, St-Amand J. A Single Intraperitoneal Secreted Protein Acidic and Rich in Cysteine Injection in Mice Is Towards an Exercise-like Phenotype. Biology. 2025; 14(4):398. https://doi.org/10.3390/biology14040398
Chicago/Turabian StyleGhanemi, Abdelaziz, Mayumi Yoshioka, and Jonny St-Amand. 2025. "A Single Intraperitoneal Secreted Protein Acidic and Rich in Cysteine Injection in Mice Is Towards an Exercise-like Phenotype" Biology 14, no. 4: 398. https://doi.org/10.3390/biology14040398
APA StyleGhanemi, A., Yoshioka, M., & St-Amand, J. (2025). A Single Intraperitoneal Secreted Protein Acidic and Rich in Cysteine Injection in Mice Is Towards an Exercise-like Phenotype. Biology, 14(4), 398. https://doi.org/10.3390/biology14040398







