Exploring the Cardiovascular Potential of Artichoke—A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Composition of Artichoke Extracts
3. Cardiovascular Effects of the Artichoke
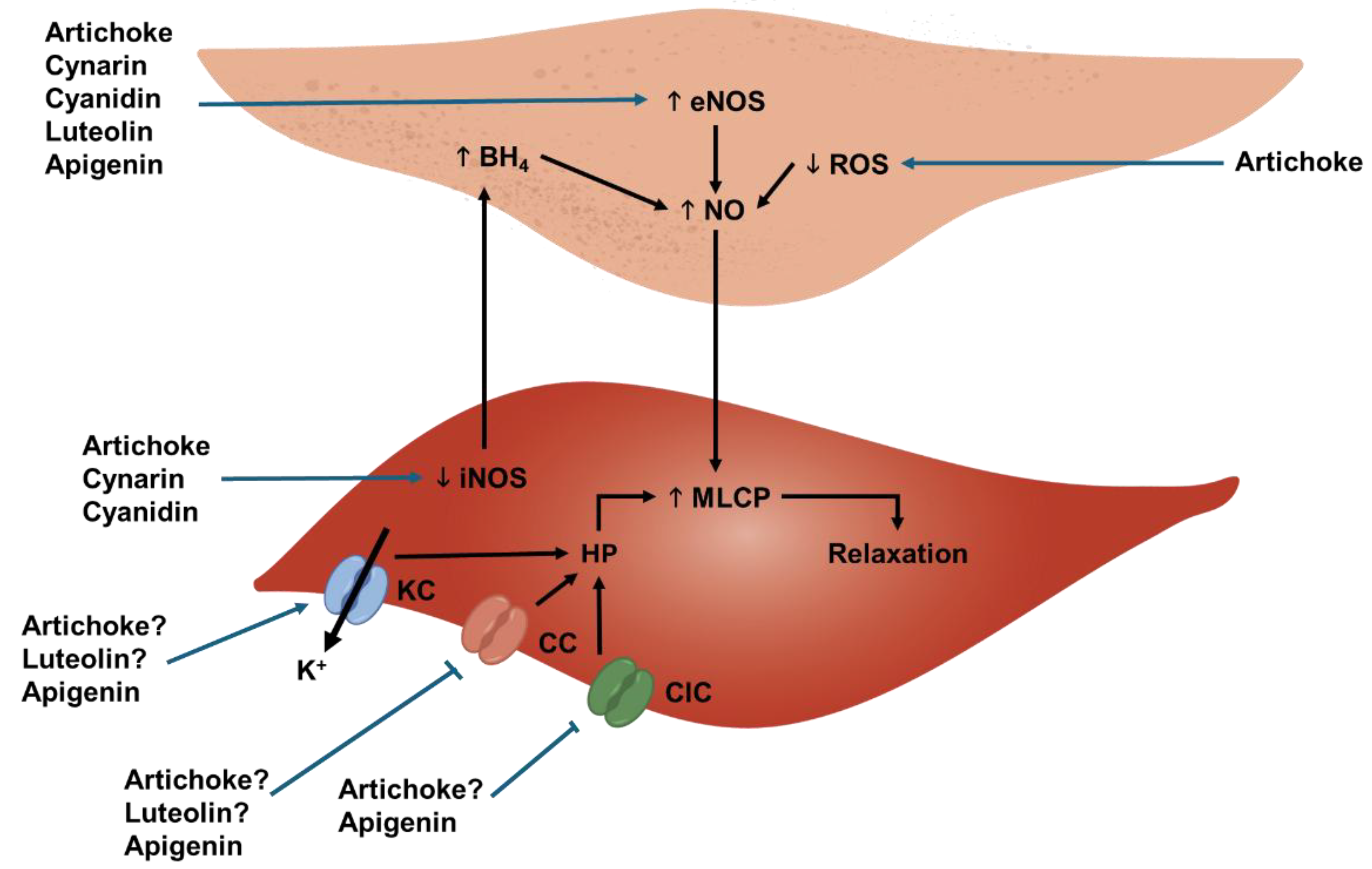
3.1. Endothelium-Protecting Effects In Vitro
3.2. Endothelium-Protecting Effects Ex Vivo and In Vivo
3.3. Modulation of the Renin–Angiotensin–Aldosterone Axis
3.4. Improvement in Flow-Mediated Dilation
3.5. Possible Effect of Reduction in Body Weight and Insulin Resistance
3.6. Antihypertensive Activity
3.7. Safety Profile of Artichokes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Elkind, M.S.; Deutsch, A.; Dowd, W.N.; Heidenreich, P.; Khavjou, O.; Mark, D.; Mussolino, M.E.; Ovbiagele, B.; Patel, S.S.; et al. Forecasting the Economic Burden of Cardiovascular Disease and Stroke in the United States Through 2050: A Presidential Advisory from the American Heart Association. Circulation 2024, 150, e89–e101. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic burden of cardiovascular diseases in the European Union: A population-based cost study. Eur. Hear. J. 2023, 44, 4752–4767. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Y.; Zhang, L.; Zhou, L.; Li, D.; Quan, H.; Zhu, L.; Hu, F.; Li, X.; Meng, S.; et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J. Am. Hear. Assoc. 2020, 9, e016804. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Godos, J.; Guglielmetti, M.; Ferraris, C.; Frias-Toral, E.; Azpíroz, I.D.; Lipari, V.; Di Mauro, A.; Furnari, F.; Castellano, S.; Galvano, F.; et al. Mediterranean Diet and Quality of Life in Adults: A Systematic Review. Nutrients 2025, 17, 577. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Wyer, S. Peeling back the artichoke leaves: Symbolism and origin stories in Jewish-Roman Cuisine. Food Cult. Soc. 2024, 27, 537–554. [Google Scholar] [CrossRef]
- Sonnante, G.; Pignone, D.; Hammer, K. The domestication of artichoke and cardoon: From Roman times to the genomic age. Ann. Bot. 2007, 100, 1095–1100. [Google Scholar] [CrossRef]
- Pignone, D.; Sonnante, G. Wild artichokes of south Italy: Did the story begin here? Genet. Resour. Crop. Evol. 2004, 51, 577–580. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Gostin, A.I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT 2020, 132, 109883. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Huarte, E.; Serra, G.; Monteagudo-Mera, A.; Spencer, J.; Cid, C.; De Peña, M.P. Raw and Sous-Vide-Cooked Red Cardoon Stalks (Cynara cardunculus L. var. altilis DC): (Poly)phenol Bioaccessibility, Anti-inflammatory Activity in the Gastrointestinal Tract, and Prebiotic Activity. J. Agric. Food Chem. 2021, 69, 9270–9286. [Google Scholar] [CrossRef]
- Lombardo, S.; Scavo, A.; Pandino, G.; Cantone, M.; Mauromicale, G. Improvement in the Cynaropicrin, Caffeoylquinic Acid and Flavonoid Content of Globe Artichokes with Gibberellic Acid Treatment. Plants 2022, 11, 1845. [Google Scholar] [CrossRef]
- Saez, V.; Fasoli, E.; D’Amato, A.; Simó-Alfonso, E.; Righetti, P.G. Artichoke and Cynar liqueur: Two (not quite) entangled proteomes. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 119–126. [Google Scholar] [CrossRef]
- Willey, D. Caravaggio’s Crimes Exposed in Rome’s Police Files. 2011. Available online: https://www.bbc.com/news/world-europe-12497978 (accessed on 21 February 2025).
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) Byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef]
- López-Molina, D.; Navarro-Martínez, M.D.; Melgarejo, F.R.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive Compounds from Cardoon as Health Promoters in Metabolic Disorders. Foods 2022, 11, 336. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; Palma MDe Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive Compounds from Artichoke and Application Potential. Food Technol. Biotechnol. 2023, 61, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Almela, L.; Guardiola, L.; Gabaldón, J.A. Microbiological and sensory quality of fresh ready-to-eat artichoke hearts packaged under modified atmosphere. J. Food Prot. 2017, 80, 740–749. [Google Scholar] [CrossRef]
- Miceli, A.; De Leo, P. Short Communication Extraction, Characterization and Utilization of Artichoke-seed Oil. Bioresour. Technol. 1996, 57, 301–302. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M. State of the art of Cynara cardunculus L. as an energy crop. In Proceedings of the 14th European Conference and Technology Exhibition on Biomass for Energy, Industry and Climate Protection, Paris, France, 17–21 October 2005. [Google Scholar]
- Bravo Bolívar Michael Steven Pasini, F.; Marzocchi, S.; Ravagli, C.; Tedeschi, P. Future Perspective and Technological Innovation in Cheese Making Using Artichoke (Cynara scolymus) as Vegetable Rennet: A Review. Foods 2023, 12, 3032. [Google Scholar] [CrossRef]
- European Medicines Agency. Artichoke leaf. 2018. Available online: www.ema.europa.eu/contact (accessed on 12 February 2025).
- Porro, C.; Benameur, T.; Cianciulli, A.; Vacca, M.; Chiarini, M.; De Angelis, M.; Panaro, M.A. Functional and Therapeutic Potential of Cynara scolymus in Health Benefits. Nutrients 2024, 16, 872. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Rezazadeh, F.; Ebrahimi-Mameghani, M. The effect of artichoke leaf extract supplementation on lipid and CETP response in metabolic syndrome with respect to Taq 1B CETP polymorphism: A randomized placebo-controlled clinical trial. Eur. J. Integr. Med. 2018, 17, 112–118. [Google Scholar] [CrossRef]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytotherapy Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Veronesi, M.; Borghi, C. Short-Term Effects of Dry Extracts of Artichokeand Berberis in Hypercholesterolemic Patients Without Cardiovascular Disease. Am. J. Cardiol. 2018, 123, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mameghani, M.; Asghari-Jafarabadi, M.; Rezazadeh, K. TCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2018, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hemati, N.; Venkatakrishnan, K.; Yarmohammadi, S.; Moradi, M.; Moravejolahkami, A.R.; Hadi, A.; Moradi, S.; Aneva, I.Y.; Farzaei, M.H. The effects of supplementation with Cynara scolymus L. on anthropometric indices: A systematic review and dose-response meta-analysis of clinical trials. Vol. 56, Complementary Therapies in Medicine. Complement. Ther. Med. 2020, 56, 102612. [Google Scholar] [CrossRef]
- Olas, B. An Overview of the Versatility of the Parts of the Globe Artichoke (Cynara scolymus L.), Its By-Products and Dietary Supplements. Nutrients 2024, 16, 599. [Google Scholar] [CrossRef]
- Salem MBen Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef]
- Arnedo-Pena, A.; Puig-Barberà, J.; Bellido-Blasco, J.; Romeu-Garcia, M.A.; Pac-Sa, M.R.; Guillen-Grima, F. Production of vegetables and artichokes is associated with lower cardiovascular mortality: An ecological study. Int. J. Environ. Res. Public Health 2020, 17, 6583. [Google Scholar] [CrossRef]
- Moradi, M.; Sohrabi, G.; Golbidi, M.; Yarmohammadi, S.; Hemati, N.; Campbell, M.S.; Moradi, S.; Kermani, M.A.H.; Farzaei, M.H. Effects of artichoke on blood pressure: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 57, 102668. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Alvani, M.; Shoura, S.M.S.; Sohrabnavi, A.; Heidarian, E.; Hekmatdoost, A. Anti-hypertensive Effects of Artichoke Supplementation in Adults: A Systematic Review and Dose-response Meta-analysis of Randomized Controlled Trials. Clin. Nutr. Res. 2022, 11, 214. [Google Scholar] [CrossRef]
- Phimarn, W.; Sungthong, B.; Wichiyo, K. Effect of Cynara scolymus L. on Cardiometabolic Outcomes: An Updated Meta-analysis of Randomized Controlled Trials and Meta-regression. Pharmacogn. Mag. 2024, 20, 372–388. [Google Scholar] [CrossRef]
- Colombo, R.; Moretto, G.; Pellicorio, V.; Papetti, A. Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties. Foods 2024, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory effects of cynaropicrin and related sesquiterpene lactones from leaves of artichoke (Cynara scolymus L.) on induction of iNOS in RAW264.7 cells and its high-affinity proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Dejonghe, W.; Mariniello, L.; Porta, R. Enzymatic milk clotting activity in artichoke (Cynara scolymus) leaves and alpine thistle (Carduus defloratus) flowers. Immobilization of alpine thistle aspartic protease. Food Chem. 2016, 204, 115–121. [Google Scholar] [CrossRef]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; Russo, M.; Rastrelli, L. Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis 2018, 39, 1899–1907. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crop. Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Cynara cardunculus L., a potential source of inulin in the Mediterranean environment: Screening of genetic variability. Aust. J. Agric. Res. 2004, 55, 693. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Durazzo, A.; Foddai, M.S.; Temperini, A.; Azzini, E.; Venneria, E.; Lucarini, M.; Finotti, E.; Maiani, G.; Crinò, P.; Saccardo, F.; et al. Antioxidant properties of seeds from lines of artichoke, cultivated cardoon and wild cardoon. Antioxidants 2013, 2, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, P.; Quizhpe, J.; de los Rosell, M.Á.; Peñalver, R.; Nieto, G. Antioxidant and Nutritional Potential of Artichoke (Cynara scolymus L.) By-Product Extracts in Fat-Replaced Beef Burgers with Hydrogel Emulsions from Olive Oil. Appl. Sci. 2024, 14, 10123. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Biomass and grain oil yields in Cynara cardunculus L. genotypes grown in a Mediterranean environment. Field Crop. Res. 2006, 101, 187–197. [Google Scholar] [CrossRef]
- Ferracane, R.; Pellegrini, N.; Visconti, A.; Graziani, G.; Chiavaro, E.; Miglio, C.; Fogliano, V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J. Agric. Food Chem. 2008, 56, 8601–8608. [Google Scholar] [CrossRef]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; RéMéSy, C.; Scalbert, A. Nutrient Metabolism Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Dupas, C.; Baglieri, A.M.; Ordonaud, C.; Tomè, D.; Maillard, M.N. Chlorogenic acid is poorly absorbed, independently of the food matrix: A Caco-2 cells and rat chronic absorption study. Mol. Nutr. Food Res. 2006, 50, 1053–1060. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Lucchini, F.; Lucini, L. Polyphenols and sesquiterpene lactones from artichoke heads: Modulation of starch digestion, gut bioaccessibility, and bioavailability following in vitro digestion and large intestine fermentation. Antioxidants 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Müller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [PubMed]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Grande, S.; Bogani, P.; De Saizieu, A.; Schueler, G.; Galli, C.; Visioli, F. Vasomodulating potential of Mediterranean wild plant extracts. J. Agric. Food Chem. 2004, 52, 5021–5026. [Google Scholar] [CrossRef]
- Rossoni, G.; Grande, S.; Galli, C.; Visioli, F. Wild artichoke prevents the age-associated loss of vasomotor function. J. Agric. Food Chem. 2005, 53, 10291–10296. [Google Scholar] [CrossRef]
- Juzyszyn, Z.; Czerny, B.; Pawlik, A.; Drozdzik, M.; Juzyszyn, Z. The Effect of Artichoke (Cynara scolymus L.) Extract on ROS Generation in HUVEC Cells. Phytother. Res. 2008, 22, 1159–1161. [Google Scholar]
- Kim DBin Unenkhuu, B.; Kim, G.J.; Kim, S.W.; Kim, H.S. Cynarin attenuates LPS-induced endothelial inflammation via upregulation of the negative regulator MKP-3. Anim. Cells Syst. 2022, 26, 119–128. [Google Scholar]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Hakkou, Z.; Maciuk, A.; Leblais, V.; Bouanani, N.E.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Rauf, A.; Hadda, T.B.; et al. Antihypertensive and vasodilator effects of methanolic extract of Inula viscosa: Biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed. Pharmacother. 2017, 93, 62–69. [Google Scholar] [CrossRef]
- Thilavech, T.; Abeywardena, M.Y.; Adams, M.; Dallimore, J.; Adisakwattana, S. Naturally occurring anthocyanin cyanidin-3-rutinoside possesses inherent vasorelaxant actions and prevents methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed. Biomed. Pharmacother. 2017, 95, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, H.; Jandaghi, P.; Meraji, A.; Hassanpour Moghadam, M. The Effect of Cynara scolymus on Blood Pressure and BMI in Hypertensive Patients: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Complement. Med. Res. 2019, 27, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gunnett, C.A.; Lund, D.D.; McDowell, A.K.; Faraci, F.M.; Heistad, D.D. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arter. Thromb. Vasc. Biol. 2005, 25, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Bauersachs, J.; Busse, R.; Schini-Kerth, V.B. Inhibition of Inducible Nitric Oxide Synthase Restores Endothelium-Dependent Relaxations in Proinflammatory Mediator-Induced Blood Vessels. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1746–1755. [Google Scholar] [CrossRef]
- Gorzalczany, S.; Moscatelli, V.; Ferraro, G. Artemisia copa aqueous extract as vasorelaxant and hypotensive agent. J. Ethnopharmacol. 2013, 148, 56–61. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhao, J.; Jin, H.T.; Cao, Y.; Ming, T.; Zhang, L.L.; Hu, M.-Y.; Hamlati, H.; Pang, S.-B.; Ma, X.-P. Vasorelaxant effects of the extracts and some flavonoids from the buds of Coreopsis tinctoria. Pharm. Biol. 2013, 51, 1158–1164. [Google Scholar] [CrossRef]
- Jiang, H.; Xia, Q.; Wang, X.; Song, J.; Affiliations, B.; Pharmazie, P.D. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie 2005, 60, 444–447. [Google Scholar]
- Yang, W.; Li, Q.; Duncan, J.W.; Bakrania, B.A.; Bradshaw, J.L.; Granger, J.P.; Rana, S.; Spradley, F.T. Luteolin-induced vasorelaxation in uterine arteries from normal pregnant rats. Pregnancy Hypertens. 2021, 23, 11–17. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Park, Y.S.; Kim, T.J.; Fang, L.H.; Ahn, H.Y.; Hong, J.; Kim, Y.; Lee, C.K.; Yun, Y.-P. Endothelium-dependent vasorelaxant and antiproliferative effects of apigenin. Gen. Pharmacol. Vasc. Syst. 2000, 35, 341–347. [Google Scholar] [CrossRef]
- Jin, B.H.; Qian, L.B.; Chen, S.; Li, J.; Wang, H.-p.; Bruce, I.C.; Lin, J.; Xia, Q. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 2009, 616, 200–205. [Google Scholar] [CrossRef]
- Jing, Y.; Chen, R.; Dong, M.; Liu, Y.; Hou, X.; Guo, P.; Li, W.; Lv, J.; Zhang, M. Apigenin relaxes rat intrarenal arteries, depresses Ca 2+ -activated Cl—currents and augments voltage-dependent K + currents of the arterial smooth muscle cells. Biomed. Pharmacother. 2019, 115, 108926. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; He, D.; Ru, X.; Chen, Y.; Cai, Y.; Bruce, I.C.; Xia, Q.; Yao, X.; Jin, J. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012, 166, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Mastantuono, T.; Battiloro, L.; Sabatino, L.; Chiurazzi, M.; Di Maro, M.; Muscariello, E.; Colantuoni, A.; Lapi, D. Effects of Citrus Flavonoids Against Microvascular Damage Induced by Hypoperfusion and Reperfusion in Rat Pial Circulation. Microcirculation 2015, 22, 378–390. [Google Scholar] [CrossRef]
- Ksiazek, S.H.; Hu, L.; Andò, S.; Pirklbauer, M.; Säemann, M.D.; Ruotolo, C.; Zaza, G.; Manna, G.; De Nicola, L.; Mayer, G.; et al. Renin–Angiotensin–Aldosterone System: From History to Practice of a Secular Topic. Int. J. Mol. Sci. 2024, 25, 4035. [Google Scholar] [CrossRef]
- Villiger, A.; Sala, F.; Suter, A.; Butterweck, V. In vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine 2015, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Recio, I.; Amigo, L. β-Lactoglobulin as source of bioactive peptides. Amino Acids 2007, 35, 257–265. [Google Scholar] [CrossRef]
- Alvarado, C.; Guerra, M. Lactosuero como fuente de péptidos bioactivos. In Anales Venezolanos de Nutrición; Fundación Bengoa: Caracas, Venezuela, 2010; Volume 23, pp. 42–49. [Google Scholar]
- Premaratna, S.D.; Manickam, E.; Begg, D.P.; Rayment, D.J.; Hafandi, A.; Jois, M.; Cameron-Smith, D.; Weisinger, R.S. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int. J. Obes. 2011, 36, 233–243. [Google Scholar] [CrossRef]
- Mellott, E.; Faulkner, J.L. Mechanisms of leptin-induced endothelial dysfunction. Curr. Opin. Nephrol. Hypertens. 2022, 32, 118–123. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 2–12. [Google Scholar] [CrossRef]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Roscini, A.R.; Trinca, F.; Gemelli, F.; Vaudo, G.; Mannarino, E. Artichoke juice improves endothelial function in hyperlipemia. Life Sci. 2004, 76, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® supplement containing chlorogenic acid and luteolin improved hepatic and cardiometabolic parameters in subjects with metabolic syndrome: A 6 month randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Amato, A.; Magán-Fernández, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Chlorogenic Acid and Luteolin Improves Cardiometabolic Parameters in Subjects with Pre-obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- Maurotti, S.; Pujia, R.; Ferro, Y.; Mare, R.; Russo, R.; Coppola, A.; Gazzaruso, C.; Montalcini, T.; Pujia, A.; Paone, S.; et al. A nutraceutical with Citrus bergamia and Cynara cardunculus improves endothelial function in adults with non-alcoholic fatty liver disease. Nutrition 2023, 118, 112294. [Google Scholar] [CrossRef]
- Fogacci, F.; Giovannini, M.; Di Micoli, A.; Fiorini, G.; Grandi, E.; Borghi, C.; Cicero, A.F.G. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial on the Effect of a Dietary Supplement Containing Dry Artichoke and Bergamot Extracts on Metabolic and Vascular Risk Factors in Individuals with Suboptimal Cholesterol Levels. Nutrients 2024, 16, 1587. [Google Scholar] [CrossRef]
- Hall, M.E.; Cohen, J.B.; Ard, J.D.; Egan, B.M.; Hall, J.E.; Lavie, C.J.; Ma, J.; Ndumele, C.E.; Schauer, P.R.; Shimbo, D.; et al. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2021, 78, E38–E50. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. Heart J. 2024, 45, 4063–4098. [Google Scholar] [CrossRef]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Kamkhah, A.F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet. Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The Effect of Artichoke Leaf Extract on Alanine Aminotransferase and Aspartate Aminotransferase in the Patients with Nonalcoholic Steatohepatitis. Int. J. Hepatol. 2016, 2016, 4030476. [Google Scholar] [CrossRef]
- Daniels, S.R.; Kimball, T.R.; Khoury, P.; Witt, S.; Morrison, J.A. Correlates of the hemodynamic determinants of blood pressure. Hypertension 1996, 28, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Borghi, C.; Rizzoli, E.; Giovannini, M.; Bove, M.; D’addato, S.; Borghi, C.; Cicero, A.F. Effect of Dietary Supplementation with Eufortyn® Colesterolo Plus on Serum Lipids, Endothelial Reactivity, Indexes of Non-Alcoholic Fatty Liver Disease and Systemic Inflammation in Healthy Subjects with Polygenic Hypercholesterolemia: The ANEMONE Study. Nutrients 2022, 14, 2099. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytotherapy Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.Y.; Tu, J.F.; Ding, Y.H.; Pang, J.; Che XDa Zou, H.; Huang, D.S. Association of blood pressure level with nonalcoholic fatty liver disease in nonhypertensive population Normal is not the new normal. Medicine 2016, 95, e4293. [Google Scholar] [CrossRef]
- Shariq, O.A.; Mckenzie, T.J. Obesity-related hypertension: A review of pathophysiology, management, and the role of metabolic surgery. Gland. Surg. 2020, 9, 80–93. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Community herbal monograph on Cynara scolymus L., folium F. 2011. Available online: www.ema.europa.eu (accessed on 12 February 2025).
- Schulz, V.; Hänsel, R.; Blumenthal, M.; Tyler, V.E. Rational Phytotherapy; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]

| Part | Compounds |
|---|---|
| Bracts | Fibers (cellulose, hemicellulose, lignin, and inulin) Proteins Phenolic acids—CQAs (e.g., chlorogenic acid) and diCQAs (e.g., cynarin) Flavones (e.g., luteolin, apigenin) Terpenoids—sesquiterpene lactones (e.g., cynaropicrin) |
| Stems | Fibers (cellulose, hemicellulose, lignin, inulin, pectin, gums, β-glucans) Peroxidase enzymes Proanthocyanidins Terpenoids (sesquiterpene lactones) |
| Residual leaves | Fibers (cellulose, hemicellulose, lignin, inulin, pectin, gums, β-glucans) Proteolytic enzymes (cardosins, cyprosins) Flavanols (proanthocyanidins) Terpenoids—sesquiterpene lactones Phenolic acids (CQAs, diCQAs) |
| Stalks | Complex carbohydrates (cellulose, hemicellulose, and lignin) |
| Roots | Fibers (e.g., inulin) |
| Seeds | Proteins Polyunsaturated and monounsaturated fatty acids (e.g., linoleic and linolenic acids) Phenolic acids (CQAs and diCQAs) Terpenoids (e.g., tocopherols) Phytosterols (e.g., β-sitosterol, campesterol, 5-stigmasterol) Minerals (calcium, potassium, magnesium, manganese, iron, sodium and zinc) |
| Authors (Year) | Population | Artichoke Product | Intervention | Main Results |
|---|---|---|---|---|
| Lupatelli et al. (2004) [96] | 28 hyperlipidemic subjects (TG: N = 18, 53 y.o.; CG: N = 10, 55 y.o.) | Artichoke leaf juice | 20 mL/day for 6 weeks | Significant increase in FMD when compared with the control group |
| Castellino et al. (2019) [97] | 100 subjects with metabolic syndrome (TG: N = 50, 63 y.o.; CG: N = 50, 63 y.o.) | Nutraceutical containing extracts of two artichoke species (Altilix®, Belpasso, Italy) | 150 mg/day for 6 months | Significant increase in FMD when compared with the control group |
| Terzo et al. (2023) [98] | 50 subjects with pre-obesity (TG: N = 28; CG: N = 22; undisclosed ages) | Nutraceutical containing extracts of two artichoke species (Altilix®, Belpasso (CT), Italy) | 150 mg/day for 6 months | Significant increase in FMD when compared with the control group |
| Maurotti et al. (2024) [99] | 32 subjects with non-alcoholic liver steatosis (TG: N = 16, 51 y.o.; CG: N = 16, 52 y.o.) | Supplement (300 mg) containing artichoke extract and bergamot polyphenol fraction (Bergacyn®, Bianco, Italy) | 1 capsule/day for 3 months | Significant increase in the reactive hyperemia index when compared with the control group |
| Fogacci et al. (2024) [100] | 90 subjects with hypercholesterolemia (TG: N = 45, 46.2 y.o.; CG: N = 45, 47 y.o.) | Nutraceutical containing a bergamot extract (1000 mg), two artichoke extracts (120 mg), coenzyme Q10 (5 mg) and zinc (5 mg) (Eufortyn® Colesterolo Plus, Milan, Italy) | 1 tablet/day for 2 months | Significant increase in endothelial reactivity when compared with the control group |
| Authors (Year) | Population | Artichoke Product | Intervention | Main Results |
|---|---|---|---|---|
| Cicero et al. (2019a) [35] | 40 subjects with pre-hypertension and dyslipidemia (TG: N = 20, 54 y.o.; CG: N = 20, 52 y.o.) | Dry extract of artichoke and Indian barberry (undisclosed composition) | 1 tablet/day for 2 months | No significant change in SBP, DBP or BMI in either group or between groups |
| Cicero et al. (2019b) [108] | 90 subjects with pre-hypertension and overweight (TG low dose: N = 30, 43 y.o.; TG high dose: N = 30, 45 y.o.; CG: N = 30, 44 y.o.) | Nutraceutical containing standardized bergamot extract [120 mg flavonoids), artichoke extract (2 mg 5-O-caffeoylquinic acid), 120 mg phytosterols and 20 mg vitamin C] | 1 tablet/day (low dose) or 2 tablets/day (high dose) for 6 months | No significant change in SBP, DBP or BMI in either group or between groups |
| Roghani-Dehkordi & Kamkhah (2009) [104] | 107 male subjects with stage 1 hypertension (TG 50 mg: N = 44 y.o.; TG 100 mg: N = 35, 44 y.o.; CG: N = 33, 44 y.o.) | Extract of concentrated artichoke leaf juice (50 mg or 100 mg) | 2 tablets/day for 3 months | Significant reduction in SBP and DBP when compared with the control group. No significant difference in BMI when compared with the control group. |
| Ardalani et al. (2018) [76] | 40 subjects with stage 1–2 hypertension and overweight/obesity medicated with captopril (TG: N = 20, 58 y.o.; CG: N = 20, 56 y.o.) | Artichoke leaf extract (500 mg) | 2 capsules/day for 2 months | Significant reduction in SBP in both treatment and control groups with no significant differences between groups. Significant reduction in BMI in the treatment group when compared with the control group. |
| Fogacci et al. (2022) [107] | 56 subjects (TG: N = 28, 54 y.o.; CG: N = 28, 54 y.o.) | Nutraceutical containing a bergamot extract (1000 mg), two artichoke extracts (120 mg), coenzyme Q10 (5 mg) and zinc (5 mg) (Eufortyn® Colesterolo Plus, Milan, Italy) | 1 tablet/day for 2 months | No significant change in SBP, DBP or BMI between treatment and control groups. Significant increase in endothelial reactivity index when compared to the control group. Significant reduction in waist circumference when compared to the control group. |
| Ferro et al. (2020) [103] | 86 subjects with NAFLD (TG: N = 45, 53 y.o.; CG: N = 41, 51 y.o.) | Nutraceutical (300 mg) containing bergamot polyphenolic fraction, wild thistle extract, PUFA, bergamot pulp and albedo derivative | 1 capsule /day for 3 months | No significant change in SBP or DBP in either group or between groups. Significant reduction in body weight and BMI in the treatment and control groups. Significant reduction when compared with the control group. |
| Panahi et al. (2018) [34] | 89 subjects with NAFLD (TG: N = 49, 45.2 y.o.; CG: N = 40, 47.2 y.o.) | Artichoke leaf extract (200 mg; Cynarol®, Brussels, Belgium, standardized to contain 2 mg cynarin) | 3 tablets/day for 2 months | Significant increase in SBP when compared with the control group. Significant decrease in BMI when compared with the control group. |
| Maurotti et al. (2024) [99] | 32 subjects with non-alcoholic liver steatosis (TG: N = 16, 51 y.o.; CG: N = 16, 52 y.o.) | Supplement (300 mg) containing artichoke extract and bergamot polyphenol fraction | 1 capsule/day for 3 months | No significant change in SBP, DBP or BMI between groups. |
| Rangboo et al. (2016) [105] | 60 subjects with NASH (TG: N = 30, 47 y.o.; CG: N = 30, 49 y.o.) | Artichoke leaf extract | 2700 mg/day (6 tablets) for 2 months | Significant reduction in SBP and body weight in treatment and control groups. No significant reduction in DBP in either group. No significant change in SBP or body weight between groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.; Daia, A.M. Exploring the Cardiovascular Potential of Artichoke—A Comprehensive Review. Biology 2025, 14, 397. https://doi.org/10.3390/biology14040397
Silva H, Daia AM. Exploring the Cardiovascular Potential of Artichoke—A Comprehensive Review. Biology. 2025; 14(4):397. https://doi.org/10.3390/biology14040397
Chicago/Turabian StyleSilva, Henrique, and Avina Mahendra Daia. 2025. "Exploring the Cardiovascular Potential of Artichoke—A Comprehensive Review" Biology 14, no. 4: 397. https://doi.org/10.3390/biology14040397
APA StyleSilva, H., & Daia, A. M. (2025). Exploring the Cardiovascular Potential of Artichoke—A Comprehensive Review. Biology, 14(4), 397. https://doi.org/10.3390/biology14040397






