Population Status of Sympatrically Breeding Skuas (Catharacta spp.) at Admiralty Bay, King George Island, Antarctica: A Case Report for 2020–2024
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
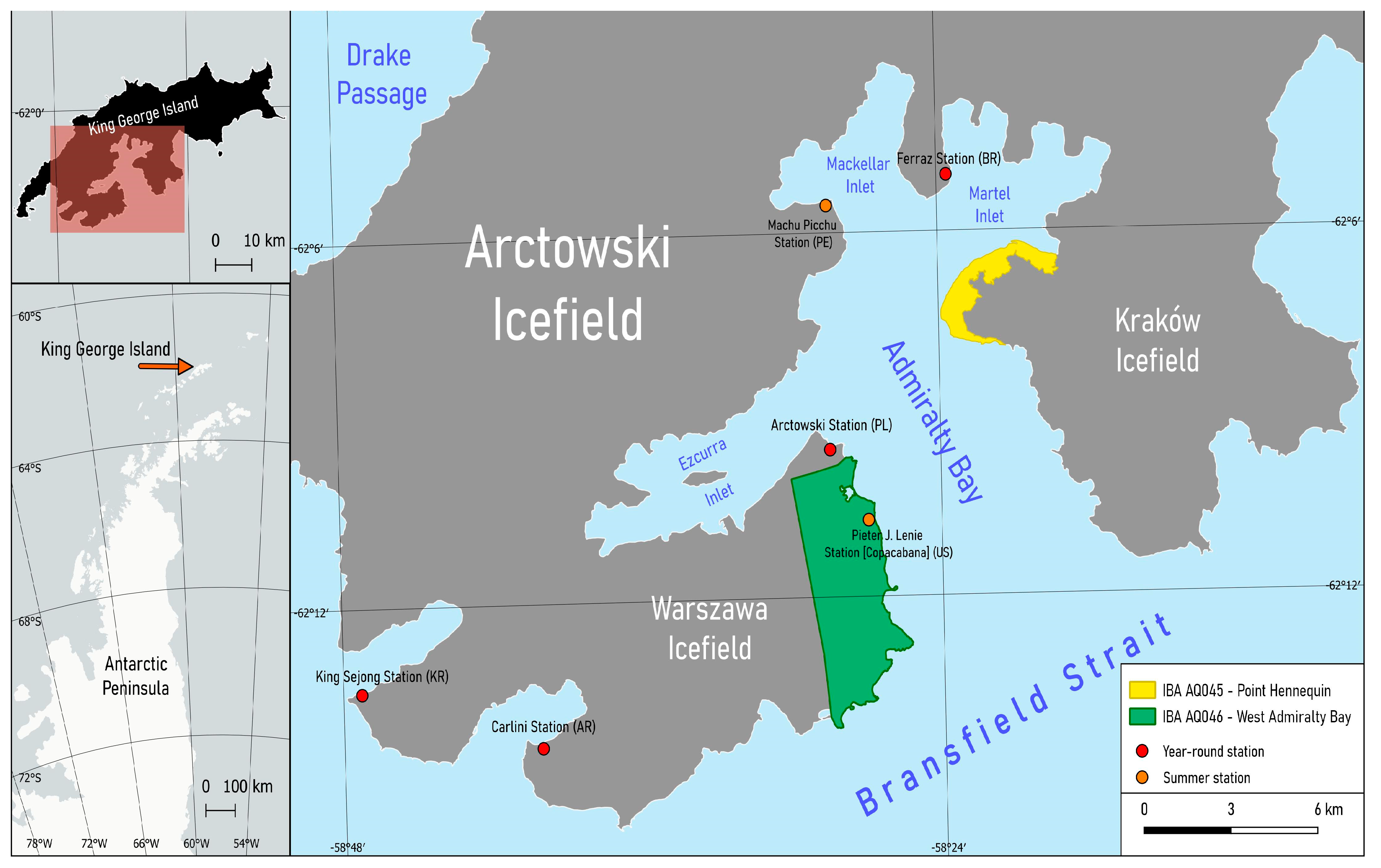
2.1. Study Area
2.2. Sampling
2.3. Discrepancies in Data Used in the Study
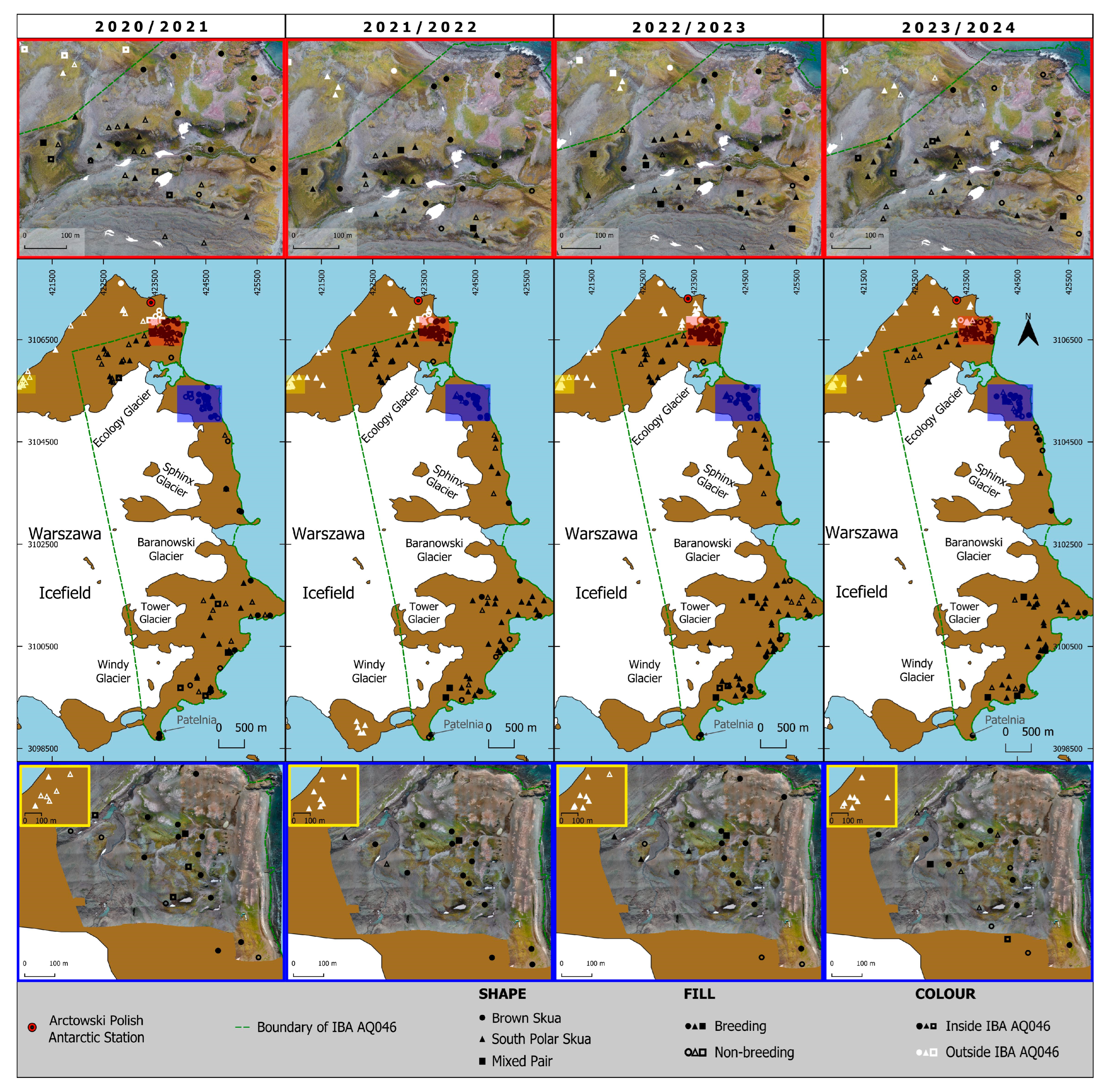
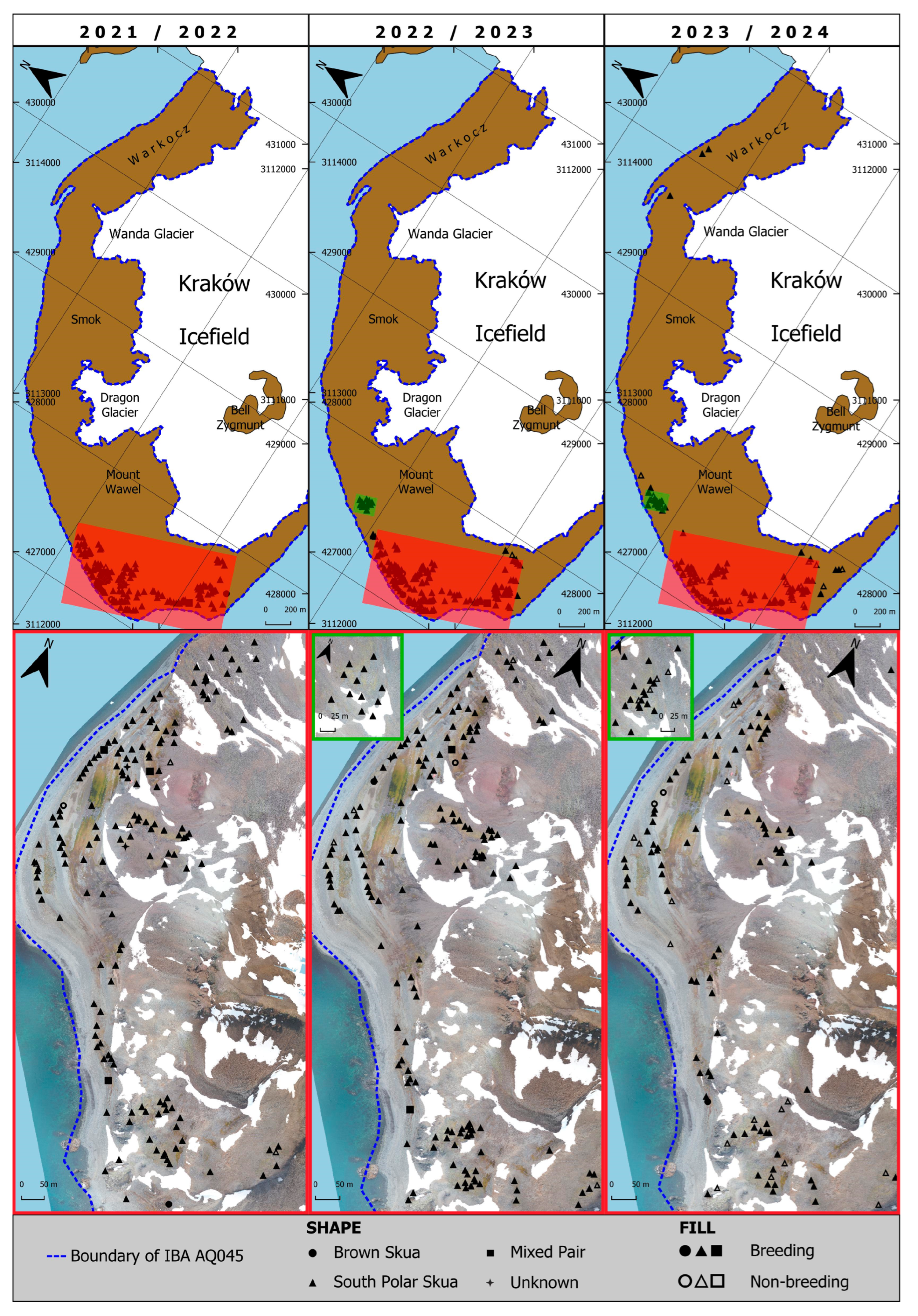
3. Results
4. Discussion
5. Conclusions
- (1)
- The total number of SPS nests in IBA PH in all three seasons (2021/22, 2022/23, and 2023/24) in which the inventory was conducted met the requirement of global criterion A4, exceeding 50 pairs, with 153, 176, and 141 pairs obtained, respectively;
- (2)
- The total number of SPS nests in IBA WAB also exceeded the required 50 pairs in all four seasons (2020/21, 2021/22, 2022/23, and 2023/24), with 63, 66, 77, and 63 recorded, respectively, thus meeting the global criterion A4 for this species. Consequently, it is recommended that IBA WAB be updated and that SPS be incorporated into the criterion as an additional trigger species;
- (3)
- The expansion of both IBA boundaries is recommended.
- (a)
- IBA PH, as the exposure of new ice-free areas, has the potential to host new SPS and BS nests;
- (b)
- In the case of IBA WAB, 24 ± 3 SPS nests have been found outside the IBA boundaries, which on their own cannot form a separate IBA but could complement the existing one, particularly in the areas of Jasnorzewski Garden and Italian Valley.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | SPS | MIXED | |||||||||||
| Census Period | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | |
| Stage of the Breeding Season * | |||||||||||||
| 1 | 31.10–20.11 | 23.10–09.11 | 03.10–04.11 | 22.10–14.11 | 22.11–12.12 | 18.11–05.12 | 17.11–14.12 | 24.11–10.12 | 10.11–04.01 | 15.11–07.12 | 05.11–06.12 | 06.11–01.12 | |
| 2 | 21.11–23.12 | 10.11–19.12 | 05.11–09.12 | 15.11–22.12 | 13.12–16.02 | 06.12–24.01 | 15.12–26.01 | 11.12–08.01 | 05.01–25.01 | 08.12–11.01 | 07.12–04.01 | 02.12–16.01 | |
| 3 | 24.12–22.01 | 20.12–24.01 | 10.12–04.01 | 23.12–27.01 | 17.02–09.03 | 25.01–31.01 | 27.01–10.02 | 09.01–28.01 | 26.01–26.02 | 12.01–09.02 | 05.01–27.01 | 17.01–28.01 | |
| 4 | 23.01–18.02 | 25.01–16.02 | 05.01–10.02 | 28.01–10.02 | 10.03–26.03 | 01.02–16.03 | 11.02–02.03 | N/A | N/A | 10.02–16.03 | 28.01–08.03 | N/A | |
| 5 | 19.02–13.04 | 17.02–31.03 | 11.02–29.03 | 11.02–26.03 | 27.03–31.03 | 17.03–30.04 | 03.03–29.03 | N/A | N/A | 17.03–31.03 | 09.03–29.03 | N/A | |
| Species | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | SPS | MIXED | |||||||||||
| Census Period | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | |
| Stage of the Breeding Season | |||||||||||||
| 1 | 8 | 2 | 9 | 10 | 2 | 4 | 33 | 34 | 0 | 0 | 4 | 2 | |
| 2 | 26 | 26 | 24 | 18 | 21 | 56 | 58 | 46 | 3 | 6 | 8 | 7 | |
| 3 | 23 | 22 | 20 | 16 | 3 | 37 | 45 | 12 | 3 | 5 | 6 | 4 | |
| 4 | 22 | 19 | 18 | 14 | 0 | 15 | 38 | 0 | 0 | 3 | 2 | 0 | |
| 5 | 18 | 16 | 15 | 13 | 0 | 7 | 26 | 0 | 0 | 2 | 2 | 0 | |
| Census Period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2021/2022 | 2022/2023 | 2023/2024 | ||||||||
| Species Timespan | BS | SPS | MIXED | UNK | BS | SPS | MIXED | UNK | BS | SPS |
| 21.12–04.02 | 1 | 139 | 2 | 2 | 2 | 176 | 2 | 1 | 2 | 117 |
| 05.02–05.03 | 0 | 50 | 1 | 1 | 1 | 42 | 1 | 0 | 0 | 25 |
| 06.03–03.04 | 0 | 33 | 0 | 0 | 1 | 32 | 1 | 0 | 0 | 0 |
References
- Mikkelsen, E.K.; Weir, J.T. Phylogenomics reveals that mitochondrial capture and nuclear introgression characterize skua species proposed to be of hybrid origin. Syst. Biol. 2023, 72, 78–91. [Google Scholar] [CrossRef]
- Furness, R.W. The Skuas; T & AD Poyser Ltd., Town Head House: Calton, UK, 1987. [Google Scholar]
- Pietz, P.J. Feeding and nesting ecology of sympatric South Polar and Brown skuas. Auk 1987, 104, 617–627. [Google Scholar] [CrossRef]
- Peter, H.U.; Kaiser, M.; Gebauer, A. Ecological and morphological investigations on south polar skuas (Catharacta maccormicki) and brown skuas (Catharacta skua lonnbergi) on Fildes Peninsula, King George Island, South Shetland Islands. Zool. Jb. Syst. 1990, 117, 201–218. [Google Scholar]
- Carneiro, A.P.B.; Polito, M.J.; Sander, M.; Trivelpiece, W.Z. Abundance and spatial distribution of sympatrically breeding Catharacta spp. (skuas) in Admiralty Bay, King George Island, Antarctica. Polar Biol. 2010, 33, 673–682. [Google Scholar] [CrossRef]
- Devillers, P. Distribution and relationships of South American skuas. Gerfaut 1978, 68, 374–417. [Google Scholar]
- Ritz, M.S.; Millar, C.; Miller, G.D.; Phillips, R.A.; Ryan, P.; Sternkopf, V.; Liebers-Helbig, D.; Peter, H.-U. Phylogeography of the southern skua complex—Rapid colonization of the southern hemisphere during a glacial period and reticulate evolution. Mol. Phylogenetics Evol. 2008, 49, 292–303. [Google Scholar] [CrossRef]
- Harrison, P.; Perrow, M.R.; Larsson, H. Seabirds. In The New Identification Guide; Lynx Edicions: Barcelona, Spain, 2021. [Google Scholar]
- Ritz, M.S.; Hahn, S.; Janicke, T.; Peter, H.-U. Hybridisation between South polar skua (Catharacta maccormicki) and Brown skua (Cantarctica lonnbergi) in the Antarctic Peninsula region. Polar Biol. 2006, 29, 153–159. [Google Scholar] [CrossRef]
- Trivelpiece, W.; Volkman, N.J. Feeding strategies of sympatric south polar Catharacta maccormicki and brown skuas C. lonnbergi. Ibis 1982, 124, 50–54. [Google Scholar] [CrossRef]
- Reinhardt, K. Food and feeding of Antarctic skua chicks Catharacta antarctica lonnbergi and C. maccormicki. J. Ornithol. 1997, 138, 199–214. [Google Scholar] [CrossRef]
- de Almeida Reis, A.O.; Costa, E.S.; Torres, J.P.M.; dos Santos Alves, M.A. Pellets and prey remains as indicators of the diet of two sympatric skuas (Aves: Stercorariidae) on King George Island, Antarctica. Oecologia Aust. 2021, 25, 674–684. [Google Scholar] [CrossRef]
- Parmelee, D.F. The hybrid skua: A southern ocean enigma. Wilson Bull. 1988, 100, 345–356. [Google Scholar]
- Young, E.C. Feeding habits of the South Polar Skua Catharacta maccormicki. Ibis 1963, 105, 301–318. [Google Scholar] [CrossRef]
- del Hoyo, J. Stercorariidae. In Birds of the World; Lynx Ed.: Barcelona, Spain, 1996; pp. 556–571. [Google Scholar]
- ATCM 46 Final Report, Management Plan for Antarctic Specially Protected Area No 128 Western Shore of Admiralty Bay, King George Island, South Shetland Islands. 2024. Available online: https://documents.ats.aq/ATCM46/fr/ATCM46_fr001_e.pdf (accessed on 4 January 2025).
- ATCP. Protocol on Environmental Protection to the Antarctic Treaty. CM 1960; Her Majesty’s Stationery Office: London, UK, 1991. [Google Scholar]
- Harris, J.W.; Woehler, E.J. Can the important bird area approach improve the Antarctic protected area system? Polar Res. 2004, 40, 97–105. [Google Scholar] [CrossRef]
- Harris, C.M.; Carr, R.; Lorenz, K.; Jones, S. Important Bird Areas in Antarctica: Antarctic Peninsula, South Shetland Islands, South Orkney Islands—Final Repor; Environmental Research & Assessment Ltd. Prepared for BirdLife International and the Polar Regions Unit of the UK Foreign & Commonwealth Office: Cambridge, UK, 2011. [Google Scholar]
- Harris, C.M.; Lorenz, K.; Fishpool, L.D.C.; Lascelles, B.; Cooper, J.; Coria, N.R.; Croxall, J.P.; Emmerson, L.M.; Fijn, R.C.; Fraser, W.L.; et al. Important Bird Areas in Antarctica 2015; BirdLife International and Environmental Research & Assessment Ltd.: Cambridge, UK, 2015. [Google Scholar]
- BirdLife International. Country Profile: Antarctica. 2025. Available online: https://datazone.birdlife.org/country/antarctica (accessed on 4 January 2025).
- BirdLife International. Important Bird Area Factsheet: Ryder Bay Islands (Antarctica). 2025. Available online: https://datazone.birdlife.org/site/factsheet/ryder-bay-islands-iba-antarctica (accessed on 4 January 2025).
- Phillips, R.A.; Silk, J.R.D.; Massey, A.; Hughes, K.A. Surveys reveal increasing and globally important populations of south polar skuas and Antarctic shags in Ryder Bay (Antarctic Peninsula). Polar Biol. 2019, 42, 423–432. [Google Scholar] [CrossRef]
- BirdLife International. Important Bird Area Factsheet: Cape Melville, King George Island (Antarctica). 2024. Available online: https://datazone.birdlife.org/site/factsheet/cape-melville-king-george-island-iba-antarctica (accessed on 29 August 2024).
- Fudala, K.; Bialik, R.J. Identifying important bird and biodiversity areas in Antarctica using RPAS surveys—A case study of Cape Melville, King George Island, Antarctica. Drones 2023, 7, 538. [Google Scholar] [CrossRef]
- BirdLife International. IBA Criteria. 2025. Available online: http://datazone.birdlife.org/site/ibacriteria (accessed on 4 January 2025).
- Costa, E.S.; Alves, M.A.S. The breeding birds of Hennequin Point: An ice-free area of Admiralty Bay (Antarctic Specially Managed Area), King George Island, Antarctica. Rev. Bras. Ornitol. 2008, 16, 137–141. [Google Scholar]
- BirdLife International. Important Bird Area Factsheet: Point Hennequin, King George Island (Antarctica). 2025. Available online: https://datazone.birdlife.org/site/factsheet/point-hennequin-king-george-island-iba-antarctica (accessed on 4 January 2025).
- BirdLife International. Important Bird Area Factsheet: West Admiralty Bay, King George Island (Antarctica). 2025. Available online: https://datazone.birdlife.org/site/factsheet/west-admiralty-bay-king-george-island-iba-antarctica (accessed on 4 January 2025).
- Shirihai, H. The Complete Guide to Antarctic Wildlife; Princetown Univ. Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Ainley, D.G.; Spear, L.B.; Wood, R.C. Sexual color and size variation in the south polar skua. Condor 1985, 87, 427–428. [Google Scholar] [CrossRef]
- Jabłoński, B. Distribution, abundance and biomass of summer community of birds in the region of the Admiralty Bay (King George Island, South Shetland Islands, Antarctica) in 1978/1979. Pol. Polar Res. 1986, 7, 217–260. [Google Scholar]
- Trivelpiece, W.; Butler, R.G.; Volkman, N.J. Feeding territories of brown skuas (Catharacta lonnbergi). Auk 1980, 97, 669–676. [Google Scholar]
- Sierakowski, K. Birds and mammals in the region of SSSI No. 8 in the season 1988/89 (South Shetlands, King George Island, Admiralty Bay). Pol. Polar Res. 1991, 12, 25–54. [Google Scholar]
- Lesiński, G. Monitoring of birds and pinnipedians on King George Island (South Shetland Islands) in 1989/1990. Pol. Polar Res. 1993, 14, 75–89. [Google Scholar]
- Sierakowski, K.; Korczak-Abshire, M.; Jadwiszczak, P. Changes in bird communities of Admiralty Bay, King George Island (West Antarctic): Insights from monitoring data (1977–1996). Pol. Polar Res. 2017, 38, 231–262. [Google Scholar] [CrossRef]
- Reinhardt, K.; Hahn, S.; Peter, H.-U. The role of skuas in the food web of Potter Cove system—A review. Ber Polarforsc. 1998, 299, 279–284. [Google Scholar]
- Jabłoński, B. Distribution and numbers of penguins in the region of King George Island (South Shetland Islands) in the breeding season 1980/1981. Pol. Polar Res. 1984, 5, 17–30. [Google Scholar]
- Hemmings, A.D. Aspects of the breeding biology of McCormick's skua Catharacta maccormicki at Signy Island, South Orkney Islands. Br. Antarct. Surv. Bull. 1984, 65, 65–79. [Google Scholar]
- Graña Grilli, M.; Montalti, D. Trophic interactions between brown and south polar skuas at Deception Island, Antarctica. Polar Biol. 2012, 35, 299–304. [Google Scholar] [CrossRef]
- Votier, S.C.; Bearhop, S.; Ratcliffe, N.; Philips, R.A.; Furness, R.W. Predation by great skuas at a large seabird colony. J. Appl. Ecol. 2004, 41, 1117–1128. [Google Scholar] [CrossRef]
- Talis, E.J.; Che-Castaldo, C.; Hart, T.; McRae, L.; Lynch, H.J. Penguindex: A Living Planet Index for Pygoscelis species penguins identifies key eras of population change. Polar Biol. 2023, 46, 707–718. [Google Scholar] [CrossRef]
- Müller-Schwarze, D.; Müller-Schwarze, C. Differential predation by South Polar Skuas in an Adelie Penguin rookery. Condor 1973, 75, 127–131. [Google Scholar]
- Trillmich, F. Feeding territories and breeding success of South Polar Skuas. Auk 1978, 95, 23–33. [Google Scholar] [CrossRef]
- Hull, C.; Carter, R.; Whitehead, M.D. Aspects of breeding chronology and success of the Antarctic skua Catharacta maccormicki at Magnetic Island, Prydz Bay, Antarctica. Corella 1994, 18, 37–40. [Google Scholar]
- Hahn, S.; Peter, H.U.; Bauer, S. Skuas at penguin carcass: Patch use and state-dependent leaving decisions in a top-predator. Proc. R. Soc. B Biol. Sci. 2005, 272, 1449–1454. [Google Scholar] [CrossRef]
- Ritz, M.S.; Hahn, S.; Peter, H.-U. Factors affecting chick growth in the South Polar Skua (Catharacta maccormicki): Food supply, weather and hatching date. Polar Biol. 2005, 29, 53–60. [Google Scholar] [CrossRef]
- Lewis, S.; Sherratt, T.N.; Hamer, K.C.; Wanless, S. Evidence for intra-specific competition for food in a pelagic seabird. Nature 2001, 412, 816–818. [Google Scholar] [CrossRef]
- Corman, A.M.; Mendel, B.; Voigt, C.C.; Garthe, S. Varying foraging patterns in response to competition? A multicolony approach in a generalist seabird. Ecol. Evol. 2016, 6, 974–986. [Google Scholar]
- Furness, R.W.; Birkhead, T.R. Seabird colony distributions suggest competition for food supplies during the breeding season. Nature 1984, 311, 655–656. [Google Scholar] [CrossRef]
- Forero, M.G.; Tella, J.L.; Hobson, K.A.; Bertellotti, M.; Blanco, G. Conspecific food competition explains variability in colony size: A test in Magellanic penguins. Ecology 2002, 83, 3466–3475. [Google Scholar] [CrossRef]
- Ainley, D.G.; Ribic, C.A.; Ballard, G.; Heath, S.; Gaffne, I.; Karl, B.J.; Barton, K.J.; Wilson PR Webb, S. Geographic structure of Ade´lie penguin populations: Overlap in colony-specific foraging areas. Ecol. Monogr. 2004, 74, 159–178. [Google Scholar] [CrossRef]
- Bitiutskii, D.G.; Samyshev, E.Z.; Minkina, N.I.; Melnikov, V.V.; Chudinovskih, E.S.; Usachev, S.I.; Salyuk, P.A.; Serebrennikov, A.N.; Zuev, O.A.; Orlov, A.M. Distribution and Demography of Antarctic Krill and Salps in the Atlantic Sector of the Southern Ocean during Austral Summer 2021–2022. Water 2022, 14, 3812. [Google Scholar] [CrossRef]
- Flores, H.; Atkinson, A.; Kawaguchi, S.; Krafft, B.A.; Milinevsky, G.; Nicol, S.; Reiss, C.; Tarling, G.A.; Werner, R.; Rebolledo, E.L.B.; et al. Impact of Climate Change on Antarctic Krill. Mar. Ecol. Prog. Ser. 2012, 458, 1–19. [Google Scholar] [CrossRef]
- McCormack, S.A.; Melbourne-Thomas, J.; Trebilco, R.; Blanchard, J.L.; Raymond, B.; Constable, A. Decades of dietary data demonstrate regional food web structures in the Southern Ocean. Ecol. Evol. 2021, 11, 227–241. [Google Scholar] [CrossRef]
- Constable, A.J.; Costa, D.P.; Schofield, O.; Newman, L.; Urban, E.R.; Fulton, E.A.; Melbourne-Thomas, J.; Ballerini, T.; Boyd, P.W.; Brandt, A.; et al. Developing priority variables (“ecosystem Essential Ocean Variables”—eEOVs) for observing dynamics and change in Southern Ocean ecosystems. J. Mar. Syst. 2016, 161, 26–41. [Google Scholar] [CrossRef]
- Mota, A.C.M.; Costa, E.S.; Torres, J.P.M.; de Araujo, J.; Tormena, L.C.; Dantas, G.P.d.M. Brown Skua and south polar Skua (Aves: Stercorariidae) a hybridization case or same species? Polar Biol. 2023, 46, 1191–1201. [Google Scholar] [CrossRef]
- Bennison, A.; Adlard, S.; Banyard, A.C.; Blockley, F.; Blyth, M.; Browne, E.; Day, G.; Dunn, M.J.; Falchieri, M.; Fitzcharles, E.; et al. A case study of highly pathogenic avian influenza (HPAI) H5N1 at Bird Island, South Georgia: The first documented outbreak in the subantarctic region. Bird Study 2024, 1–12. [Google Scholar] [CrossRef]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef]
- Camphuysen, C.J.; Gear, S.C.; Furness, R.W. Avian influenza leads to mass mortality of adult Great Skuas in Foula in summer 2022. Scott Birds 2022, 42, 312–323. [Google Scholar]



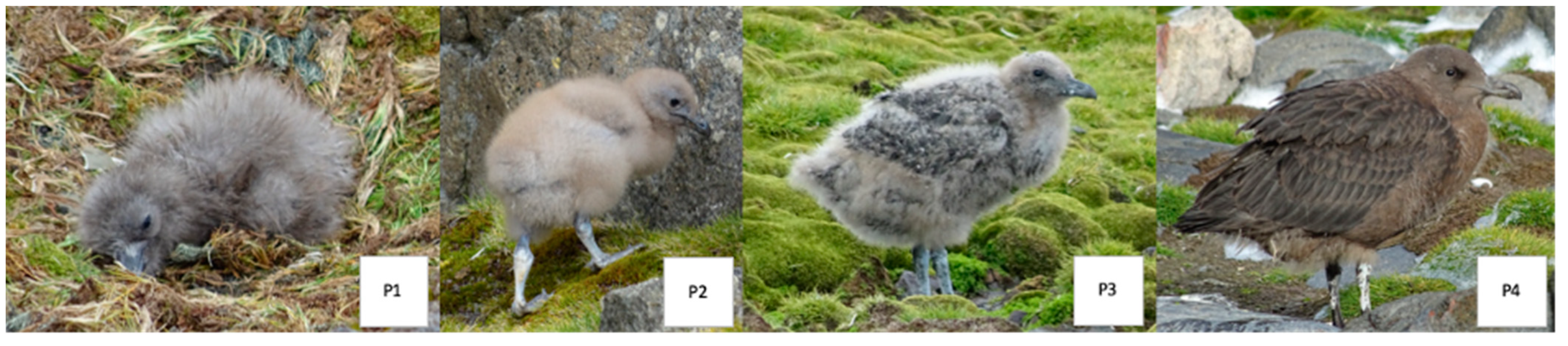
| Species | 1977/78 1 | 1978/79 2 | 1988/89 3 | 1989/90 4 | 1990/91 5 | 1991/92 5 | 1992/93 5 | 1994/95 5 | 2004/05 6 | 2020/21 | 2021/22 | 2022/23 | 2023/24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | at least 28 | 47 | 42 | 29 | 28 | 32 | 20 | ||||||
| SPS | at least 13 | 12 | 72 | 24 | 58 | 64 | 47 | ||||||
| MIX | at least 4 | 10 | 16 | 4 | 7 | 9 | 7 | ||||||
| Total | at least 45 | 69 | 38 | 57 | 62 | 71 | 75 | 64 | 130 | 57 | 93 | 105 | 74 |
| Census Period | ||||||
|---|---|---|---|---|---|---|
| Species | 1978/79 1 | 2004/05 2 | 2004/05 3 | 2021/22 | 2022/23 | 2023/24 |
| BS | 12 | 2 | 1 | 1 | 1 | 1 |
| SPS | 10 | 116 | 113 | 149 | 171 | 117 |
| MIX | 0 | 8 | 15 | 3 | 2 | 0 |
| UNK | - | - | - | 3 | 1 | 0 |
| Total | 22 | 126 | 129 | 156 | 175 | 118 |
| Census Period | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | ||||||||||
| Sp. | IBA | BR | NBR | Total Pairs | BR | NBR | Total Pairs | BR | NBR | Total Pairs | BR | NBR | Total Pairs |
| BS | WAB | 29 | 13 | 42 | 28 | 7 | 35 | 32 | 8 | 40 | 20 | 10 | 30 |
| PH | - | - | - | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 3 | |
| SPS | WAB | 24 | 39 | 63 | 58 | 8 | 66 | 64 | 13 | 77 | 47 | 16 | 63 |
| PH | - | - | - | 149 | 4 | 153 | 171 | 5 | 176 | 117 | 24 | 141 | |
| MIX | WAB | 4 | 12 | 16 | 7 | 2 | 9 | 9 | 2 | 11 | 7 | 3 | 10 |
| PH | - | - | - | 3 | 0 | 3 | 2 | 0 | 2 | 0 | 0 | 0 | |
| UNK | PH | - | - | - | 3 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 |
| Census Period | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | ||||||
| Species | Location | BR | NBR | BR | NBR | BR | NBR | BR | NBR |
| BS | N | 1 | 3 | 2 | 3 | 4 | 0 | 1 | 1 |
| S | - | - | 0 | 0 | - | - | - | - | |
| SPS | N | 7 | 12 | 24 | 0 | 25 | 2 | 21 | 5 |
| S | - | - | 5 | 0 | - | - | - | - | |
| MIX | N | 0 | 3 | 1 | 0 | 2 | 0 | 0 | 0 |
| S | - | - | 0 | 0 | - | - | - | - | |
| Census Period | Species | Number of Pairs with Two Fledglings | Number of Pairs with One Fledgling | Number of Fledglings |
|---|---|---|---|---|
| 2020/2021 | BS | 3 | 15 | 21 |
| SPS | 0 | 0 | 0 | |
| MIX | 0 | 0 | 0 | |
| 2021/2022 | BS | 8 | 8 | 24 |
| SPS | 0 | 7 | 7 | |
| MIX | 0 | 2 | 2 | |
| 2022/2023 | BS | 2 | 13 | 17 |
| SPS | 0 | 26 | 26 | |
| MIX | 0 | 2 | 2 | |
| 2023/2024 | BS | 4 | 9 | 17 |
| SPS | 0 | 0 | 0 | |
| MIX | 0 | 0 | 0 |
| Census Period | Species | Number of Pairs with Two Fledglings | Number of Pairs with One Fledgling | Number of Pairs with Two Chicks | Number of Pairs with One Chick | Number of Pairs with at Least One Chick or Fledgling |
|---|---|---|---|---|---|---|
| 2021/2022 | BS | 0 | 0 | 0 | 0 | 0 |
| SPS | 0 | 30 | 1 | 9 | 40 | |
| MIX | 0 | 0 | 0 | 0 | 0 | |
| UNK | 0 | 0 | 0 | 0 | 0 | |
| 2022/2023 | BS | 0 | 1 | 0 | 0 | 1 |
| SPS | 2 | 30 | 1 | 5 | 38 | |
| MIX | 0 | 1 | 0 | 0 | 1 | |
| UNK | 0 | 0 | 0 | 0 | 0 | |
| 2023/2024 | BS | 0 | 0 | 0 | 0 | 0 |
| SPS | 0 | 0 | 0 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarowska, K.; Fudala, K.; Dziembowski, M.; Hagge, A.; Bialik, R.J. Population Status of Sympatrically Breeding Skuas (Catharacta spp.) at Admiralty Bay, King George Island, Antarctica: A Case Report for 2020–2024. Biology 2025, 14, 305. https://doi.org/10.3390/biology14030305
Komarowska K, Fudala K, Dziembowski M, Hagge A, Bialik RJ. Population Status of Sympatrically Breeding Skuas (Catharacta spp.) at Admiralty Bay, King George Island, Antarctica: A Case Report for 2020–2024. Biology. 2025; 14(3):305. https://doi.org/10.3390/biology14030305
Chicago/Turabian StyleKomarowska, Katarzyna, Katarzyna Fudala, Michał Dziembowski, Alexander Hagge, and Robert Józef Bialik. 2025. "Population Status of Sympatrically Breeding Skuas (Catharacta spp.) at Admiralty Bay, King George Island, Antarctica: A Case Report for 2020–2024" Biology 14, no. 3: 305. https://doi.org/10.3390/biology14030305
APA StyleKomarowska, K., Fudala, K., Dziembowski, M., Hagge, A., & Bialik, R. J. (2025). Population Status of Sympatrically Breeding Skuas (Catharacta spp.) at Admiralty Bay, King George Island, Antarctica: A Case Report for 2020–2024. Biology, 14(3), 305. https://doi.org/10.3390/biology14030305






