Triptolide Affects the Function of Hepatocellular Drug Uptake Transporter Organic Anion Transporting Polypeptide 1B1 Through the Suppression of SGK1
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture of HEK293 Cells Stably Overexpressing Human SLCO1B1
2.3. Uptake Assay
2.4. RNA Isolation and RT-PCR
2.5. Construction of Plasmid DNA with Various Fragments of the Upstream Regulatory Sequence of SGK1
2.6. Analysis of Luciferase Activity
2.7. Chromatin Immunoprecipitation (ChIP)
2.8. Statistical Analysis
3. Results
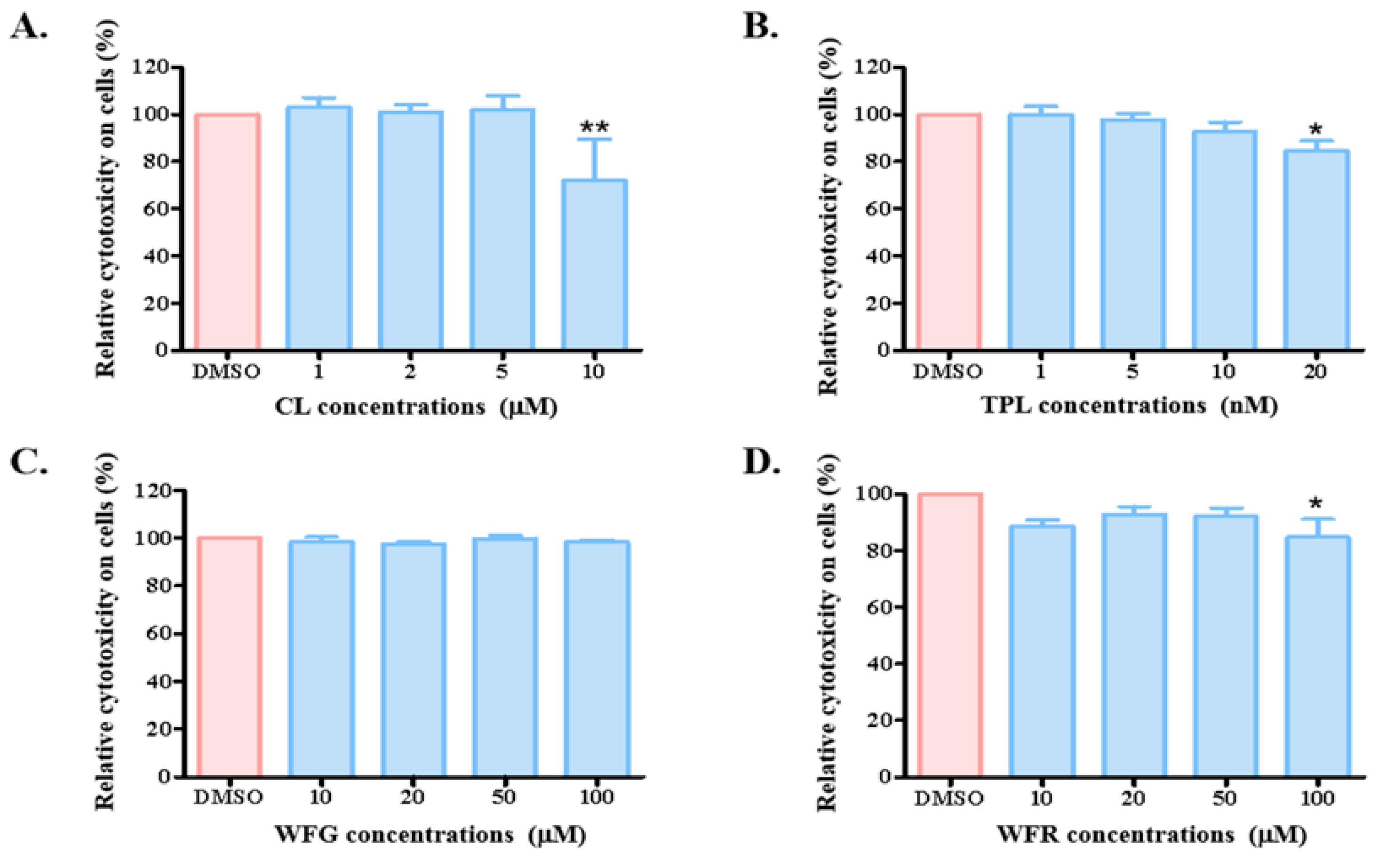
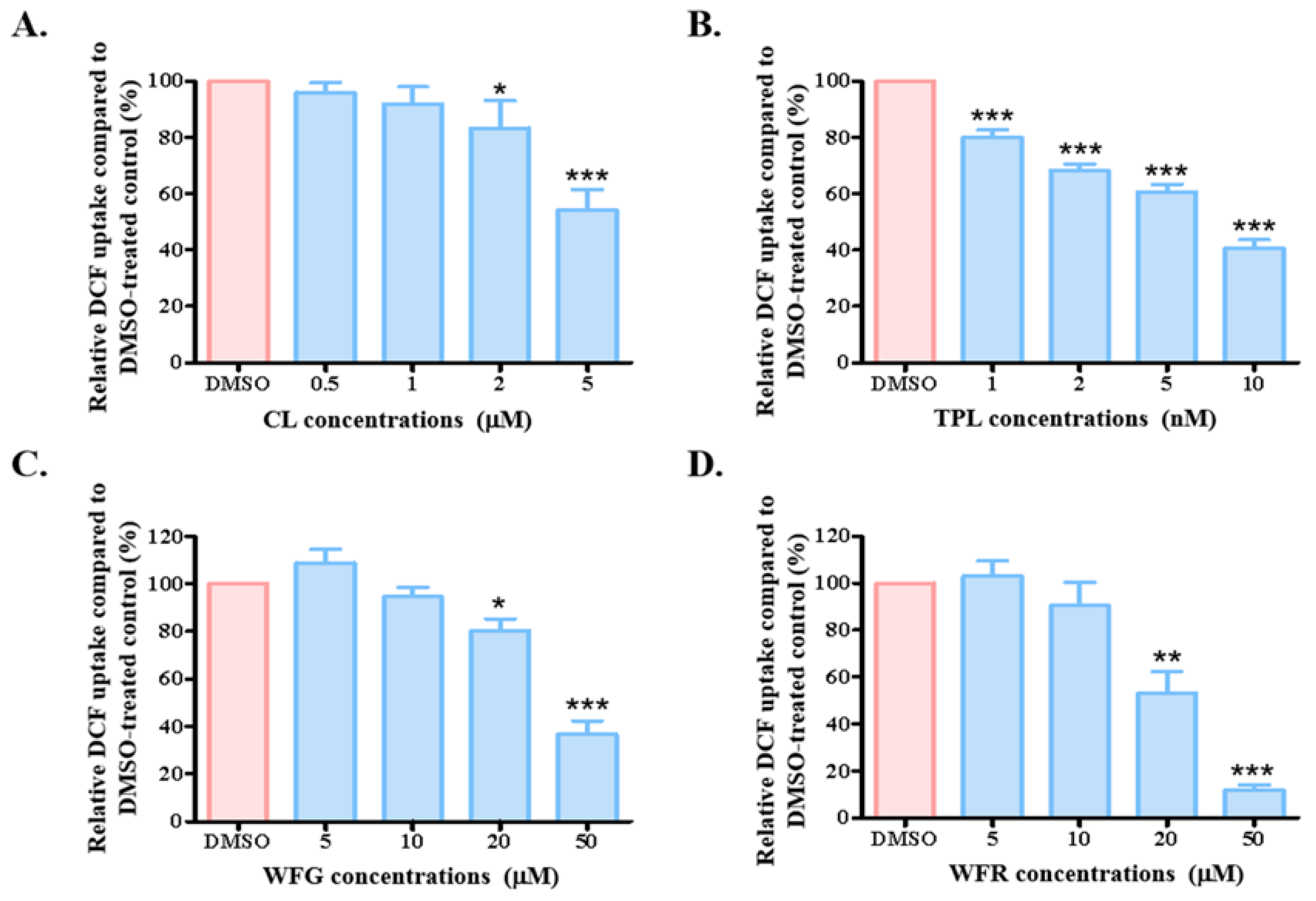
3.1. Long-Term Incubation of TWHF Components Affected Uptake Function of OATP1B1
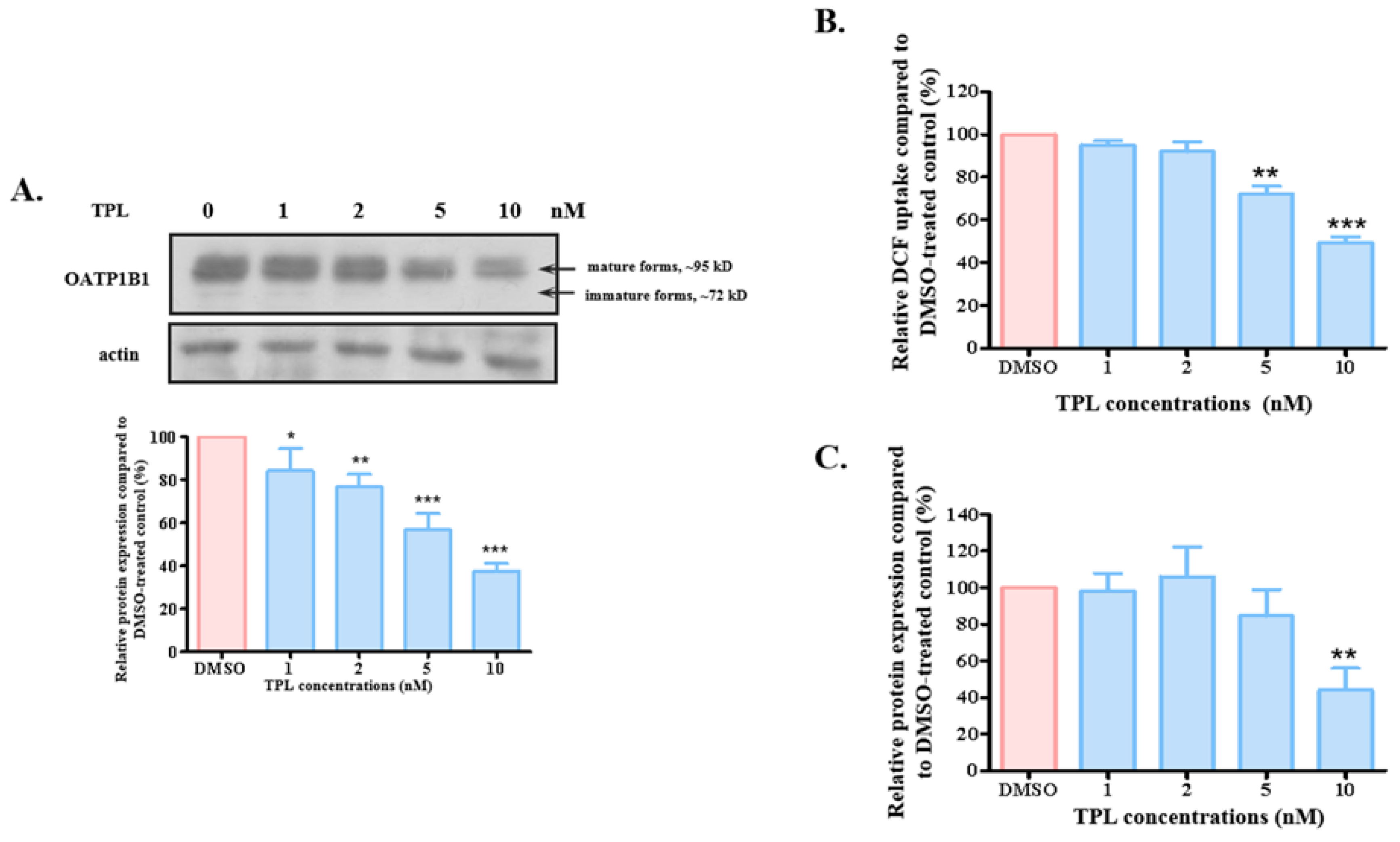
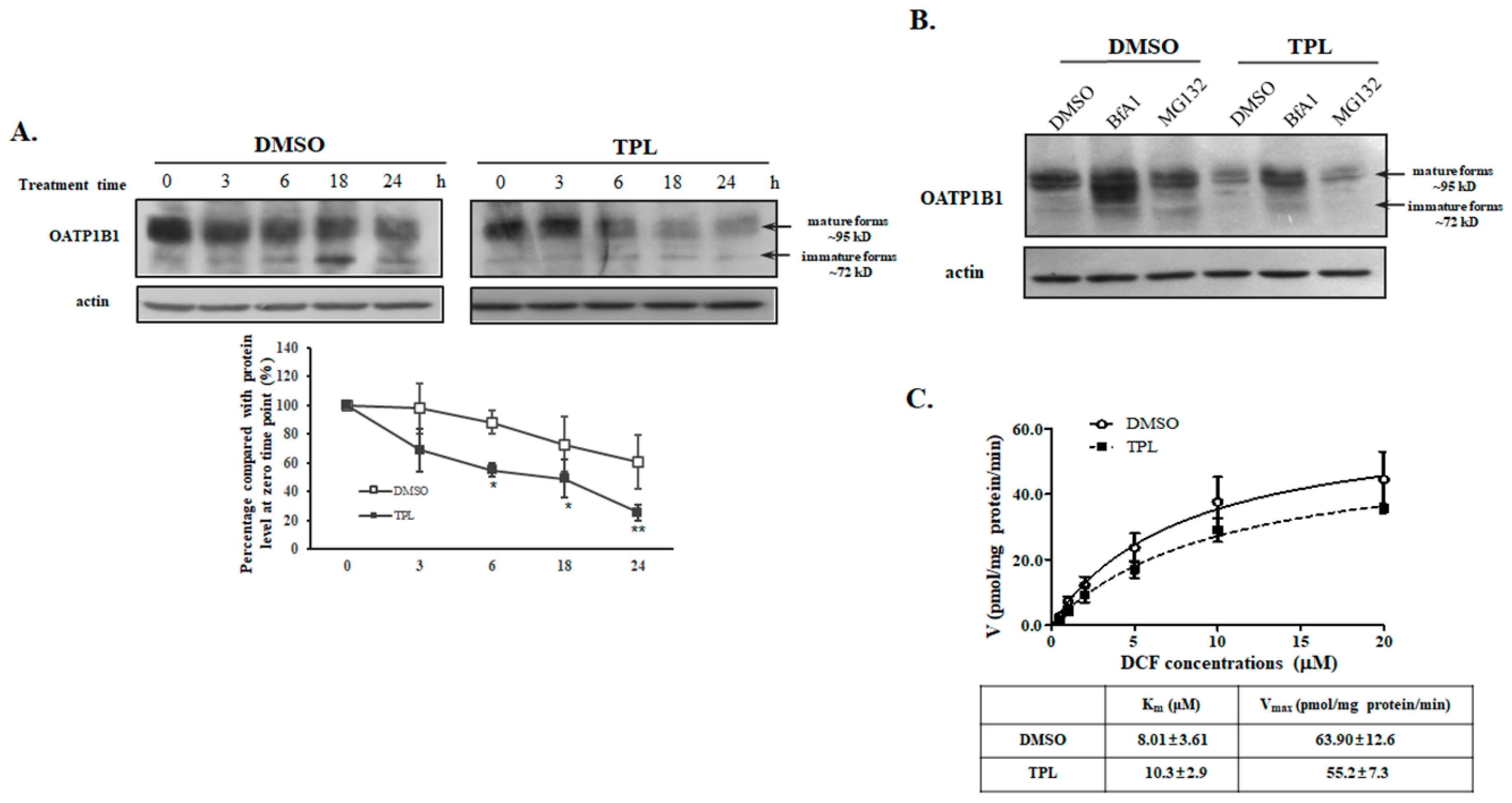
3.2. TPL Accelerated the Degradation of OATP1B1
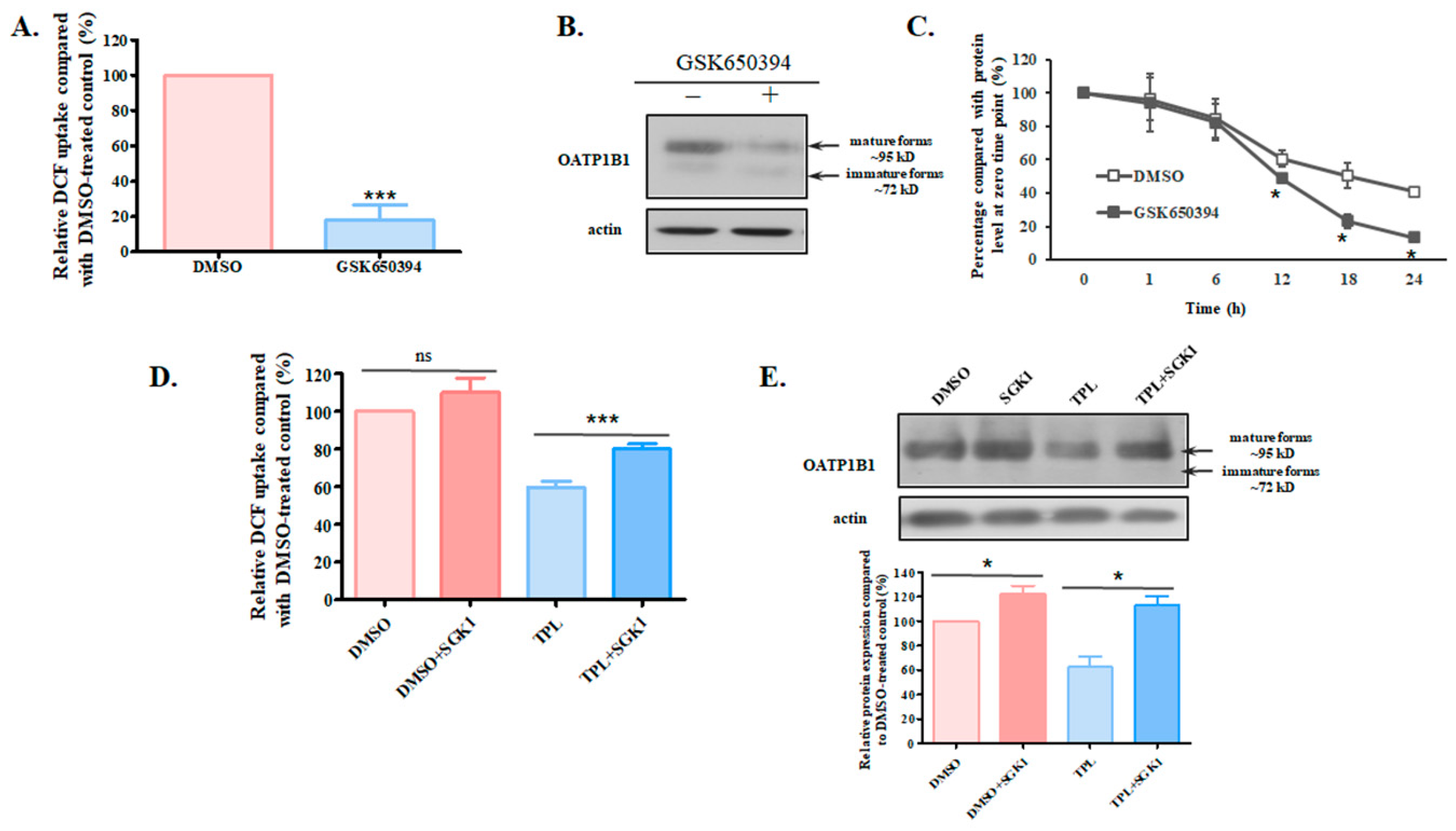
3.3. SGK1 Is Involved in the Effect of TPL on OATP1B1
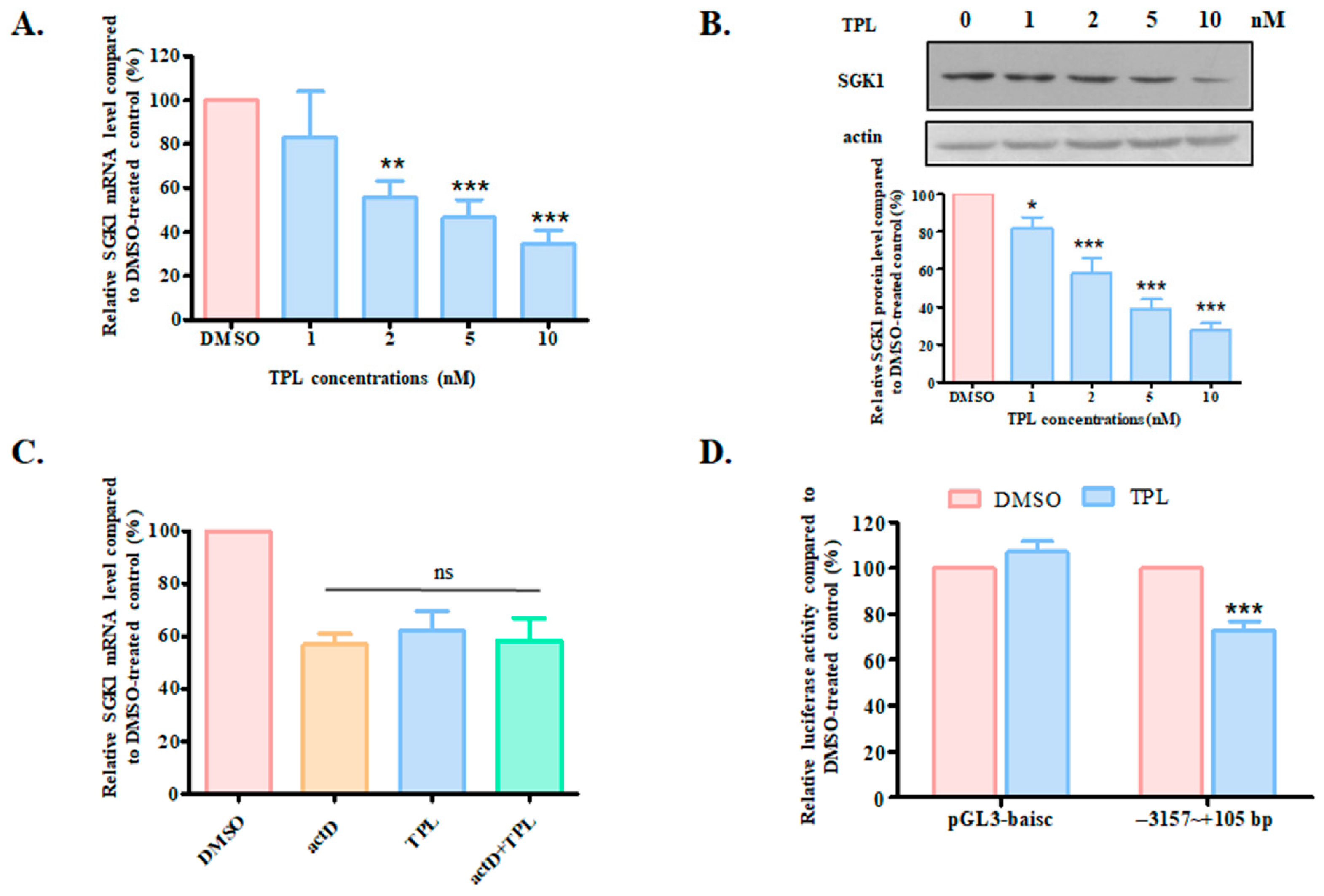
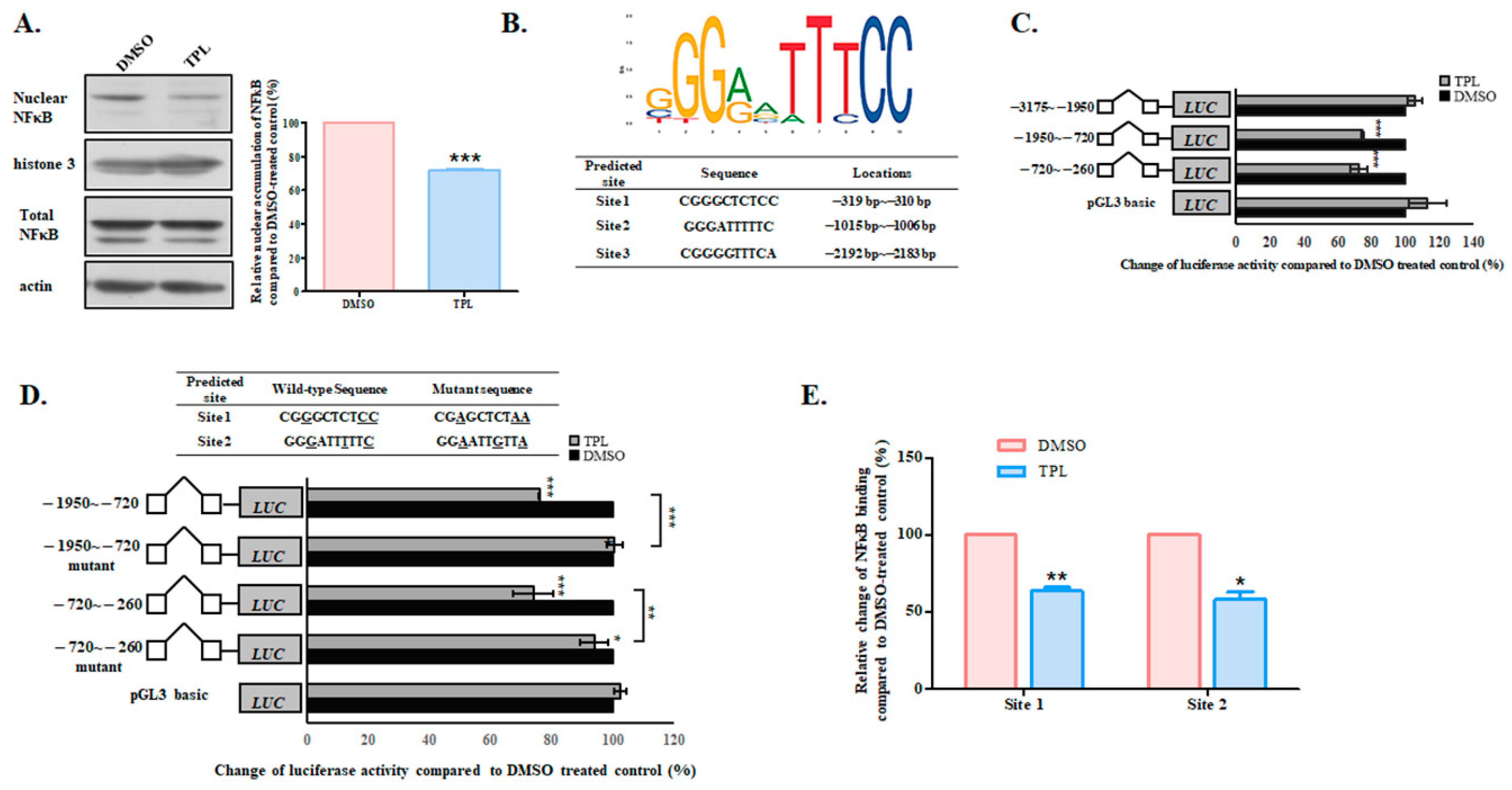
3.4. TPL Downregulated SGK1 Through NFκB
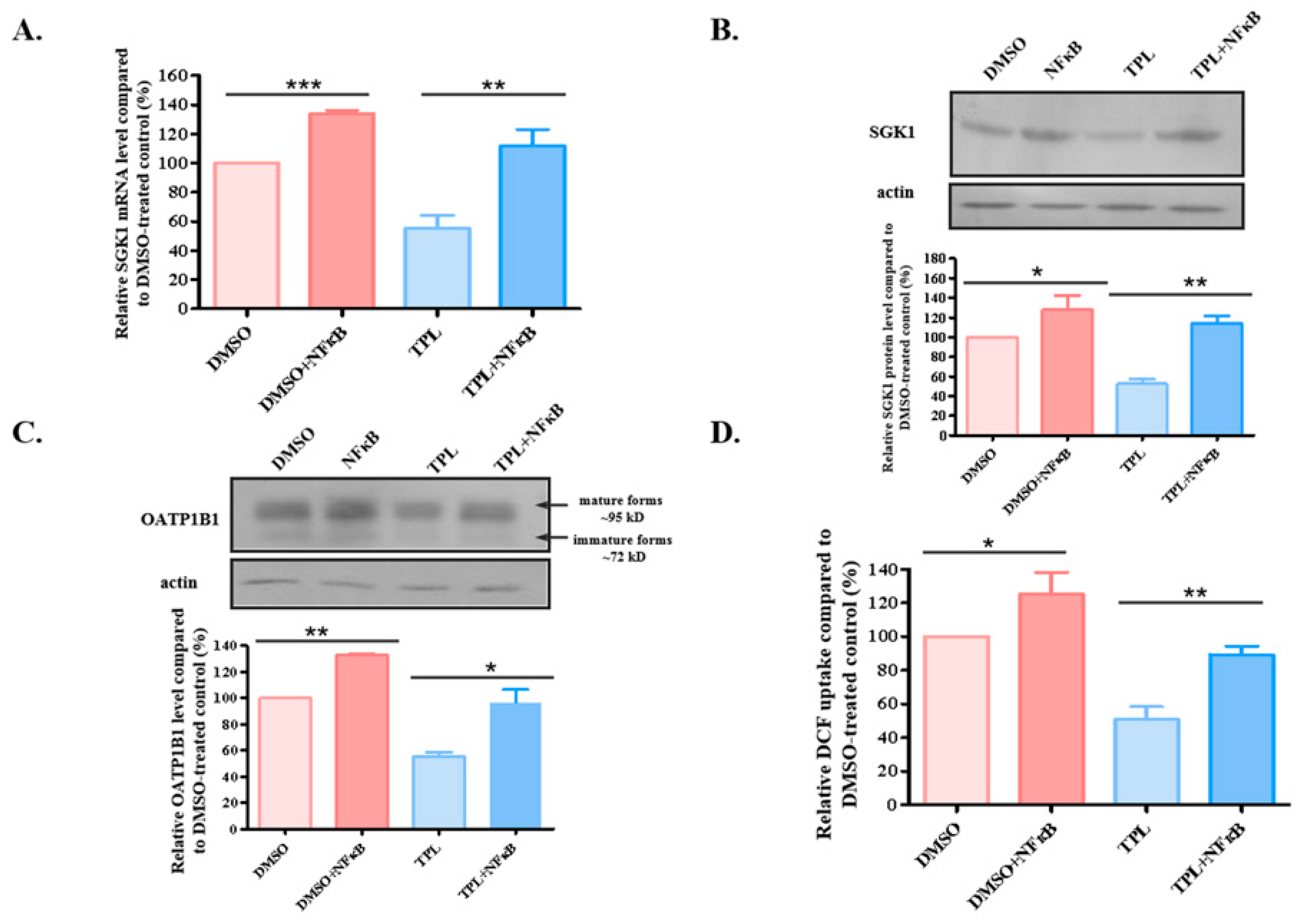
3.5. Overexpression of NFκB Attenuated the Effect of TPL on OATP1B1 Protein Expression and Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagenbuch, B.; Gui, C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 2008, 38, 778–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, M. Protein kinases and cross-talk between post-translational modifications in the regulation of drug transporters. Mol. Pharmacol. 2023, 103, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Crowe, A.; Wang, X.; Zhang, P.; Ding, K.; Li, L.; Yue, W. Regulation of organic anion transporting polypeptides (OATP) 1B1- and OATP1B3-mediated transport: An updated review in the context of OATP-mediated drug-drug interactions. Int. J. Mol. Sci. 2018, 19, 855. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biol. Pharm. Bull. 2015, 38, 155–168. [Google Scholar] [CrossRef]
- Wu, X.; Ma, J.; Ye, Y.; Lin, G. Transporter modulation by Chinese herbal medicines and its mediated pharmacokinetic herb-drug interactions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 236–253. [Google Scholar] [CrossRef]
- Köck, K.; Xie, Y.; Hawke, R.L.; Oberlies, N.H.; Brouwer, K.L. Interaction of silymarin flavonolignans with organic anion-transporting polypeptides. Drug Metab. Dispos. 2013, 41, 958–965. [Google Scholar] [CrossRef]
- Lynch, K.D.; Montonye, M.L.; Tian, D.D.; Arman, T.; Oyanna, V.O.; Bechtold, B.J.; Graf, T.N.; Oberlies, N.H.; Paine, M.F.; Clarke, J.D. Hepatic organic anion transporting polypeptides mediate disposition of milk thistle flavonolignans and pharmacokinetic silymarin-drug interactions. Phytother. Res. 2021, 35, 3286–3297. [Google Scholar] [CrossRef]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef]
- Qu, L.; Xiao, Y.; Jia, Z.X.; Wang, Z.; Wang, C.H.; Hu, T.; Wu, C.S.; Zhang, J.L. Comprehensive two-dimensional liquid chromatography coupled with quadrupole time-of-flight mass spectrometry for chemical constituents analysis of Tripterygium glycosides tablet. J. Chromatogr. A 2015, 1400, 65–73. [Google Scholar] [CrossRef]
- Chen, J.; Xue, Y.; Shuai, X.; Ni, C.; Fang, Z.; Ye, L.; Hong, M. Effect of major components of Tripterygium wilfordii Hook. f on the uptake function of organic anion transporting polypeptide 1B1. Toxicol. Appl. Pharmacol. 2022, 435, 115848. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, Y.; Zheng, Y. Therapeutic potential of triptolide in autoimmune diseases and strategies to reduce its toxicity. Chin. Med. 2021, 16, 114. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Wang, X.; Liu, Y.G. Robust one-tube Ω-PCR strategy accelerates precise sequence modification of plasmids for functional genomics. Plant Cell Physiol. 2013, 54, 634–642. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, W.; Wang, L.; Yi, J.; Chen, K.; Hong, M. Identification of a NF κ B Inhibition site on the proximal promoter region of human organic anion transporting polypeptide 1A2 coding gene SLCO1A2. Drug Metab. Dispos. 2018, 46, 643–651. [Google Scholar] [CrossRef]
- Dai, J.; Sun, Y.; Chen, D.; Zhang, Y.; Yan, L.; Li, X.; Wang, J. Negative regulation of PI3K/AKT/mTOR axis regulates fibroblast proliferation, apoptosis and autophagy play a vital role in triptolide-induced epidural fibrosis reduction. Eur. J. Pharmacol. 2019, 864, 172724. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, S.; Lai, J. Triptolide restrains the growth, invasion, stemness, and glycolysis of non-small cell lung cancer cells by PFKFB2-mediated PI3K/AKT pathway. Chem. Biol. Drug Des. 2024, 103, e14450. [Google Scholar] [CrossRef]
- Cristofano, A.D. SGK1: The Dark Side of PI3K Signaling. Curr. Top. Dev. Biol. 2017, 123, 49–71. [Google Scholar]
- Dai, Y.Q.; Jin, D.Z.; Zhu, X.Z.; Lei, D.L. Triptolide inhibits COX-2 expression via NF-kappa B pathway in astrocytes. Neurosci. Res. 2006, 55, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lee, J.Y.; Oh, D.H.; Park, S.Y.; Woo, T.M.; Kim, M.K.; Park, M.H.; Jang, Y.H.; Do, S.M. Triptolide-induced suppression of phospholipase D expression inhibits proliferation of MDA-MB-231 breast cancer cells. Exp. Mol. Med. 2009, 41, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Hou, Q.; Zhu, X.; Chen, Z.; Liu, Z. Triptolide attenuates proteinuria and podocyte apoptosis via inhibition of NF-kappaB/GADD45B. Sci. Rep. 2018, 8, 10843. [Google Scholar]
- König, J. Uptake transporters of the human OATP family: Molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 201, pp. 1–28. [Google Scholar]
- Ali, Y.; Shams, T.; Wang, K.; Cheng, Z.; Li, Y.; Shu, W.; Bao, X.; Zhu, L.; Murray, M.; Zhou, F. The involvement of human organic anion transporting polypeptides (OATPs) in drug-herb/food interactions. Chin. Med. 2020, 15, 71. [Google Scholar] [CrossRef]
- Belova, L.; Sharma, S.; Brickley, D.R.; Nivolstdrn, J.R.; Patterson, C.; Conzen, S.D. Ubiquitin-proteasome degradation of serum- and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP. Biochem. J. 2006, 400, 235–244. [Google Scholar] [CrossRef]
- Brickley, D.R.; Mikosz, C.A.; Hagan, C.R.; Conzen, S.D. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1). J. Biol. Chem. 2002, 277, 43064–43070. [Google Scholar] [CrossRef]
- Wang, H.; You, G. SGK1/Nedd4-2 signaling pathway regulates the activity of human organic anion transporters 3. Biopharm. Drug Dispos. 2017, 38, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wan, F.; Lian, H.; Lu, Z.; Li, X.; Cao, D.; Jiang, Y.; Li, J. Friend or foe? The dual role of triptolide in the liver, kidney, and heart. Biomed. Pharmacother. 2023, 161, 114470. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhang, Y.; Pan, J.; Chen, S.; Lu, S.; Shen, F.; Huang, Z. Triptolide dysregulates glucose uptake via inhibition of IKKβ-NF-κB pathway by p53 activation in cardiomyocytes. Toxicol. Lett. 2020, 318, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Nan, X.X.; Yang, J.R.; Liang, B.L.; Wei, Y.B.; Zhu, N.; Hu, S.B.; Zhang, H.; Luo, Y.; Xu, Y.F. Triptolide inhibits the multidrug resistance in prostate cancer cells via the downregulation of MDR1 expression. Neoplasma 2013, 60, 598–604. [Google Scholar] [CrossRef]
- Yi, J.M.; Huan, X.J.; Song, S.S.; Zhou, H.; Wang, Y.Q.; Miao, Z.H. Triptolide induces cell killing in multidrug-resistant tumor cells via CDK7/RPB1 rather than XPB or p44. Mol. Cancer Ther. 2016, 15, 1495–1503. [Google Scholar] [CrossRef]
- Lin, J.; Lin, B.; Song, H. Research progress on in vivo pharmacokinetics of triptolide and celastrol. Chin. Tradit. Herbal Drugs 2016, 47, 528–532. [Google Scholar]
| Fragments | Forward Primer | Reverse Primer |
|---|---|---|
| −3157 bp~−1950 bp | CAAGGAGTTGAGACTAGAGCAGC CGATGGAGACTGATAAC | GTTATCAGTCTCCATCGGCTGCT CTAGTCTCAACTCCTTG |
| −1950 bp~−720 bp | CGAGCTCTTACGCGTGCTAGCCAA GGAGTTGAGACTAGAGC | GCTCTAGTCTCAACTCCTTGGCT AGCACGCGTAAGAGCTCG |
| −720 bp~−260 bp | CGAGCTCTTACGCGTGCTAGCAG CGAGTCCTTCCTGCTGA | TCAGCAGGAAGGACTCGCTGCT AGCACGCGTAAGAGCTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Pan, C.; Chen, J.; Shuai, X.; Hong, M. Triptolide Affects the Function of Hepatocellular Drug Uptake Transporter Organic Anion Transporting Polypeptide 1B1 Through the Suppression of SGK1. Biology 2025, 14, 1618. https://doi.org/10.3390/biology14111618
Li Z, Pan C, Chen J, Shuai X, Hong M. Triptolide Affects the Function of Hepatocellular Drug Uptake Transporter Organic Anion Transporting Polypeptide 1B1 Through the Suppression of SGK1. Biology. 2025; 14(11):1618. https://doi.org/10.3390/biology14111618
Chicago/Turabian StyleLi, Zichong, Chaomin Pan, Jieru Chen, Xiaoyu Shuai, and Mei Hong. 2025. "Triptolide Affects the Function of Hepatocellular Drug Uptake Transporter Organic Anion Transporting Polypeptide 1B1 Through the Suppression of SGK1" Biology 14, no. 11: 1618. https://doi.org/10.3390/biology14111618
APA StyleLi, Z., Pan, C., Chen, J., Shuai, X., & Hong, M. (2025). Triptolide Affects the Function of Hepatocellular Drug Uptake Transporter Organic Anion Transporting Polypeptide 1B1 Through the Suppression of SGK1. Biology, 14(11), 1618. https://doi.org/10.3390/biology14111618








