Storytelling of Myocardial Biopsy
Simple Summary
Abstract
1. Introduction: History of Cardiac Biopsy
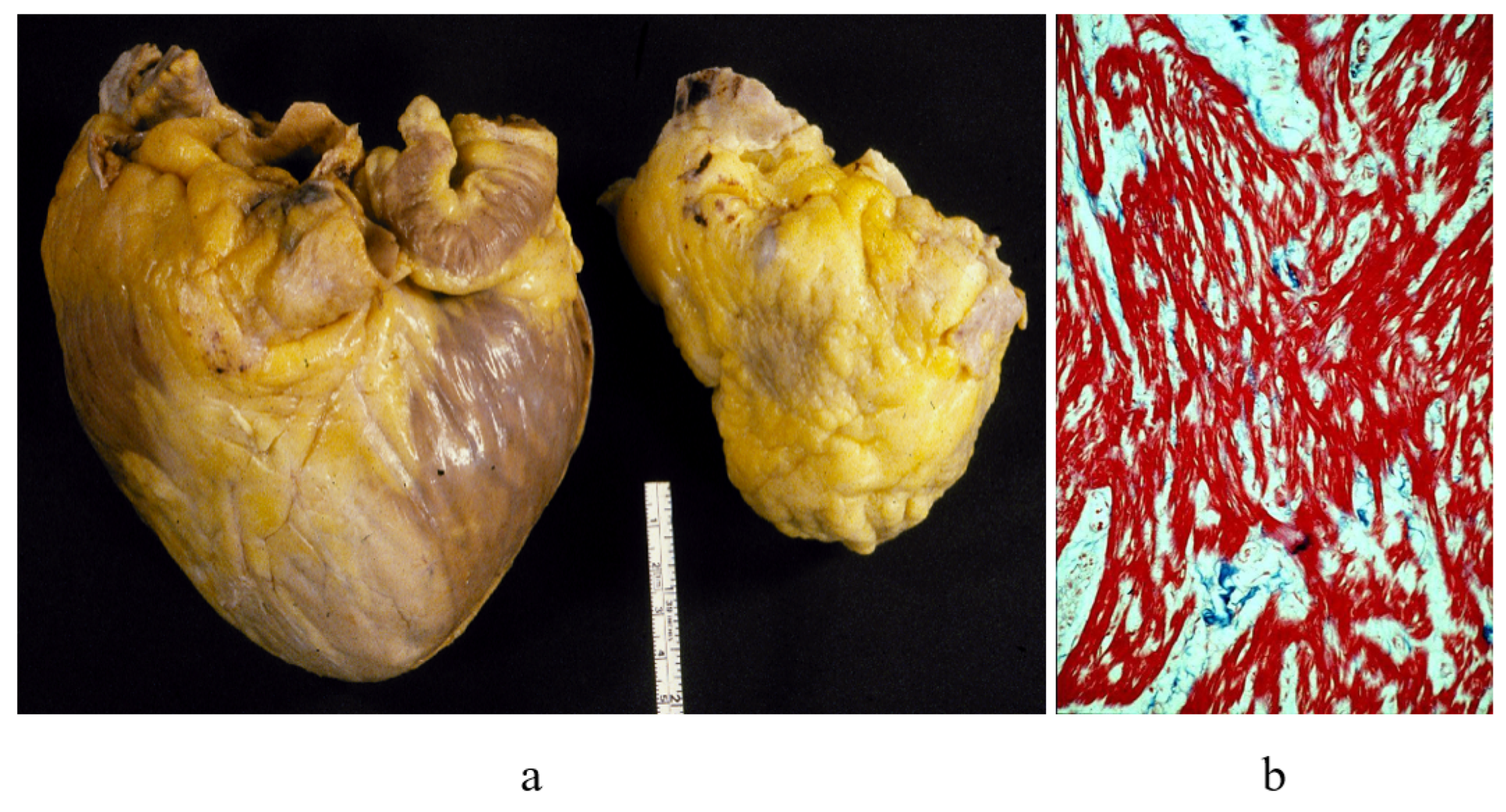
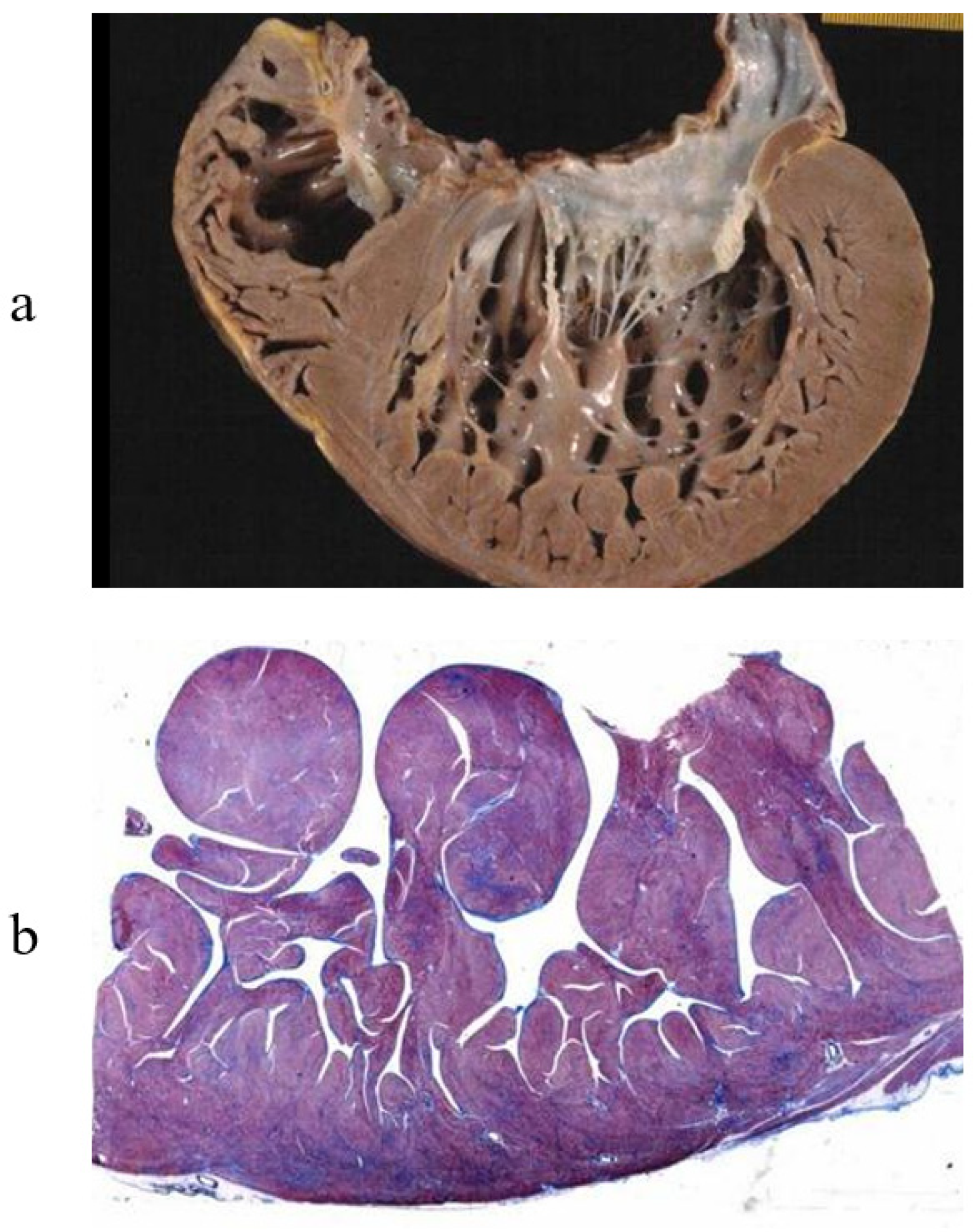
2. Cardiac Transplantation and Cardiomyopathies
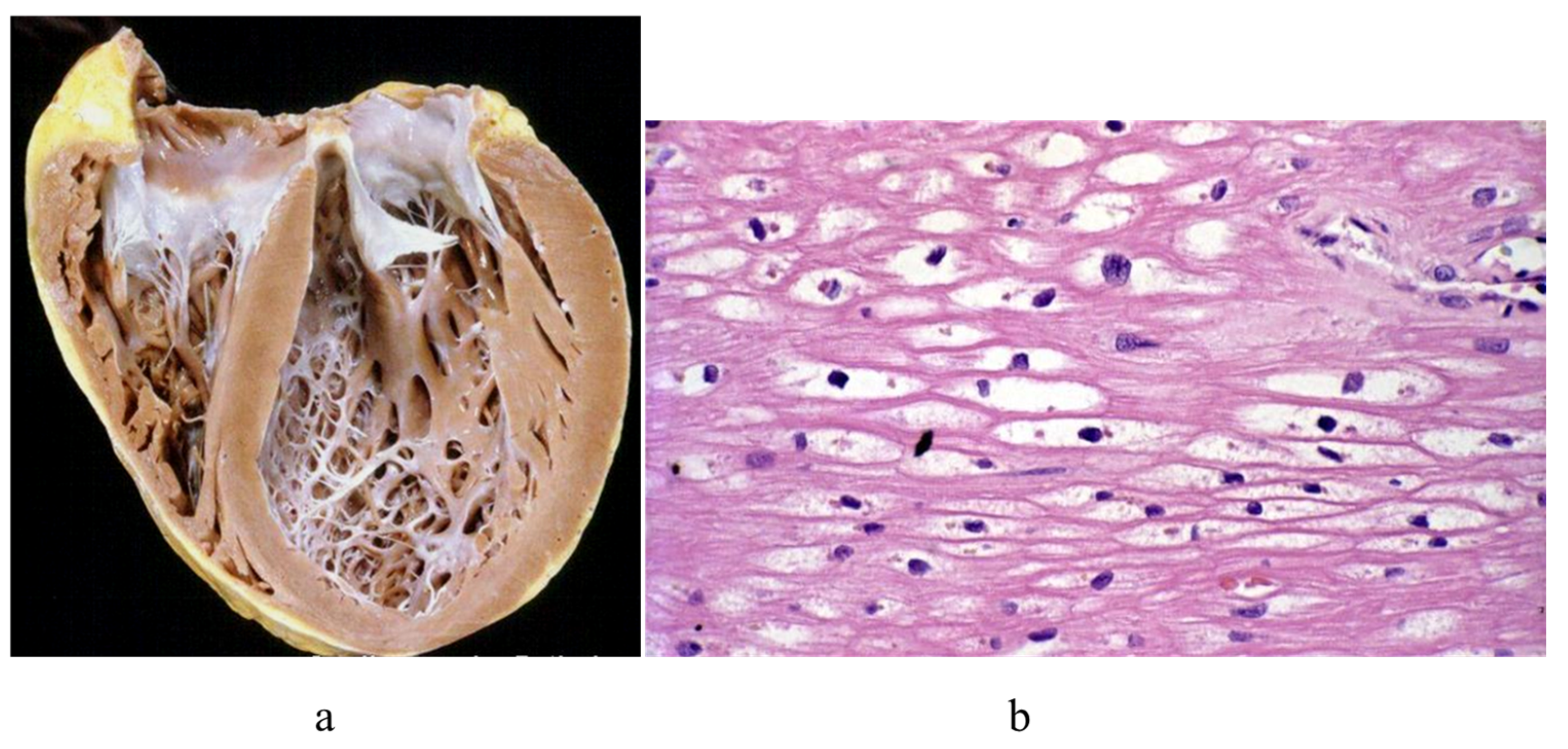
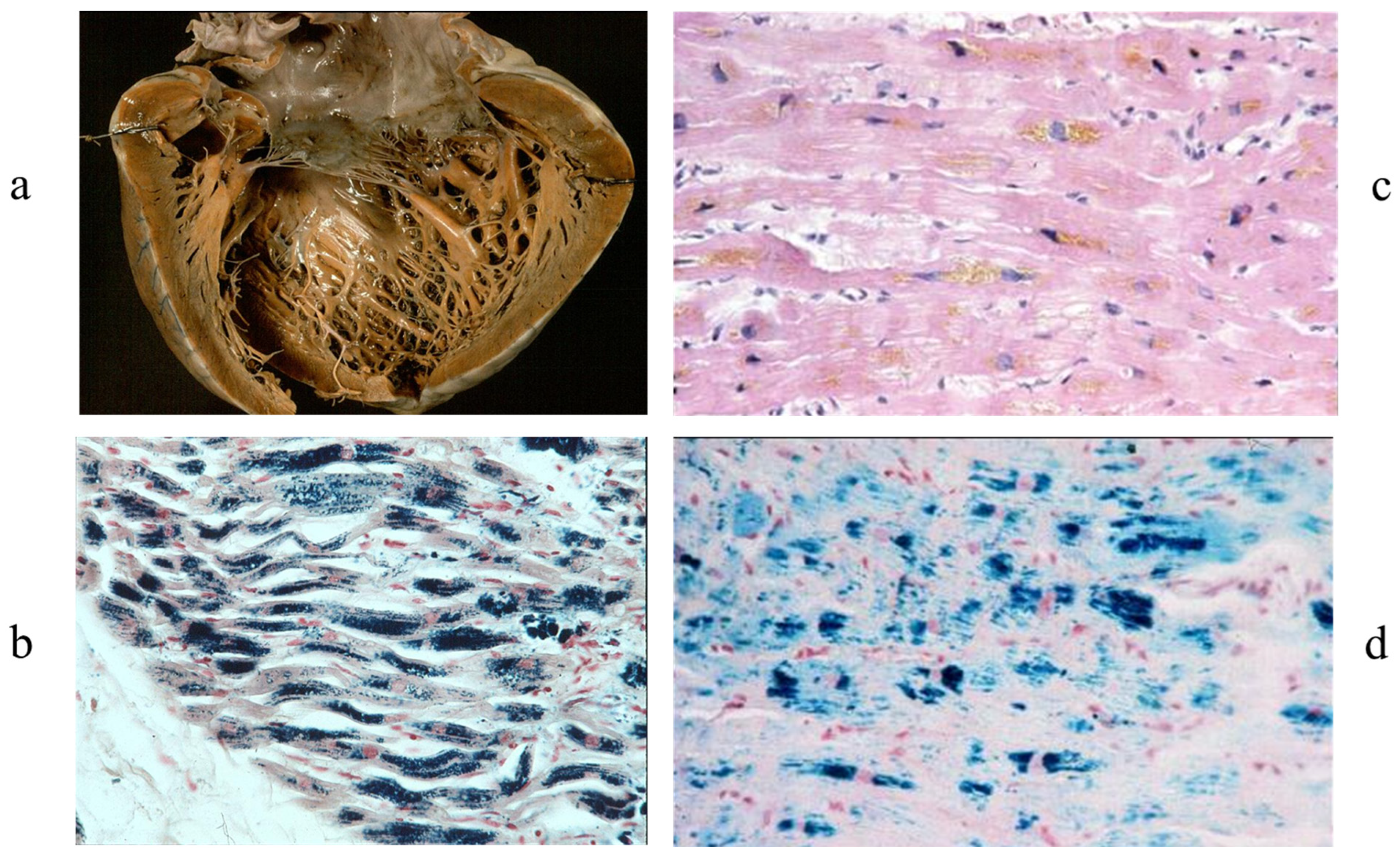
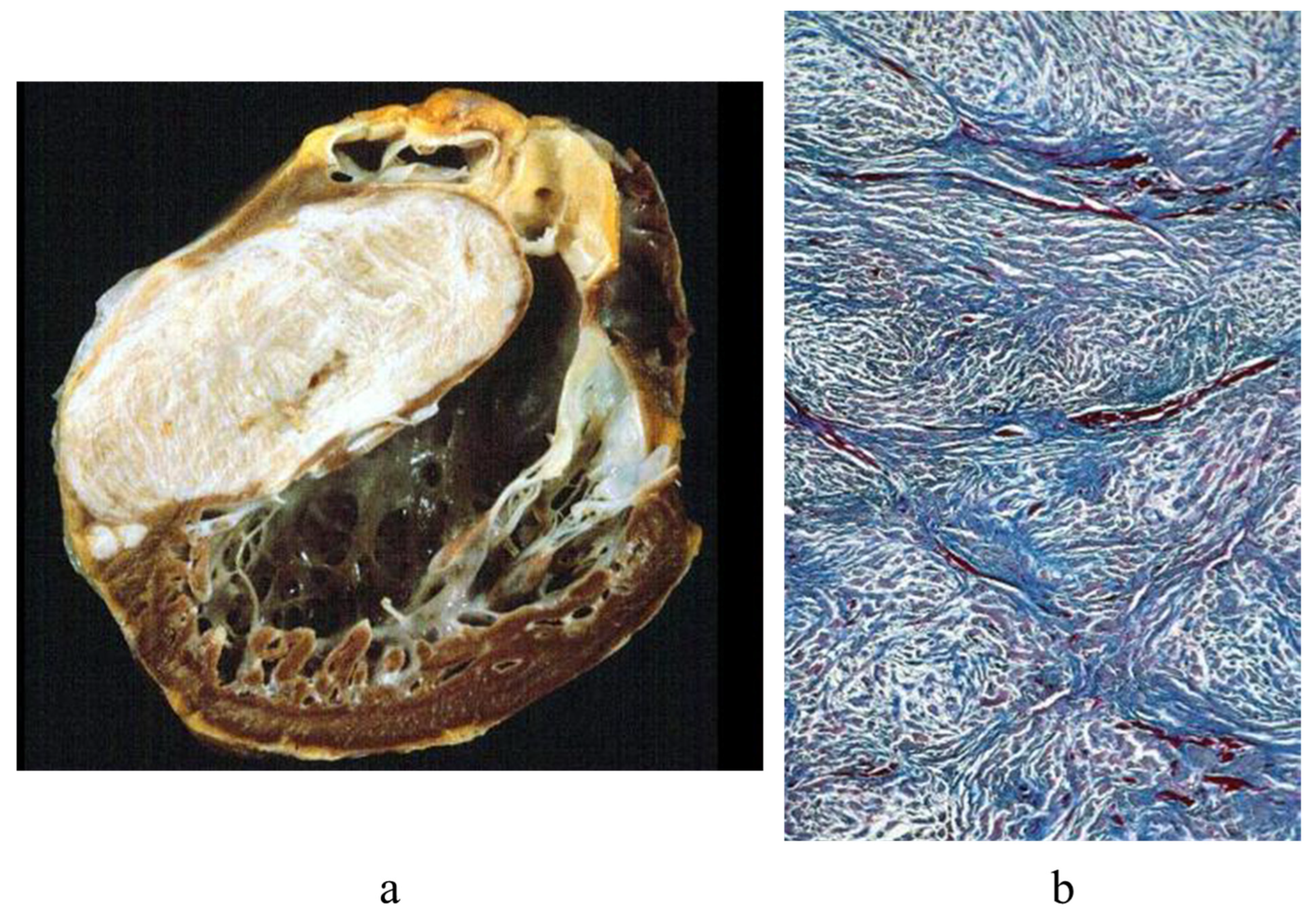
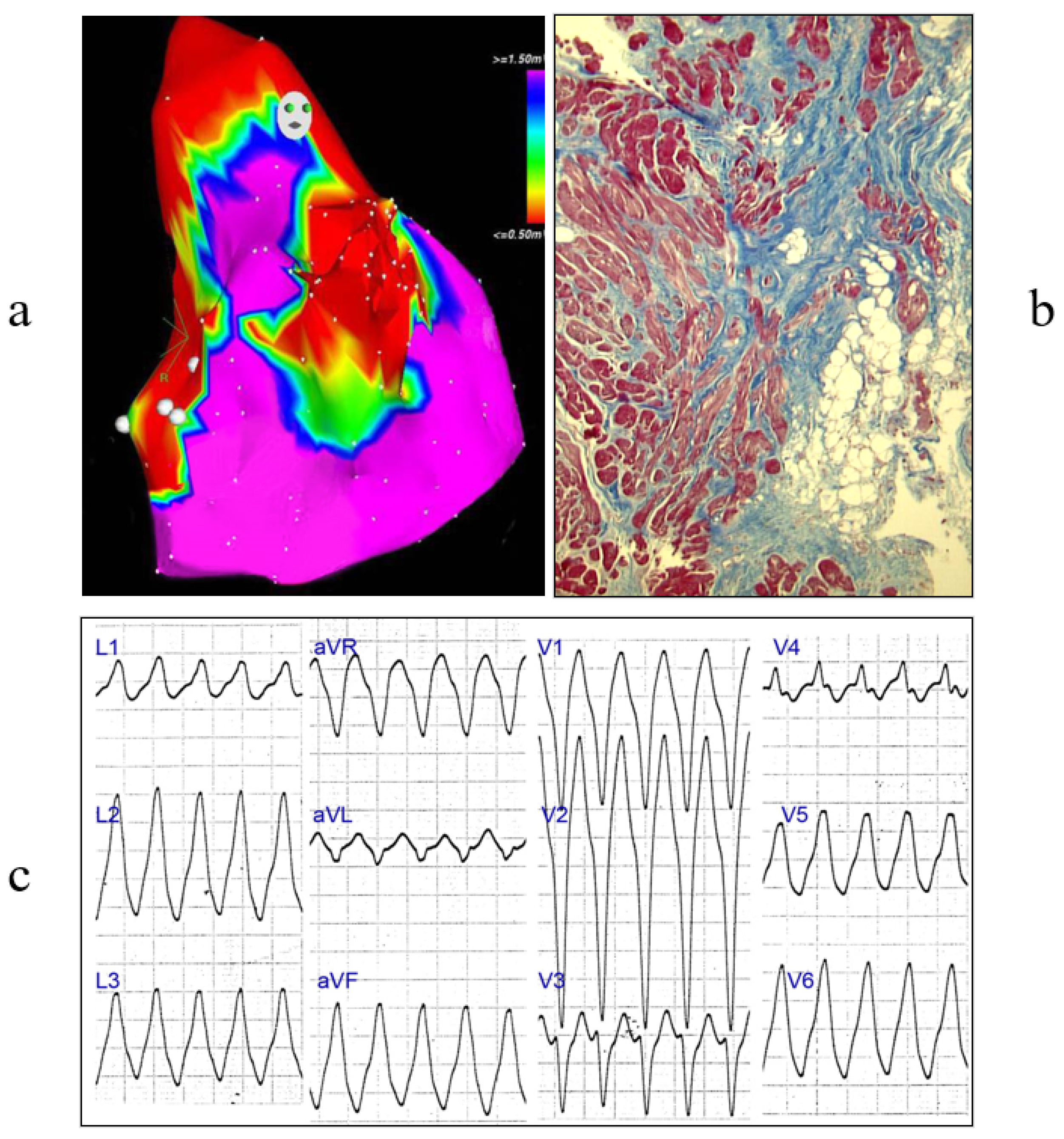
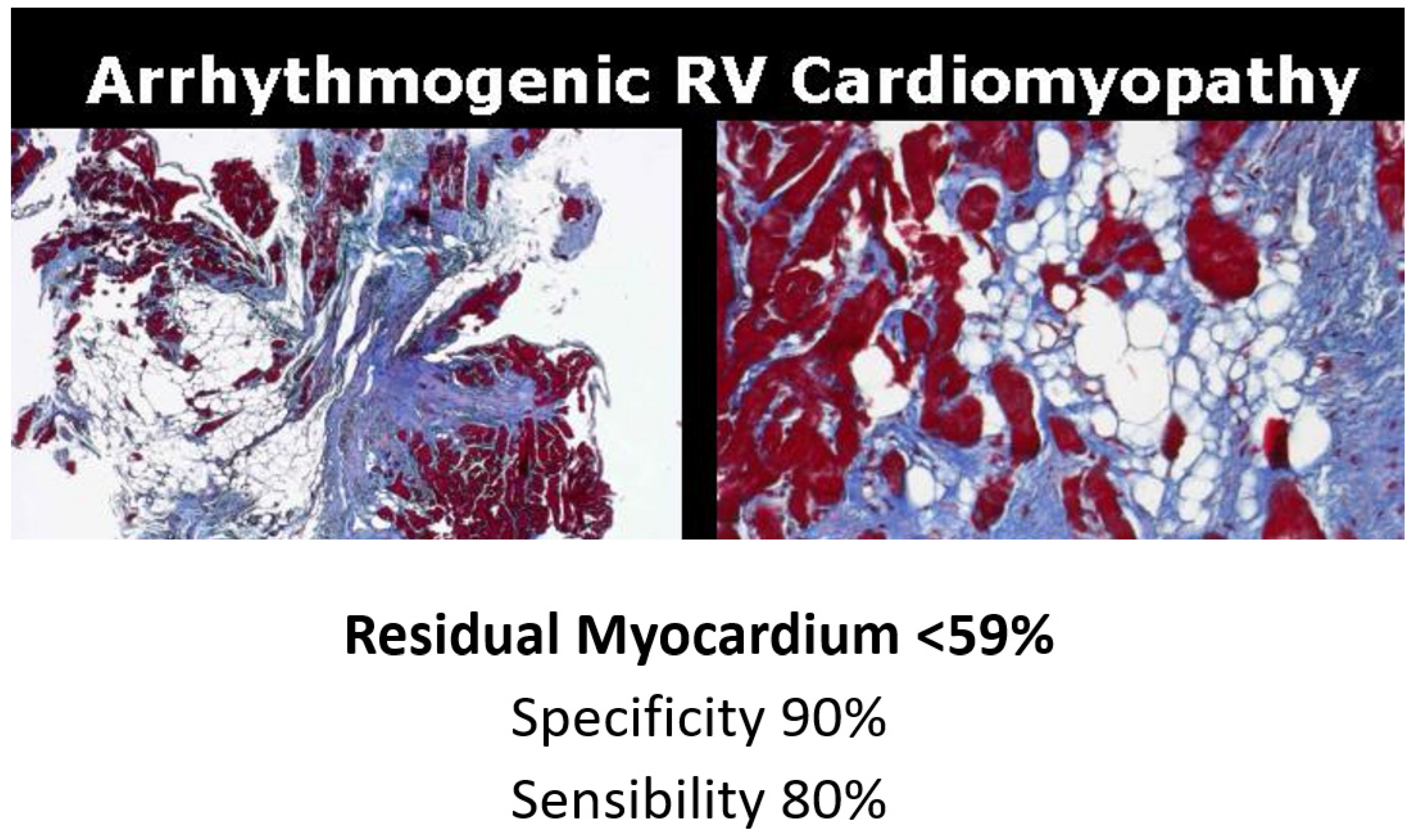
3. Arrhythmogenic Cardiomyopathy
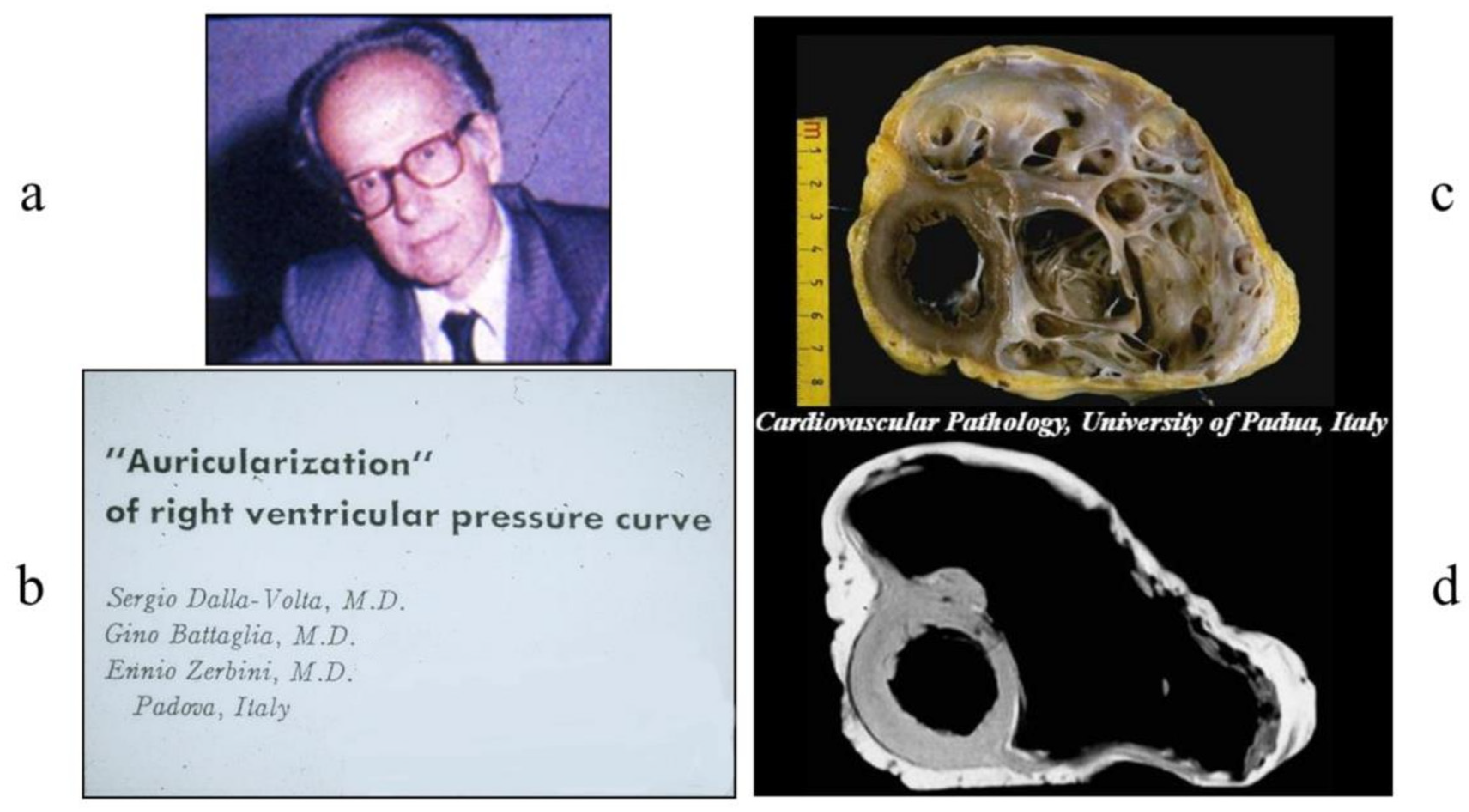
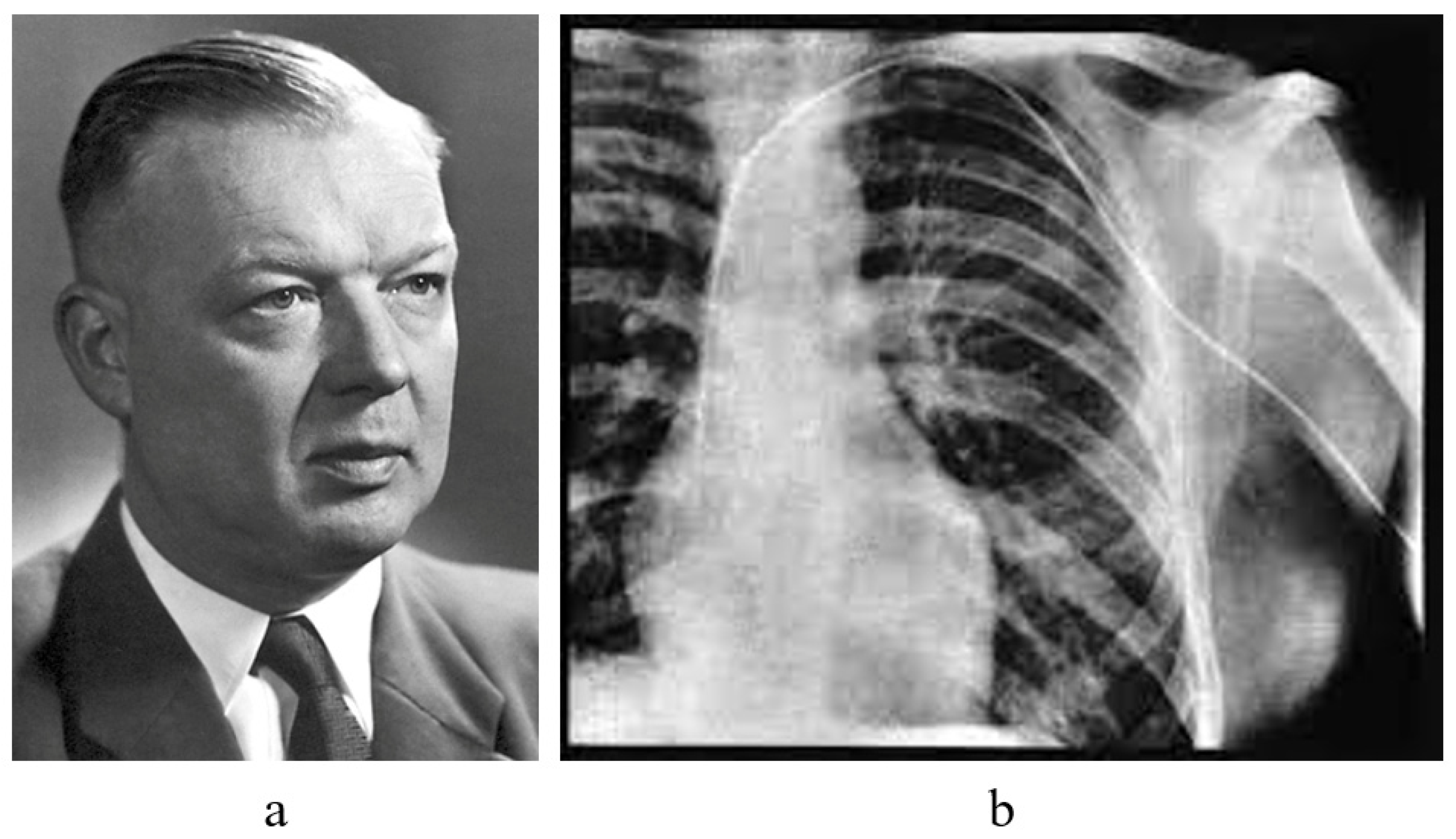
4. History of Cardiac Catheterization
5. The Discovery of Microscope
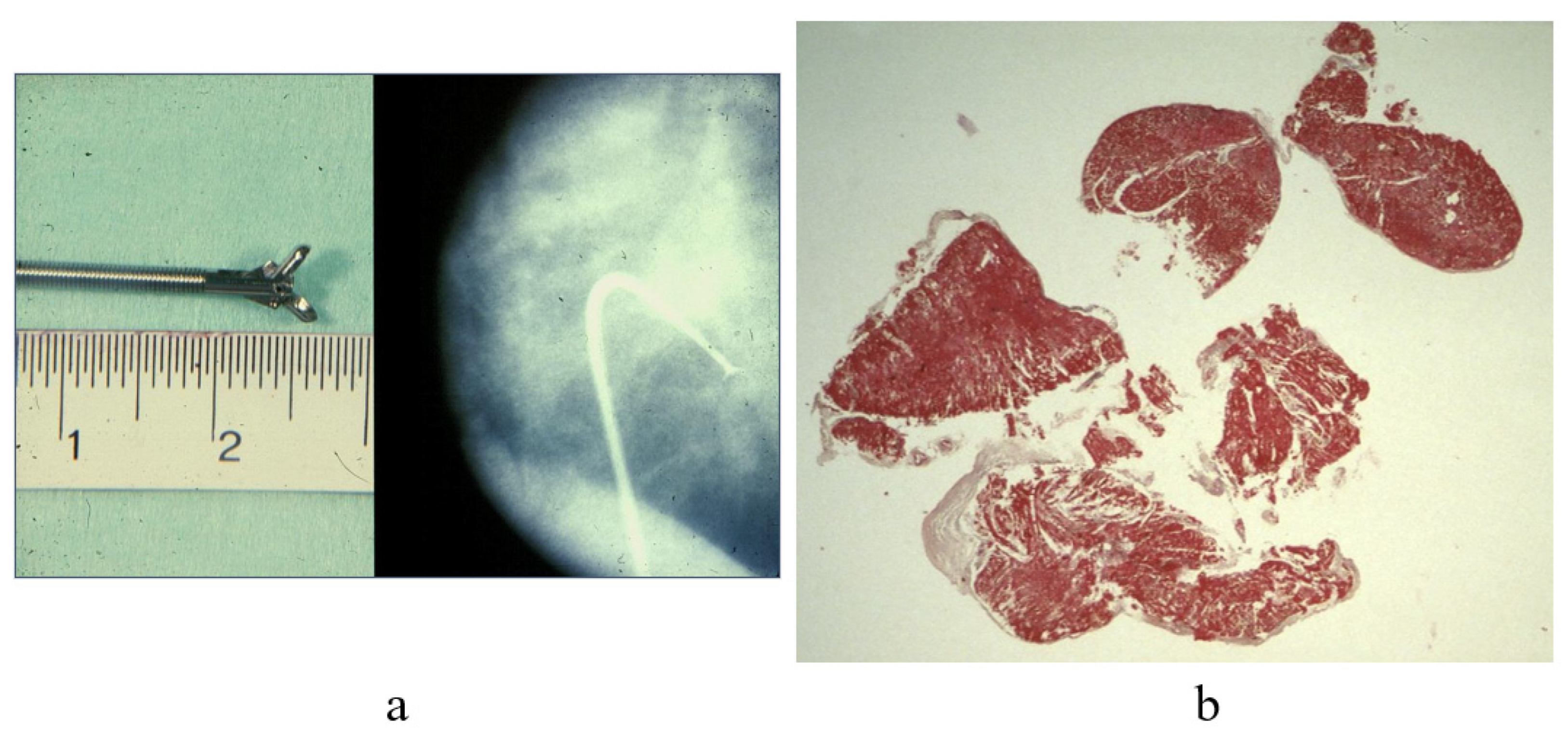
6. Monitoring of Cardiac Transplant Rejection by Endomyocardial Biopsy
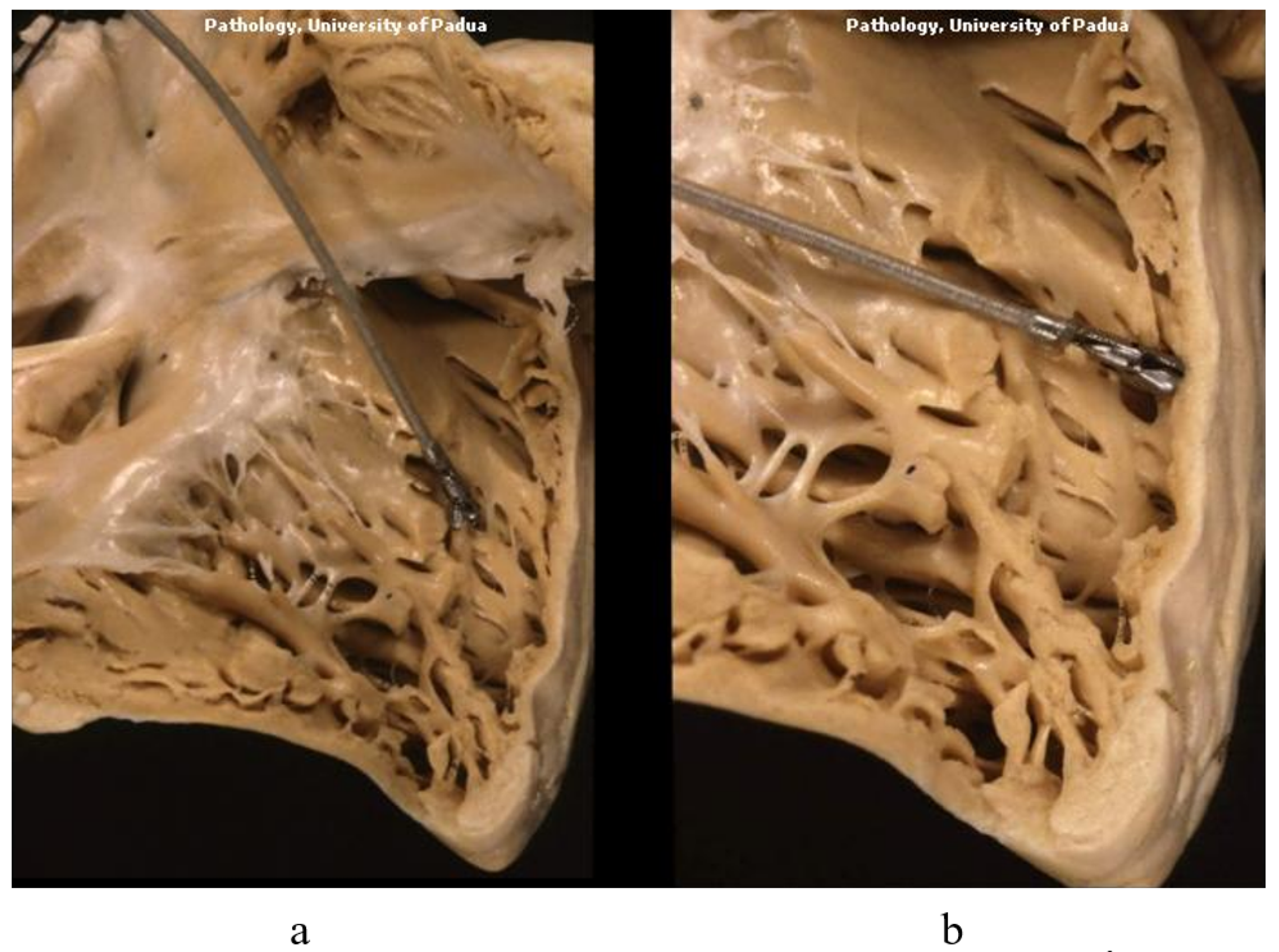
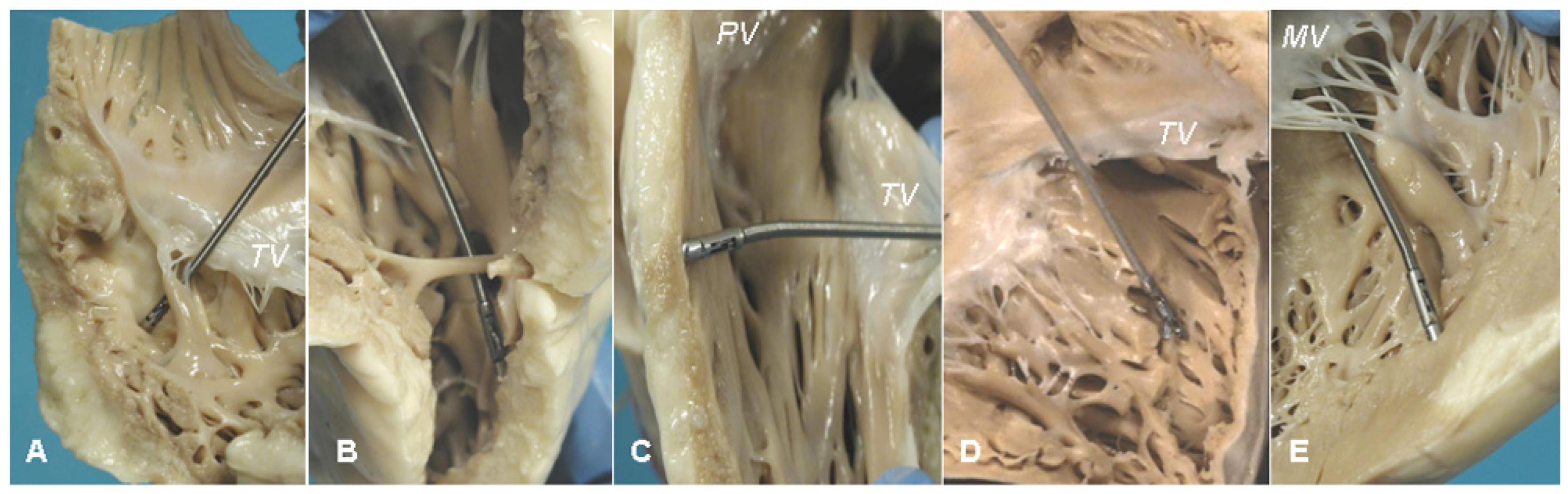
7. Clinical Indications of Endomyocardial Biopsy
8. The Invention of Polymerase Chain Reaction: Molecular Pathology
9. Diagnosis of ACM Through Endomyocardial Biopsy and Infiltrative Disease as Amyloidosis
10. EMB and Electron Microscopy
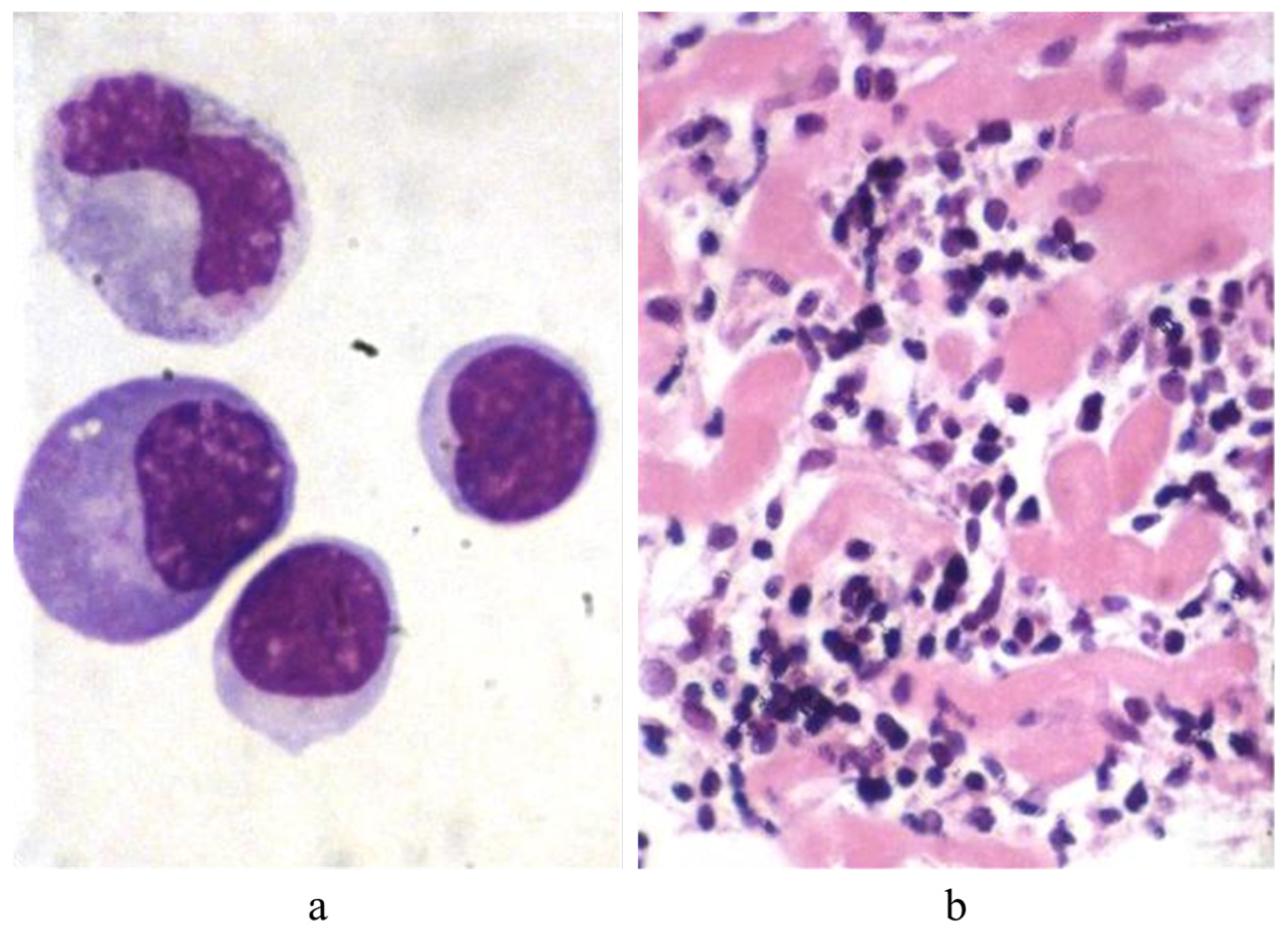
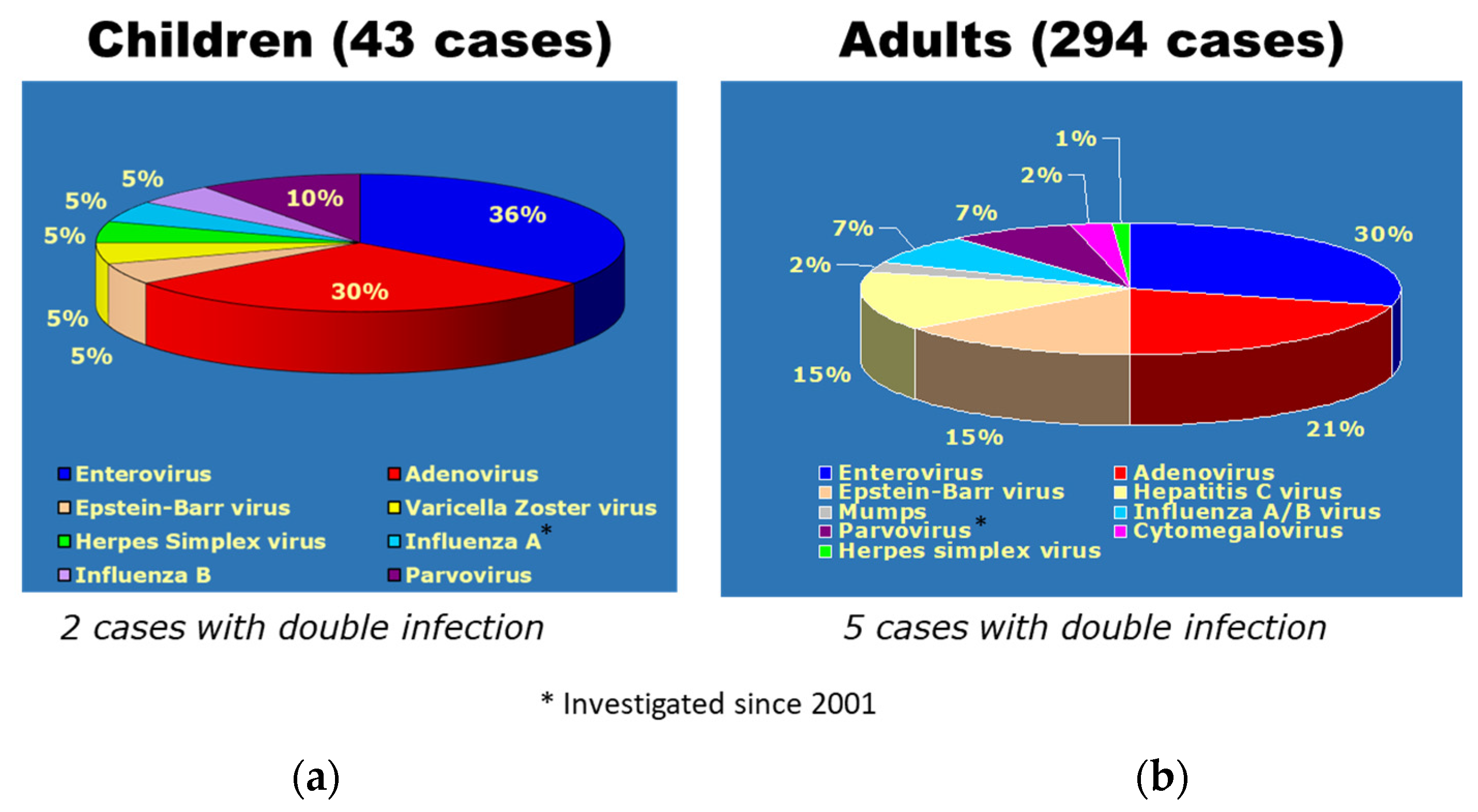
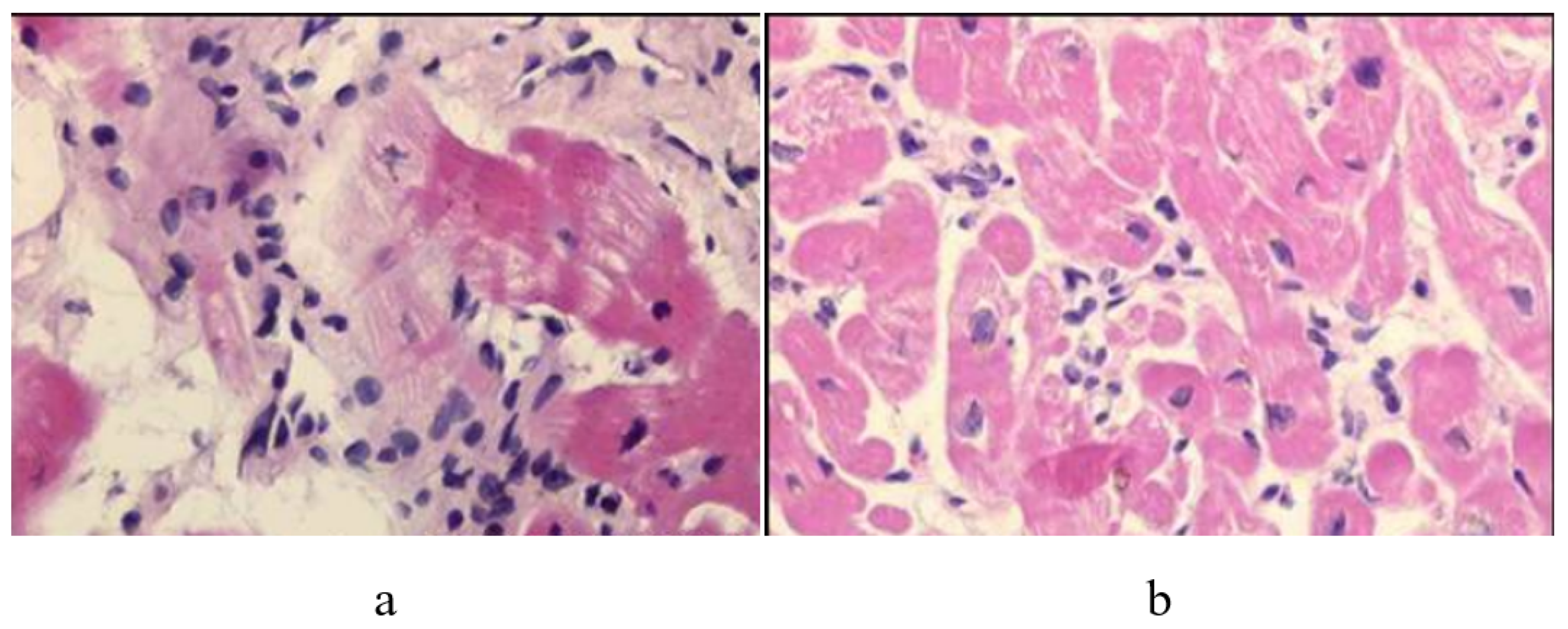
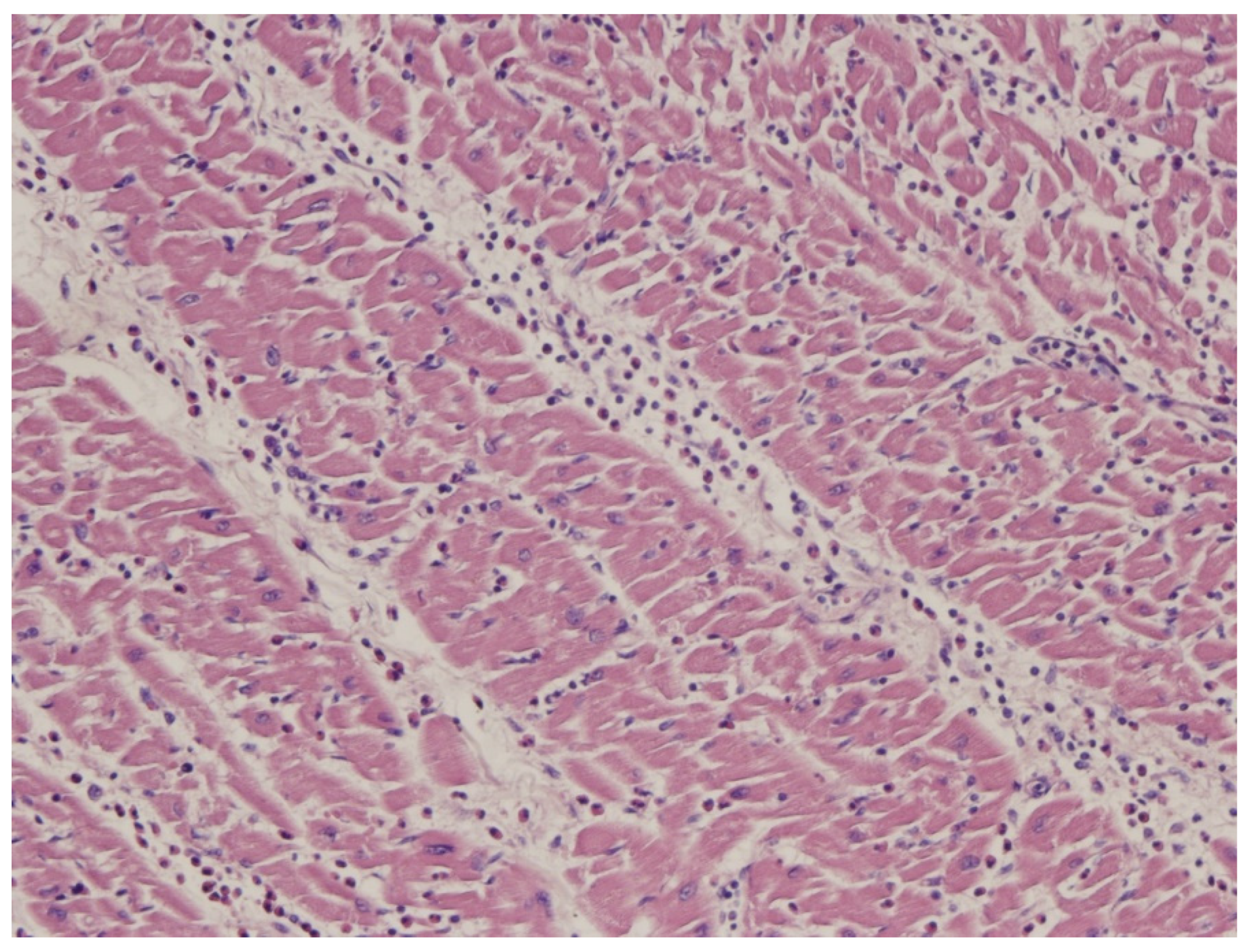
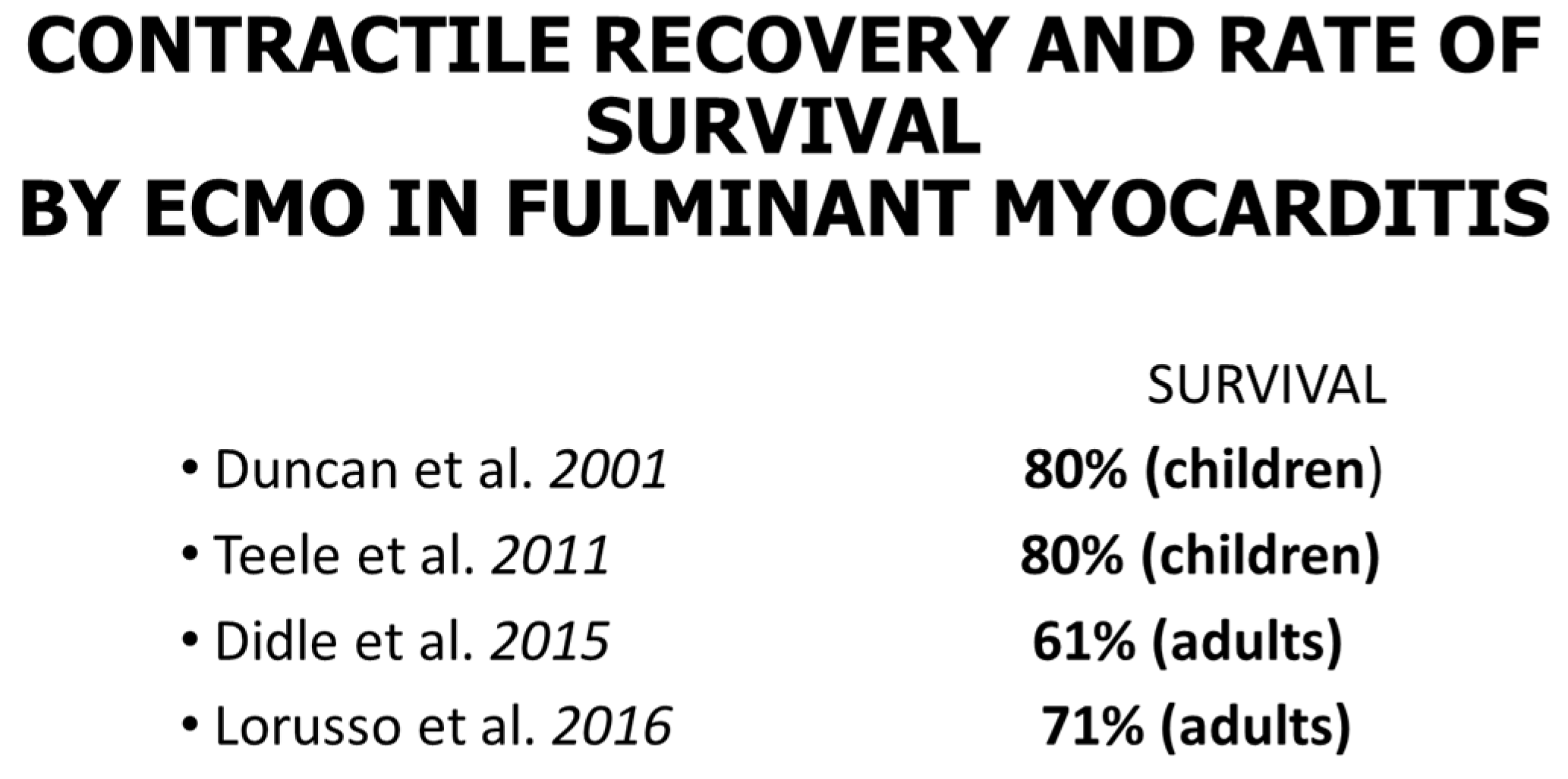
11. Endomyocardial Biopsy and Myocarditis: The Invention of Extracorporeal Membrane Oxygenation and Its Efficacy
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACM | Arrhythmogenic right ventricular cardiomyopathy. |
| DCM | Dilated cardiomyopathy. |
| ECMO | Extracorporeal membrane oxygenation. |
| EMB | Endomyocardial biopsy. |
| HCM | Hypertrophic cardiomyopathy. |
| MCR | Magnetic Cardiac Resonance. |
| PCR | Polymerase chain reaction. |
| RCM | Restrictive cardiomyopathy. |
| RV | Right ventricle. |
References
- Casten, G.G.; Marsh, J.B. Metabolic Studies on Cardiac Tissue Obtained by Needle Biopsy in the Intact Unanesthetized Dog. Circ. Res. 1953, 1, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Kent, G.; Sutton, D.C.; Sutton, G.C. Needle Biopsy of the Human Ventricular Myocardium. Q. Bull. Northwest Univ. Med. Sch. 1956, 30, 213–214. [Google Scholar]
- Sakakibara, S.; Konno, S. Endomyocardial Biopsy. Jpn. Heart J. 1962, 3, 537–543. [Google Scholar] [CrossRef]
- Richardson, P.J. King’s Endomyocardial Bioptome. Lancet 1974, 1, 660–661. [Google Scholar] [CrossRef]
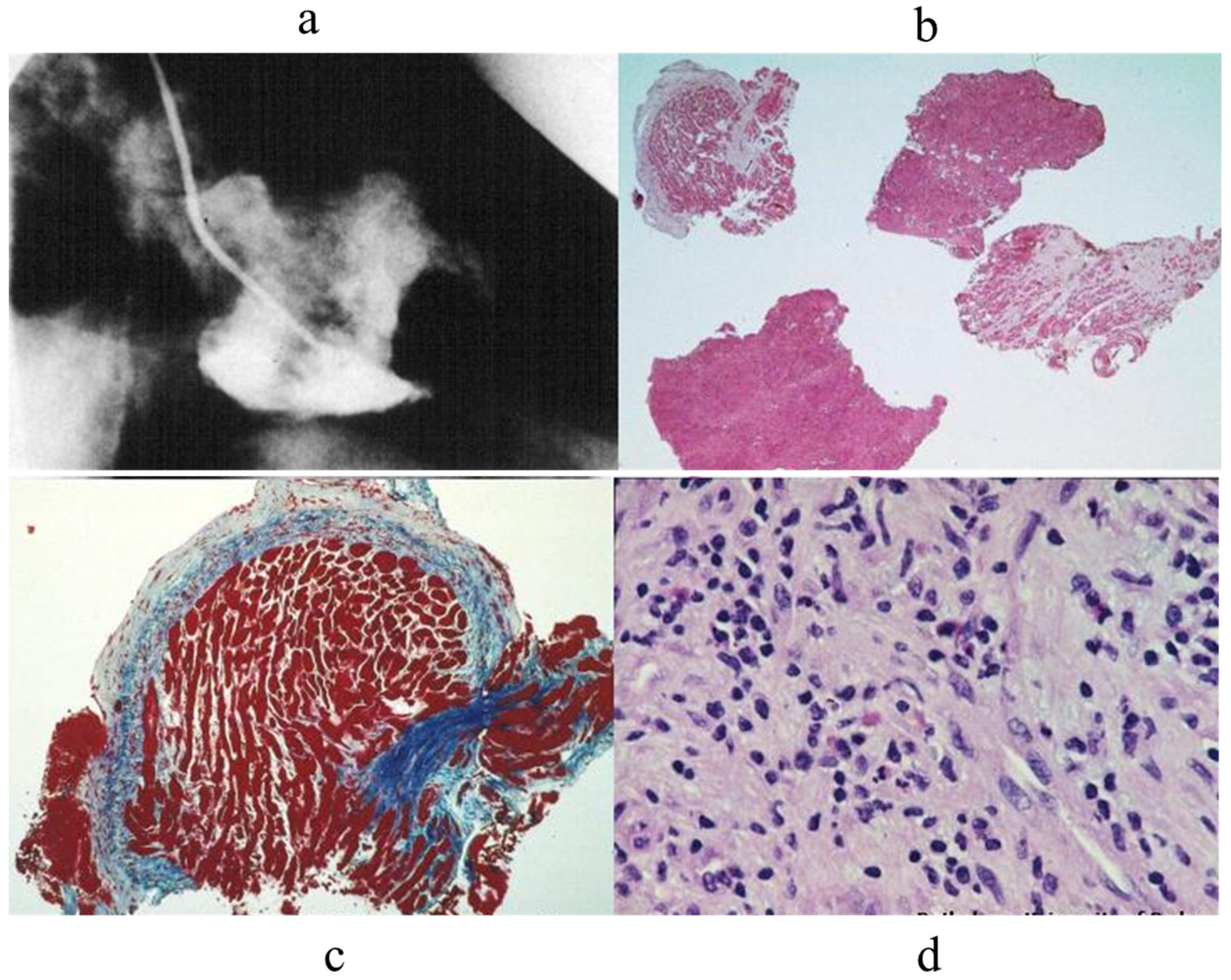
- Valente, M.; Cocco, P.; Thiene, G.; Casula, R.; Poletti, A.; Milanesi, O.; Fasoli, G.; Livi, U. Cardiac Fibroma and Heart Transplantation. J. Thorac. Cardiovasc. Surg. 1993, 106, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Dalla Volta, S.; Battaglia, G.; Zerbini, E. ‘Auricularization’ of Right Ventricular Pressure Curve. Am. Heart J. 1961, 61, 25–33. [Google Scholar] [CrossRef]
- Forssmann, W. Die Sondierung des rechten Herzens. Klin. Wochenschr. 1929, 8, 2085–2087. [Google Scholar] [CrossRef]
- Hooke, R. Micrographia, or, Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses with Observations and Inquiries Thereupon; Martyn, J., Allestry, J., Eds.; Royal Society: London, UK, 1665. [Google Scholar]
- Ferrans, V.J.; Roberts, W.C. Myocardial Biopsy: A Useful Diagnostic Procedure or Only a Research Tool? Am. J. Cardiol. 1978, 41, 965–967. [Google Scholar] [CrossRef]
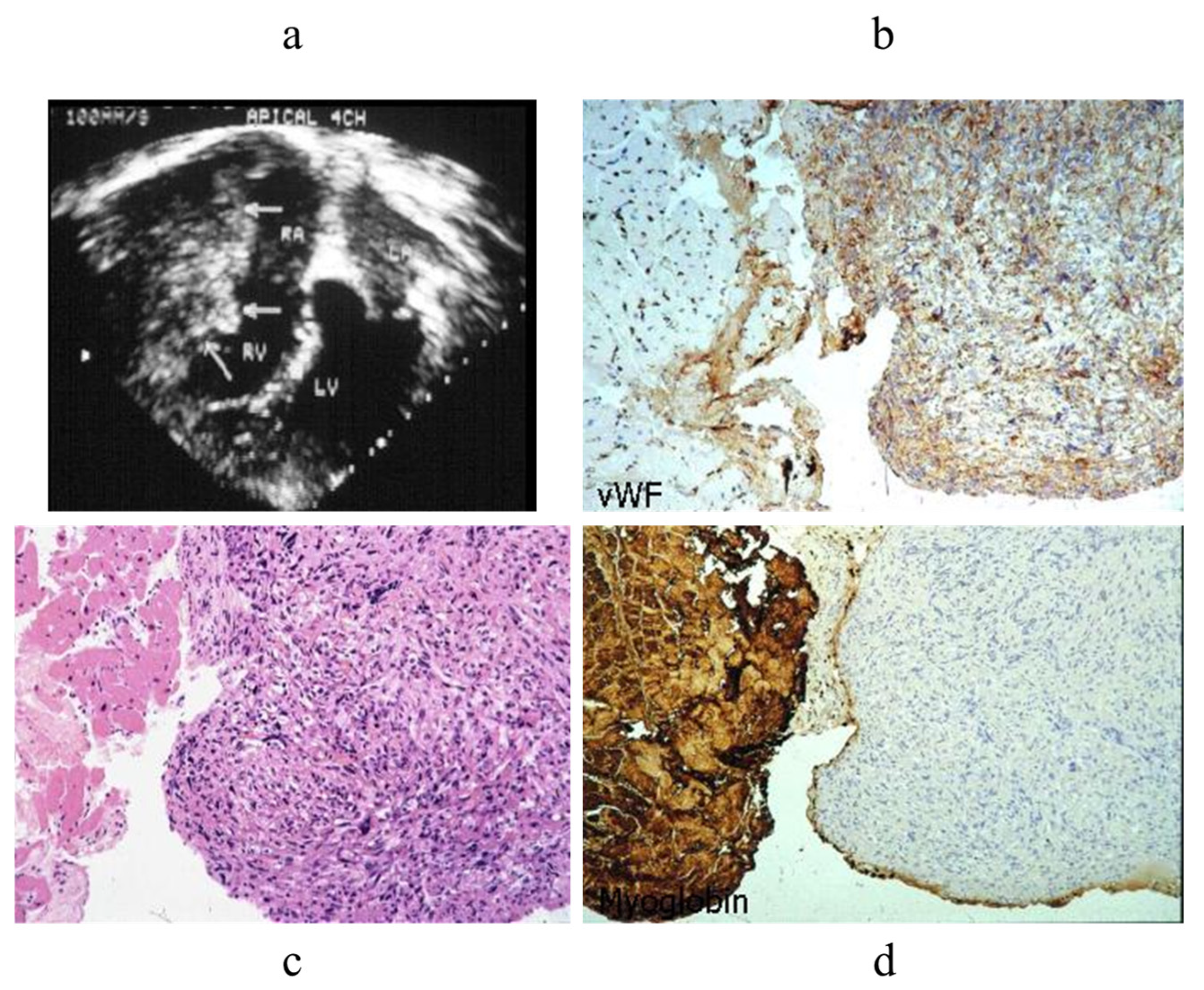
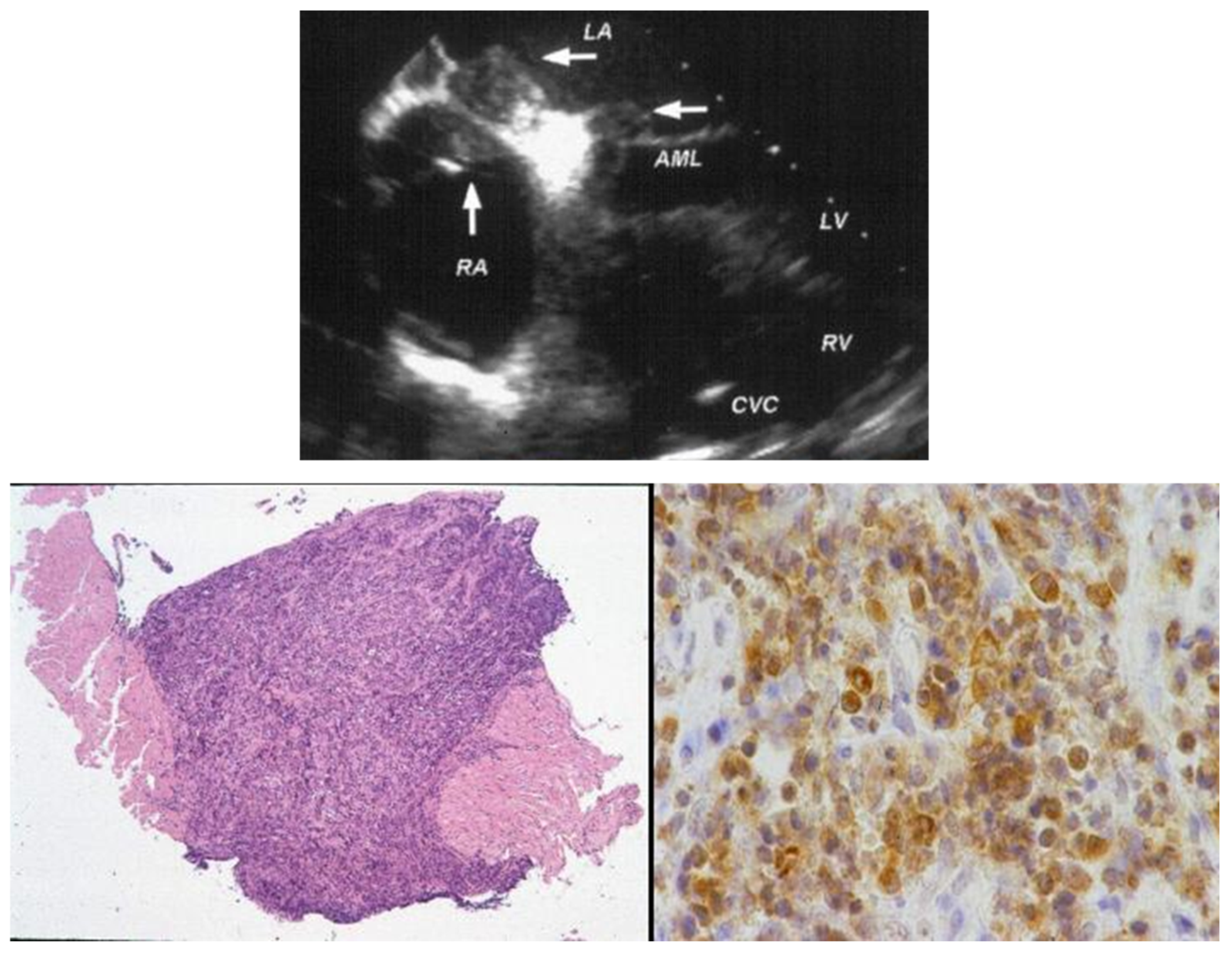
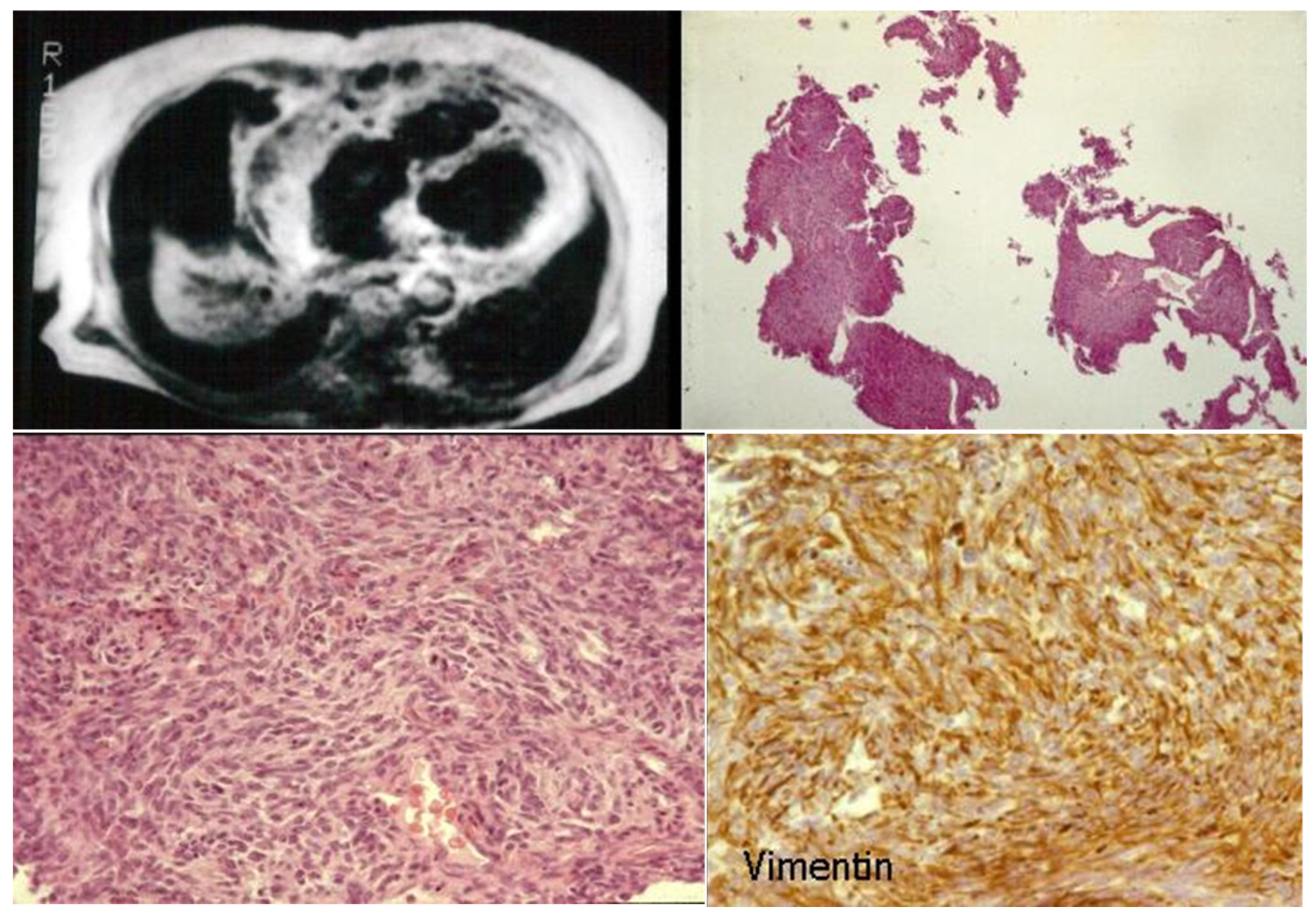
- Poletti, A.; Cocco, P.; Valente, M.; Fasoli, G.; Chioin, R.; Thiene, G. In Vivo Diagnosis of Cardiac Angiosarcoma by Endomyocardial Biopsy. Cardiovasc. Pathol. 1993, 2, 89–91. [Google Scholar] [CrossRef]
- Testolin, L.; Basso, C.; Pittarello, D.; Casarotto, D.; Valente, M. Cardiogenic Shock Due to Metastatic Cardiac Lymphoma: Still a Diagnostic and Therapeutic Challenge. Eur. J. Cardiothorac. Surg. 2001, 19, 365–368. [Google Scholar] [CrossRef]
- Basso, C.; Stefani, A.; Calabrese, F.; Fasoli, G.; Valente, M. Primary Right Atrial Fibrosarcoma Diagnosed by Endocardial Biopsy. Am. Heart J. 1996, 131, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Thiene, G.; Rizzo, S.; De Lazzari, M.; Carturan, E.; Tona, F.; Caforio, A.L.; Cacciavillani, L.; Marcolongo, R.; Tarantini, G.; et al. Cardiac Magnetic Resonance Features of Biopsy-Proven Endomyocardial Diseases. JACC Cardiovasc. Imaging 2014, 7, 309–312. [Google Scholar] [CrossRef]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus Statement on Endomyocardial Biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef]
- Mullis, K.B.; Erlich, H.A.; Gelfand, D.H.; Horn, G.; Saiki, R.K. Process for Amplifying, Detecting, and/or Cloning Nucleic Acid Sequences. U.S. Patent 4,683,195, 7 February 1986. [Google Scholar]
- Basso, C.; Calabrese, F.; Angelini, A.; Carturan, E.; Thiene, G. Classification and Histological, Immunohistochemical, and Molecular Diagnosis of Inflammatory Myocardial Disease. Heart Fail. Rev. 2013, 18, 673–681. [Google Scholar] [CrossRef]
- Thiene, G.; Bruneval, P.; Veinot, J.; Leone, O. Diagnostic Use of the Endomyocardial Biopsy: A Consensus Statement. Virchows Arch. 2013, 463, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Ronco, F.; Marcus, F.; Abudureheman, A.; Rizzo, S.; Frigo, A.C.; Bauce, B.; Maddalena, F.; Nava, A.; Corrado, D.; et al. Quantitative Assessment of Endomyocardial Biopsy in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An In Vitro Validation of Diagnostic Criteria. Eur. Heart J. 2008, 29, 2760–2771. [Google Scholar] [CrossRef]
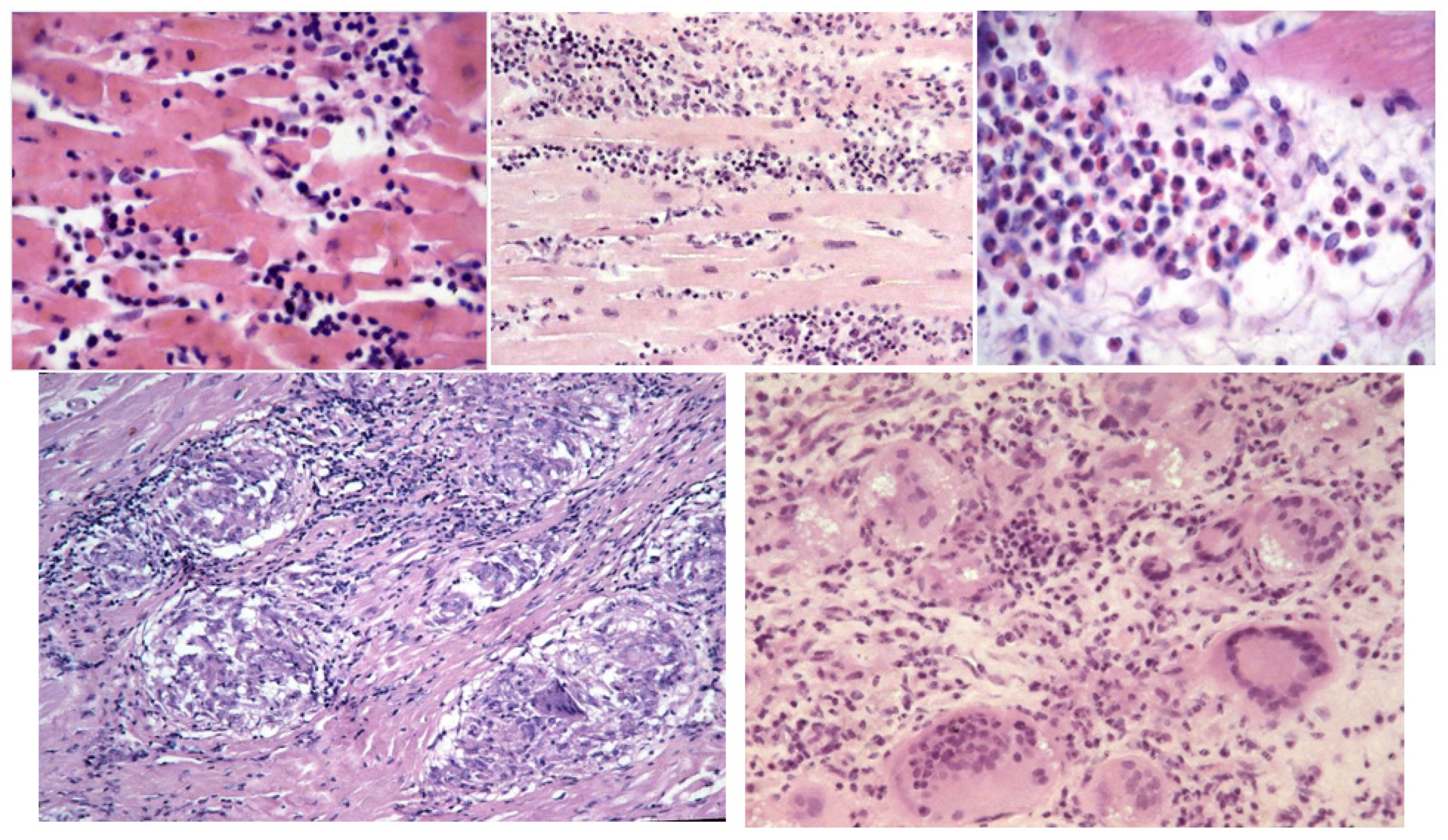
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J., Jr.; Olsen, E.G.; Schoen, F.J. Myocarditis: A Histopathologic Definition and Classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar]
- Baughman, K.L. Diagnosis of Myocarditis: Death of Dallas Criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Bartlett, R.H.; Kittredge, D.; Noyes, B.S., Jr.; Willard, R.H., III; Drinker, P.A. Development of a Membrane Oxygenator: Overcoming Blood Diffusiolimitation. J. Thorac. Cardiovasc. Surg. 1969, 58, 795–800. [Google Scholar] [CrossRef]
- Duncan, B.W.; Bohn, D.J.; Atz, A.M.; French, J.W.; Laussen, P.C.; Wessel, D.L. Mechanical Circulatory Support for the Treatment of Children with Acute Fulminant Myocarditis. J. Thorac. Cardiovasc. Surg. 2001, 122, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Teele, S.A.; Allan, C.K.; Laussen, P.C.; Newburger, J.W.; Gauvreau, K.; Thiagarajan, R.R. Management and Outcomes in Pediatric Patients Presenting with Acute Fulminant Myocarditis. J. Pediatr. 2011, 158, 638–643.e1. [Google Scholar] [CrossRef] [PubMed]
- Diddle, J.W.; Almodovar, M.C.; Rajagopal, S.K.; Rycus, P.T.; Thiagarajan, R.R. Extracorporeal Membrane Oxygenation for the Support of Adults with Acute Myocarditis. Crit. Care Med. 2015, 43, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Centofanti, P.; Gelsomino, S.; Barili, F.; Di Mauro, M.; Orlando, P.; Milazzo, F.; Dato, G.A.; Casabona, R.; Casali, G.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann. Thorac. Surg. 2016, 101, 919–926. [Google Scholar] [CrossRef]



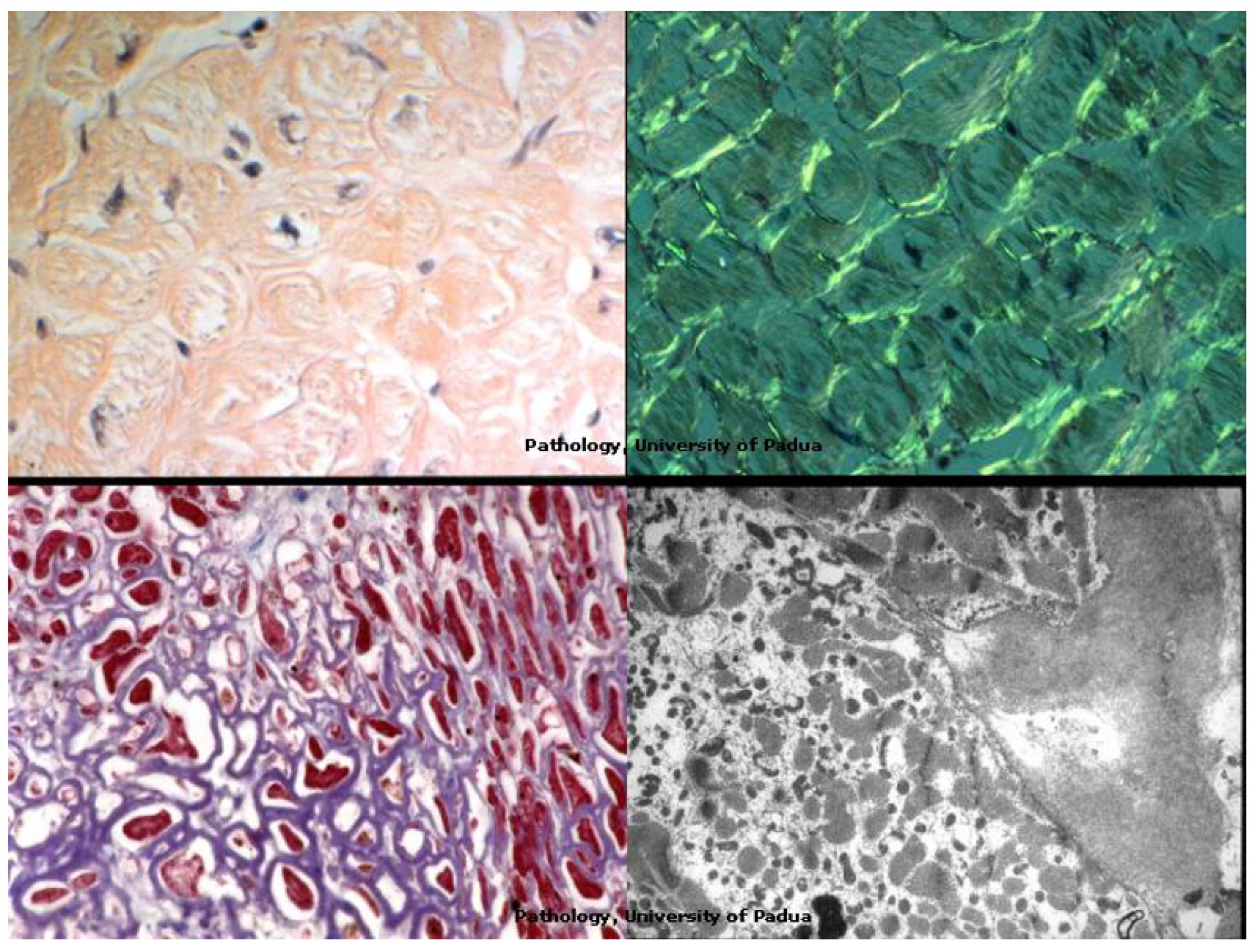
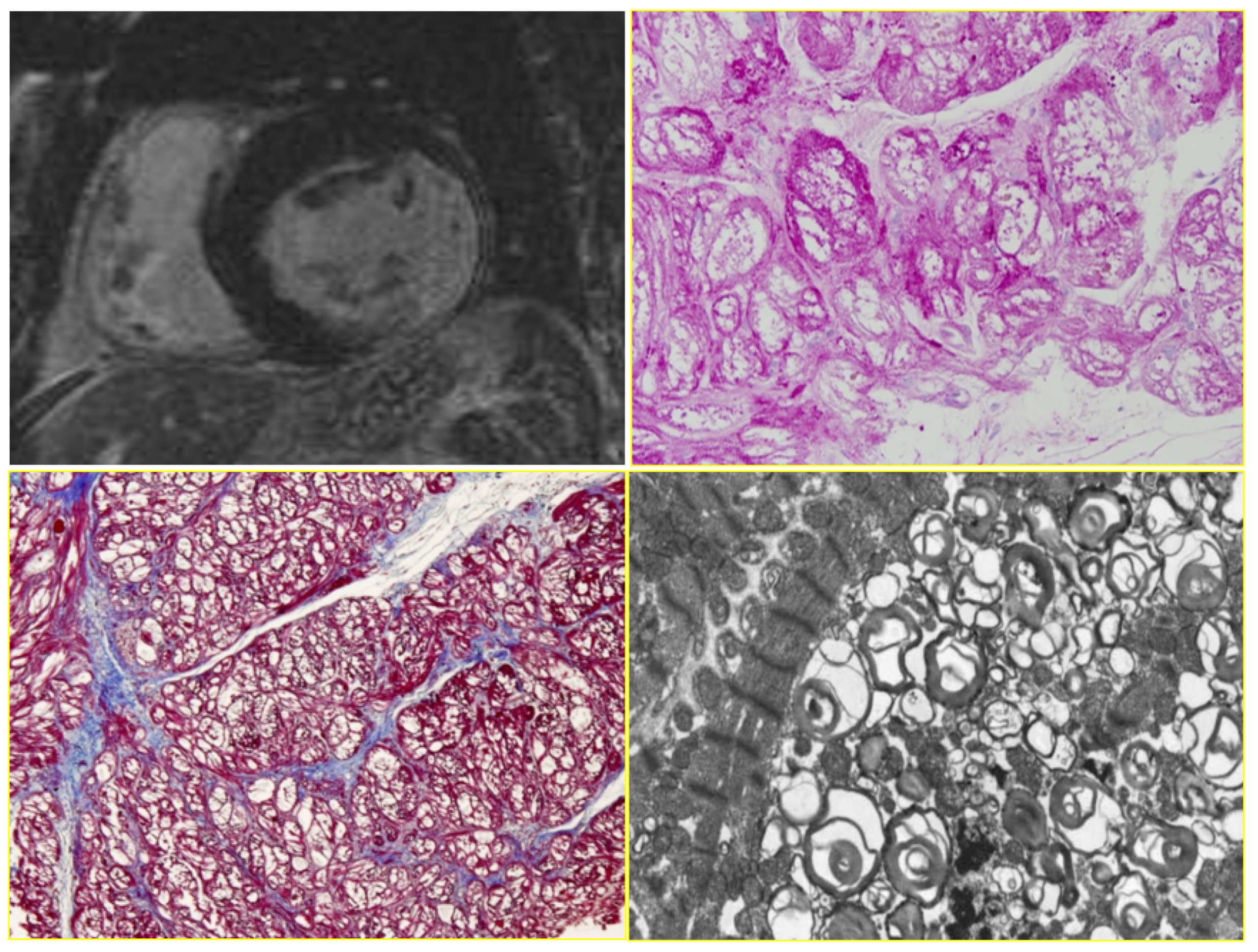
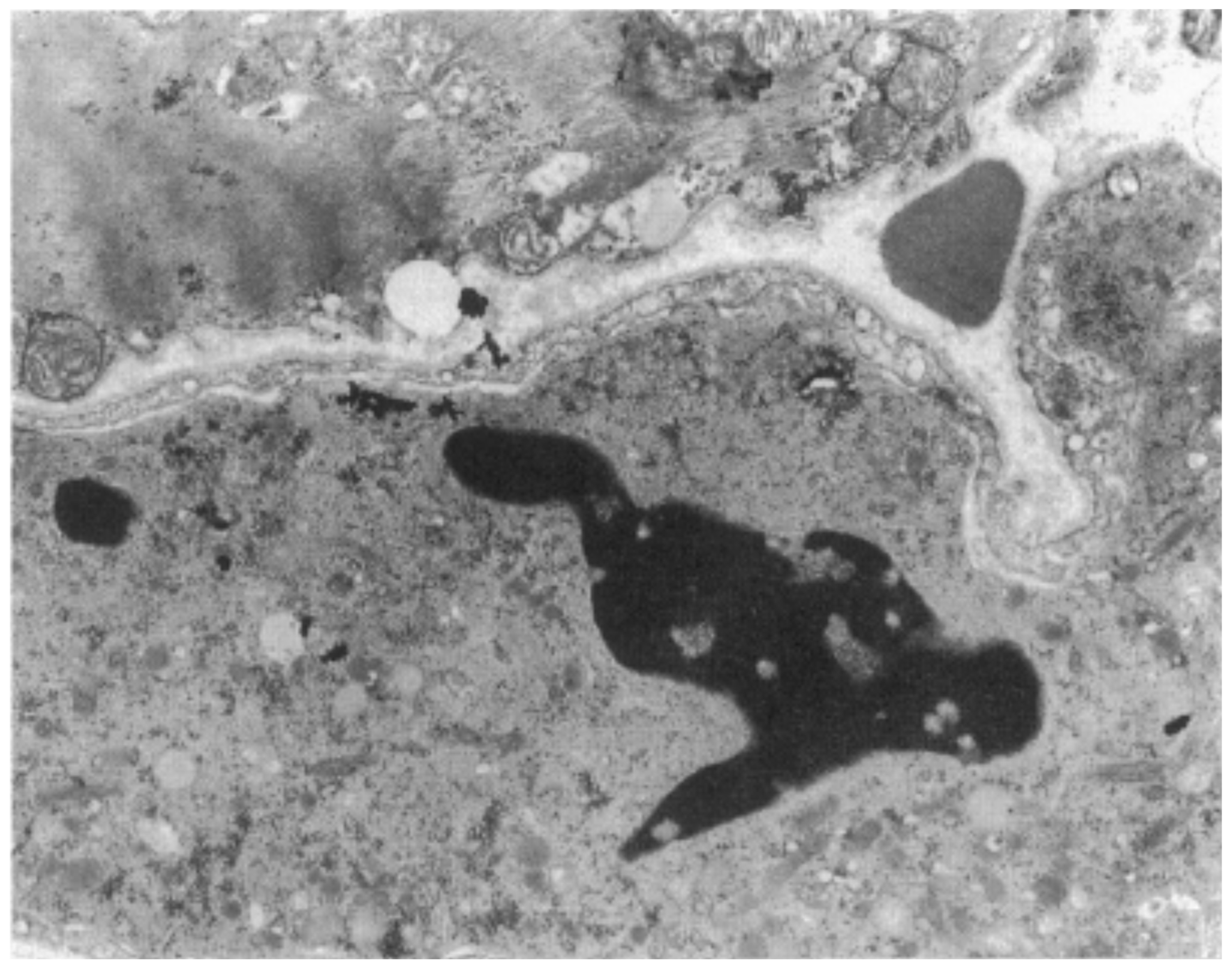
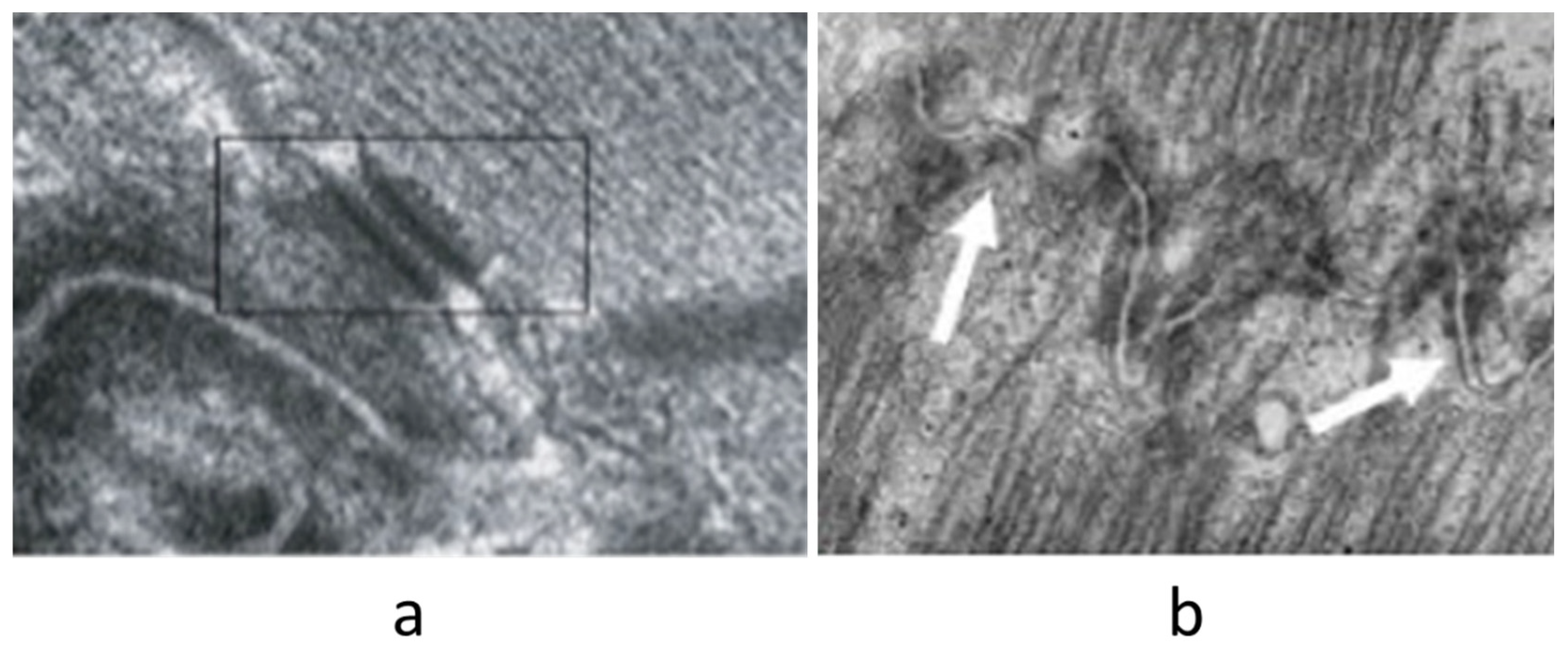

|
|
|
|
|
|
|
|
| Molecular Analysis | Histology | Immunohistochemistry |
|---|---|---|
| ↘ | ↓ | ↙ |
| Gold Standard |
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiene, G. Storytelling of Myocardial Biopsy. Biology 2025, 14, 306. https://doi.org/10.3390/biology14030306
Thiene G. Storytelling of Myocardial Biopsy. Biology. 2025; 14(3):306. https://doi.org/10.3390/biology14030306
Chicago/Turabian StyleThiene, Gaetano. 2025. "Storytelling of Myocardial Biopsy" Biology 14, no. 3: 306. https://doi.org/10.3390/biology14030306
APA StyleThiene, G. (2025). Storytelling of Myocardial Biopsy. Biology, 14(3), 306. https://doi.org/10.3390/biology14030306






